Abstract
Understanding escape behavior in cryptic and venomous reef fishes is critical for both ecological theory and public safety in coastal environments. We quantified the Flight Initiation Distance (FID) of 65 individual stonefish (Synanceia spp.) across four public beaches in Eilat, Israel, between March and May 2025. Initial Identification Distance (Initial ID) ranged from 0.5 to 3.5 m, whereas FID was consistently short (0.0–0.6 m), with 62% of individuals (n = 40) showing no flight response. Logistic regression revealed that the probability of fleeing was positively predicted by Alert behavior (p = 0.005), while Initial ID and site were not significant. Among individuals that did flee (n = 25), FID remained short and showed no significant spatial variation. A linear model confirmed Alert as the only positive predictor of FID (p = 0.045), while other variables were non-significant. These findings demonstrate that stonefish predominantly rely on crypsis and venom rather than active escape, resulting in minimal or absent flight responses. This lack of FID highlights their unique defensive strategy among reef fishes but also increases the risk of accidental human envenomation in areas of high recreational activity. Monitoring FID patterns may serve as a behavioral indicator of anthropogenic disturbance, while also informing conservation and public safety strategies in urban reef environments.
1. Introduction
Understanding the behavioral responses of marine organisms to human presence is increasingly critical for biodiversity conservation and the management of human–wildlife interactions in coastal ecosystems [1]. One widely applied behavioral metric is the Flight Initiation Distance (FID), which quantifies the distance at which an animal begins to flee from an approaching threat—typically a human observer [2,3]. FID serves as a proxy for an individual’s risk assessment and tolerance to disturbance, offering insight into species’ antipredator strategies and the impact of human encroachment [4,5].
Numerous intrinsic and extrinsic factors influence FID, including body size, group size, escape strategy, prior experience, predator speed, and distance to refuge [6,7]. Larger-bodied animals often show greater FID, though group dynamics can modulate this; some taxa tolerate closer approaches in larger groups, while others exhibit the opposite pattern [6]. Morphological traits such as armor and camouflage are associated with reduced FID, presumably because these defenses reduce perceived vulnerability [1]. However, this is not always consistent: physiological traits such as body temperature in reptiles show mixed or negligible effects on FID [6]. Risk assessment is dynamic and context-dependent—approach speed and starting distance strongly affect response in birds, mammals [8] and fish [9].
Recent studies further highlight that anthropogenic factors—especially tourism—can shape wildlife responses. For example, during the COVID-19 pandemic, Nubian ibex (Capra nubiana) in the Eilat mountains exhibited altered escape behaviors in response to face masks, illustrating the sensitivity even in habituated wildlife [10]. In marine systems, where animals may be exposed to repeated non-lethal encounters from snorkelers and divers, FID provides a valuable, non-invasive means to assess behavioral plasticity and potential human impact [11].
Among marine species, few are as ecologically enigmatic or potentially dangerous as the stonefish (Synanceia spp.), which includes some of the most venomous fishes known to science [12]. Stonefish are cryptic, benthic ambush predators that rely on camouflage and venom rather than flight for defense [13]. Despite their sedentary nature, these fish may exhibit flight behaviors under sustained disturbance or when concealment fails. Their dual defense strategy—camouflage and venom—makes them compelling but underexplored models for examining FID in cryptic, dangerous reef species [14]. The presence of these fish in coral reef systems frequented by recreational swimmers poses both ecological and public health concerns.
The Gulf of Eilat (Aqaba), located at the northern tip of the Red Sea, is a globally recognized coral reef system and a hotspot for human marine recreation. Its Israeli section includes multiple urban beaches with varying degrees of anthropogenic pressure, ranging from intensively used tourist hubs like Royal Beach and Aqua Sport to quieter research-oriented or semi-protected sites such as Migdal Or and KATZA. This natural gradient of human activity makes the region an ideal laboratory for evaluating spatial variation in FID and understanding how cryptic venomous fish, like stonefish, respond to different levels of human disturbance.
Stonefish venom is evolutionarily adapted for deterrence and defense. Delivered via robust dorsal fin spines, the venom contains a complex cocktail of proteins, including verrucotoxin—a potent neurotoxin that can cause neuromuscular paralysis and, in severe cases, death [15,16]. Envenomation leads to excruciating pain, edema, cardiovascular effects, and systemic shock [17]. The extreme danger posed by stonefish underscores the necessity for both ecological monitoring and public education in reef regions.
Despite their biological significance and hazard potential, stonefish remain largely absent from behavioral studies of escape responses. A systematic review of 33 studies on reef fish escape behavior found that FID was the most commonly measured metric; however, research focused overwhelmingly on conspicuous taxa such as wrasses (family Labridae), with minimal data on cryptic or venomous fishes like Synanceia [9]. The few existing studies on cryptic reef species note that camouflage and venom generally reduce the reliance on rapid flight, resulting in shorter FIDs [1,12]. Nevertheless, these works typically address theoretical frameworks rather than empirical measurements. A recent experimental study in freshwater systems measured FID in cryptic species such as Crenuchus spilurus, but the absence of venom and the ecological differences from reef systems limit the relevance to stonefish [18]. This gap highlights the urgent need for species-specific field data.
Quantifying FID in stonefish is therefore valuable not only for understanding behavioral ecology but also for informing marine management. FID variation may serve as a sensitive, non-invasive indicator of human pressure and habitat disruption [19]. Moreover, identifying where stonefish shift from cryptic immobility to escape behavior may assist in public safety protocols, particularly in densely visited reef zones.
This study was conducted in Eilat, Israel, because the northern Gulf of Aqaba represents a unique convergence of ecological importance and intense human use. The region harbors exceptionally high coral reef biodiversity within a relatively narrow coastal zone [20], while also being a major hub for tourism, diving, and recreational activities [21]. These overlapping ecological and anthropogenic factors create conditions in which interactions between venomous cryptic species, such as stonefish, and humans are both frequent and consequential. Thus, Eilat is an ideal natural laboratory for investigating variation in Flight Initiation Distance (FID) under gradients of human presence, with findings relevant to both conservation and public safety.
This study aims to quantify and analyze the spatial variation in FID of stonefish across four beaches in Eilat and contextualize these patterns in relation to human disturbance levels. By integrating behavioral ecology with conservation and public safety concerns, the research contributes to a broader understanding of how cryptic, dangerous reef species respond to growing anthropogenic pressures.
2. Methods
2.1. Study Species
This study focused on two species in the family Synanceiidae: the reef stonefish (Synanceia verrucosa) and the Red Sea stonefish (Synanceia nana). Both are found in the Gulf of Eilat and along the Red Sea coast of Israel [22,23]. The reef stonefish is widely distributed across the Indo-Pacific, including the Red Sea, and typically occupies coral reefs, rubble flats, lagoons, and tide pools up to 30 m deep. The Red Sea stonefish, by contrast, is endemic to the northwestern Indian Ocean, restricted to the Red Sea and the Persian Gulf, and inhabits coral and rocky reefs at depths of 3.5–18 m.
In situ species identification was based on visible morphological traits such as coloration, skin texture, and body size [24]. However, given the challenge of reliably distinguishing the two species, based on relative body size (length 24–40 cm; https://www.fishbase.org/summary/5825, accessed on 1 February 2025) and dorsal fin morphology, during underwater surveys and their overlapping ecology and habitat preferences, individuals were treated as a single operational taxonomic unit in data analysis (Figure 1).

Figure 1.
A stonefish on the coral reef at Eilat, Israel. Photo Tom Younger.
Both species were observed using standardized underwater visual census techniques (search image [25]). Experienced divers approached each fish slowly to minimize disturbance and recorded the distance at which the fish initiated escape behavior [26,27].
None of the studied individuals were handled, harmed, or marked for individual identification. Distances were measured using a calibrated measuring stick, which featured color-coded markings: the proximal third was marked in 0.5 m intervals, while the distal two-thirds—closest to the fish—were marked with 10 cm wide color bands to enable precise distance estimation underwater.
2.2. Study Beaches
We compared stonefish FID across four popular beaches in Eilat: Royal Beach, Aqua Sport, Migdal Or (Lighthouse), and KATZA (also known as EAPC—Europe-Asia Pipeline Company Ltd., Ashkelon, Israel). These sites differ not only in their proximity to urban infrastructure but also in the frequency and type of human activity. Royal Beach and Aqua Sport are among the most frequented tourist areas, characterized by high snorkeler density, water sports, and close proximity to hotels. Migdal Or and KATZA, in contrast, are relatively quieter, with KATZA serving as a semi-protected area due to its use by researchers and marine guides [28]. These distinctions provide a natural gradient of human disturbance, which allowed us to evaluate the variation in FID observed in the stonefish populations surveyed.
The FID parameters we evaluated included: (1) the Initial Identification Distance (the distance at which the observer first detected the fish), (2) the behavioral response to the observer’s approach, quantified as an Alert score on a 0–1 scale (0 = no visible change in posture, fin, or eye movement; 1 = clear and rapid postural changes or fin responses), and (3) the outcome of the interaction, i.e., whether the stonefish actively fled or remained unresponsive to the observer’s presence in proximity.
2.3. Statistical Analyses
We carried out all analyses in R 4.5.1 using two-sided tests with a 0.05 significance level. Because many fish did not flee at all (FID = 0), we used a two-step strategy that matches the biology of the response.
First, we modeled whether a fish fled (FID > 0) using a binomial GLM (logit link). Predictors were Initial Identification Distance (Initial ID), Alert, and beach (site). This estimates the effects of Initial ID and Alert and checks for differences among sites. We report Wald z tests and Tjur’s R2.
Second, for fish that fled, we modeled FID on the log scale with the same predictors (linear model of log (FID)). This lets us interpret effects as percentage changes. We then converted predictions back to meters for summaries and plots. For the positive-distance model we report coefficient tests (t tests for the log-scale linear model), model R2 and ad-adjusted R2 for the log-scale linear model, and in all cases summarize predictive accuracy on the meter scale using mean absolute error (MAE) and R2 computed from back-transformed predictions. We also tested the association between Initial ID and Alert using Spearman’s rho (ρ). For site-level proportions of fish that fled, we report Wilson 95% confidence intervals. Zeros were genuine no-flight events, so a two-part model is more appropriate than zero-inflated count models.
To examine temporal stability over the March–May sampling period, we added sur-vey day as a simple time index to the flight/no-flight model and to the log(FID) model (for fleeing fish only) and tested whether trends differed from zero. We report effect estimates with two-sided p-values and 95% confidence intervals. For site-level proportions of fish that fled we show Wilson 95% intervals. Next, for the flight/no-flight model we give coefficient tests (Wald z), an likelihood-ratio test for the site term, and Tjur’s R2 as a measure of discrimination; for the distance model on log(FID) we report coefficient tests (t), model R2, adjusted R2, and the overall F-test, and we summarize predictive accuracy on the original meter scale using mean absolute error (MAE) (with variance explained on that scale calculated from back-transformed predictions).
3. Results
We analyzed 65 stonefish encounters across four beaches in Eilat (Royal Beach = 24, Mig-dal Or = 19, KATZA = 13, Aqua Sport = 9). Initial Identification Distance (Initial ID) ranged from 0.5 to 3.5 m. Flight Initiation Distance (FID) ranged from 0.0 to 0.6 m, with many no-flight events (n = 40, FID = 0). Initial ID and Alert were not correlated (Spearman ρ = 0.058, p = 0.646; Figure S1).
In the binomial model for probability of flight (flee ~ Initial ID + Alert + site), Alert in-creased the chance of flight (z = 2.803, p = 0.005). Initial ID was not significant (z = −1.806, p = 0.071). Sites did not differ when comparing them at the same values of Initial ID and Alert (relative to Aqua Sport: KATZA z = −0.692, p = 0.489; Migdal Or z = −1.313, p = 0.189; Royal Beach z = −1.214, p = 0.226). Model discrimination was high (Tjur’s R2 = 0.849). The fraction of fish that fled (FID > 0) was broadly similar among sites (Figure 2A). In a logistic regression of the probability of fleeing (FID ~ Initial ID + Alert + site), Alert was a positive predictor (z = 2.803, p = 0.005), Initial ID was not significant (z = −1.806, p = 0.071), as well as site terms were also not significant relative to Aqua Sport (KATZA: z = −0.692, p = 0.489; Migdal Or: z = −1.313, p = 0.189; Royal Beach: z = −1.214, p = 0.226); model discrimination was high (Tjur’s R2 = 0.849). Among individuals that fled (n = 25), observed FID values were short at all sites (0.1–0.6 m; Figure 2B).
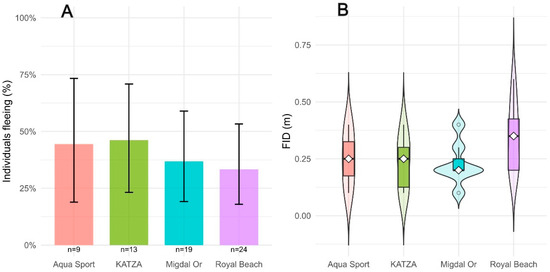
Figure 2.
Stonefish escape responses, (A) Proportion of individuals that initiated flight (FID > 0) at each beach; bars show percent fleeing with Wilson 95% confidence intervals, (B) Flight-initiation distance (FID, m) by beach. Plot shows the full distribution (kernel density; wider = more observations) with overlaid boxplots (interquartile range) and a marker for the median.
In the second model for fish that fled (n = 25), FID values were short at all sites (0.1–0.6 m; Figure 2B). In the log-scale linear model (log(FID) ~ Initial ID + Alert + site), Alert was a positive predictor of distance (β = 1.200 ± 0.558, t = 2.148, p = 0.045). Initial ID was not significant (β = 0.122 ± 0.197, t = 0.616, p = 0.545), and site effects were also not significant (KAT-ZA: p = 0.387; Migdal Or: p = 0.925; Royal Beach: p = 0.342). Overall fit on the log scale was R2 = 0.482 (adjusted R2 = 0.346; F(5,19) = 3.541, p = 0.0198). Back-transformed predictions had MAE = 0.065 m and explained 57% of variance on the meter scale (R2 = 0.570; Figure 3).
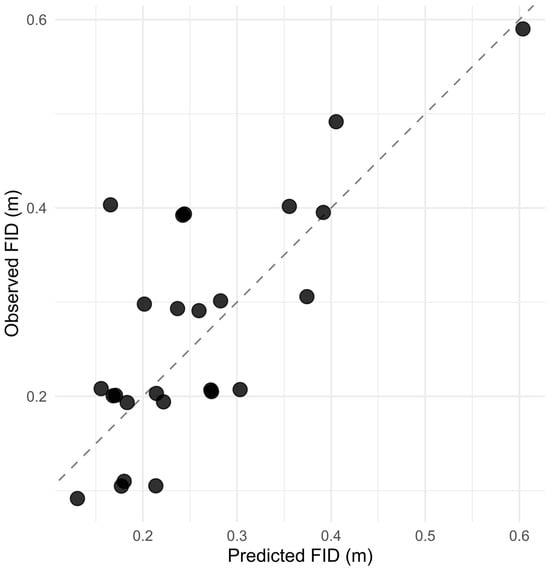
Figure 3.
Observed vs. predicted flight-initiation distance (FID) for stonefish. Each point represents an individual fish; the dashed line marks perfect agreement between prediction and observation. Predictions come from the conditional model for FID > 0 using Initial ID, Alert, and site.
4. Discussion
This study provides new insights into the escape behavior of reef stonefish in a heavily visited reef system. By modeling Flight Initiation Distance (FID) based on Initial Detection Distance (Initial ID) and behavioral alertness, we not only quantified key predictors of antipredator behavior but also examined spatial and temporal consistency across ecologically distinct beaches in Eilat, Israel.
The remarkably scarce Flight Initiation Distance (FID) observed in stonefish—often ranging between 0.0 and 0.6 m, with 40 out of 65 individuals showing no flight at all—suggests a behavioral ecology shaped by their potent venom, cryptic lifestyle, and the trade-offs predicted by optimal escape theory [29].
Interestingly, stonefish FID was not significantly affected by beach type, despite variation in human activity and habitat structure. This may be explained by their reliance on crypsis and venom, which likely reduce sensitivity to external environmental factors, and by threshold-based escape behavior, where flight is triggered only by proximate threats [2,30]. Moreover, if predators or disturbances are relatively uniform across sites, behavioral responses may remain consistent [31]. These findings suggest that for cryptic, venomous, solitary species, intrinsic defenses can outweigh external environmental cues in shaping antipredator behavior, contrasting with social, non-toxic species whose escape responses are more context-dependent.
Stonefish are among the most venomous fish, possessing robust dorsal spines that deliver a lethal cocktail of neurotoxins and cytotoxins, including verrucotoxin, which can cause paralysis and even mortality in predators or humans [15,16]. Such highly effective chemical deterrence fundamentally alters the cost–benefit dynamics of fleeing: with an assured and powerful defense already in place, the necessity—and thus frequency—of initiating flight is significantly reduced. The sedentary and cryptic nature of both species was consistent with prior descriptions of their camouflage-and-ambush predation strategy and their reliance on venom and camouflage for defense rather than rapid escape [1,11].
Stonefish are also exceptionally cryptic (Figure 1), blending into coral rubble and sandy substrates. Camouflage provides strong protection by reducing the likelihood of detection, which in turn lowers the need for escape. Similar patterns have been reported in cryptic reef fishes, where structural complexity and coloration allow fish to minimize FID [30]. For stonefish, crypsis and venom work synergistically, leading to a consistent strategy of immobility unless direct disturbance occurs.
Optimal escape theory [2] predicts that animals should flee when the costs of escape outweigh the costs of remaining in place. For stonefish, however, fleeing may incur higher costs—including the energetic expenditure, loss of camouflage, and exposure to other threats—than remaining immobile. Given their potent venom and cryptic morphology, flight is rarely initiated, which explains the dominance of zero FID in our dataset. Notably, the lack of strong correlation between Initial Identification Distance and Alert further supports the idea that flight is a last-resort option in this species.
Studies on FID in venomous fishes are quite limited, but research on reef fishes reveals that their responses to predators are adaptable. These responses are significantly shaped by factors like body structure, human interference, and the type of habitat they occupy. Meta-analyses indicate that fish with effective defense mechanisms or those that inhabit complex environments tend to have shorter FIDs when compared to more vulnerable or noticeable species [6,30]. Our findings regarding stonefish expand on this understanding, emphasizing how their physical characteristics and chemical defenses allow them to rely less on fleeing.
The remarkably short FIDs recorded in stonefish during our study (ranging from 0.0 to 0.6 m, with most showing no flight response) stand in stark contrast to what is generally observed in many other reef fish. Usually, for various reef-associated species, FIDs are much longer, often over 1 m, and vary greatly depending on the surrounding ecological conditions and levels of human disturbance. For example, a comprehensive review found that the FID for reef fishes typically falls between 1 and 3 m, especially in areas with heavy fishing and snorkeling activities [31]. Januchowski-Hartley et al. [32] also found that mesopredatory reef fishes in Papua New Guinea had average FIDs of 1.5 to 2.0 m, with larger fish fleeing further from predators. Later work by these researchers indicated that fish subjected to spearfishing exhibited even longer FIDs compared to those in protected environments [33].
Experimental studies have confirmed these trends. For instance, Goetze et al. [34] reported that mesopredatory reef fishes reacted to the approach of snorkelers and shark models with average FIDs of about 1.4 to 1.5 m, showcasing the impact of perceived threat levels. Habitat complexity plays an important role too, as Nunes et al. [30] found that labrid reef fishes had FIDs ranging between 1 and 3 m, influenced by the availability of refuges—more complex habitats allowed for shorter escape distances. These insights reinforce the notion that most conspicuous reef fishes tend to use early flight strategies when they perceive potential threats.
In contrast, the significantly shorter FID of stonefish indicates a distinctive strategy for avoiding predators, which combines extreme camouflage with their venomous defenses. Unlike more mobile reef fish, stonefish remain mostly still and blend in with their surroundings to avoid being noticed, while their potent dorsal spines serve as a deterrent against predators [15]. This approach lessens their energy costs related to fleeing, reduces the risk of exposure to predators during movement, and highlights an evolutionary trade-off between fleeing and defending. However, the very characteristics that help them evade predation also pose increased risks to humans, as stonefish can tolerate close approaches without fleeing unless they are nearly touched. In this sense, stonefish occupy a unique position on the spectrum of antipredatory behaviors within reef ecosystems, contrasting sharply with the quicker escape reactions seen in other reef species.
When comparing FIDs, it is clear that stonefish exhibit significantly smaller distances compared to other brightly colored, non-toxic reef species. However, such comparisons must take into account the ecological and behavioral contexts surrounding each species.
Ecological Context and Group Behavior—Stonefish are solitary, cryptic predators that depend on camouflage and their venomous spines for protection. Living alone means they bear individual risks of predation, which could contribute to their shorter FIDs as a means of staying hidden [2].
On the other hand, many brightly colored reef fish, like damselfishes (Pomacentridae) and wrasses (Labridae), often exist in shoals. This group living offers various advantages, including collective awareness and the dilution effect, which decreases individual chances of being preyed upon [35,36]. These species usually form schools, which enhance predator detection through teamwork and reduce individual risk as group size increases. Consequently, they often display longer FIDs, as their strategies balance the benefits of group living with the necessity of evading predators.
Assessing Personal Risk and Adjusting FID Comparisons—When comparing the FIDs of solitary versus group-living species, it is crucial to adjust for the varying levels of perceived personal risk [27]. One way to do this is by normalizing FID according to group size; larger groups typically face less individual threat, allowing for a fairer comparison of FIDs among species with different social structures.
Empirical Evidence and Limitations—While studies have documented FID in various fish species, including reef fish, the influence of group size on FID has shown mixed results. For instance, Sbragaglia et al. [37] found weak evidence linking group size to FID in response to underwater human presence, suggesting that other factors may also play significant roles in determining FID. Hence, despite documentation of FIDs across various fish species, including reef fish, there remain limitations in the research surrounding these distance responses, preventing a full understanding of the factors that influence FID in these species.
In our study on the escape behavior of stonefish, we uncovered some interesting findings; however, we also recognize a few limitations. Firstly, our sample size of 65 was relatively small and limited to just four beaches in Eilat, which restricts the applicability of our results to the wider Red Sea region. Additionally, we did not distinguish between the species Synanceia verrucosa and S. nana in situ, which might obscure any interspecific differences in escape behavior. The observed individuals were neither measured for size nor individually marked for identification. Environmental factors, including microhabitats such as substrate type, wave action, and time of day—known to influence predator-prey interactions—were also not considered in our models [38]. Lastly, the presence of observers, despite our attempts to maintain consistency in the approach, may have inadvertently influenced the behavior of the fish, particularly those accustomed to frequent human interaction in popular recreational areas.
Our research has important implications for conservation management and public safety in the Gulf of Eilat/Aqaba:
Public Health and Tourism Safety—The extremely low fish avoidance distance (FID) indicates a heightened risk of accidental encounters between humans and stonefish, especially in popular swimming and snorkeling areas. Implementing public awareness campaigns and placing informative signage about the stonefish’s cryptic nature can help reduce the risk of envenomations. Additionally, training for lifeguards and dive operators could be beneficial in managing these risks [17].
Indicator of Anthropogenic Disturbance—The stonefish’s FID may serve as a useful indicator of anthropogenic impacts. In general, fish in reef environments tend to exhibit higher FIDs in response to increased fishing pressure and disturbances. The consistently low FID observed in stonefish may indicate their resilience, attributed to their venomous nature and cryptic behavior. However, any noticeable alterations in FID over time might reflect changes in their risk perception due to escalating human activities.
Ecosystem and Conservation Significance—Stonefish play a crucial role as ambush predators, helping to manage populations of small reef fishes and invertebrates [13]. Preserving their populations is vital for maintaining balance within reef ecosystems. It is essential to protect their habitats from degradation, especially in urbanized regions like Eilat’s coastline, as this not only supports ecological health but also enhances public safety.
Marine Spatial Planning—Our findings suggest that areas frequented by tourists, where stonefish are known to reside, need careful management. Zoning strategies, such as limiting barefoot walking in shallow coral reefs or establishing designated swimming areas, could minimize harmful encounters while allowing stonefish to continue fulfilling their ecological role.
5. Conclusions
The consistently low FID seen in stonefish highlights how their potent venom and camouflage reduce their need for active escape. While this strategy is ecologically beneficial, it also increases the chances of human envenomation in busy coastal areas. Future studies should look at a broader geographical range and consider differences between species, environmental factors, and experimental approaches to various threats for a more complete understanding of this issue. At the same time, it is important for management strategies to combine biological knowledge with public safety measures to ensure both conservation and human safety in reef ecosystems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jmse13091789/s1, Figure S1: The temporal linear trends of the initial ID of the stonefish, the Alert status, and flight response. The dates are represented in in Julian Days—60 (1 March) to 123 (3 May).
Author Contributions
Conceptualization—R.Y. and L.Y.; Methodology—L.Y. and S.P.; Software R.Y.; Validation—R.Y. and L.Y.; Formal analysis—R.Y.; Investigation—S.P. and L.Y.; Data curation—S.P.; Writing—original draft—S.P., L.Y. and R.Y.; Writing—review and editing—R.Y.; Visualization—R.Y.; Supervision—L.Y. and R.Y.; Project administration—R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Available from corresponding author with reasonable request.
Acknowledgments
We thank Jakub Z. Kosicki of Adam Mickiewicz University in Poznań, Poland, for his assistance with the data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Willis, T.J.; Anderson, M.J. Structure of cryptic reef fish assemblages: Relationships with habitat characteristics and predator density. Mar. Ecol. Prog. Ser. 2003, 257, 209–221. [Google Scholar] [CrossRef]
- Ydenberg, R.C.; Dill, L.M. The economics of fleeing from predators. Adv. Study Behav. 1986, 16, 229–249. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Blumstein, D.T. Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manag. 2003, 67, 852–857. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Blumstein, D.T.; Díaz, M.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Møller, A.P. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 2015, 6, 8877. [Google Scholar] [CrossRef]
- Stankowich, T.; Blumstein, D.T. Fear in animals: A meta-analysis and review of risk assessment. Proc. R. Soc. B Biol. Sci. 2005, 272, 2627–2634. [Google Scholar] [CrossRef]
- Samia, D.S.; Nomura, F.; Blumstein, D.T. Do animals generally flush early and avoid the rush? A meta-analysis. Biol. Lett. 2013, 9, 20130016. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Individual consistency in flight initiation distances in burrowing owls: A new hypothesis on disturbance-induced habitat selection. Biol. Lett. 2010, 6, 167–170. [Google Scholar] [CrossRef]
- Nunes, J.A.C.; Costa, Y.; Blumstein, D.T.; Leduc, A.O.; Dorea, A.C.; Benevides, L.J.; Sampaio, C.L.; Barros, F. Global trends on reef fishes’ ecology of fear: Flight initiation distance for conservation. Mar. Environ. Res. 2018, 136, 153–157. [Google Scholar] [CrossRef]
- Yosef, R.; Hershko, M.; Zduniak, P. Anti COVID-19 face-masks increases vigilance in Nubian ibex (Capra nubiana). Biol. Conserv. 2021, 263, 109339. [Google Scholar] [CrossRef]
- Parsons, D.F.; Suthers, I.M.; Cruz, D.O.; Smith, J.A. Effects of habitat on fish abundance and species composition on temperate rocky reefs. Mar. Ecol. Prog. Ser. 2016, 561, 155–171. [Google Scholar] [CrossRef]
- Harris, R.J.; Jenner, R.A. Evolutionary ecology of fish venom: Adaptations and consequences of evolving a venom system. Toxins 2019, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.R.; Erdmann, M.V. Reef Fishes of the East Indies; Tropical Reef Research: Perth, Australia, 2012. [Google Scholar]
- Lennox-Bulow, D.; Smout, M.; Loukas, A.; Seymour, J. Stonefish (Synanceia spp.) Ichthyocrinotoxins: An ecological review and prospectus for future research and biodiscovery. Toxicon 2023, 236, 107329. [Google Scholar] [CrossRef] [PubMed]
- Church, J.E.; Hodgson, W.C. The pharmacological activity of fish venoms. Toxicon 2002, 40, 1083–1093. [Google Scholar] [CrossRef]
- Church, J.E.; Hodgson, W.C. Dose-dependent cardiovascular and neuromuscular effects of stonefish (Synanceja trachynis) venom. Toxicon 2000, 38, 391–407. [Google Scholar] [CrossRef]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Pinheiro, J.V.; Albuquerque, A.; Ferreira, D.; Goncalves, D.M.; Giglio, V.J. Number of individuals, but not habitat complexity, influences the antipredator behavior of an Amazonian floodplain fish. Neotrop. Ichthyol. 2024, 22, e240044. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L.; Kool, J.T. Coral reef recovery dynamics in a changing world. Coral Reefs 2011, 30, 283–294. [Google Scholar] [CrossRef]
- Loya, Y. The coral reefs of Eilat—Past, present and future: Three decades of coral community structure studies. In Coral Health and Disease; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–34. [Google Scholar]
- Fine, M.; Gildor, H.; Genin, A. A coral reef refuge in the Red Sea. Glob. Chang. Biol. 2013, 19, 3640–3647. [Google Scholar] [CrossRef]
- Randall, J.E. Coastal Fishes of Oman; University of Hawai’i Press: Honolulu, HI, USA, 1995. [Google Scholar]
- Golani, D.; Bogorodsky, S.V. The Fishes of the Red Sea–Reappraisal and Updated Checklist. Zootaxa 2010, 2463, 1–135. [Google Scholar] [CrossRef]
- Smith, W.L.; Wheeler, W.C. Venom evolution widespread in fishes: A phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef]
- Gendron, R.P. Searching for cryptic prey: Evidence for optimal search rates and the formation of search images in quail. Anim. Behav. 1986, 34, 898–912. [Google Scholar] [CrossRef]
- Cooper, W.E.; Blumstein, D.T. Escaping from Predators: An Integrative View of Escape Decisions; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Frid, A.; Dill, L.M. Human-Caused Disturbance Stimuli as A Form of Predation Risk. Conserv. Ecology 2002, 6, 11. Available online: http://www.consecol.org/vol6/iss1/art11 (accessed on 15 February 2025). [CrossRef]
- Shabi, T.; Ziv, Y.; Yosef, R.; Shashar, N. Deciphiring preferences of shelter volume and distribution by coral reef fishes, using systematic and functional grouping. J. Mar. Sci. Eng. 2024, 12, 186. [Google Scholar] [CrossRef]
- Nunes, J.A.C.C.; Sampaio, C.L.; Barros, F. The influence of structural complexity and reef habitat types on flight initiation distance and escape behaviors in labrid fishes. Mar. Biol. 2015, 162, 493–499. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr. Risk factors affecting escape behavior by the desert iguana, Dipsosaurus dorsalis: Speed and directness of predator approach, degree of cover, direction of turning by a predator, and temperature. Can. J. Zool. 2003, 81, 979–984. [Google Scholar] [CrossRef]
- Kruschel, C.; Schultz, S. Lure-assisted visual census: A new method for quantifying fish abundance, behaviour, and predation risk in shallow coastal habitats. Mar. Freshw. Res. 2010, 61, 1349–1359. [Google Scholar] [CrossRef]
- Januchowski-Hartley, F.A.; Nash, K.L.; Lawton, R.J. Influence of spear guns, dive gear and observers on estimating fish flight initiation distance on coral reefs. Mar. Ecol. Prog. Ser. 2012, 469, 113–119. [Google Scholar] [CrossRef]
- Januchowski-Hartley, F.A.; Graham, N.A.; Cinner, J.E.; Russ, G.R. Spillover of fish naïveté from marine reserves. Ecol. Lett. 2013, 16, 191–197. [Google Scholar] [CrossRef]
- Goetze, J.S.; Januchowski-Hartley, F.A.; Claudet, J.; Langlois, T.J.; Wilson, S.K.; Jupiter, S.D. Fish wariness is a more sensitive indicator to changes in fishing pressure than abundance, length or biomass. Ecol. Appl. 2017, 27, 1178–1189. [Google Scholar] [CrossRef]
- Hamilton, W.D. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Pitcher, T.J. Functions of shoaling behaviour in teleosts. In The Behaviour of Teleost Fishes; Springer: Boston, MA, USA, 1993; pp. 294–337. [Google Scholar] [CrossRef]
- Sbragaglia, V.; Morroni, L.; Blumstein, D.T. Weak evidence for a relationship between group size and flight initiation distance in response to underwater human presence in an exploited fish species. Behav. Ecol. Sociobiol. 2025, 7, 22. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Frederick, W.G. Optimal flight initiation distance. J. Theor. Biol. 2007, 244, 59–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).