1. Introduction
Driven by stringent environmental regulations from the International Maritime Organization (IMO) and the need to reduce greenhouse gas emissions, the marine fuel market is undergoing a significant transformation [
1]. The implementation of IMO 2020 sulfur cap regulations (limiting sulfur content to 0.5% wt.) has accelerated the shift from high-sulfur fuel oil (HSFO) to low-sulfur alternatives [
2,
3]. Consequently, HSFO’s market share plummeted from 97% in 2015 to 65% in 2023, while low-sulfur fuel oil (LSFO) surged to 32% [
4,
5,
6]. Marine fuel blending technology, particularly the blending process based on hydrogenated residue oil, has garnered increasing attention. Hydrogenated residue oil boasts advantages such as low sulfur content, high energy density, and good compatibility with existing marine engines. These attributes make it a significant contender in meeting the increasingly stringent emission standards [
7,
8]. It is estimated that by 2025, the global marine fuel blending market will exceed USD 12 billion, with the hydrogenated residue oil-based blending process taking the dominant position. Therefore, the application of hydrogenated residual oil is of great significance for promoting the sustainable development of the shipping industry.
Marine fuel instability during long storage periods can lead to stratification, sedimentation, and particulate aggregation, causing filter clogging, engine damage, and operational failures [
9]. Factors influencing this instability include the fuel’s chemical composition, presence of contaminants, storage conditions, and time [
10,
11]. The colloidal stability of marine fuels directly governs vessel reliability, economic efficiency, and environmental compliance. Robust stability management reduces operational risks, extends equipment lifespan, and ensures adherence to stringent emission standards [
12,
13].
Extensive research has explored factors governing marine fuel stability. The type and proportion of chemical functional groups in the fuel significantly affect its stability [
11]. The composition of marine residual fuel influences sedimentation due to incompatibility [
14]. Ilyin et al. [
15] identified correlations between fuel density, viscosity, and resin/asphaltene concentrations, noting asphaltenes’ disproportionate influence on sulfur distribution. Asphaltene molecular architecture dictates colloidal behavior: pyrolysis-derived asphaltenes with short alkyl chains increase sedimentation compared to crude-derived counterparts [
16,
17]. Kondrasheva et al. [
18] demonstrated that ternary blends of vacuum residue, ultra-low sulfur diesel (ULSD), and light-cycle gas oil (LCGO) could balance sulfur reduction (via ULSD) and stability preservation (via LCGO aromatics). Biomass-derived blending components further enhance viscosity reduction and component compatibility [
19,
20], though scalability challenges persist [
21].
Asphaltene aggregation is a critical factor in fuel stability, influenced by the chemical composition and intermolecular interactions of asphaltenes [
22]. Asphaltenes are complex, polyaromatic molecules containing heteroatoms (nitrogen, sulfur, oxygen) and metals such as nickel and vanadium [
23]. The aromaticity and heteroatom content of asphaltenes play a significant role in governing their aggregation kinetics, with high-aromaticity asphaltenes tending to form larger aggregates [
22,
24]. Pinheiro et al. [
25] established that aromaticity and heteroatom content govern aggregation kinetics, with high-aromaticity asphaltenes forming larger aggregates. The dominant aggregation mechanism involves π–π stacking of polycyclic aromatic cores [
26], though hydrogen bonding and acid-base interactions contribute significantly [
27]. Wang et al. [
28] further revealed that asphaltene nanoaggregate sizes remain concentration-independent, suggesting self-assembly follows critical nanoaggregate concentration (CNAC) principles. Metal porphyrins (Ni/V) exhibit strong coupling with asphaltenes, catalyzing aggregation via coordination bonding [
29]. Ding et al. [
30] observed that metal porphyrins, particularly nickel and vanadium, exhibit strong interactions with asphaltenes and can catalyze aggregation through coordination bonding. These metal-containing compounds can act as bridging ligands, linking asphaltene molecules together and promoting the formation of larger aggregate [
31]. Efimov et al. [
32] established a UNIFAC-based model to predict sedimentation stability in VLSFO, integrating NMR and elemental SARA data to prevent asphaltene precipitation from component incompatibility. Kuzmin et al. [
33] demonstrated through multidimensional analysis that recycled cooking oil as a bio-additive enhances both performance and stability of residual marine fuels. Zvereva et al. [
34] provides theoretical and experimental support for standard-compliant VLSFO production, highlighting the critical role of component selection and stabilizer application. Separately, Frank et al. [
35] demonstrated that a resorcinarene-based macrocyclic dispersant effectively suppresses asphaltene deposition, even in highly precipitative environments such as n-heptane.
Residue desulfurization (RDS) is a crucial process in petroleum refining for producing low-sulfur fuel oil (LSFO) [
36]. Residue oil, when subjected to deep hydrodesulfurization to meet stringent sulfur regulations, can experience changes in its composition that affect its stability [
37]. However, deep hydrodesulfurization can lead to over-hydrotreatment, which may reduce the concentration of natural stabilizers like resins and aromatics, vital for maintaining asphaltene peptization and overall fuel stability [
38]. This over-hydrotreatment can cause stability issues when RDS heavy oil is blended with high-conversion FCC slurry oil, mainly due to the high concentrations of unstable asphaltenes [
39]. The resulting incompatibility manifests as excessive total sediment potential (TSP), jeopardizing fuel quality and engine safety. Despite its technical urgency, the mechanistic interplay between FCC slurry oil–RDS heavy oil blending ratios, asphaltene colloidal stability, and aggregation evolution remains inadequately understood.
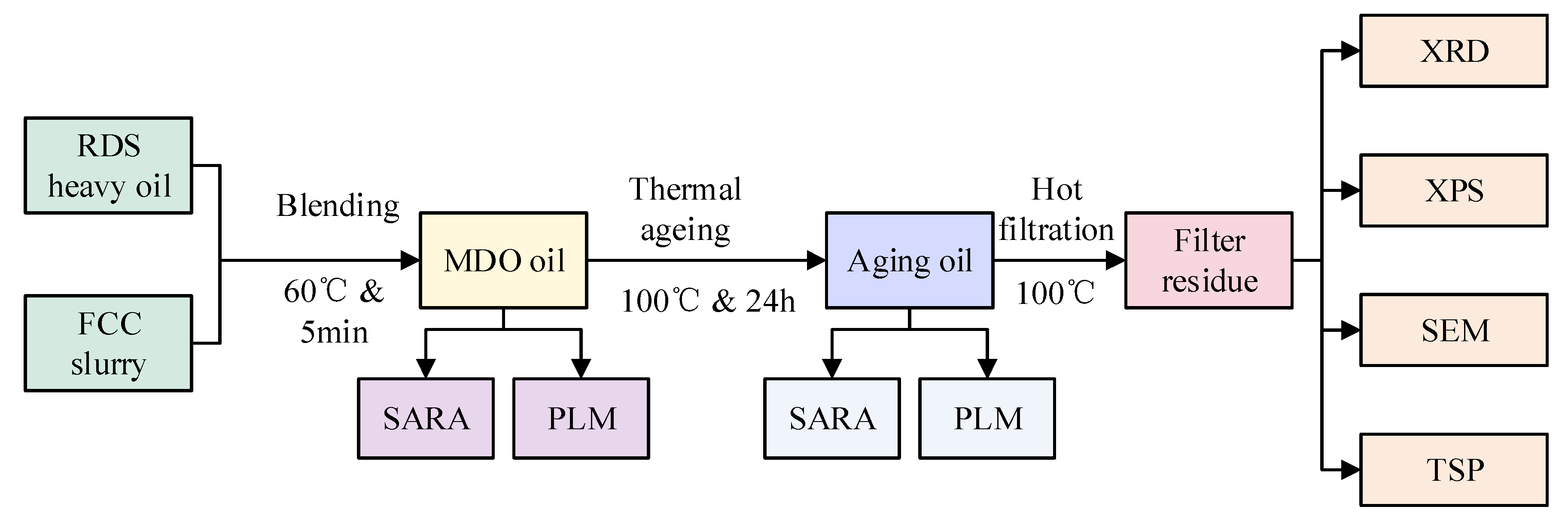
While most studies focus on the compatibility of high-sulfur residues with distillates, the fundamental pairwise interaction between a low-sulfur residue (RDS heavy oil) and a low-sulfur aromatic stabilizer (FCC slurry oil) remains less explored. This study is therefore designed as a systematic investigation into the independent and interactive behaviors of these two key streams. We aim to elucidate the intrinsic mechanisms governing colloidal stability and asphaltene aggregation in this binary system. The insights gained from this fundamental work are expected to provide a critical knowledge base for solving the practical problem of formulating stable, compliant marine fuels from modern refinery streams. This study investigates the effects of different FCC slurry oil blending ratios (5–25% wt.) on the colloidal stability and asphaltene aggregation behavior of RDS-based marine fuels. This study employs advanced characterization techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) to analyze the distribution characteristics, structural changes, and evolution behaviors of asphaltenes in blended fuels. These analyses aim to elucidate the colloidal stability and aggregation mechanisms of asphaltenes, providing critical insights into the causes of excessive TSP. By systematically investigating the colloidal stability and structural evolution of asphaltenes, this research offers both theoretical and technical support for optimizing the marine fuel oil blending process. The findings have significant implications for petrochemical enterprises seeking to enhance the stability and quality of marine fuels while complying with stringent sulfur regulations.
4. Conclusions
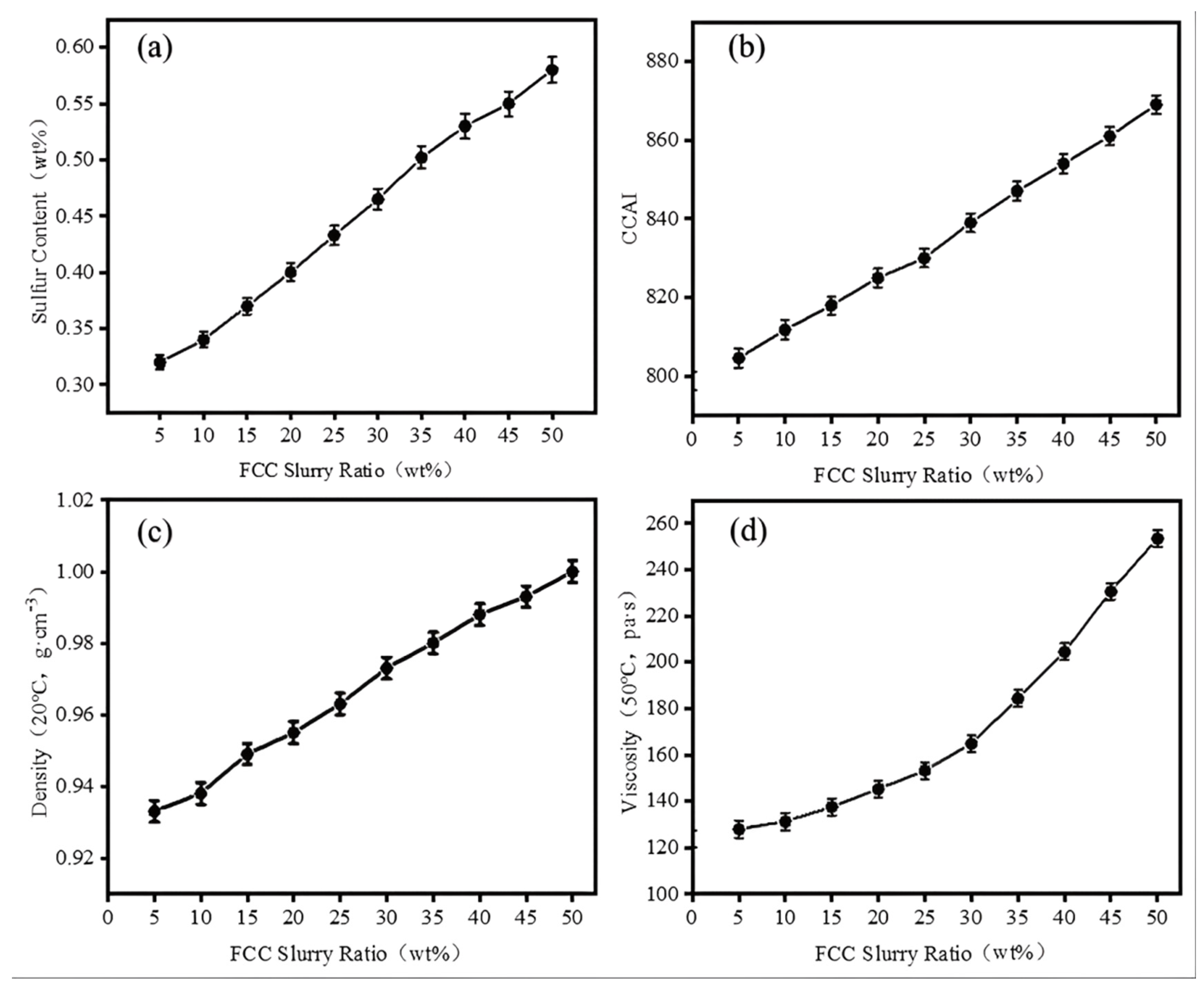
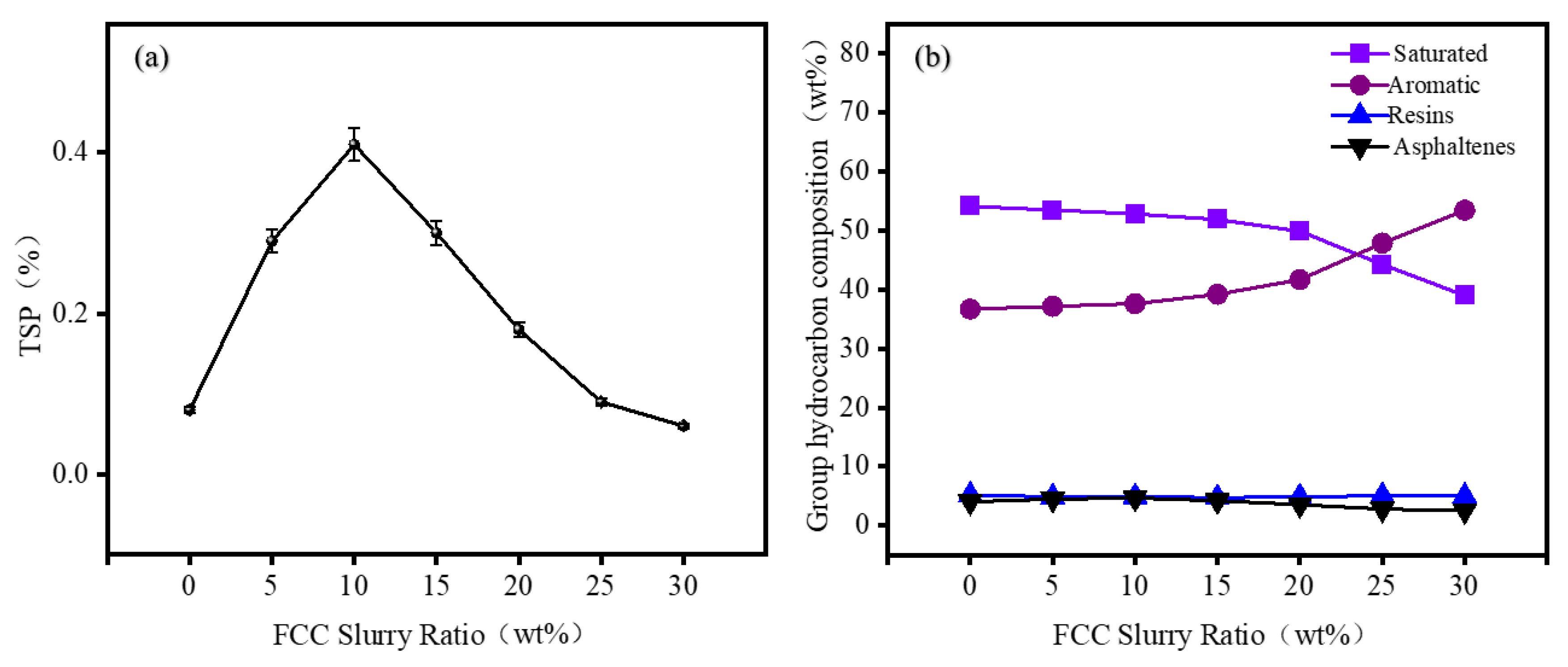
This study demonstrates that the blending ratio of FCC slurry oil plays a critical role in the colloidal stability of low-sulfur marine fuels. Specifically, it highlights the instability issues associated with blending excessively hydrotreated RDS heavy oil, which is produced in the residue hydrotreating units of full-conversion refineries. Total Sediment Potential (TSP) shows a non-monotonic response to FCC addition. Initially, TSP increases, peaking at 0.41% when the FCC ratio reaches 10%, due to destabilization of FCC-derived asphaltenes by high-saturation hydrocarbons in RDS heavy oil. Increasing the FCC ratio to 25% reduces TSP to 0.09%, attributed to the solubilizing effect of aromatic fractions on asphaltenes.
SARA analysis demonstrated that the ω (Asp/Res) ratio and ω (Asp + Res) show consistent trends with TSP, reaching a maximum at 10% FCC incorporation, indicating weakened peptization capacity of resins. Beyond this threshold, the enhanced aromatic content of FCC slurry oil improved colloidal stability by suppressing asphaltene aggregation.
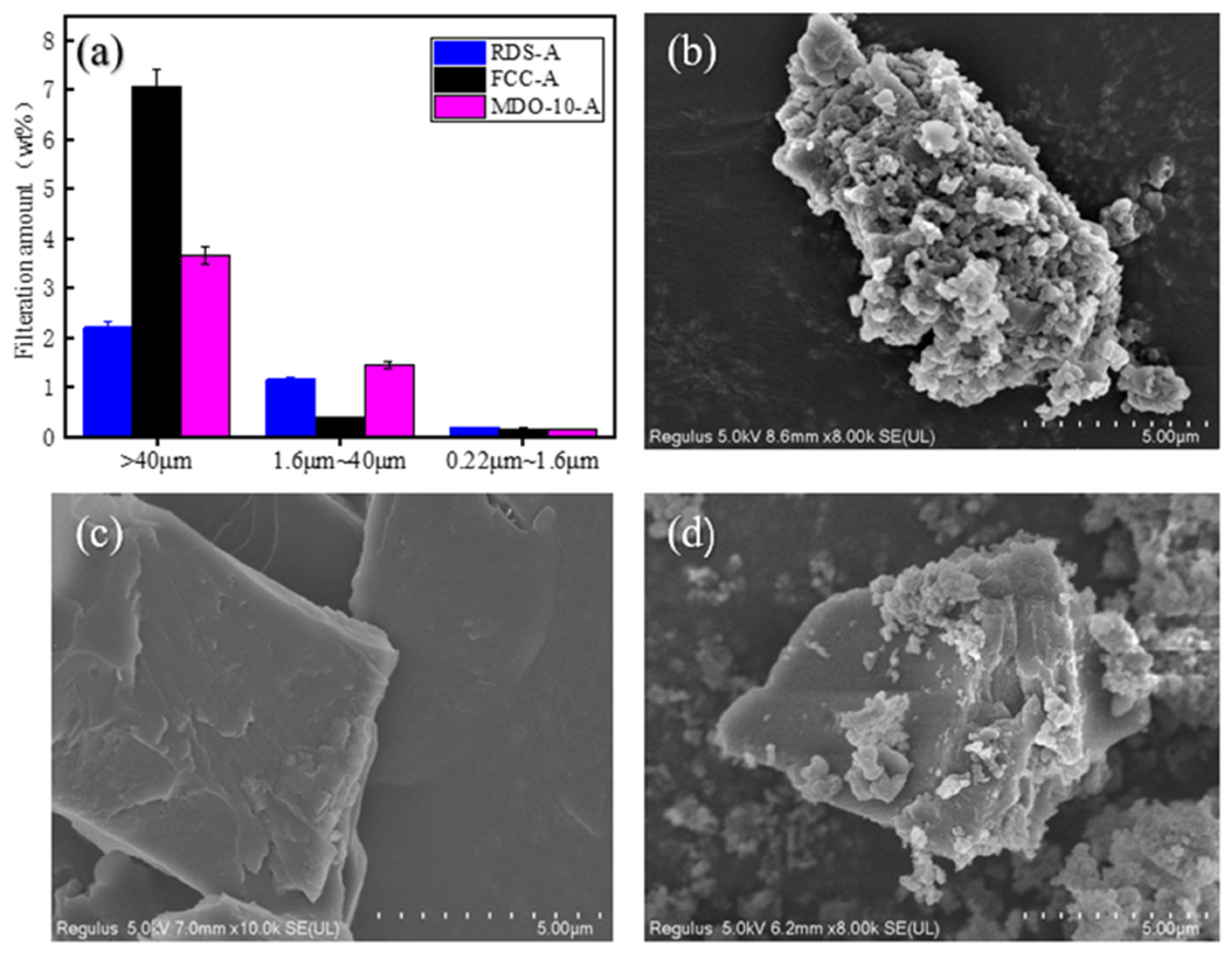
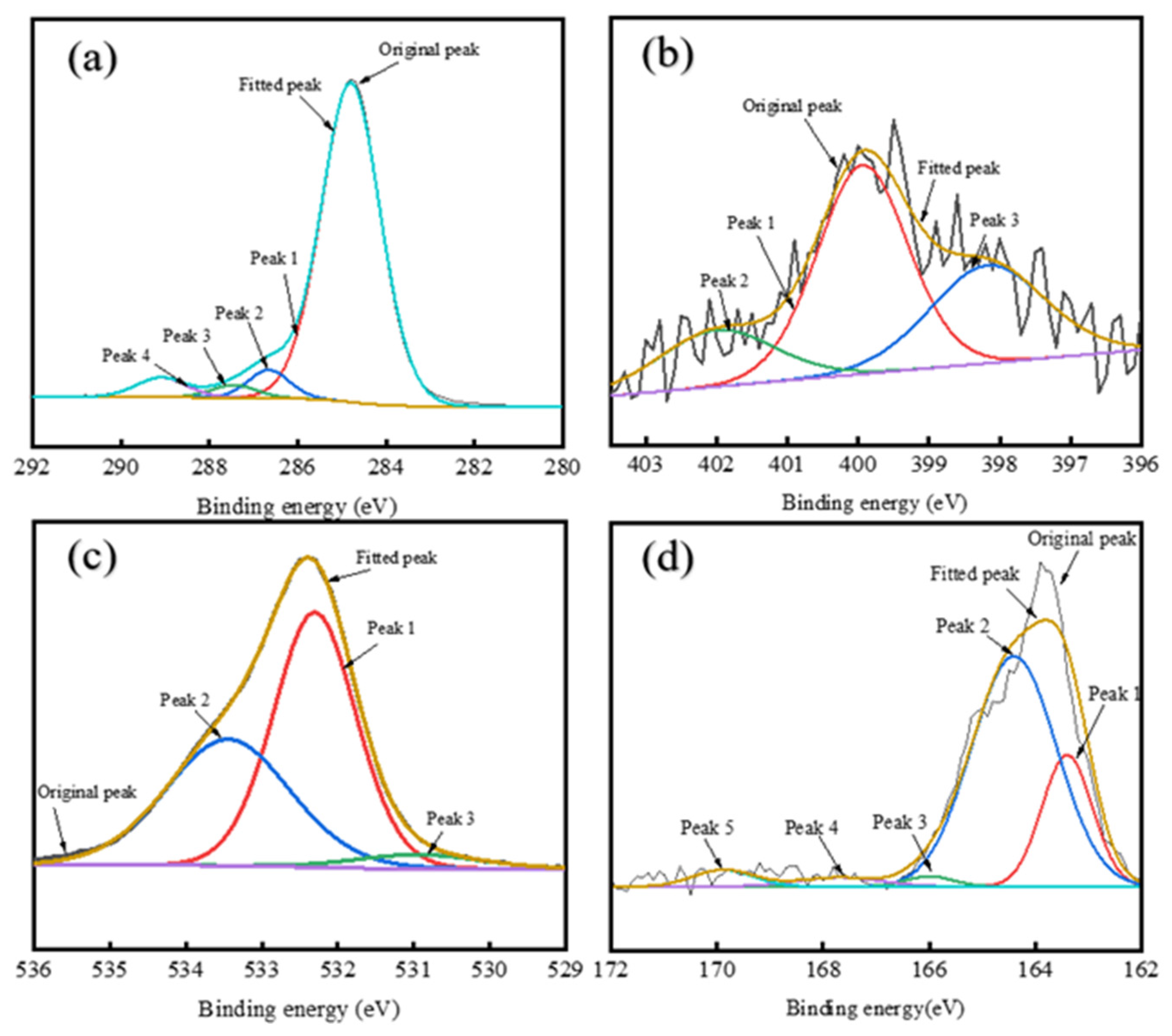
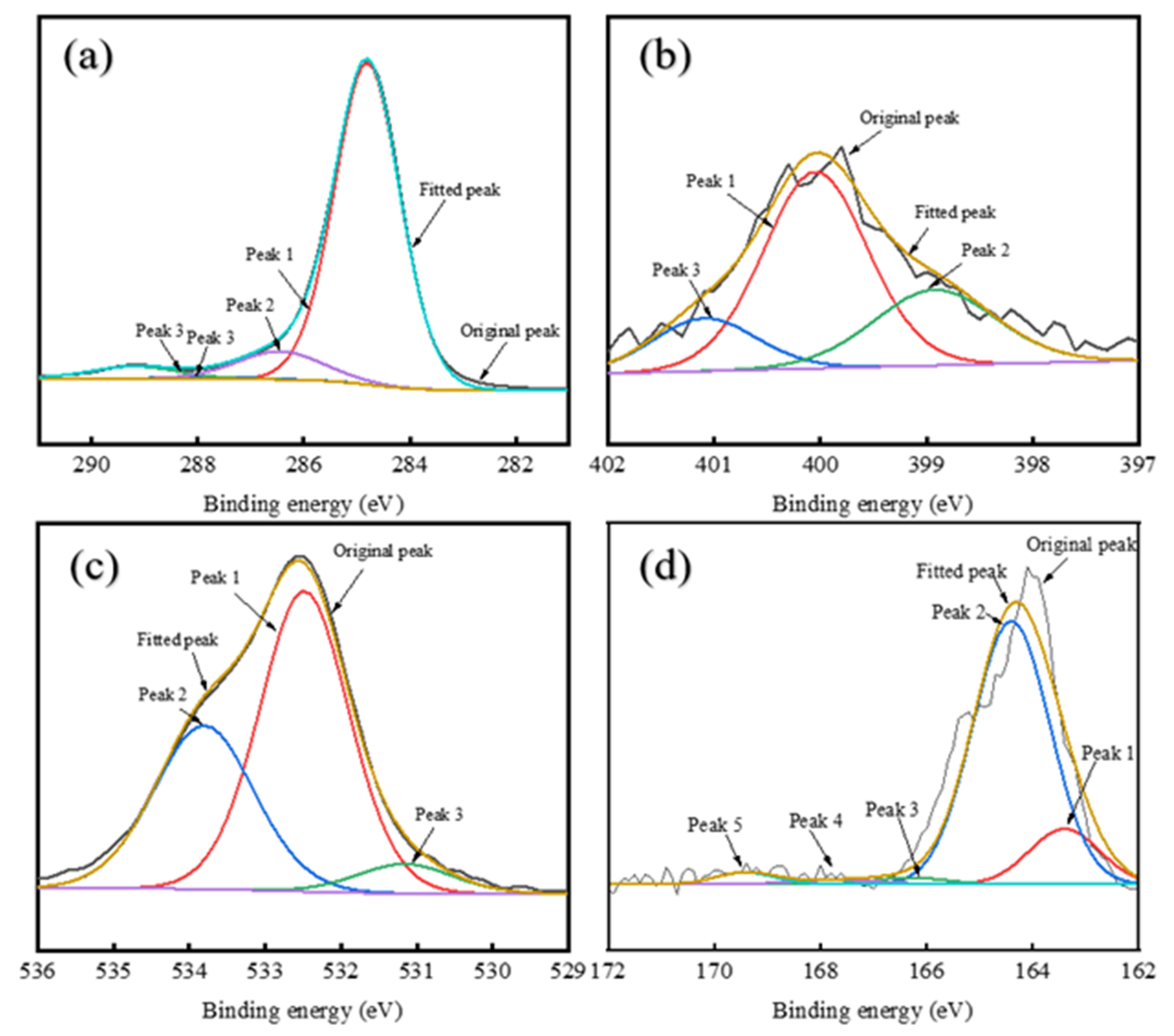
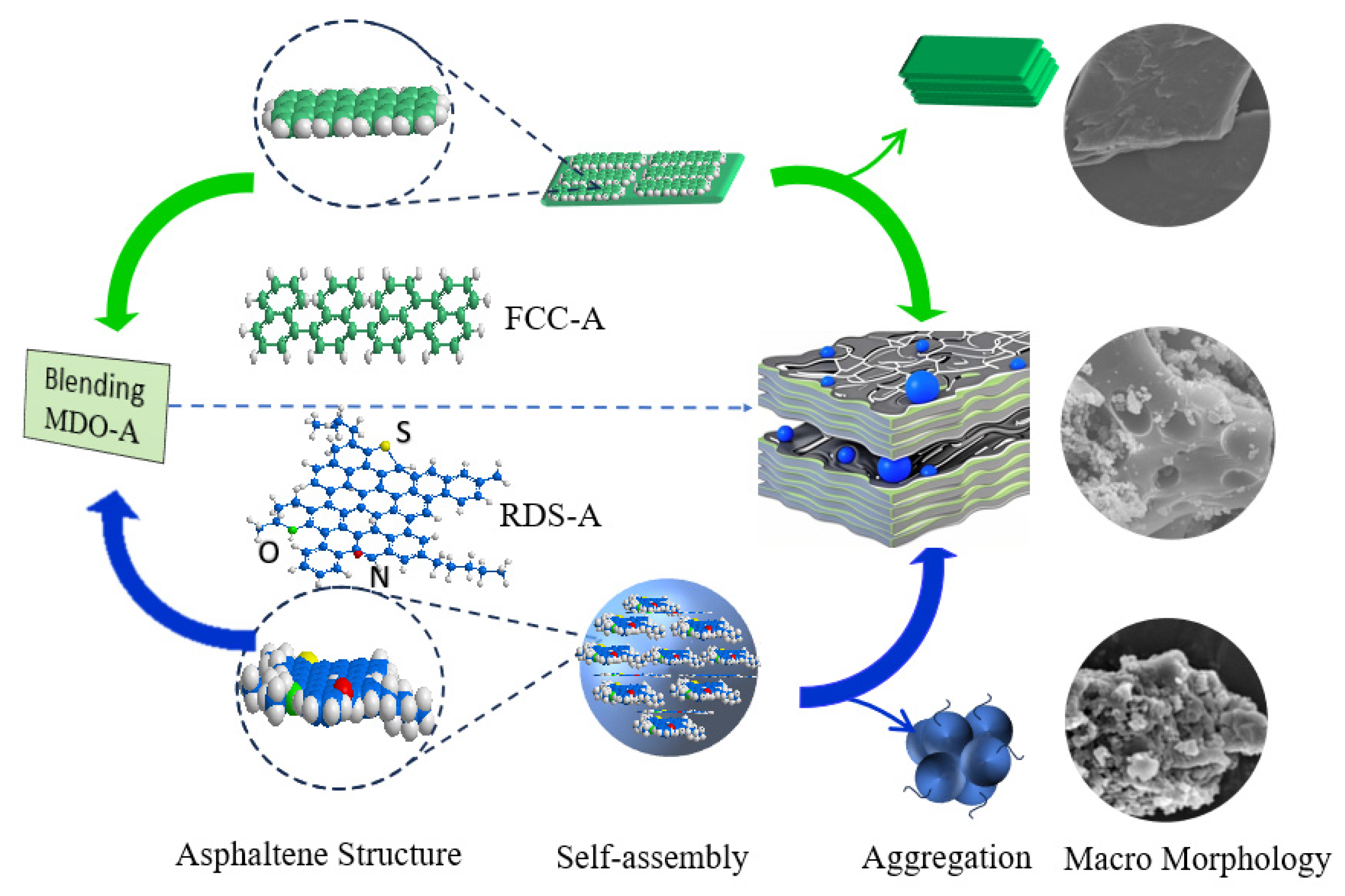
XRD and SEM analyses showed distinct structural differences between RDS-derived and FCC-derived asphaltenes. FCC-A had smaller molecular dimensions and higher aromaticity, forming planar aggregates, while RDS-A were larger and heteroatom-rich, leading to spherical aggregation. MDO-A exhibited FCC-dominated sediment architecture, with spherical RDS-A particles embedded in FCC-A matrices, confirming FCC asphaltenes as the primary sediment precursors. XPS confirmed that heteroatoms (N, S, O) in MDO-A asphaltenes were mainly derived from RDS-A.
This study delves into the compatibility between RDS heavy oil and FCC slurry oil—a binary system critical to industrial fuel formulation yet often overlooked. Our results reveal that marine fuel stability depends fundamentally on the balance between saturate and aromatic components. The non-monotonic variation in TSP with FCC slurry oil ratio, correlated with asphaltene structural changes, delivers practical guidance for optimizing blends to minimize sedimentation. This insight facilitates the production of stable low-sulfur marine fuels and improves operational flexibility for refineries employing deeply hydrotreated residues.