Towards Scalable Ecological Monitoring: Assessing AI-Based Annotation of Benthic Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Image Collection
2.2. Annotation Workflow
2.2.1. Manual Annotation
2.2.2. AI-Assisted Tool Selection
2.2.3. AI-Assisted Tool Description
2.2.4. Data Import Bridge
2.3. Training Dataset Preparation
2.3.1. Annotation Process
2.3.2. Number of Images Used in Training Sets
2.4. Reef-EBQI Index Calculations
2.5. Additional CoralNet Training for Accuracy Improvement
3. Results
3.1. Automated Annotation Classifier Training
3.2. Comparison of Ecosystem Health Index Outputs
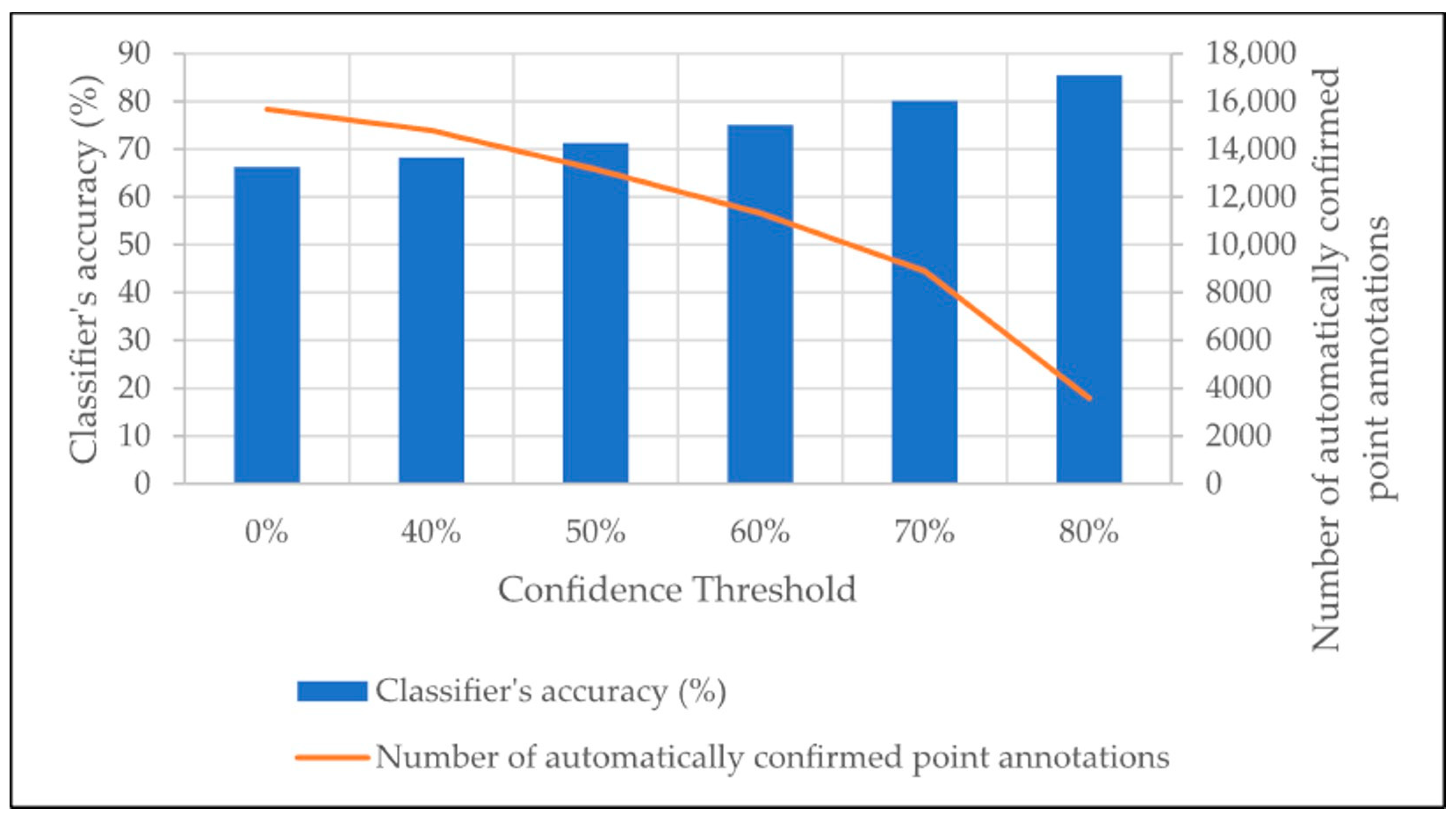
3.3. Additional CoralNet Training for Accuracy Improvement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sala, E.; Giakoumi, S. No-Take Marine Reserves Are the Most Effective Protected Areas in the Ocean. ICES J. Mar. Sci. 2018, 75, 1166–1168. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Airoldi, L.; Ballesteros, E.; Benedetti-Cecchi, L.; Boero, F.; Bulleri, F.; Cebrian, E.; Cerrano, C.; Claudet, J.; Colloca, F.; et al. Mediterranean Rocky Reefs in the Anthropocene: Present Status and Future Concerns. Adv. Mar. Biol. 2021, 89, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Paine, R.T. Intertidal Community Structure: Experimental Studies on the Relationship between a Dominant Competitor and Its Principal Predator. Oecologia 1974, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, E. Mediterranean Coralligenous Assemblages: A Synthesis of Present Knowledge. In Oceanography and Marine Biology: An Annual Review; Taylor & Francis Group: Boca Raton, FL, USA, 2006; Volume 44, pp. 123–195. [Google Scholar]
- Prato, G.; Guidetti, P.; Bartolini, F.; Mangialajo, L.; Francour, P. The Importance of High-Level Predators in Marine Protected Area Management: Consequences of Their Decline and Their Potential Recovery in the Mediterranean Context. Adv. Oceanogr. Limnol. 2013, 4, 176–193. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E.; Anderson, M.J.; Iveša, L.; Cardona, E. Biogeographical Patterns of Algal Communities in the Mediterranean Sea: Cystoseira Crinita-dominated Assemblages as a Case Study. J. Biogeogr. 2012, 39, 140–152. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfune, A.; Boudouresque, C.-F.; Verlaque, M. Decline and Local Extinction of Fucales in French Riviera: The Harbinger of Future Extinctions? Mediterr. Mar. Sci. 2014, 16, 206. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Personnic, S.; Ruitton, S.; Ballesteros, E.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Cebrian, E.; et al. An Ecosystem-Based Approach to Assess the Status of Mediterranean Algae-Dominated Shallow Rocky Reefs. Mar. Pollut. Bull. 2017, 117, 311–329. [Google Scholar] [CrossRef]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.-M.; Francour, P. Nursery Value of Cystoseira Forests for Mediterranean Rocky Reef Fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Piazzi, L.; Bonaviri, C.; Castelli, A.; Ceccherelli, G.; Costa, G.; Curini-Galletti, M.; Langeneck, J.; Manconi, R.; Montefalcone, M.; Pipitone, C.; et al. Biodiversity in Canopy-Forming Algae: Structure and Spatial Variability of the Mediterranean Cystoseira Assemblages. Estuar. Coast. Shelf Sci. 2018, 207, 132–141. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate Change Effects on a Miniature Ocean: The Highly Diverse, Highly Impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef]
- Dailianis, T.; Smith, C.J.; Papadopoulou, N.; Gerovasileiou, V.; Sevastou, K.; Bekkby, T.; Bilan, M.; Billett, D.; Boström, C.; Carreiro-Silva, M.; et al. Human Activities and Resultant Pressures on Key European Marine Habitats: An Analysis of Mapped Resources. Mar. Policy 2018, 98, 1–10. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The Structure of Mediterranean Rocky Reef Ecosystems across Environmental and Human Gradients, and Conservation Implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef] [PubMed]
- Tornero, V.; Hanke, G. Chemical Contaminants Entering the Marine Environment from Sea-Based Sources: A Review with a Focus on European Seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion Impacts on Biodiversity, Ecosystem Services, and Human Health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Meinesz, A.; Lefevre, J.R.; Astier, J.M. Impact of Coastal Development on the Infralittoral Zone along the Southeastern Mediterranean Shore of Continental France. Mar. Pollut. Bull. 1991, 23, 343–347. [Google Scholar] [CrossRef]
- Guidetti, P. The Destructive Date-Mussel Fishery and the Persistence of Barrens in Mediterranean Rocky Reefs. Mar. Pollut. Bull. 2011, 62, 691–695. [Google Scholar] [CrossRef]
- Rilov, G. Multi-Species Collapses at the Warm Edge of a Warming Sea. Sci. Rep. 2016, 6, 36897. [Google Scholar] [CrossRef]
- Chatzimentor, A.; Doxa, A.; Katsanevakis, S.; Mazaris, A.D. Are Mediterranean Marine Threatened Species at High Risk by Climate Change? Glob. Change Biol. 2023, 29, 1809–1821. [Google Scholar] [CrossRef]
- Nikolaou, A.; Katsanevakis, S. Marine Extinctions and Their Drivers. Reg. Environ. Change 2023, 23, 88. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine Heatwaves Drive Recurrent Mass Mortalities in the Mediterranean Sea. Glob. Change Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bulleri, F.; Cinelli, F. Density Dependent Foraging of Sea Urchins in Shallow Subtidal Reefs on the West Coast of Italy (Western Mediterranean). Mar. Ecol. Prog. Ser. 1998, 163, 203–211. [Google Scholar] [CrossRef]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien Marine Fishes Deplete Algal Biomass in the Eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef] [PubMed]
- Rindi, F.; Gavio, B.; Díaz-Tapia, P.; Di Camillo, C.G.; Romagnoli, T. Long-Term Changes in the Benthic Macroalgal Flora of a Coastal Area Affected by Urban Impacts (Conero Riviera, Mediterranean Sea). Biodivers. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The Fate of Cystoseira crinita, a Forest-Forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf Sci. 2016, 181, 196–208. [Google Scholar] [CrossRef]
- European Commission; Directorate General for the Environment. European Red List of Habitats: Part 1. Marine Habitats; Publications Office: Luxembourg, 2016. [Google Scholar]
- Gerovasileiou, V.; Smith, C.J.; Sevastou, K.; Papadopoulou, N.; Dailianis, T.; Bekkby, T.; Fiorentino, D.; McOwen, C.J.; Amaro, T.; Bengil, E.G.T.; et al. Habitat Mapping in the European Seas—Is It Fit for Purpose in the Marine Restoration Agenda? Mar. Policy 2019, 106, 103521. [Google Scholar] [CrossRef]
- Fraschetti, S.; Fabbrizzi, E.; Tamburello, L.; Uyarra, M.C.; Micheli, F.; Sala, E.; Pipitone, C.; Badalamenti, F.; Bevilacqua, S.; Boada, J.; et al. An Integrated Assessment of the Good Environmental Status of Mediterranean Marine Protected Areas. J. Environ. Manage. 2022, 305, 114370. [Google Scholar] [CrossRef]
- EU. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; EU: Brussels, Belgium, 2000. [Google Scholar]
- EU. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive); EU: Brussels, Belgium, 2008. [Google Scholar]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; De Torres, M. A New Methodology Based on Littoral Community Cartography Dominated by Macroalgae for the Implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Ugland, K. Ecological Evaluation Index Continuous Formula (EEI-c) Application: A Step Forward for Functional Groups, the Formula and Reference Condition Values. Mediterr. Mar. Sci. 2011, 12, 199. [Google Scholar] [CrossRef]
- D’Archino, R.; Piazzi, L. Macroalgal Assemblages as Indicators of the Ecological Status of Marine Coastal Systems: A Review. Ecol. Indic. 2021, 129, 107835. [Google Scholar] [CrossRef]
- Berov, D.; Hiebaum, G.; Vasilev, V.; Karamfilov, V. An Optimised Method for Scuba Digital Photography Surveys of Infralittoral Benthic Habitats: A Case Study from the SW Black Sea Cystoseira-Dominated Macroalgal Communities. Underw. Technol. 2016, 34, 11–20. [Google Scholar] [CrossRef]
- Van Rein, H.; Schoeman, D.S.; Brown, C.J.; Quinn, R.; Breen, J. Development of Benthic Monitoring Methods Using Photoquadrats and Scuba on Heterogeneous Hard-substrata: A Boulder-slope Community Case Study. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 676–689. [Google Scholar] [CrossRef]
- Preskitt, L.B.; Vroom, P.S.; Smith, C.M. A Rapid Ecological Assessment (REA) Quantitative Survey Method for Benthic Algae Using Photoquadrats with Scuba. Pac. Sci. 2004, 58, 201–209. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L.; Rindi, F. Testing a New Classification of Morphological Functional Groups of Marine Macroalgae for the Detection of Responses to Stress. Mar. Biol. 2011, 158, 2459–2469. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The Evolution of Thallus Form and Survival Strategies in Benthic Marine Macroalgae: Field and Laboratory Tests of a Functional Form Model. Am. Nat. 1980, 116, 25–44. [Google Scholar] [CrossRef]
- Steneck, R.S.; Dethier, M.N. A Functional Group Approach to the Structure of Algal-Dominated Communities. Oikos 1994, 69, 476. [Google Scholar] [CrossRef]
- González-Rivero, M.; Bongaerts, P.; Beijbom, O.; Pizarro, O.; Friedman, A.; Rodriguez-Ramirez, A.; Upcroft, B.; Laffoley, D.; Kline, D.; Bailhache, C.; et al. The Catlin Seaview Survey—Kilometre-scale Seascape Assessment, and Monitoring of Coral Reef Ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 184–198. [Google Scholar] [CrossRef]
- The Influence of Nearshore Waters on Corals of the Florida Reef Tract. In The Everglades, Florida Bay, and Coral Reefs of the Florida Keys; CRC Press: Boca Raton, FL, USA, 2001; pp. 795–812. [CrossRef]
- Langenkämper, D.; Zurowietz, M.; Schoening, T.; Nattkemper, T.W. BIIGLE 2.0-Browsing and Annotating Large Marine Image Collections. Front. Mar. Sci. 2017, 4, 83. [Google Scholar] [CrossRef]
- Rivera-Sosa, A.; Muñiz-Castillo, A.I.; Charo, B.; Asner, G.P.; Roelfsema, C.M.; Donner, S.D.; Bambic, B.D.; Bonelli, A.G.; Pomeroy, M.; Manzello, D.; et al. Six Decades of Global Coral Bleaching Monitoring: A Review of Methods and Call for Enhanced Standardization and Coordination. Front. Mar. Sci. 2025, 12, 1547870. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.-Y.; Tan, C.-J.; et al. Towards Automated Annotation of Benthic Survey Images: Variability of Human Experts and Operational Modes of Automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Curtis, E.J.; Durden, J.M.; Bett, B.J.; Huvenne, V.A.I.; Piechaud, N.; Walker, J.; Albrecht, J.; Massot-Campos, M.; Yamada, T.; Bodenmann, A.; et al. Improving Coral Monitoring by Reducing Variability and Bias in Cover Estimates from Seabed Images. Prog. Oceanogr. 2024, 222, 103214. [Google Scholar] [CrossRef]
- Shihavuddin, A.S.M.; Gracias, N.; Garcia, R.; Gleason, A.; Gintert, B. Image-Based Coral Reef Classification and Thematic Mapping. Remote Sens. 2013, 5, 1809–1841. [Google Scholar] [CrossRef]
- Weinstein, B.G. Scene-specific Convolutional Neural Networks for Video-based Biodiversity Detection. Methods Ecol. Evol. 2018, 9, 1435–1441. [Google Scholar] [CrossRef]
- Dawkins, M.; Sherrill, L.; Fieldhouse, K.; Hoogs, A.; Richards, B.; Zhang, D.; Prasad, L.; Williams, K.; Lauffenburger, N.; Wang, G. An Open-Source Platform for Underwater Image and Video Analytics. In Proceedings of the 2017 IEEE Winter Conference on Applications of Computer Vision (WACV), Santa Rosa, CA, USA, 24–31 March 2017; IEEE: Santa Rosa, CA, USA, 2017; pp. 898–906. [Google Scholar] [CrossRef]
- González-Rivero, M.; Beijbom, O.; Rodriguez-Ramirez, A.; Bryant, D.E.P.; Ganase, A.; Gonzalez-Marrero, Y.; Herrera-Reveles, A.; Kennedy, E.V.; Kim, C.J.S.; Lopez-Marcano, S.; et al. Monitoring of Coral Reefs Using Artificial Intelligence: A Feasible and Cost-Effective Approach. Remote Sens. 2020, 12, 489. [Google Scholar] [CrossRef]
- Hermanto, B.; Bourne, D.G.; Smith, H. Comparative Image Analysis Approaches to Assess Ecological Effects of Macroalgal Removal on Inshore Reefs of Magnetic Island, Australia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1137, 012052. [Google Scholar] [CrossRef]
- González-Rivero, M.; Beijbom, O.; Rodriguez-Ramirez, A.; Holtrop, T.; González-Marrero, Y.; Ganase, A.; Roelfsema, C.; Phinn, S.; Hoegh-Guldberg, O. Scaling up Ecological Measurements of Coral Reefs Using Semi-Automated Field Image Collection and Analysis. Remote Sens. 2016, 8, 30. [Google Scholar] [CrossRef]
- Pavoni, G.; Corsini, M.; Ponchio, F.; Muntoni, A.; Edwards, C.; Pedersen, N.; Sandin, S.; Cignoni, P. TagLab: AI-assisted Annotation for the Fast and Accurate Semantic Segmentation of Coral Reef Orthoimages. J. Field Robot. 2022, 39, 246–262. [Google Scholar] [CrossRef]
- Günzel, L.; Monk, J.; Jackett, C.; Friedman, A.; Bastiaansen, A.; Najafi, A.; Garcia-Ortiz, A. Harnessing the Power of Squidle+ to Develop Flexible Machine Learning Models; Elsevier BV: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Trygonis, V.; Sini, M. photoQuad: A Dedicated Seabed Image Processing Software, and a Comparative Error Analysis of Four Photoquadrat Methods. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 99–108. [Google Scholar] [CrossRef]
- Savin, A.; Sini, M.; Xynogala, I.; Lioupa, V.; Vougioukalou, K.; Stamatis, K.; Noè, S.; Ragkousis, M.; Gerovasileiou, V.; Dailianis, T.; et al. Assessment of Macroalgal Communities on Shallow Rocky Reefs in the Aegean Sea Indicates an Impoverished Ecological Status. Mediterr. Mar. Sci. 2023, 24, 241–258. [Google Scholar] [CrossRef]
- Sini, M.; Katsanevakis, S.; Koukourouvli, N.; Gerovasileiou, V.; Dailianis, T.; Buhl-Mortensen, L.; Damalas, D.; Dendrinos, P.; Dimas, X.; Frantzis, A.; et al. Assembling Ecological Pieces to Reconstruct the Conservation Puzzle of the Aegean Sea. Front. Mar. Sci. 2017, 4, 347. [Google Scholar] [CrossRef]
- Lykousis, V.; Chronis, G.; Tselepides, A.; Price, N.B.; Theocharis, A.; Siokou-Frangou, I.; Van Wambeke, F.; Danovaro, R.; Stavrakakis, S.; Duineveld, G.; et al. Major Outputs of the Recent Multidisciplinary Biogeochemical Researches Undertaken in the Aegean Sea. J. Mar. Syst. 2002, 33–34, 313–334. [Google Scholar] [CrossRef]
- Zervakis, V.; Georgopoulos, D.; Karageorgis, A.P.; Theocharis, A. On the Response of the Aegean Sea to Climatic Variability: A Review. Int. J. Climatol. 2004, 24, 1845–1858. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. Ecological Evaluation of Transitional and Coastal Waters: A Marine Benthic Macrophytes-Based Model. Mediterr. Mar. Sci. 2001, 2, 45. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. An Insight to the Ecological Evaluation Index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Katsanevakis, S.; Micheli, F.; Sala, E.; Rilov, G.; Sarà, G.; Malak, D.A.; Abdulla, A.; Gerovasileiou, V.; Gissi, E.; et al. The Status of Coastal Benthic Ecosystems in the Mediterranean Sea: Evidence From Ecological Indicators. Front. Mar. Sci. 2020, 7, 475. [Google Scholar] [CrossRef]
- Pavoni, G.; Corsini, M.; Pedersen, N.; Petrovic, V.; Cignoni, P. Challenges in the Deep Learning-Based Semantic Segmentation of Benthic Communities from Ortho-Images. Appl. Geomat. 2021, 13, 131–146. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, J.; Li, C.; Jiang, Q.; Zhou, M.; Lam, K.-M.; Zhang, W.; Fu, X. HCLR-Net: Hybrid Contrastive Learning Regularization with Locally Randomized Perturbation for Underwater Image Enhancement. Int. J. Comput. Vis. 2024, 132, 4132–4156. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Gruen, A.; Li, D. A Survey on Underwater Coral Image Segmentation Based on Deep Learning. Geo-Spat. Inf. Sci. 2025, 28, 472–496. [Google Scholar] [CrossRef]
- Althaus, F.; Hill, N.; Ferrari, R.; Edwards, L.; Przeslawski, R.; Schönberg, C.H.L.; Stuart-Smith, R.; Barrett, N.; Edgar, G.; Colquhoun, J.; et al. A Standardised Vocabulary for Identifying Benthic Biota and Substrata from Underwater Imagery: The CATAMI Classification Scheme. PLoS ONE 2015, 10, e0141039. [Google Scholar] [CrossRef]
- Williams, I.D.; Couch, C.S.; Beijbom, O.; Oliver, T.A.; Vargas-Angel, B.; Schumacher, B.D.; Brainard, R.E. Leveraging Automated Image Analysis Tools to Transform Our Capacity to Assess Status and Trends of Coral Reefs. Front. Mar. Sci. 2019, 6, 222. [Google Scholar] [CrossRef]



| CoralNet Labels | Morphofunctional Group | Taxonomic Group | Description | Example Species |
|---|---|---|---|---|
| Algal turf | Turf/Encrusting | Algae | Low-lying species of macroalgae | Cladophora sp., Pseudochlorodesmis furcellata |
| Encrusting calcareous algae | Turf/Encrusting | Algae | Heavily calcified thalli with stone-like texture and prostrate growth, forming flat, but sometimes multi-layered epilithic crusts | Lithophyllum spp., Mesophyllum spp., Peyssonnelia squamaria, Peyssonnelia rosa-marina |
| Non-calcareous encrusting algae | Turf/Encrusting | Algae | Thin, soft-textured thalli lacking calcium carbonate, typically forming smooth or slightly uneven crusts, with a flexible, often gelatinous or membranous consistency | Palmophyllum spp. |
| Articulated calcareous algae 1 | Shrubby | Algae | Heavily calcified branched/multilayered/articulated algae | Liagora spp., Jania spp., Corallina spp., Multilayered forms of Peyssonellia |
| Articulated calcareous algae 2 | Shrubby | Algae | Semi-calcified erect | Halimeda tuna, Flabellia petiolata, Peyssonnelia rubra |
| Shrubby algae 1 | Shrubby | Algae | Foliose macroalgae with large thalli forming pseudo-canopies | Padina pavonica, Zonaria tournefortii, Stypopodium schimperi |
| Shrubby algae 2 | Shrubby | Algae | Foliose macroalgae with thin thalli forming pseudo-canopies | Dictyota spp., Dictyopteris spp. |
| Shrubby algae 3 | Shrubby | Algae | Upright, well-developed thalli of moderate height, forming bushy aggregations | Laurencia spp., Halopteris spp. |
| Canopy-forming macrophytes 1 | Arborescent perennial | Algae | Perennial stems, upright, tree-like thalli with thick blades and branches, forming dense canopies found primarily in pristine environments | Cystoseira spp., Gongolaria spp. |
| Canopy-forming macrophytes 2 | Arborescent perennial | Algae | Perennial stems, upright, tree-like thalli with thick blades and branches, forming dense canopies. Present high-adaptive plasticity and can survive in adverse conditions; found in pristine and moderately degraded environments | Cystoseira compressa, Sargassum spp. |
| Massive algae | Shrubby | Algae | Wide cauloid | Codium bursa |
| Mucilaginous | Turf/Encrusting | Algae | Mucus-like phenotype | Chrysophyceae |
| Animal turf | Animals | Low-lying, turf-like growth form, typically not higher than 2–3 cm | Aglaophenia sp. | |
| Perennial animal boring | Animals | Species that bore into the substrate | Rocellaria dubia, Lithophaga lithophaga, Cliona spp. | |
| Perennial animal cup | Animals | Cup-like or tooth-like animals | Balanophyllia europaea, Caryophyllia inornata | |
| Perennial animal encrusting | Animals | Species growing as crusts over hard substrate, typically not higher than 2–3 cm | Crambe crambe, Phorbas spp., Reptadeonella violacea | |
| Perennial animal massive | Animals | Large invertebrates with an upright growth form | Sarcotragus foetidus, Ircinia spp. | |
| Perennial animal tree | Animals | Branching or tree-like invertebrates | Axinella spp., Adeonella spp., Myriapora truncata | |
| Perennial tube forming animals | Animals | Organisms creating calcareous tubes | Polychaeta | |
| Bare rock | Substrate | Bare rock areas | ||
| Substrate pebbles/sand | Substrate | Soft motile sediment: pebbles, sand, biogenic substrate | ||
| Substrate holes | Substrate | Holes in the substrate | ||
| Unidentified substrate | Substrate | Unclear parts of the image |
| Iteration | Classifier Number | Number of Confirmed Images the Classifiers Were Trained On | Number of Confirmed Images Added to Trigger the Next Classifier | Classifier ID | Classifier’s Accuracy |
|---|---|---|---|---|---|
| 1 | - | 36 | 122 | 54,712 | - |
| 2 | 1 | 158 | 123 | 54,735 | 55% |
| 3 | 2 | 281 | 126 | 54,737 | 58% |
| 4 | 3 | 407 | 126 | 54,741 | 60% |
| 5 | 4 | 533 | 126 | 54,759 | 61% |
| 6 | * | 659 | 126 | - | - |
| 7 | 5 | 785 | 126 | 54,863 | 63% |
| 8 | ** | 911 | 128 | - | - |
| 9 | 6 | 1039 | 124 | 54,890 | 64% |
| 10 | 7 | 1163 | 122 | 54,905 | 65% |
| 11 | 8 | 1285 | - | 54,922 | 66% |
| Manually Annotated | ||||||
|---|---|---|---|---|---|---|
| AI Annotated | Ecological Status | Bad | Poor | Moderate | High | Very High |
| Bad | 64 | 1 | 2 | |||
| Poor | 5 | 4 | 1 | |||
| Moderate | 3 | 2 | ||||
| High | 2 | |||||
| Very High | 1 | |||||
| Turf algae | 5385 | 406 | 122 | 102 | 77 | 102 | 30 | 32 | 25 | 25 | 4 | 1 | 1 | 3 | 0 | 1 | 0 |
| Encrusting calcareous algae | 962 | 1148 | 119 | 37 | 30 | 11 | 12 | 3 | 12 | 2 | 3 | 0 | 2 | 0 | 0 | 0 | 0 |
| Substrate: bare rock | 653 | 187 | 775 | 13 | 6 | 75 | 3 | 0 | 11 | 1 | 7 | 0 | 1 | 0 | 0 | 0 | 0 |
| Shrubby algae 2 | 291 | 20 | 2 | 718 | 20 | 4 | 6 | 19 | 0 | 14 | 1 | 5 | 2 | 0 | 0 | 5 | 0 |
| Articulated calcareous algae 1 | 223 | 19 | 11 | 17 | 524 | 7 | 0 | 16 | 1 | 4 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Substrate: pebbles/sand | 339 | 22 | 75 | 13 | 1 | 326 | 5 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Perennial animal massive | 57 | 9 | 0 | 5 | 1 | 1 | 596 | 0 | 10 | 2 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Canopy-forming macrophytes 1 | 181 | 19 | 0 | 4 | 6 | 0 | 0 | 361 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perennial animal encrusting | 215 | 43 | 17 | 4 | 1 | 4 | 55 | 0 | 214 | 0 | 7 | 1 | 0 | 1 | 0 | 0 | 0 |
| Shrubby algae 1 | 68 | 5 | 1 | 15 | 6 | 1 | 1 | 0 | 0 | 261 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perennial animal boring | 92 | 8 | 18 | 1 | 1 | 6 | 4 | 1 | 20 | 0 | 37 | 0 | 0 | 0 | 0 | 0 | 0 |
| Articulated calcareous algae 2 | 28 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 23 | 0 | 0 | 0 | 0 | 0 |
| Shrubby algae 3 | 40 | 5 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Mucilaginous | 15 | 0 | 1 | 10 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| Unidentified | 12 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Animal turf | 5 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Canopy forming macrophytes 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Turf algae | Encrusting calcareous algae | Substrate: bare rock | Shrubby algae 2 | Articulated calcareous algae 1 | Substrate: pebbles/sand | Perennial animal massive | Canopy forming macrophytes 1 | Perennial animal encrusting | Shrubby algae 1 | Perennial animal boring | Articulated calcareous algae 2 | Shrubby algae 3 | Mucilaginous | Unidentified | Animal turf | Canopy-forming macrophytes 2 |
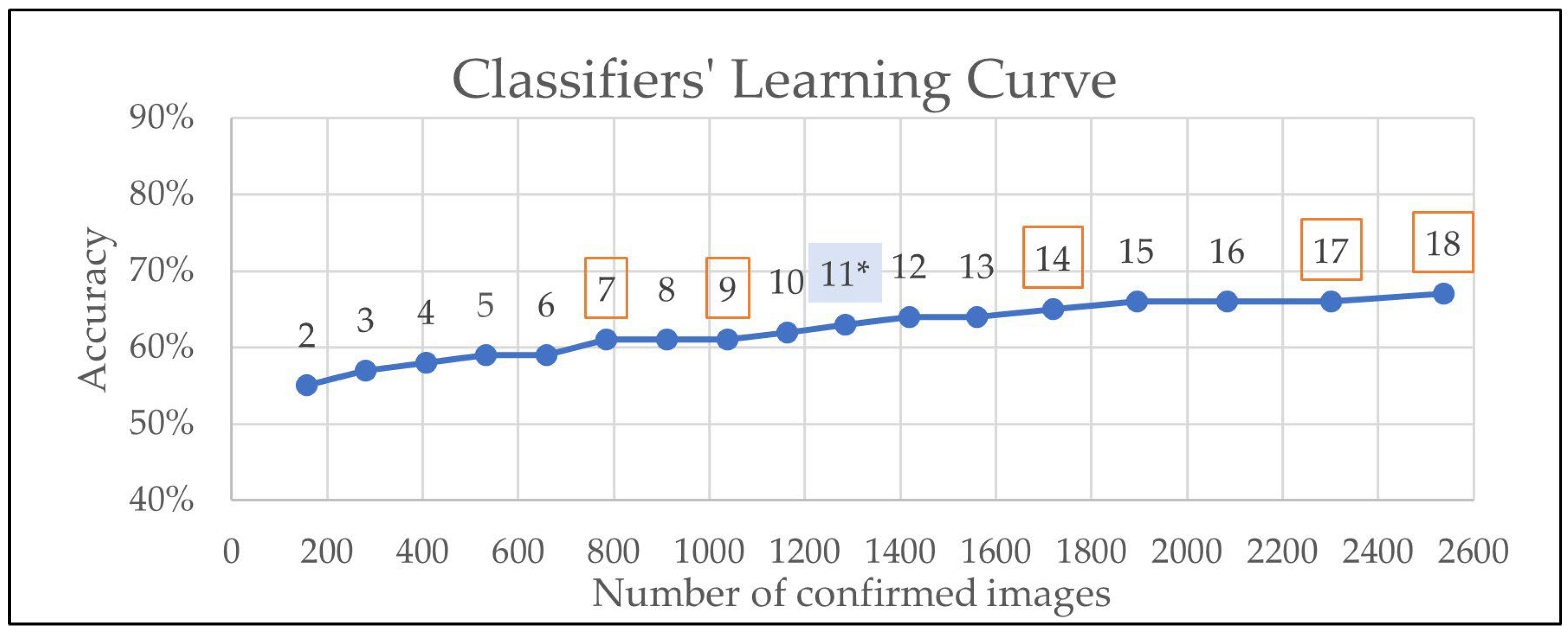
| Iteration | Classifier Number | Number of Confirmed Images the Classifiers Were Trained on | Classifier’s Accuracy |
|---|---|---|---|
| 1 | NA | 36 | - |
| 2 | 1 | 158 | 55% |
| 3 | 2 | 281 | 57% |
| 4 | 3 | 407 | 58% |
| 5 | 4 | 533 | 59% |
| 6 | NA | 659 | 59% |
| 7 | 5 | 785 | 61% |
| 8 | NA | 911 | 61% |
| 9 | 6 | 1039 | 61% |
| 10 | 7 | 1163 | 62% |
| 11 * | 8 | 1285 | 63% |
| 12 | 9 | 1419 | 64% |
| 13 | NA | 1561 | 64% |
| 14 | 10 | 1720 | 65% |
| 15 | 11 | 1895 | 66% |
| 16 | NA | 2085 | 66% |
| 17 | NA | 2302 | 66% |
| 18 | 12 | 2537 | 67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotou, M.; Sini, M.; Trygonis, V.; Greggio, N.; Mazaris, A.D.; Katsanevakis, S. Towards Scalable Ecological Monitoring: Assessing AI-Based Annotation of Benthic Images. J. Mar. Sci. Eng. 2025, 13, 1721. https://doi.org/10.3390/jmse13091721
Zotou M, Sini M, Trygonis V, Greggio N, Mazaris AD, Katsanevakis S. Towards Scalable Ecological Monitoring: Assessing AI-Based Annotation of Benthic Images. Journal of Marine Science and Engineering. 2025; 13(9):1721. https://doi.org/10.3390/jmse13091721
Chicago/Turabian StyleZotou, Maria, Maria Sini, Vasilis Trygonis, Nicola Greggio, Antonios D. Mazaris, and Stelios Katsanevakis. 2025. "Towards Scalable Ecological Monitoring: Assessing AI-Based Annotation of Benthic Images" Journal of Marine Science and Engineering 13, no. 9: 1721. https://doi.org/10.3390/jmse13091721
APA StyleZotou, M., Sini, M., Trygonis, V., Greggio, N., Mazaris, A. D., & Katsanevakis, S. (2025). Towards Scalable Ecological Monitoring: Assessing AI-Based Annotation of Benthic Images. Journal of Marine Science and Engineering, 13(9), 1721. https://doi.org/10.3390/jmse13091721








