Review of Eastern Adriatic Hydromedusae: Unravelling Two Centuries of Records
Abstract
1. Introduction
Historical Background and Recent Research
2. Methodology
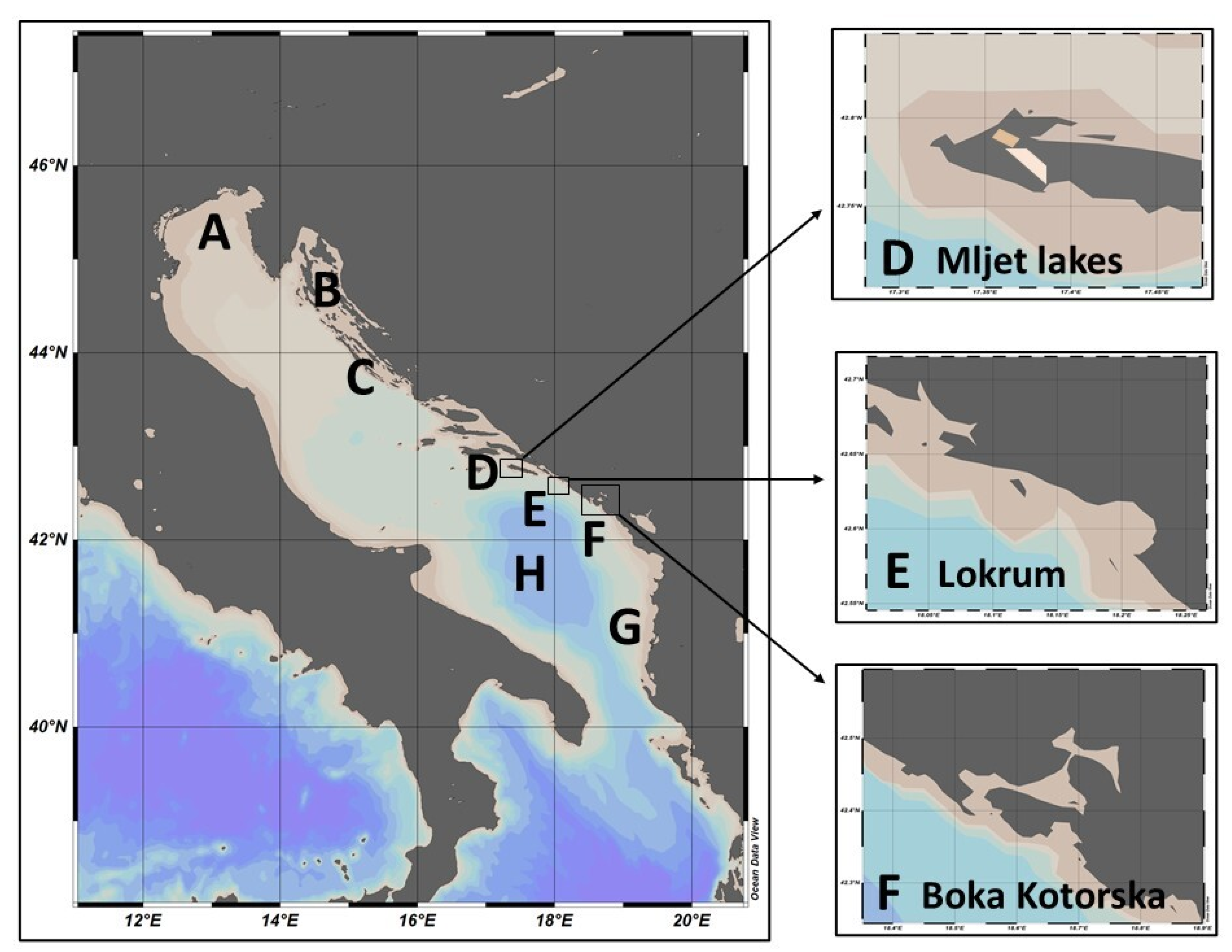
Study Area Eastern Adriatic Sea
- A.
- North Adriatic (NA)
- B.
- Kvarner and Velebit Channel
- C.
- Middle Adriatic
- D.
- South Station 100 (Lokrum) (≈42°37′ N, 18°07′ E)
- E.
- Mljet Lakes (≈42.78° N, 17.35° E)
- F.
- Boka Kotorska Bay
- G.
- Albania (coastal margin)
- H.
- South Adriatic Pit (Open South Pit)
3. Results and Discussion
3.1. Study Areas
3.1.1. Northern Adriatic (NA)
3.1.2. Kvarner and Velebit Channel
3.1.3. Middle Adriatic
3.1.4. South Station 100 (Lokrum)
3.1.5. Mljet Lakes
3.1.6. Boka Kotorska Bay
3.1.7. Albania (Coastal Margin)
3.1.8. South Adriatic Pit
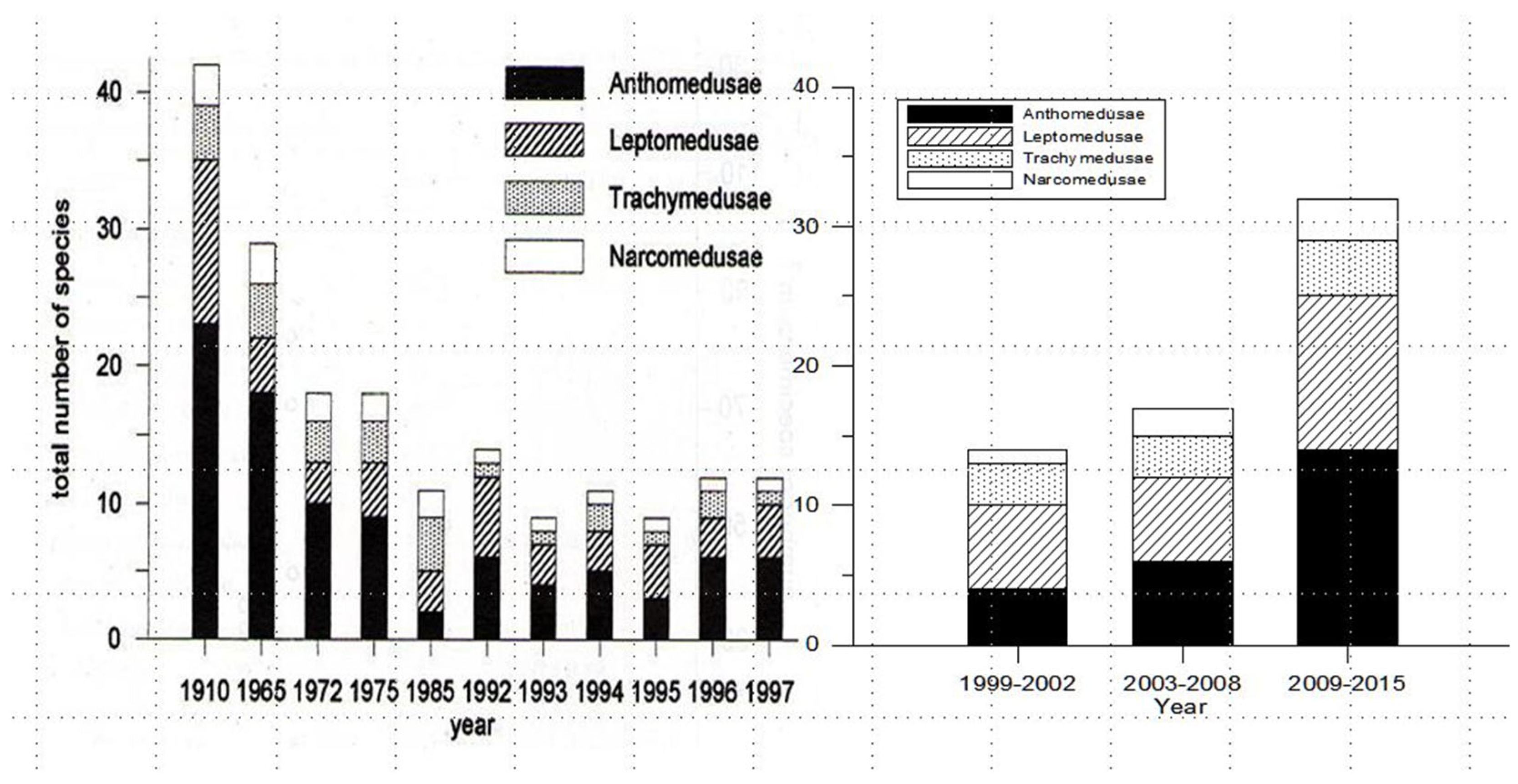
3.2. Bloom Phenomena
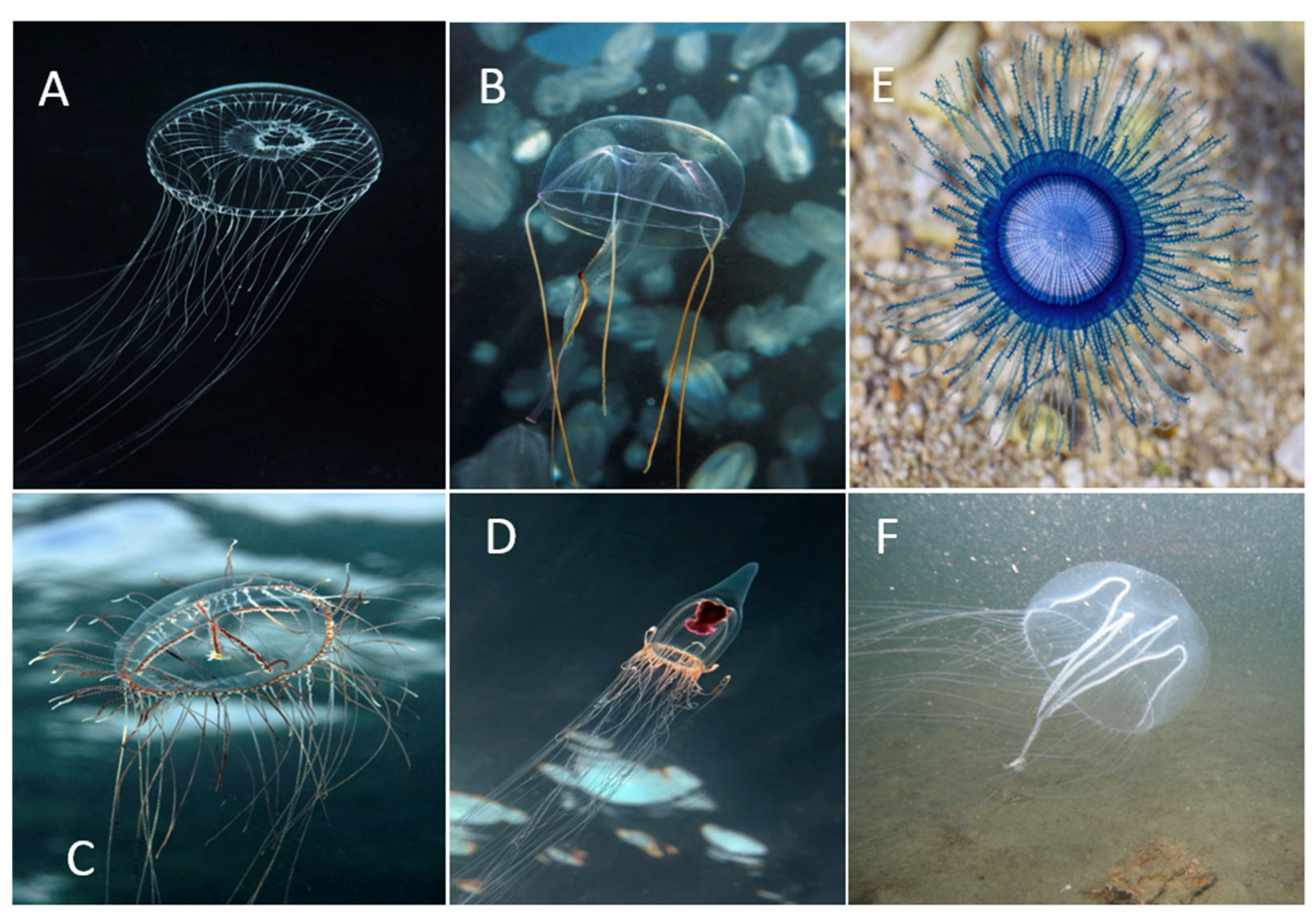
3.3. Larger Species Notes and Recent Bloom Events
3.3.1. Craspedacusta sowerbii Lankester, 1880
3.3.2. Aequorea forskalea Péron & Lesueur, 1810
3.3.3. Geryonia proboscidalis (Forsskål, 1775)
3.3.4. Olindias muelleri Haeckel, 1879
3.3.5. Neoturris pileata (Forsskål, 1775)
3.3.6. Porpita porpita (Linnaeus, 1758)
3.3.7. Neotima lucullana (Delle Chiaje, 1823)
3.4. Cryptic, Doubtful and Recently Introduced Species
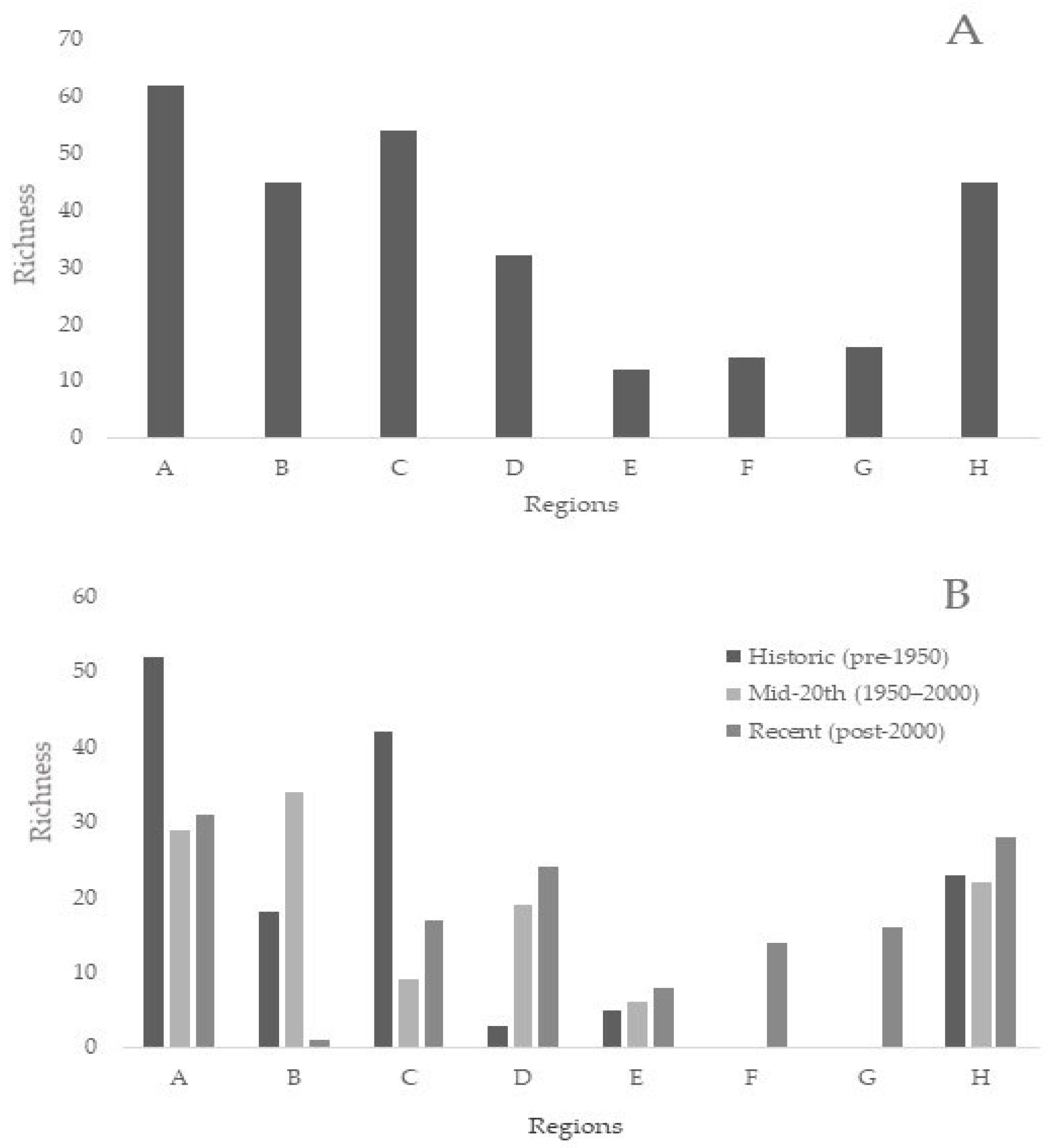
3.5. Biodiversity and Taxa Richness by Region Summarised
3.6. Methodological Constraints: Gear Selectivity and the “Recovery” Signal
4. Conclusions
- (i)
- Benthic polyp gap: The most conspicuous gap concerns the benthic polyp stage of meroplanktonic species. The decline of medusae has been inferred largely from their absence in plankton samples, yet long-term ecological studies of hydroid communities are virtually lacking. Priority should be given to mapping hydroid populations, assessing their condition and reproductive output, and experimentally determining their response to stressors such as hypoxia and temperature change. Such work is crucial for confirming mechanisms of decline, identifying refugia and evaluating recovery potential.
- (ii)
- Taxonomy identification challenge: accurate species identification, particularly for small or damaged specimens collected with traditional nets. Many historical and recent datasets aggregate taxa at the genus level (e.g., Obelia spp., Clytia spp.), which limits diagnostic and comparative value. Routine molecular integration, especially DNA barcoding, is therefore essential. Recent confirmations of B. triestina and N. lucullana demonstrate how genetic tools resolve taxonomic ambiguities, reveal cryptic diversity, and provide a stable framework for tracking biodiversity over time. Inconsistent nomenclature (e.g., Dipurena/Stauridiosarsia, Janiopsis/Merga) also requires revision. A clear example is Bougainvillia muscus in the Northern Adriatic: modern records report it only in 2009–2014, yet earlier occurrences were typically published as B.ramosa, B. autumnalis and B. nana now synonymized with B. muscus. Reconciling synonymy is therefore essential to avoid false novelty signals in long-term biodiversity assessments.
- (iii)
- Spatial and sampling gaps: significant structural gaps include the lack of post-1970s data from the Kvarner and Velebit Channel and sparse recent coverage of Boka Kotorska Bay and the Albanian margin leaving large parts of the historical assemblage unassessed under present-day conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BiOS | Bimodal Oscillating System |
| WoRMS | World Register of Marine Species |
| DVM | Diel Vertical Migration |
| WP-2 net | UNESCO Working Party 2 (WP-2) standard plankton net |
| VJ | Veliko Jezero (Mljet Island, Mljet National Park), a semi-enclosed marine lake |
| MJ | Malo Jezero (Mljet Island, Mljet National Park), the smaller inner marine lake |
| Syn | Synonym |
| NA | North Adriatic |
| CS | Citizen Science Record |
| NIS | Non-Indigenous Species |
| Mero | Meroplanktonic |
| Holo | Holoplanktonic |
| TQ | Taxon Inquirendum (Doubtful validity) |
| FW | Freshwater |
| PE | Putatively Extinct |
References
- Llorente-Vega, J.L.; Cedeño-Posso, C.; Quirós-Rodríguez, J.A. First Record of Two Leptothecata Medusae (Cnidaria, Hydrozoa) in Colombia with Annotations on Their Distribution and Ecology. Biodivers. Data J. 2025, 13, e138523. [Google Scholar] [CrossRef]
- Purcell, J.E. Predation on Fish Larvae and Eggs by the Hydromedusa Aequorea Victoria at a Herring Spawning Ground in British Columbia. Can. J. Fish. Aquat. Sci. 1989, 46, 1415–1427. [Google Scholar] [CrossRef]
- Graham, W.M.; Pagès, F.; Hamner, W.M. A Physical Context for Gelatinous Zooplankton Aggregations: A Review. Hydrobiologia 2001, 451, 199–212. [Google Scholar] [CrossRef]
- Purcell, J.; Uye, S.; Lo, W. Anthropogenic Causes of Jellyfish Blooms and Their Direct Consequences for Humans: A Review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Baxter, E.J.; Rodger, H.D.; McAllen, R.; Doyle, T.K. Gill disorders in marine-farmed salmon: Investigating the role of hydrozoan jellyfish. Aquacult. Environ. Interact. 2011, 1, 245–257. [Google Scholar] [CrossRef]
- Malej, A.; Lučić, D.; Bojanić, N.; Vodopivec, M.; Paliaga, P.; Pestorić, B.; Violić, I.; Supić, N. The Occurrence of the Jellyfish Aequorea cf. forskalea in the Adriatic Sea: Comparison of Historical and Recent Data. Acta Adriat. 2022, 63, 175–192. [Google Scholar] [CrossRef]
- Meech, R.W. Phylogenetics of Swimming Behaviour in Medusozoa: The Role of Giant Axons and Their Possible Evolutionary Origin. J. Exp. Biol. 2022, 225, jeb243382. [Google Scholar] [CrossRef] [PubMed]
- Pruski, S.; Miglietta, M.P. Fluctuation and Diversity of Hydromedusae (Hydrozoa, Cnidaria) in a Highly Productive Region of the Gulf of Mexico Inferred from High Frequency Plankton Sampling. PeerJ 2019, 7, e7848. [Google Scholar] [CrossRef] [PubMed]
- Condon, R.H.; Graham, W.M.; Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Haddock, S.H.D.; Sutherland, K.R.; Robin-son, K.L.; Dawson, M.N.; Decker, M.B.; et al. Questioning the Rise of Gelatinous Zooplankton in the World’s Oceans. BioScience 2012, 62, 160–169. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef]
- Licandro, P.; Blackett, M.; Fischer, A.; Hosia, A.; Kennedy, J.; Kirby, R.R.; Raab, K.; Stern, R.; Tranter, P. Biogeography of Jellyfish in the North Atlantic, by Traditional and Genomic Methods. Earth Syst. Sci. Data 2015, 7, 173–191. [Google Scholar] [CrossRef]
- Giachini Tosetto, E.; Neumann-Leitão, S.; Nogueira Júnior, M. Sampling Planktonic Cnidarians with Paired Nets: Implications of Mesh Size on Community Structure and Abundance. Estuar. Coast. Shelf Sci. 2019, 220, 48–53. [Google Scholar] [CrossRef]
- García-Barón, I.; Giakoumi, S.; Santos, M.B.; Granado, I.; Louzao, M. The Value of Time-Series Data for Conservation Planning. J. Appl. Ecol. 2021, 58, 608–619. [Google Scholar] [CrossRef]
- Tinta, T.; Klun, K.; Herndl, G.J. The Importance of Jellyfish–Microbe Interactions for Biogeochemical Cycles in the Ocean. Limnol. Oceanogr. 2021, 66, 2011–2032. [Google Scholar] [CrossRef]
- Pestorić, B.; Lučić, D.; Bojanić, N.; Vodopivec, M.; Kogovšek, T.; Violić, I.; Paliaga, P.; Malej, A. Scyphomedusae and Ctenophora of the Eastern Adriatic: Historical Overview and New Data. Diversity 2021, 13, 186. [Google Scholar] [CrossRef]
- Benović, A.; Lučić, D. Comparison of hydromedusae findings in the northern and southern Adriatic Sea. Sci. Mar. 1996, 60, 129–135. [Google Scholar]
- Will, J.G.F. Horae Tergestinae Oder Beschreibung Und Anatomie der im Herbste 1843 bei Triest Beobachteten Akalephen; Leopold Voss: Leipzig, Germany, 1844. [Google Scholar]
- Busch, W. Beobachtungen über Anatomie und Entwickelung Einiger Wirbellosen Seethiere; Abraham Hirschwald: Berlin, Germany, 1851. [Google Scholar]
- Claus, C. Studien Über Polypen Und Quallen Der Adria. Acalephen (Discomedusen). Denkschr. Akad. Wiss. Wien 1877, 38, 13–97. [Google Scholar]
- Claus, C. Grundzüge Der Zoologie. Zum Wissenschaftlichen Gebrauche, 4th ed.; Elwert: Marburg, Germany, 1880. [Google Scholar]
- Graeffe, E. Übersicht Der Seethierfauna Des Golfes von Triest. III. Colenteraten. Arb. Zool. Inst. Univ. Wien 1884, 5, 333–362. [Google Scholar]
- Metchnikoff, E. Embryologische Studien an Medusen: Ein Beitrag zur Genealogie der Primitiv-Organe; Alfred Hölder: Wien, Austria, 1886. [Google Scholar]
- Cori, C.J.; Steuer, A. Beobachtungen Über Das Plankton Des Triester Golfes in Den Jahren 1899 Und 1900. Zool. Anz. 1901, 24, 111–117. [Google Scholar]
- Steuer, A. Beobachtungen Über Das Plankton Des Triester Golfes Im Jahre 1901. Zool. Anz. 1902, 25, 369–372. [Google Scholar]
- Steuer, A. Beobachtungen Über Das Plankton Des Triester Golfes Im Jahre 1902. Zool. Anz. 1903, 27, 145–149. [Google Scholar]
- Mayer, A.G. Medusae of the World; Carnegie Institution of Washington: Washington, DC, USA, 1910. [Google Scholar]
- Neppi, V.; Stiasny, G. Die Hydromedusen Des Golfes von Triest. Arb. Zool. Inst. Univ. Wien 1913, 21, 23–92. [Google Scholar]
- Joseph, H. Ein Gonionemus Aus Der Adria. Sitzungsber. Kais. Akad. Wiss. Wien Math.-Naturwissenschaftliche Kl. 1918, 127, 95–158. [Google Scholar]
- Neppi, V. Adriatische Hydromedusen. Sitzungsber. Kais. Akad. Wiss. Wien. Math.-Naturwissenschaftliche Kl. 1912, 121, 709–734. [Google Scholar]
- Neppi, V. Meduse Adriatiche (Risultato dell’esame del materiale raccolto durante le crociere adriatiche delle nave Najade negli anni 1911–14). Mem. R. Com. Talass. Ital. 1922, 101, 1–38. [Google Scholar]
- Babnik, P. Hidromeduze Iz Srednjega in Juznega Jadrana v Letih 1939 in 1940. Acta Adriat. 1948, 3, 275–340. [Google Scholar]
- Pell, M. The hydromedusae of the Adriatic, collected by the “Najade”. Mat. Termész Ert. 1938, 57, 919–930. [Google Scholar]
- Car, L.; Hadži, J. Izveštaji o Naučnom Istraživanju Jadranskog Mora God. 1913 i 1914. Prir. Istraž. Hrvat. Slav. 1914, 2, 1–34. [Google Scholar]
- Babić, K. Planktonički Celenterati Iz Jadranskog Mora. Rad. Jazu Zagreb. 1913, 55, 186–202. [Google Scholar]
- Hadži, J. Ljetni Plankton Bakarskog Zaljeva (1918.) te Puljskog i Riječkog (1913.). Prir. Istraž. Kralj. Jugosl. 1930, 16, 172–192. [Google Scholar]
- Schmidt, H.-E.; Benović, A. Notes on Hydromedusae (Cnidaria) from the Adriatic Sea. J. Mar. Biol. Assoc. UK 1977, 57, 635–640. [Google Scholar] [CrossRef]
- Schmidt, H.E.; Benović, A. Cruises of R/V “Vila Velebita” in the Kvarner Region of the Adriatic Sea, XII.-Hydromedusae (Cnidaria). Thalass. Jugosl. 1979, 15, 193–202. [Google Scholar]
- Benović, A. Idromedusae Dell’ Adriatico Settentrionale Nell’ Anno 1965. Boll. Pesca Piscic. Idrobiol. 1973, 2128, 59–70. [Google Scholar]
- Benović, A.; Bender, A. Medusae from the Open Waters of the Adriatic Sea. Stud. Mar. 1986, 17–18, 55–74. [Google Scholar]
- Benović, A.; Bender, A. Seasonal Distribution of Medusae in the Adriatic Sea. In Modern Trends in the Systematics, Ecology and Evolution of Hydroids and Hydromedusae; Bouillon, J., Boero, F., Cicogna, F., Cornelius, P.F.S., Eds.; Oxford University Press: Oxford, UK, 1987; pp. 117–131. [Google Scholar]
- Malej, A. Biomasa in Struktura Zooplanktona Vzhodnega Dela Tržaškega Zaliva. Master’s Thesis, University of Ljubljana, Ljubljana, Slovenia, 1977. [Google Scholar]
- Benović, A.; Justić, D.; Bender, A. Enigmatic changes in the hydromedusan fauna of the Northern Adriatic Sea. Nature 1987, 326, 597–600. [Google Scholar] [CrossRef]
- Crema, R.; Castelli, A.; Prevedelli, D. Long Term Eutrophication Effects on Macrofaunal Communities in Northern Adriatic Sea. Mar. Pollut. Bull. 1991, 22, 503–508. [Google Scholar] [CrossRef]
- Benović, A.; Lučić, D.; Onofri, V. Does Change in an Adriatic Hydromedusan Fauna Indicate an Early Phase of Marine Ecosystem Destruction? Mar. Ecol. 2000, 21, 221–231. [Google Scholar] [CrossRef]
- Miloš, Č.; Malej, A. Gelatinous Zooplankton Assemblages in Temperate Coastal Waters Seasonal Variations (Gulf of Trieste, Adriatic Sea). Ann. Ser. Hist. Nat. 2005, 15, 11–20. [Google Scholar]
- Mozetič, P.; Solidoro, C.; Cossarini, G.; Socal, G.; Precali, R.; Francé, J.; Bianchi, F.; De Vittor, C.; Smodlaka, N.; Fonda Umani, S. Recent Trends Towards Oligotrophication of the Northern Adriatic: Evidence from Chlorophyll a Time Series. Estuaries Coasts 2010, 33, 362–375. [Google Scholar] [CrossRef]
- Cozzi, S.; Giani, M. River Water and Nutrient Discharges in the Northern Adriatic Sea: Current Importance and Long Term Changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent Changes in the Marine Ecosystems of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Onofri, I.; Hure, M.; Gangai Zovko, B.; Supić, N.; Ivančić, I.; Lučić, D. Repopulation of Hydromedusans in the Northern Adriatic Sea. In Proceedings of the 5th International Jellyfish Bloom Symposium, Barcelona, Spain, 30 May–3 June 2016. [Google Scholar]
- Pierson, J.; Camatti, E.; Hood, R.; Kogovšek, T.; Lučić, D.; Tirelli, V.; Malej, A. Mesozooplankton and Gelatinous Zooplankton in the Face of Environmental Stressors. In Coastal Ecosystems in Transition; American Geophysical Union (AGU): Washington, DC, USA, 2021; pp. 105–127. [Google Scholar] [CrossRef]
- Batistić, M.; Garić, R.; Molinero, J.C. Interannual Variations in Adriatic Sea Zooplankton Mirror Shifts in Circulation Regimes in the Ionian Sea. Clim. Res. 2014, 61, 231–240. [Google Scholar] [CrossRef]
- Benović, A.; Lučić, D.; Onofri, V.; Pehardia, M.; Carić, M.; Jasprica, N.; Bobanović-Čolić, S. Ecological Characteristics of the Mljet Islands Seawater Lakes (South Adriatic Sea) with Special Reference to Their Resident Populations of Medusae. Sci. Mar. 2000, 64, 197–206. [Google Scholar] [CrossRef]
- Benović, A. Diurnal Vertical Migration of Solmissus Albescens (Hydromedusae) in the Southern Adriatic. Mar. Biol. 1973, 18, 298–301. [Google Scholar] [CrossRef]
- Benović, A. Hydromedusae (Cnidaria) from Two Stations in the Southern Adriatic and Tyrrhenian Seas in the Year 1967–1968. Proc. Zool. Soc. Lond. Ser. I Mar. Ecol. 1976, 40, 1–10. [Google Scholar]
- Batistić, M.; Kršinić, F.; Jasprica, N.; Carić, M.; Viličić, D.; Lučić, D. Gelatinous Invertebrate Zooplankton of the South Adriatic: Species Composition and Vertical Distribution. J. Plankton Res. 2004, 26, 459–474. [Google Scholar] [CrossRef]
- Benović, A.; Lučić, D.; Onofri, V.; Batistić, M.; Njire, J. Bathymetric Distribution of Medusae in the open Waters of the Middle and South Adriatic Sea during Spring 2002. J. Plankton Res. 2005, 27, 79–89. [Google Scholar] [CrossRef]
- Lučić, D.; Benović, A.; Morović, M.; Batistić, M.; Onofri, I. Diel vertical migration of medusae in the open South-ern Adriatic Sea over a short time period (July 2003). Mar. Ecol. Evol. Persp. 2009, 30, 16–32. [Google Scholar] [CrossRef]
- Batistić, M.; Garić, R.; Morović, M. Changes in the Non-Crustacean Zooplankton Community in the Middle Adriatic Sea during the East Mediterranean Transient. Period. Biol. 2016, 118, 21–28. [Google Scholar] [CrossRef]
- Batistić, M.; Garić, R. The Case of Bougainvillia Triestina Hartlaub 1911 (Hydrozoa, Cnidaria): A 100-Year-Long Struggle for Recognition. Mar. Ecol. 2016, 37, 145–154. [Google Scholar] [CrossRef]
- Hure, M.; Lučić, D.; Gangai Zovko, B.; Onofri, I.; Violić, I. Dynamics of Mesozooplankton Along the Eastern Coast of the South Adriatic Sea. Ann. Ser. Hist. Nat. 2022, 32, 411–430. [Google Scholar]
- Vukanić, V. Note on Appearance of Scyphomedusa Chrysaora Hysoscella (Linné, 1766) at Bay of Kotor (Southern Adriatic). Nat. Montenegrina 2006, 5, 49–53. [Google Scholar]
- Pestorić, B.; Krpo-Ćetković, J.; Gangai Zovko, B.; Lučić, D. Pelagic Cnidarians in the Boka Kotorska Bay, Monte-Negro (South Adriatic). Acta Adriat. 2012, 52, 289–300. [Google Scholar]
- Miloslavić, M.; Lučić, D.; Njire, J.; Gangai Zovko, B.; Onofri, I.; Garić, R.; Žarić, M.; Osmani, F.; Pestorić, B.; Nikleka, E.; et al. Zooplankton composition and distribution across coastal and offshore waters off Albania (Southern Adriatic) in late spring. Acta Adriat. 2012, 53, 155–230. [Google Scholar]
- Belmonte, G.; Moscatello, S.; Hajdëri, E.; Vaglio, I.; Denitto, F. Composition and Spatial Distribution of Mesozooplankton along Confinement and Anthropogenic-Impact Gradients in the Gulf of Vlorë (Albania). J. Coast. Res. 2018, 34, 174–184. [Google Scholar] [CrossRef]
- Babić, K. Prilog Fauni Jadranskog Mora. Rad. Jazu Zagreb. 1910, 48, 207–235. [Google Scholar]
- Grobben, K. Adriatic Medusae Collected by S.M.S. “Najade”. Anz. Akad. Wiss. Wien 1915, 52, 2–5. [Google Scholar]
- WoRMS World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 23 November 2025).
- Batistić, M.; Jasprica, N.; Carić, M.; Lučić, D. Annual Cycle of the Gelatinous Invertebrate Zooplankton of the Eastern South Adriatic Coast (NE Mediterranean). J. Plankton Res. 2007, 29, 671–686. [Google Scholar] [CrossRef]
- Lučić, D.; Onofri, I.; Garić, R.; Violić, I.; Vranješ, M.; Zovko, B.G.; Jurinović, J.; Njire, J.; Hure, M. Ingression of the Hydromedusa Neotima lucullana (Delle Chiaje, 1822) into the Ecosystem of the Neretva River Estuary (South-Eastern Adriatic, Croatia. Acta Adriat. 2022, 63, 165–174. [Google Scholar] [CrossRef]
- Schuchert, P.; Cartwright, P.; Choong, H.; Galea, H. World Hydrozoa Database 2025. Available online: https://www.marinespecies.org/hydrozoa (accessed on 23 November 2025).
- Bouillon, J.; Medel, M.D.; Pagès, F.; Gili, J.M.; Boero, F.; Gravili, C. Fauna of the Mediterranean Hydrozoa. Sci. Mar. 2004, 68, 5–438. [Google Scholar] [CrossRef]
- Gravili, C.; Bevilacqua, S.; Terlizzi, A.; Boero, F. Missing species among Mediterranean non-Siphonophoran Hydrozoa. Biodivers. Conserv. 2015, 24, 1329–1357. [Google Scholar] [CrossRef]
- Gravili, C.; Vito, D.D.; Camillo, C.G.D.; Martell, L.; Piraino, S.; Boero, F. The Non-Siphonophoran Hydrozoa (Cnidaria) of Salento, Italy with Notes on Their Life-Cycles: An Illustrated Guide. Zootaxa 2015, 3908, 1–187. [Google Scholar] [CrossRef]
- Djakovac, T. Marked Reduction of Eutrophication Pressure in the Northeastern Adriatic in the Period 2000–2009. Estuar. Coast. Shelf Sci. 2012, 115, 25–32. [Google Scholar] [CrossRef]
- Djakovac, T.; Supić, N.; Bernardi Aubry, F.; Degobbis, D.; Giani, M. Mechanisms of Hypoxia Frequency Changes in the Northern Adriatic Sea during the Period 1972–2012. J. Mar. Syst. 2015, 141, 179–189. [Google Scholar] [CrossRef]
- Civitarese, G.; Gačić, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the Impact of the Bimodal Oscillating System (BiOS) on the Biogeochemistry and Biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Furlan, E.; Torresan, S.; Critto, A.; Lovato, T.; Solidoro, C.; Lazzari, P.; Marcomini, A. Cumulative Impact Index for the Adriatic Sea: Accounting for Interactions among Climate and Anthropogenic Pressures. Sci. Total Environ. 2019, 670, 379–397. [Google Scholar] [CrossRef]
- Brush, M.J.; Giani, M.; Totti, C.; Testa, J.M.; Faganeli, J.; Ogrinc, N.; Kemp, W.M.; Umani, S.F. Eutrophication, Harmful Algae, Oxygen Depletion, and Acidification. In Coastal Ecosystems in Transition; American Geophysical Union (AGU): Washington, DC, USA, 2021; pp. 75–104. [Google Scholar] [CrossRef]
- Fuks, D.; Ivančić, I.; Najdek, M.; Lučić, D.; Njire, J.; Godrijan, J.; Marić, D.; Šilović, T.; Paliaga, P.; Blažina, M.; et al. Changes in the Planktonic Community Structure Related to Trophic Conditions: The Case Study of the Northern Adriatic Sea. J. Mar. Syst. 2012, 96–97, 95–102. [Google Scholar] [CrossRef][Green Version]
- Boicourt, W.C.; Ličer, M.; Li, M.; Vodopivec, M.; Malačič, V. Sea State. In Coastal Ecosystems in Transition; American Geophysical Union (AGU): Washington, DC, USA, 2021; pp. 21–48. [Google Scholar] [CrossRef]
- Vilibić, I.; Zemunik, P.; Šepić, J.; Dunić, N.; Marzouk, O.; Mihanović, H.; Denamiel, C.; Precali, R.; Djakovac, T. Present Climate Trends and Variability in Thermohaline Properties of the Northern Adriatic Shelf. Ocean Sci. 2018, 15, 1351–1362. [Google Scholar] [CrossRef]
- Šantić, D.; Šestanović, S.; Šolić, M.; Krstulović, N.; Kušpilić, G.; Ordulj, M.; Gladan, Ž.N. Dynamics of pico-plankton community from coastal waters to the open sea in the Central Adriatic. Mediterr. Mar. Sci. 2013, 15, 179–188. [Google Scholar] [CrossRef]
- RAC/SPA-UNEP/MAP. Marine Biodiversity of Boka Kotorska Bay-Pilot Project on Testing Ecosystem Approach (EcAp) Application in Boka Kotorska Bay (Montenegro); Petovic, S., Batakovic, M., Eds.; RAC/SPA-MedMPAnet Project: Tunis, Tunisia, 2014. [Google Scholar]
- Campanelli, A.; Bulatovic, M.; Cabrini, F.; Grilli, Z.; Kljajic, R.; Mosetti, E.; Paschini, P.; Penna, P.; Marini, M. Spatial Distribution of Physical, Chemical and Biological Oceanographic Properties, Phytoplankton, Nutrients and Coloured Dissolved Organic Matter (CDOM) in the Boka Kotorska Bay (Adriatic Sea). Geofizika 2009, 29, 215–228. [Google Scholar]
- Vilibić, I.; Šepić, J.; Pasarić, M. The Adriatic Sea: A Long-Standing Laboratory for Sea Level Studies. Pure Appl. Geophys. 2017, 174, 3765–3811. [Google Scholar] [CrossRef]
- Gačić, M.; Civitarese, G.; Miserocchi, S.; Cardin, V.; Crise, A.; Mauri, E. The Open-Ocean Convection in the Southern Adriatic: A Controlling Mechanism of the Spring Phytoplankton Bloom. Cont. Shelf Res. 2002, 22, 1897–1908. [Google Scholar] [CrossRef]
- Manca, B.B.; Kovaĉević, V.; Gaĉić, M.; Viezzoli, D. Dense Water Formation in the Southern Adriatic Sea and Spreading into the Ionian Sea in the Period 1997–1999. J. Mar. Syst. 2002, 33–34, 133–154. [Google Scholar] [CrossRef]
- Krom, M.D.; Herut, B.; Mantoura, R.F.C. Nutrient Budget for the Eastern Mediterranean: Implications for Phosphorus Limitation. Limnol. Oceanogr. 2004, 49, 1582–1592. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera d’Alcalá, M.; Vaqué, D.; Zingone, A. Plankton in the Open Mediterranean Sea: A Review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef]
- Justić, D. Hypoxic Conditions in the Northern Adriatic Sea: Historical Development and Ecological Significance. In Modern and Ancient Continental Shelf Anoxia; Tyson, R.V., Pearson, T.H., Eds.; Geological Society Special Publication 58; Geological Society of London: London, UK, 1991; pp. 95–105. [Google Scholar] [CrossRef]
- Mozetič, P.; Francé, J.; Kogovšek, T.; Talaber, I.; Malej, A. Plankton Trends and Community Changes in a Coastal Sea (Northern Adriatic): Bottom-up vs. Top-down Control in Relation to Environmental Drivers. Estuar. Coast. Shelf Sci. 2012, 115, 138–148. [Google Scholar] [CrossRef]
- Genzano, G.N.; Mianzan, H.W.; Bouillon, J. Hydromedusae (Cnidaria: Hydrozoa) from the temperate southwestern Atlantic Ocean: A review. Zootaxa 2008, 1750, 1–18. [Google Scholar] [CrossRef]
- Lucas, C.H.; Williams, D.W.; Wiliams, J.A.; Sheader, M. Seasonal Dynamics and Production of the Hydromedusan Clytia hemisphaerica (Hydromedusa: Leptomedusa) in Southampton Water. Estuaries 1995, 18, 362–372. [Google Scholar] [CrossRef]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar] [CrossRef]
- Ramšak, A.; Ščančar, J.; Horvat, M. Evaluation of Metallothioneins in Blue Mussels (Mytilus galloprovincialis) as a Biomarker of Mercury and Cadmium Exposure in the Slovenian Waters (Gulf of Trieste): A Long-Term Field Study. Acta Adriat. 2012, 53, 71–86. [Google Scholar]
- Malej, A.; Turk, V.; Lučić, D.; Benović, A. Direct and Indirect Trophic Interactions of Aurelia sp. (Scyphozoa) in a Stratified Marine Environment (Mljet Lakes, Adriatic Sea). Mar. Biol. 2007, 151, 827–841. [Google Scholar] [CrossRef]
- Alvarez Colombo, G.; Benović, A.; Malej, A.; Lučić, D.; Makovec, T.; Onofri, V.; Acha, M.; Madirolas, A.; Mianzan, H. Acoustic Survey of a Jellyfish-Dominated Ecosystem (Mljet Island, Croatia). Hydrobiologia 2009, 616, 99–111. [Google Scholar] [CrossRef]
- Lučić, D.; Pestorić, B.; Malej, A.; Lopez-Lopez, L.; Drakulović, D.; Onofri, V.; Miloslavić, M.; Gangai, B.; Onofri, I.; Benović, A. Mass Occurrence of the Ctenophore Bolinopsis vitrea (L. Agassiz, 1860) in the nearshore northern Adriatic Sea (Kotor Bay, Montenegro). Environ. Monit. Assess. 2012, 184, 4777–4785. [Google Scholar] [CrossRef]
- Shiganova, T.A.; Dumont, H.J.; Mikaelyan, A.; Glazov, D.M.; Bulgakova, Y.V.; Musaeva, E.I.; Sorokin, P.Y.; Pautova, L.A.; Mirzoyan, Z.A.; Studenikina, E.I. Interactions between the Invading Ctenophores Mnemiopsis leidyi (A. Agassiz) and Beroe Ovata Mayer 1912, and Their Influence on the Pelagic Ecosystem of the Northeastern Black Sea. In Aquatic Invasions in the Black, Caspian, and Mediterranean Seas; Nato Science Series: IV: Earth and Environmental Sciences; Dumont, H., Shiganova, T.A., Niermann, U., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 35, pp. 33–70. [Google Scholar] [CrossRef]
- Boero, F.; Belmonte, G.; Bracale, R.; Fraschetti, S.; Piraino, S.; Zampardi, S. A Salp Bloom (Tunicata, Thaliacea) along the Apulian Coast and in the Otranto Channel between March–May 2013. F1000Research 2013, 2, 181. [Google Scholar] [CrossRef]
- Ciriaco, S.; Faresi, L.; Segarich, M.; Ciriaco, S.; Faresi, L.; Segarich, M. Observations on the Feeding of Drymonema Dalmatinum in the Gulf of Trieste. Diversity 2021, 13, 163. [Google Scholar] [CrossRef]
- Bouillon, J. Hydroméduses de La Mer de Bismarck (Papouasie, Nouvelle-Guinée). III. Anthomedusae Filifera (Hydrozoa-Cnidaria). Cah. Biol. Mar. 1980, 21, 307–344. [Google Scholar]
- Schuchert, P.; Schuchert, P. The European Athecate Hydroids and Their Medusae (Hydrozoa, Cnidaria): Capitata Part 2. Rev. Suisse Zool. 2010, 117, 337–555. [Google Scholar] [CrossRef]
- Šilović, T.; Ljubešić, Z.; Mihanović, H.; Olujić, G.; Terzić, S.; Jakšić, Ž.; Viličić, D. Picoplankton Composition Related to Thermohaline Circulation: The Albanian Boundary Zone (Southern Adriatic) in Late Spring. Estuar. Coast. Shelf Sci. 2011, 91, 519–525. [Google Scholar] [CrossRef]
- Viličić, D.; Šilović, T.; Kuzmić, M.; Mihanović, H.; Bosak, S.; Tomažić, I.; Olujić, G. Phytoplankton Distribution across the Southeast Adriatic Continental and Shelf Slope to the West of Albania (Spring Aspect). Environ. Monit. Assess. 2011, 177, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous Plankton: Irregularities Rule the World (Sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The Jellyfish Joyride: Causes, Consequences and Management Responses to a More Gelatinous Future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Violić, I.; Lučić, D.; Bojanić, N.; Pestorić, B.; Gangai Zovko, B.; Onofri, I.; Hure, M. Long-term Monitoring of Carnivorous Gelatinous Macrozooplankton in the Area of Dubrovnik-Neretva County (Croatia). Naše More 2022, 69, 22–29. [Google Scholar] [CrossRef]
- Aytan, Ü.; Aksu, İ.; Bektaş, Y. Recent occurrence of Olindias muelleri Haeckel, 1879 (Cnidaria, Hydrozoa, Limnomedusae, Olindiidae) in the Aegean Sea. Plankton Benthos Res. 2019, 14, 22–28. [Google Scholar] [CrossRef]
- Schuchert, P. Systematic Notes on Some Leptomedusa Species with a Description of Neotima galeai n. Spec. (Hydrozoa, Cnidaria). Rev. Suisse Zool. 2017, 124, 351–375. [Google Scholar] [CrossRef]
- Vodopivec, M.; Giordano, F.; Ličer, M.; Salon, S.; Querin, S. Hidden Heath: The Case of 2023 Gulf of Trieste Bot-Tom Marine Heatwave. In Proceedings of the OCS Meeting, Glasgow, UK, 22–27 February 2026. [Google Scholar]
| Species/Taxon (Authority) | Life Cycle | Historic (Pre-1950) | Mid-20th (1950–2000) | Recent (Post-2000) | Ecological Flags and Notes |
|---|---|---|---|---|---|
| ORDER ANTHOATHECATA (Anthomedusae) | |||||
| Mero | A, B, C, E | A, B, D, H | A, F, G | Syn. B. ramosa, B. autumnalis, B. nana |
| Mero | A, C | A, C | Re-established species [59] | |
| Mero | A, B, H | Syn. Rathkea fasciculata | ||
| Mero | D | NIS; Episodic advection due to propagulation | ||
| Mero | A, C | B | A, H, E | Reappeared NA record |
| Mero | A, B | No recent records | ||
| Mero | A | Single record, historic | ||
| Mero | B | NIS; Single record; Syn. Euphysora annulata | ||
| Mero | A, H, C, D | A, B, C | A, C, D, | Persistent NA taxon; Syn. Steenstrupia nutans |
| Mero | A, C | A, D | A, D, F | |
| Mero | A | A, D | TQ; Syn. S. producta | |
| Mero | A, C | A, B | Syn. Dipurena halterata | |
| Mero | A, C | A, B, C, D | A, F, G | Syn. Sarsia gemmifera |
| Mero | D | |||
| Mero | A, C | TQ | ||
| Mero | C | A | No recent records | |
| Mero | A | B | D | |
| Mero | D | D, G | ||
| Mero | A | B, C | D | Syn. P. hartlaubi, Hydractinia areolata |
| Mero | A, C | D | A, H | Reappeared NA taxon |
| Mero | C | Syn. Cytaeis exigua | ||
| Mero | A, B, H | A, D, F | Bloom; Syn. Podocoryne minima | |
| Mero | C, H | H | H | Oceanic; Middle/South Adriatic |
| Mero | A | D | Historically recorded as T. nutricula | |
| Mero | A, E | A, B | A, H | |
| Mero | H | DVM; Syn. Amphinema rubra | ||
| Mero | C | A, B, H | ||
| Mero | A, C | No recent records | ||
| Mero | G | Syn. Janiopsis costata | ||
| Mero | A, C, H, | A, B, C | Syn. Tiara tergestina | |
| Mero | A, B, C, H | A, B, H | A | CS; Syn. Turris coeca, Tiara pileata |
| Mero | B | Ref. [34] Senj (considered to be only record for the Adriatic Sea) | ||
| Mero | A | |||
| Holo | B | H | CS; Syn. Porpita umbella | |
| Mero | A, B, C | No recent records | ||
| Mero | C | PE ([72]; C = 49); TQ; Single record | ||
| Mero | A, H | A, B, | A, D, C | Bloom; Persistent NA taxon Syn. Podocoryna minuta |
| Mero | A | PE ([72]; C = 78) | ||
| Mero | A, C, H | D | ||
| Mero | B | NIS; Single finding | ||
| Mero | A | A | H | TQ |
| Mero | A | A, B, C | A | Bloom |
| Mero | A | A, B, E | A, C | |
| Mero | A, C, H | D | ||
| Mero | A, H | A, B, H | A, G, H, | Oceanic; DVM; Syn. Rhysomedusa pomponina |
| Holo | C | H | H | Deep |
| ORDER LEPTOTHECATA (Leptomedusae) | |||||
| Mero | A, B | A | Bloom; CS; Reappeared NA taxon | |
| Mero | C | Ref. [31] as Zygocanna sp. (single badly preserved specimen); PE ([72]; C = 100) | ||
| Mero | B | Single record | ||
| Mero | B | Single record | ||
| Mero | A, B, C, H | A, B, D, E, H | A, C, D, E, G, H | Bloom; Recurrent; Syn. Phialidium hemisphericum |
| Mero | A, E | Bloom | ||
| Mero | A, C | TQ | ||
| Mero | B, E | D, E, H | D, E, F, G | |
| Mero | A, B, C, E | D, E, H | C, D, E, F, G | Bloom |
| Mero | A, C | B, E | E, F, G | |
| Mero | A, B, C | A, B, H | A | |
| Mero | C, H | A, B, E | A, C, D, E, H | Syn. Eutima insignis, Saphenia gracilis |
| Mero | A | PE ([72]; C = 50) | ||
| Mero | A | A | A, D, F | Syn. H. schulzei/schulzii; Reconfirmed taxon in Mediterranean |
| Mero | A | E, C | Bloom; Mass occurrence Neretva; Syn. Tima lucullana (As Tima. Sp. In Mljet Lakes) | |
| Mero | C | C, B | A | TQ; New NA record Morphological ID |
| Mero | A, C, H | B, D | A, D, H | Reappeared NA taxon |
| Mero | H | PE ([72]; C = 100) | ||
| Mero | C | Single record, genus level | ||
| Mero | A, C | PE ([72]; C = 96) | ||
| Mero | B | Single record | ||
| Mero | C, H | H | DVM | |
| Mero | A | A | TQ; PE ([72]; C = 46); Records in North Adriatic after 2000 | |
| Mero | C | No recent records | ||
| Mero | B | Single record | ||
| Mero | A, H | H | ||
| Mero | H | PE ([72]; C = 83) | ||
| Mero | C | PE ([72]; C = 73); Single record | ||
| ORDER LIMNOMEDUSAE | |||||
| Holo | A, B | A, B, G, H | Bloom; CS; Stinging | |
| Holo | A, C, D, H | A, B, C, D, H | A, C, D, F, G, H | Oceanic dominant (previously counted in Trachymedusae) Syn. Liriope eurybia |
| Mero | A | C | FW; Bloom; Lake Kuti, Reka (entrance of Škocjan caves) | |
| Mero | A | Stinging; Single record as Gonionemus vindobonensis H. Joseph 1918 [28] (now treated as a synonym of G. vertens) | ||
| Mero | A, B, C | A | A, C, D, F | Bloom; CS; Stinging Previous records as Olindias phosphorica plausible after descriptions |
| ORDER NARCOMEDUSAE | |||||
| Holo | C, H | |||
| Holo | A | |||
| Holo | H | H | F, D, G, H | Deep; DVM; Mesopelagic |
| Holo | C | Rare | ||
| Holo | B, C, E | A | No recent records; Syn. Solmaris vanhoeffeni | |
| Holo | A, C, H | A, D, H | C, D, H | DVM |
| Holo | A, B | B, D, H | A | Bloom |
| Holo | A, B, H | B, D, H | D, F, G, H | Oceanic |
| ORDER TRACHYMEDUSAE | |||||
| Holo | H | Deep; First record 2002 | ||
| Holo | C | Deep; offshore Single record | ||
| Holo | C | Single record | ||
| Holo | B | H | Rare | |
| Holo | A, C, D, H | A, D, H | A, C, D, F, G, H | Oceanic dominant |
| Holo | H | PE ([72]; C = 69); Deep; Single Mediterranean record; Syn. Isonema najadis | ||
| Holo | H | Deep; South Adriatic | ||
| Holo | B, D, H | A, C, D, G, H | DVM; Oceanic; Newly in NA | |
| Holo | H | H | H | South Adriatic |
| Holo | A, B, C, H | A, B, D | A, C, D, F, G, H | Oceanic dominant |
| Holo | H | H | C, H | Oceanic |
| Subregion | Historic (Pre-1950) Mero | Historic Holo | Mid-20th (1950–2000) Mero | Mid-20th Holo | Recent (Post-2000) Mero | Recent Holo |
|---|---|---|---|---|---|---|
| A (North Adriatic) | 45 | 7 | 24 | 5 | 24 | 7 |
| B (Kvarner and Velebit Channel) | 12 | 6 | 28 | 6 | 0 | 1 |
| C (Middle Adriatic) | 34 | 8 | 7 | 2 | 10 | 7 |
| D (South Station 100 Lokrum) | 1 | 2 | 12 | 7 | 17 | 7 |
| E (Mljet Lakes) | 4 | 1 | 6 | 0 | 8 | 0 |
| F (Boka Kotorska Bay) | 0 | 0 | 0 | 0 | 9 | 5 |
| G (Albania) | 0 | 0 | 0 | 0 | 9 | 7 |
| H (South Adriatic Pit) | 14 | 9 | 12 | 10 | 12 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Onofri, I.; Lučić, D.; Malej, A.; Gangai Zovko, B. Review of Eastern Adriatic Hydromedusae: Unravelling Two Centuries of Records. J. Mar. Sci. Eng. 2026, 14, 288. https://doi.org/10.3390/jmse14030288
Onofri I, Lučić D, Malej A, Gangai Zovko B. Review of Eastern Adriatic Hydromedusae: Unravelling Two Centuries of Records. Journal of Marine Science and Engineering. 2026; 14(3):288. https://doi.org/10.3390/jmse14030288
Chicago/Turabian StyleOnofri, Ivona, Davor Lučić, Alenka Malej, and Barbara Gangai Zovko. 2026. "Review of Eastern Adriatic Hydromedusae: Unravelling Two Centuries of Records" Journal of Marine Science and Engineering 14, no. 3: 288. https://doi.org/10.3390/jmse14030288
APA StyleOnofri, I., Lučić, D., Malej, A., & Gangai Zovko, B. (2026). Review of Eastern Adriatic Hydromedusae: Unravelling Two Centuries of Records. Journal of Marine Science and Engineering, 14(3), 288. https://doi.org/10.3390/jmse14030288







