Mangrove Transplantation to the North: Carbon Sequestration Capacity—Drivers and Strategies
Abstract
1. Introduction
2. Materials and Methods
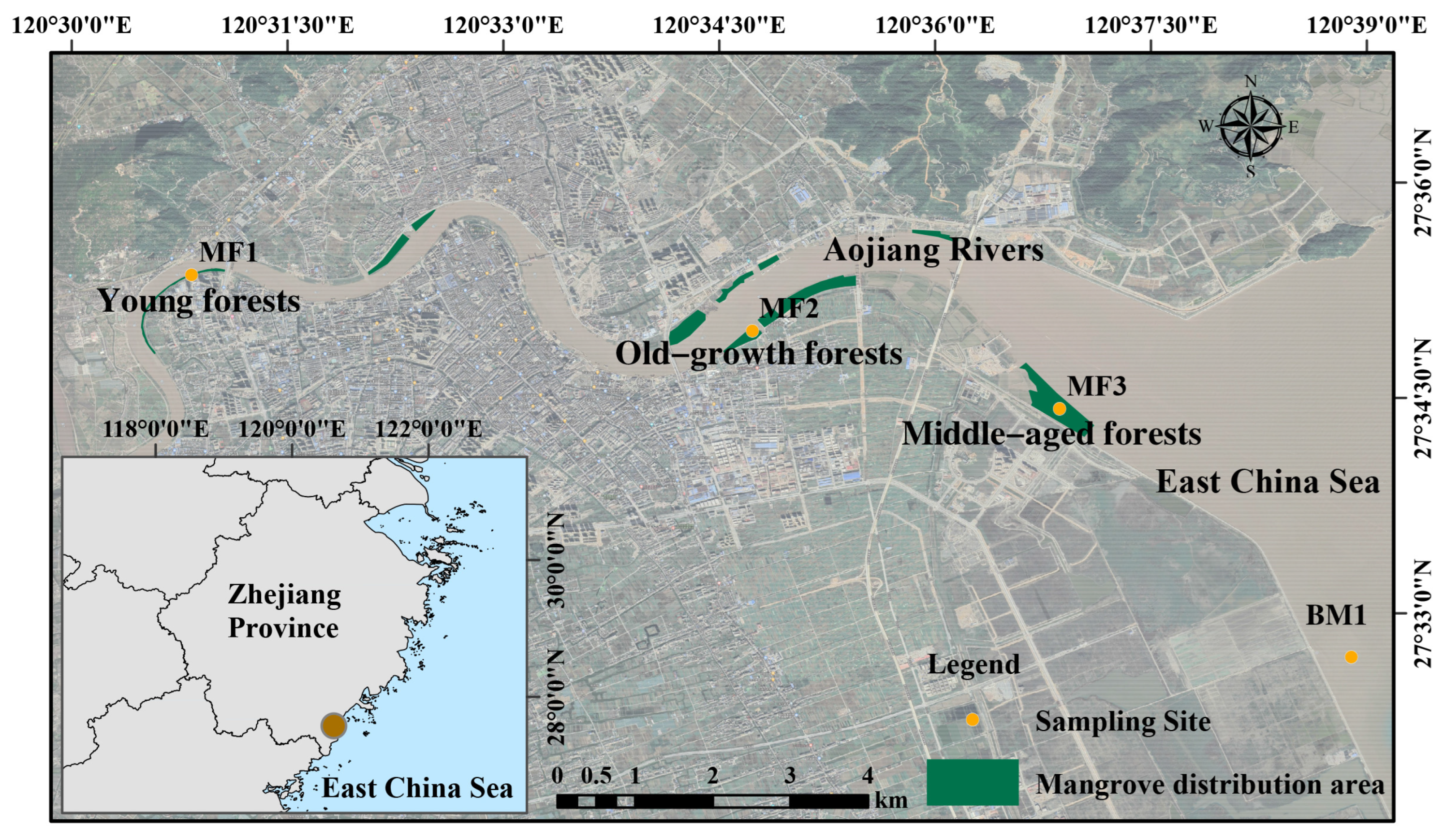
2.1. Study Area and Sampling
2.2. Measurement of Soil Properties
2.3. Analysis of Carbon Stock
2.4. Statistical Analysis
3. Results
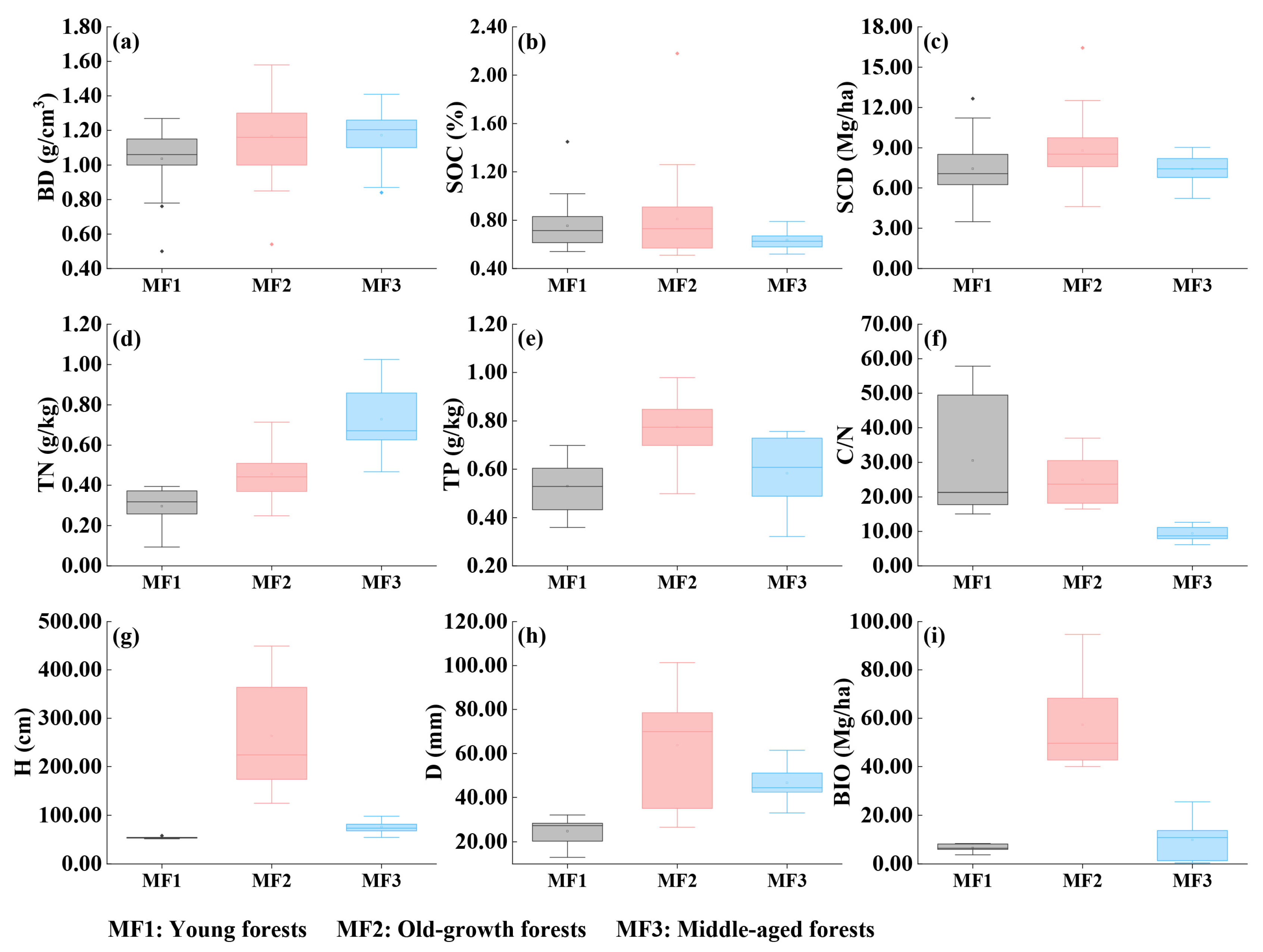
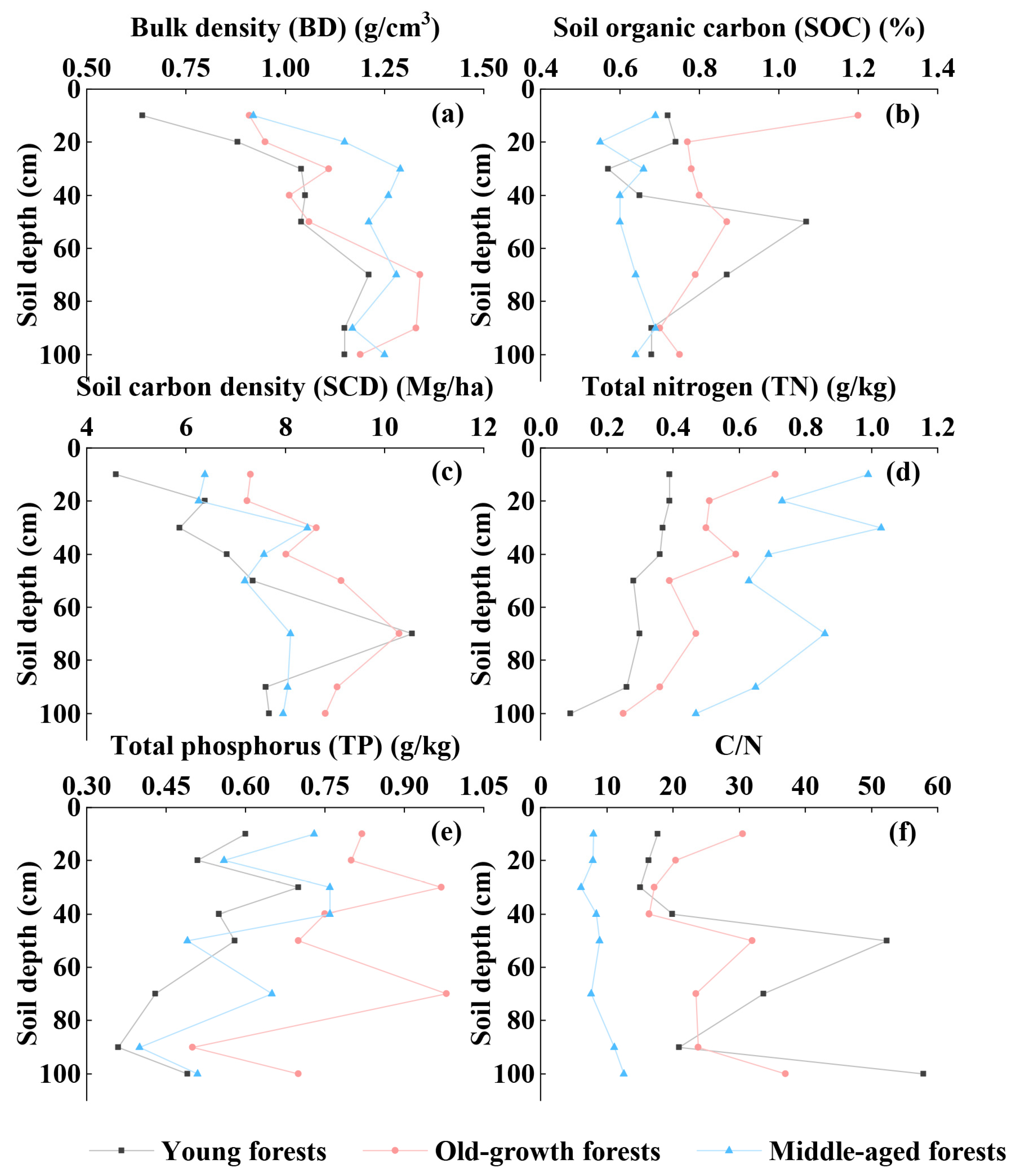
3.1. Physical and Chemical Characteristics of Mangrove Soil
3.2. Morphological Characteristics of Mangrove Plants
3.3. Allocation Pattern of Mangrove Carbon Stock
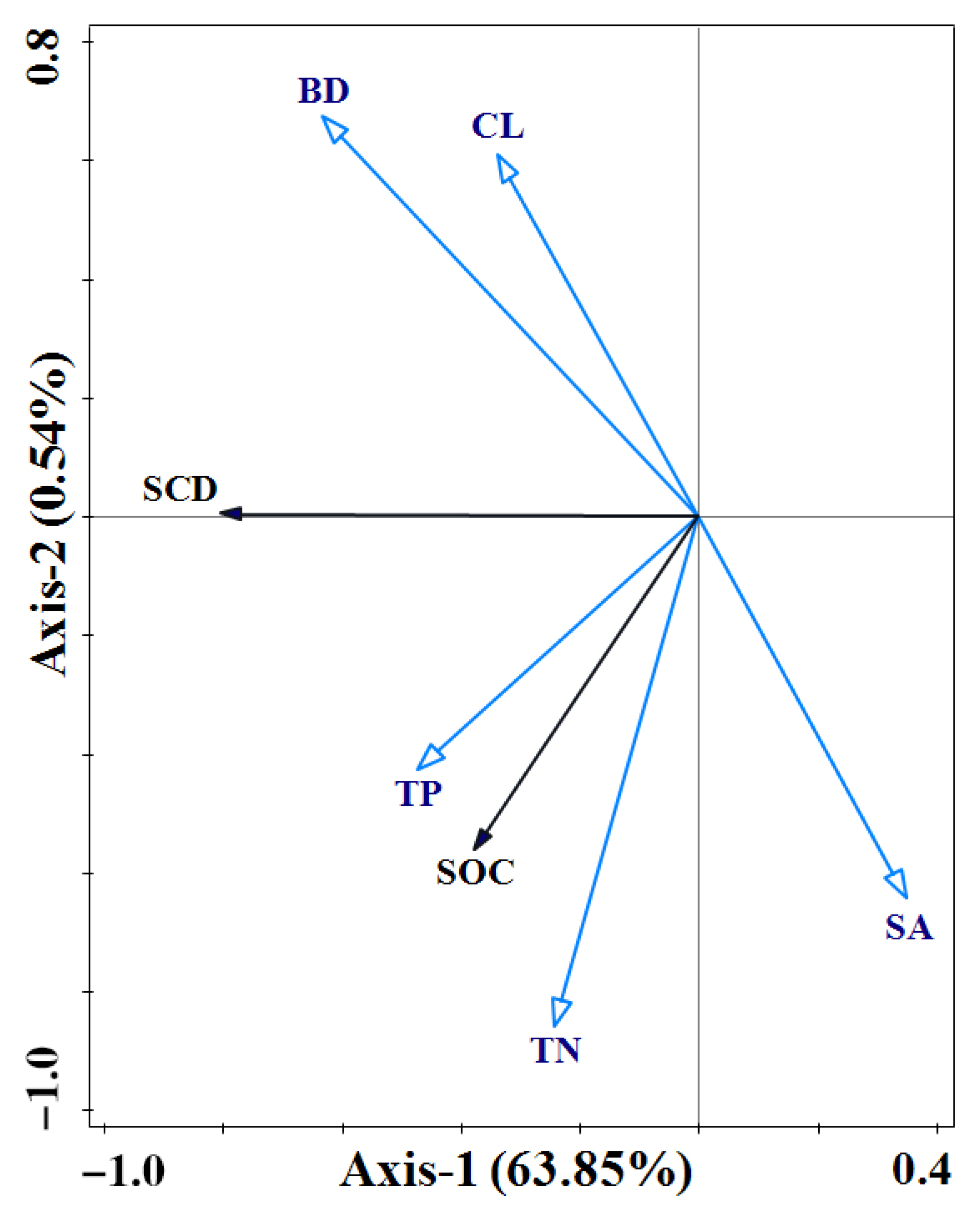
3.4. Relationship Between Mangrove Soil Carbon Sequestration and Environmental Factors
4. Discussion
4.1. Comparison of Carbon Sequestration Characteristics of Planted and Natural Mangroves
4.2. Drivers of Mangrove Carbon Sequestration
4.3. Difference Between Short-Term and Long-Term Carbon Accumulation in Planted Mangrove Forests
4.4. Suggestions for the Management and Protection of Transplanted Rehabilitated Planted Mangrove Forests and Implications for the Northward Migration of Mangrove Forests in China
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fest, B.J.; Swearer, S.E.; Arndt, S.K. A review of sediment carbon sampling methods in mangroves and their broader impacts on stock estimates for blue carbon ecosystems. Sci. Total Environ. 2022, 816, 151618. [Google Scholar] [CrossRef]
- Sahari, M.S.I.; Mohd Razali, N.A.; Redzuan, N.S.; Shah, A.M.; Awang, N.A.; Lee, L.H.; Juahir, H.; Muhammad Nor, S.M. Carbon stock variability of Setiu Lagoon mangroves and its relation to the environmental parameters. Glob. Ecol. Conserv. 2024, 53, e02994. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Change 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Rahman, C.A.; Tuahatu, J.W.; Lokollo, F.F.; Supusepa, J.; Hulopi, M.; Permatahati, Y.I.; Lewerissa, Y.A.; Wardiatno, Y. Mangrove ecosystems in Southeast Asia region: Mangrove extent, blue carbon potential and CO(2) emissions in 1996–2020. Sci. Total Environ. 2024, 915, 170052. [Google Scholar] [CrossRef]
- Stankovic, M.; Mishra, A.K.; Rahayu, Y.P.; Lefcheck, J.; Murdiyarso, D.; Friess, D.A.; Corkalo, M.; Vukovic, T.; Vanderklift, M.A.; Farooq, S.H.; et al. Blue carbon assessments of seagrass and mangrove ecosystems in South and Southeast Asia: Current progress and knowledge gaps. Sci. Total Environ. 2023, 904, 166618. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, B. Blue carbon sink function and carbon neutrality potential of mangroves. Sci. Total Environ. 2022, 822, 153438. [Google Scholar] [CrossRef]
- Yu, C.; Feng, J.; Yue, W.; Wei, L.; Ma, Y.; Huang, X.; Ling, J.; Dong, J. The role of blue carbon stocks becomes more labile with mangrove development. Ecol. Indic. 2023, 154, 110634. [Google Scholar] [CrossRef]
- Rani, V.; Schwing, P.T.; Jayachandran, P.R.; Preethy, C.M.; Sreelekshmi, S.; Joseph, P.; Bijoy Nandan, S. Carbon stocks and sequestration rate in mangroves and its major influencing factors from highly urbanised port city, southern India. J. Environ. Manag. 2023, 335, 117542. [Google Scholar] [CrossRef]
- Tang, D.; Liu, X.; Xia, Z.; Hou, J.; Yang, X.; Li, P.; Yuan, X. Sources of organic matter and carbon stocks in two mangrove sediment cores and surface sediment samples from Qinglan Bay, China. Sci. Total Environ. 2023, 893, 164897. [Google Scholar] [CrossRef]
- Palacios Peñaranda, M.L.; Cantera Kintz, J.R.; Peña Salamanca, E.J. Carbon stocks in mangrove forests of the Colombian Pacific. Estuar. Coast. Shelf Sci. 2019, 227, 106299. [Google Scholar] [CrossRef]
- Allais, L.; Thibodeau, B.; Khan, N.S.; Crowe, S.A.; Cannicci, S.; Not, C. Salinity, mineralogy, porosity, and hydrodynamics as drivers of carbon burial in urban mangroves from a megacity. Sci. Total Environ. 2024, 912, 168955. [Google Scholar] [CrossRef]
- Manoj, K.; Arumugam, T.; Prakash, A. A comparative study on carbon sequestration potential of disturbed and undisturbed mangrove ecosystems in Kannur district, Kerala, South India. Results Eng. 2024, 21, 101716. [Google Scholar]
- Pham, V.T.H.; Lu, P.; Aagaard, P.; Zhu, C.; Hellevang, H. On the potential of CO2–water–rock interactions for CO2 storage using a modified kinetic model. Int. J. Greenh. Gas Control 2011, 5, 1002–1015. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shu, Q.S.; Xie, L.Y.; Liu, Z.X.; Si, B.C. Joint Multifractal Analysis of Scaling Relationships Between Soil Water-Retention Parameters and Soil Texture. Pedosphere 2011, 21, 373–379. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X. Nanoscale soil-water retention mechanism of unsaturated clay via MD and machine learning. Comput. Geotech. 2023, 163, 105678. [Google Scholar] [CrossRef]
- Pan, J.; Wang, J.; Zhang, R.; Tian, D.; Cheng, X.; Wang, S.; Chen, C.; Yang, L.; Niu, S. Microaggregates regulated by edaphic properties determine the soil carbon stock in Tibetan alpine grasslands. Catena 2021, 206, 105570. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Vittal, K.P.R.; Venkateswarlu, B.; Wani, S.P.; Sahrawat, K.L.; Marimuthu, S.; Kundu, S. Carbon Stocks in Different Soil Types under Diverse Rainfed Production Systems in Tropical India. Commun. Soil Sci. Plant Anal. 2009, 40, 2338–2356. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Y.; Zhao, B.; Hong, H.; Liao, R.; Li, J.; Liu, J.; Lu, H.; Yan, C. Coastal soil texture controls soil organic carbon distribution and storage of mangroves in China. Catena 2021, 207, 105709. [Google Scholar] [CrossRef]
- Zeng, R.; Wei, Y.; Huang, J.; Chen, X.; Cai, C. Soil organic carbon stock and fractional distribution across central-south China. Int. Soil Water Conserv. Res. 2021, 9, 620–630. [Google Scholar] [CrossRef]
- Strukelj, M.; Parker, W.; Corcket, E.; Augusto, L.; Khlifa, R.; Jactel, H.; Munson, A.D. Tree species richness and water availability interact to affect soil microbial processes. Soil Biol. Biochem. 2021, 155, 108180. [Google Scholar] [CrossRef]
- Huang, W.; Hall, S.J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter. Nat. Commun. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Zhang, W.; Kolbe, H.; Zhang, R. Research Progress of SOC Functions and Transformation Mechanisms. Sci. Agric. Sin. 2020, 53, 317–331. [Google Scholar]
- Xie, Z.; Li, H.; Yuan, Y.; Hu, W.; Luo, G.; Huang, L.; Chen, M.; Wu, W.; Yan, G.; Sun, X. The spatial patterns and driving mechanisms of blue carbon loss and gain in a typical mangrove ecosystem: A case study of Beihai, Guangxi Province of China. Sci. Total Environ. 2023, 905, 167241. [Google Scholar] [CrossRef]
- Cameron, C.; Kennedy, B.; Tuiwawa, S.; Goldwater, N.; Soapi, K.; Lovelock, C.E. High variance in community structure and ecosystem carbon stocks of Fijian mangroves driven by differences in geomorphology and climate. Environ. Res. 2021, 192, 110213. [Google Scholar] [CrossRef]
- Ouyang, X.; Guo, F.; Lee, S.Y. Multiple drivers for carbon stocks and fluxes in different types of mangroves. Sci. Total Environ. 2024, 906, 167511. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Sillanpaa, M.; Hayes, M.A.; Bachri, S.; Saragi-Sasmito, M.F.; Sidik, F.; Hanggara, B.B.; Mofu, W.Y.; Rumbiak, V.I.; Hendri; et al. Mangrove blue carbon stocks and dynamics are controlled by hydrogeomorphic settings and land-use change. Glob. Change Biol. 2020, 26, 3028–3039. [Google Scholar] [CrossRef]
- Xu, M.; Sun, C.; Du, Z.; Zhu, X. Impacts of aquaculture on the area and soil carbon stocks of mangrove: A machine learning study in China. Sci. Total Environ. 2023, 859, 160173. [Google Scholar] [CrossRef]
- Merecí-Guamán, J.; Casanoves, F.; Delgado-Rodríguez, D.; Ochoa, P.; Cifuentes-Jara, M. Impact of Shrimp Ponds on Mangrove Blue Carbon Stocks in Ecuador. Forests 2021, 12, 816. [Google Scholar] [CrossRef]
- Bourgeois, C.F.; Mackenzie, R.A.; Sharma, S.; Bhomia, R.K.; Johnson, N.G.; Rovai, A.S.; Worthington, T.A.; Krauss, K.W.; Analuddin, K.; Bukoski, J.J.; et al. Four decades of data indicate that planted mangroves stored up to 75% of the carbon stocks found in intact mature stands. Sci. Adv. 2024, 10, 12. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Guan, D.; Xiao, L.; Singh, M. The potential of mature Sonneratia apetala plantations to enhance carbon stocks in the Zhanjiang Mangrove National Nature Reserve. Ecol. Indic. 2021, 133, 108415. [Google Scholar] [CrossRef]
- Silva, G.R.D.; Lazaro, M.L.; Queiroz, H.M.; Ferreira, A.D.; Diaz Ramos, R.A.; Machado, W.T.V.; Otero, X.L.; Ferreira, T.O.; Nobrega, G.N. How do replanted mangroves affect phosphorus dynamics in their soils? J. Environ. Manag. 2024, 366, 121915. [Google Scholar] [CrossRef]
- Lu, R. Soil Agrochemical Analytical Methods; Chinese Agriculture and Technology Press Publisher: Beijing, China, 1999. [Google Scholar]
- Guo, L.; Sun, Z.; Ouyang, Z.; Han, D.; Li, F. A comparison of soil quality evaluation methods for Fluvisol along the lower Yellow River. Catena 2017, 152, 135–143. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Cornforth, I.S.; Walmsley, D. Methods of measuring available nutrients in West Indian soils. Plant Soil 1971, 35, 389–399. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; An, S. Testing association between soil bacterial diversity and soil carbon storage on the Loess Plateau. Sci. Total Environ. 2018, 626, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Bai, J.; Gou, R.; Cui, X.; Feng, J.; Dai, Z.; Diao, X.; Zhu, X.; Lin, G. Relationships between above- and below-ground carbon stocks in mangrove forests facilitate better estimation of total mangrove blue carbon. Carbon Balance Manag. 2021, 16, 8. [Google Scholar] [CrossRef]
- Jin, C.; Wang, J.; Zheng, J.; Chen, Q.; Li, J.; Lu, X. An assessment method of Kandelia obovata population biomass. Acta Ecol. Sin. 2012, 32, 3414–3422. [Google Scholar]
- Meng, Y.; Gou, R.; Bai, J.; Moreno-Mateos, D.; Davis, C.C.; Wan, L.; Song, S.; Zhang, H.; Zhu, X.; Lin, G. Spatial patterns and driving factors of carbon stocks in mangrove forests on Hainan Island, China. Glob. Ecol. Biogeogr. 2022, 31, 1692–1706. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Peart, M.R.; Chen, Y.; Peng, Y. Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of South China. For. Ecol. Manag. 2013, 310, 539–546. [Google Scholar] [CrossRef]
- Malik, A.; Rahim, A.; Jalil, A.R.; Amir, M.F.; Arif, D.S.; Rizal, M.; Husain, J.; William, D.; Jihad, N. Mangrove blue carbon stocks estimation in South Sulawesi Indonesia. Cont. Shelf Res. 2023, 269, 105139. [Google Scholar] [CrossRef]
- Trettin, C.C.; Dai, Z.; Tang, W.; Lagomasino, D.; Thomas, N.; Lee, S.K.; Simard, M.; Ebanega, M.O.; Stoval, A.; Fatoyinbo, T.E. Mangrove carbon stocks in Pongara National Park, Gabon. Estuar. Coast. Shelf Sci. 2021, 259, 107432. [Google Scholar] [CrossRef]
- Nam, V.N.; Sasmito, S.D.; Murdiyarso, D.; Purbopuspito, J.; MacKenzie, R.A. Carbon stocks in artificially and naturally regenerated mangrove ecosystems in the Mekong Delta. Wetl. Ecol. Manag. 2016, 24, 231–244. [Google Scholar] [CrossRef]
- Buczko, U.; Cruz-García, R.; Harmuth, J.; Kalbe, J.; Scharnweber, T.; Stoll, A.; Wilmking, M.; Jurasinski, G. Soil and vegetation factors affecting carbon storage in a coastal forest in NE Germany. Geoderma Reg. 2023, 33, e00629. [Google Scholar] [CrossRef]
- Yu, C.; Feng, J.; Liu, K.; Wang, G.; Zhu, Y.; Chen, H.; Guan, D. Changes of ecosystem carbon stock following the plantation of exotic mangrove Sonneratia apetala in Qi’ao Island, China. Sci. Total Environ. 2020, 717, 137–142. [Google Scholar] [CrossRef]
- Carnell, P.E.; Palacios, M.M.; Waryszak, P.; Trevathan-Tackett, S.M.; Masque, P.; Macreadie, P.I. Blue carbon drawdown by restored mangrove forests improves with age. J. Environ. Manag. 2022, 306, 114301. [Google Scholar] [CrossRef]
- Kusumaningtyas, M.A.; Hutahaean, A.A.; Fischer, H.W.; Pérez-Mayo, M.; Ransby, D.; Jennerjahn, T.C. Variability in the organic carbon stocks, sources, and accumulation rates of Indonesian mangrove ecosystems. Estuar. Coast. Shelf Sci. 2019, 218, 310–323. [Google Scholar] [CrossRef]
- Raffeld, A.M.; Bradford, M.A.; Jackson, R.D.; Rath, D.; Sanford, G.R.; Tautges, N.; Oldfield, E.E. The importance of accounting method and sampling depth to estimate changes in soil carbon stocks. Carbon Balance Manag. 2024, 19, 2. [Google Scholar] [CrossRef]
- Bai, J.; Meng, Y.; Gou, R.; Lyu, J.; Dai, Z.; Diao, X.; Zhang, H.; Luo, Y.; Zhu, X.; Lin, G.; et al. Mangrove diversity enhances plant biomass production and carbon storage in Hainan island, China. Funct. Ecol. 2021, 35, 774–786. [Google Scholar] [CrossRef]
- Jiang, Z.; Sanders, C.J.; Xin, K.; Wang, F.; Sheng, N.; Xiong, Y. Increasing carbon and nutrient burial rates in mangroves coincided with coastal aquaculture development and water eutrophication in NE Hainan, China. Mar. Pollut. Bull. 2024, 199, 115934. [Google Scholar] [CrossRef] [PubMed]
- Kida, M.; Watanabe, I.; Kinjo, K.; Kondo, M.; Yoshitake, S.; Tomotsune, M.; Iimura, Y.; Umnouysin, S.; Suchewaboripont, V.; Poungparn, S.; et al. Organic carbon stock and composition in 3.5-m core mangrove soils (Trat, Thailand). Sci. Total Environ. 2021, 801, 149682. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Pourebrahim, S.; Khorasani, N.; Danehkar, A.; Etemadi, H.; Tanha Ziyarati, M.; Moeinaddini, M. Carbon stock in three mangrove forests in north Persian Gulf. Environ. Earth Sci. 2021, 81, 20. [Google Scholar] [CrossRef]
- Slamet, N.S.; Dargusch, P.; Aziz, A.A.; Wadley, D. Mangrove vulnerability and potential carbon stock loss from land reclamation in Jakarta Bay, Indonesia. Ocean Coast. Manag. 2020, 195, 105283. [Google Scholar] [CrossRef]
- Lunstrum, A.; Chen, L. Soil carbon stocks and accumulation in young mangrove forests. Soil Biol. Biochem. 2014, 75, 223–232. [Google Scholar] [CrossRef]
- Harishma, K.M.; Sandeep, S.; Sreekumar, V.B. Biomass and carbon stocks in mangrove ecosystems of Kerala, southwest coast of India. Ecol. Process. 2020, 9, 31. [Google Scholar] [CrossRef]
- Morrissette, H.K.; Baez, S.K.; Beers, L.; Bood, N.; Martinez, N.D.; Novelo, K.; Andrews, G.; Balan, L.; Beers, C.S.; Betancourt, S.A.; et al. Belize Blue Carbon: Establishing a national carbon stock estimate for mangrove ecosystems. Sci. Total Environ. 2023, 870, 161829. [Google Scholar] [CrossRef]
- Nobrega, G.N.; Otero, X.L.; Macias, F.; Ferreira, T.O. Phosphorus geochemistry in a Brazilian semiarid mangrove soil affected by shrimp farm effluents. Environ. Monit. Assess. 2014, 186, 5749–5762. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.; Queiroz, H.M.; Nobrega, G.N.; de Oliveira Filho, R.L.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Harbo, L.S.; Olesen, J.E.; Liang, Z.; Christensen, B.T.; Elsgaard, L. Estimating organic carbon stocks of mineral soils in Denmark: Impact of bulk density and content of rock fragments. Geoderma Reg. 2022, 30, 7. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Masqué, P.; Glass, L.; Benson, L.; Kennedy, H.; Duarte, C.M.; Garcia-Orellana, J.; Benitez-Nelson, C.R.; Humphries, M.S.; Ratefinjanahary, I.; et al. Losses of Soil Organic Carbon with Deforestation in Mangroves of Madagascar. Ecosystems 2020, 24, 1–19. [Google Scholar] [CrossRef]
- Adotey, J.; Acheampong, E.; Aheto, D.W.; Blay, J. Carbon Stocks Assessment in a Disturbed and Undisturbed Mangrove Forest in Ghana. Sustainability 2022, 14, 12782. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, E.; Li, R.; Wu, X.; Huang, Y.; Lin, G.; Li, B. The urgent need to reduce phosphorus discharges for sustainable mangrove wetland management. Water Res. 2024, 258, 121821. [Google Scholar] [CrossRef]
- Ola, A.; Staples, T.L.; Robinson, N.; Lovelock, C.E. Plasticity in the Above- and Below-Ground Development of Mangrove Seedlings in Response to Variation in Soil Bulk Density. Estuaries Coasts 2020, 43, 111–119. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Ola, A.; Gauthier, A.R.G.; Xiong, Y.; Lovelock, C.E. The roots of blue carbon: Responses of mangrove stilt roots to variation in soil bulk density. Biol. Lett. 2019, 15, 20180866. [Google Scholar] [CrossRef]
- Ola, A.; Schmidt, S.; Lovelock, C.E. The effect of heterogeneous soil bulk density on root growth of field-grown mangrove species. Plant Soil 2018, 432, 91–105. [Google Scholar] [CrossRef]
- Dajam, A.S.; Keshta, A.E.; Bindajam, A.A.; Al-Qthanin, R.N.; Arshad, M.; Eid, E.M. Mangrove (Avicennia marina) Conservation Contributed to a Higher Carbon Sequestration Rate at Protected Sites Compared to Overgrazed Mangrove Forests. J. Soil Sci. Plant Nutr. 2024, 24, 4868–4879. [Google Scholar] [CrossRef]
- Barreto, M.B.; Lo Mónaco, S.; Díaz, R.; Barreto-Pittol, E.; López, L.; Peralba, M.d.C.R. Soil organic carbon of mangrove forests (Rhizophora and Avicennia) of the Venezuelan Caribbean coast. Org. Geochem. 2016, 100, 51–61. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Jiao, N.; Wang, H.; Xu, G.; Arico, S. Blue carbon on the rise: Challenges and opportunities. Natl. Sci. Rev. 2018, 5, 464. [Google Scholar] [CrossRef]
- Thura, K.; Serrano, O.; Gu, J.; Fang, Y.; Htwe, H.Z.; Zhu, Y.; Huang, R.; Agusti, S.; Duarte, C.M.; Wang, H.; et al. Mangrove restoration built soil organic carbon stocks over six decades: A chronosequence study. J. Soils Sediments 2022, 23, 1193–1203. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W.; et al. Mangrove reforestation provides greater blue carbon benefit than afforestation for mitigating global climate change. Nat. Commun. 2023, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, X.; Du, M.; Sanders, C.J.; Li, C.; Tang, J.; Yang, H. Replacing Spartina alterniflora with northward-afforested mangroves has the potential to acquire extra blue carbon. Sci. Total Environ. 2024, 921, 170952. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Pennings, S.C.; Zhang, Y. Using a marsh organ to predict future plant communities in a Chinese estuary invaded by an exotic grass and mangrove. Limnol. Oceanogr. 2018, 63, 2595–2605. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, N.; Yang, S.; Li, Y.; Yang, L.; Cao, W. Source and stability of soil organic carbon jointly regulate soil carbon pool, but source alteration is more effective in mangrove ecosystem following Spartina alterniflora invasion. Catena 2024, 235, 107681. [Google Scholar] [CrossRef]
- Xu, T.; Li, R.; Wang, W.; Tang, L. Subtropical mangroves poleward shift to the Yangtze Estuary under different carbon emission scenarios. J. Hydrol. 2024, 637, 131356. [Google Scholar] [CrossRef]
- Xu, C.; Xue, Z.; Jiang, M.; Lyu, X.; Zou, Y.; Gao, Y.; Sun, X.; Wang, D.; Li, R. Simulating potential impacts of climate change on the habitats and carbon benefits of mangroves in China. Glob. Ecol. Conserv. 2024, 54, e03048. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gu, J.D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ricklefs, R.E.; Huang, Z.J.; Zan, Q.J.; Yu, S.X. Winter temperature structures mangrove species distributions and assemblage composition in China. Glob. Ecol. Biogeogr. 2018, 27, 1492–1506. [Google Scholar] [CrossRef]

| Parameters | Mean | SD | SE | CV | 95% Confidence Interval |
|---|---|---|---|---|---|
| Soil parameters | |||||
| BD (g/cm3) | 1.14 | 0.20 | 0.02 | 0.17 | (1.10, 1.18) |
| SOC (%) | 0.73 | 0.24 | 0.03 | 0.33 | (0.67, 0.79) |
| SCD (Mg C/ha) | 7.93 | 1.95 | 0.22 | 0.25 | (7.38, 8.48) |
| TN (g/kg) | 0.49 | 0.23 | 0.04 | 0.46 | (0.26, 0.72) |
| TP (g/kg) | 0.63 | 0.17 | 0.03 | 0.27 | (0.53, 0.73) |
| SA (%) | 2.02 | 0.88 | 0.20 | 0.44 | (1.59, 2.45) |
| SI (%) | 67.19 | 4.37 | 0.98 | 0.07 | (64.90, 69.48) |
| CL (%) | 30.79 | 4.85 | 1.08 | 0.16 | (28.48, 31.10) |
| SCS (Mg C/ha) | 78.75 | 10.76 | 2.07 | 0.14 | (74.25, 83.25) |
| Plant parameters | |||||
| H (cm) | 140.73 | 120.45 | 24.59 | 0.86 | (84.66, 196.8) |
| D (mm) | 47.52 | 22.94 | 4.68 | 0.48 | (37.27, 57.77) |
| DMF (plant/m2) | 3.50 | 3.60 | 1.27 | 1.03 | (0.20, 6.80) |
| BIO (Mg/ha) | 26.82 | 27.32 | 5.58 | 1.02 | (15.48, 38.16) |
| PCS (Mg C/ha) | 12.28 | 13.25 | 2.07 | 1.08 | (6.97, 17.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Lv, Y.; Gong, Y.; Su, J.; Wang, L.; Wu, S.; Lin, X.; Lai, Q.; Xu, Y.; Duan, X. Mangrove Transplantation to the North: Carbon Sequestration Capacity—Drivers and Strategies. J. Mar. Sci. Eng. 2025, 13, 1577. https://doi.org/10.3390/jmse13081577
Zhou K, Lv Y, Gong Y, Su J, Wang L, Wu S, Lin X, Lai Q, Xu Y, Duan X. Mangrove Transplantation to the North: Carbon Sequestration Capacity—Drivers and Strategies. Journal of Marine Science and Engineering. 2025; 13(8):1577. https://doi.org/10.3390/jmse13081577
Chicago/Turabian StyleZhou, Kewei, Yujuan Lv, Yang Gong, Jing Su, Lei Wang, Shengmin Wu, Xi Lin, Qiuying Lai, Yixin Xu, and Xingyi Duan. 2025. "Mangrove Transplantation to the North: Carbon Sequestration Capacity—Drivers and Strategies" Journal of Marine Science and Engineering 13, no. 8: 1577. https://doi.org/10.3390/jmse13081577
APA StyleZhou, K., Lv, Y., Gong, Y., Su, J., Wang, L., Wu, S., Lin, X., Lai, Q., Xu, Y., & Duan, X. (2025). Mangrove Transplantation to the North: Carbon Sequestration Capacity—Drivers and Strategies. Journal of Marine Science and Engineering, 13(8), 1577. https://doi.org/10.3390/jmse13081577





