The Geochemical Characteristics of the Fatty Acids in the Core Sediments in the Northern South Yellow Sea
Abstract
1. Introduction
2. Methods
2.1. Geological Sampling
2.2. Sample Preparation Experiments
2.3. The Chromatography–Mass Spectrometry Analysis
2.4. Quality Assurance/Quality Control
3. Results
4. Discussion
4.1. The Source of Sedimentary Organic Matter
4.2. Diagenetic Evolution of Fatty Acids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chuecas, L.; Riley, J.P. Component fatty acids of the total lipids of some marine phytoplankton. J. Mar. Biol. Assoc. U. K. 1969, 49, 97–116. [Google Scholar] [CrossRef]
- Volkman, J.K.; Johns, R.B.; Gillan, F.T.; Perry, G.J.; Bavor, H.J., Jr. Microbial lipids of an intertidal sediment—I. Fatty acids and hydrocarbon. Geochim. Cosmochim. Acta 1980, 44, 1133–1143. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I.; Mansour, M.P.; Sikes, E.L.; Gelin, F. Microalgal biomarkers: A review of recent research developments. Org. Geochem. 1998, 29, 1163–1179. [Google Scholar] [CrossRef]
- Cranwell, P.A. Monocarboxylic acids in lake sediments: Indicators derived from terrestrial and aquatic biota of palaeoenvironmental trophic levels. Chem. Geol. 1974, 14, 1–14. [Google Scholar] [CrossRef]
- Cranwell, P.A.; Eglinton, G.; Robinson, N. Lipids of aquatic organisms as potential contributors to lacustrine sediments—II. Org. Geochem. 1987, 11, 513–527. [Google Scholar] [CrossRef]
- Pearson, E.J.; Farrimond, P.; Juggins, S. Lipid geochemistry of lake sediments from semi-arid Spain: Relationships with source inputs and environmental factors. Org. Geochem. 2007, 38, 1169–1195. [Google Scholar] [CrossRef]
- Taub, R.; Ganzert, L.; Grossart, H.-P.; Gleixner, G.; Premke, K. Organic matter quality structures benthic fatty acid patterns and the abundance of fungi and bacteria in temperate lakes. Sci. Total Environ. 2018, 610, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Ficken, K.J.; Barber, K.E.; Eglinton, G. Lipid biomarker, δ13C and plant macrofossil stratigraphy of a Scottish montane peat bog over the last two millennia. Org. Geochem. 1998, 28, 217–237. [Google Scholar] [CrossRef]
- Hou, J.; Huang, Y.; Wang, Y.; Shuman, B.; Oswald, W.W.; Faison, E.; Foster, D.R. Postglacial climate reconstruction based on compound-specific D/H ratios of fatty acids from Blood Pond, New England. Geochem. Geophys. Geosyst. 2006, 7, 851. [Google Scholar] [CrossRef]
- Huguet, A.; Meador, T.B.; Laggoun-Defarge, F.; Könneke, M.; Wu, W.; Derenne, S.; Hinrichs, K.U. Production rates of bacterial tetraether lipids and fatty acids in peatland under varying oxygen concentrations. Geochim. Cosmochim. Acta 2017, 203, 103–116. [Google Scholar] [CrossRef]
- Soman, V.; Suresh, A.; Krishnankutty Remani, A.; Kalladathvalappil Venugopalan, V.; Keedakkadan, H.R. A Holistic Review on Diverse Lipid Biomarkers as Environmental Indicators: Extraction and Analysis from Sediments to Microbial Communities. Geomicrobiol. J. 2024, 41, 891–909. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, B.J.; Qian, J.S.; Xu, Y.Q. Study On organic geochemistry of fatty acids in recent sediments from Nansha sea area. Mar. Geol. Quat. Geol. 1996, 16, 23–31. [Google Scholar]
- Fu, M.Y.; Zhou, H.Y.; Wang, H.; Li, J.W.; Yi, X.J.; Yang, W.F. The fatty acids profile in core sediment in the pearl river estuary. Trans. Oceanol. Limnol. 2010, 32, 161–172. [Google Scholar]
- Jiang, S.C.; Fu, J.M.; Lin, M.F.; Yan, Z.P.; Tang, Y.Q. Fatty acid distribution in East China Sea sediments. Geochimica 1983, 12, 285–293. [Google Scholar]
- Jiang, S.C.; Fu, J.M.; Luan, Z.F. Distribution characteristics of unsaturated fatty acid of bacterial biomarkers in Okinawa trough. In Proceedings of Organic Geochemistry; Science Press: Beijing, China, 1986; pp. 168–175. [Google Scholar]
- Lin, W.D.; Zhou, Y.Z.; Shen, P. Composition, distribution and source of fatty acids in marine surface sediments from the middle of South China Sea. J. Guangxi Teach. Educ. Univ. (Nat. Sci. Ed.) 2004, 21, 11–15. [Google Scholar]
- Liu, Y.J.; Wang, J.T.; He, X.L. Distribution of fatty acid in sediments from red tide-frequent-occurrence area in East China Sea and their indications. Mar. Environ. Sci. 2012, 31, 803–807. [Google Scholar]
- Mao, S.Y.; Zhu, X.W.; Sun, Y.G.; Guan, H.X.; Wu, D.D.; Wu, N.Y. Identification of sulfate reducing bacteria and sulfur-oxidizing bacteria in marine sediments from Shenhu Area Northern South China sea: Implication from fatty acids. Mar. Geol. Quat. Geol. 2015, 35, 139–148. [Google Scholar]
- Zhao, Y.Y.; Luan, Z.F.; Sun, Z.Q. Characteristic of monatomic fatty acid and alkane of marine sediments in Beibu Gulf of the South China Sea. In Proceedings of Organic Geochemistry; Science Press: Beijing, China, 1987; pp. 1–9. [Google Scholar]
- Chen, M.; Li, D.W.; Zhang, H.; Wang, Z.; Zhao, M. Distinct variations and mechanisms for terrestrial OC 14C-ages in the Eastern China marginal sea sediments since the last deglaciation. Quat. Sci. Rev. 2023, 315, 108235. [Google Scholar] [CrossRef]
- He, J.X.; Zhang, S.Y.; Zhang, X.L.; Qian, Y.; He, H.; Wu, H. Composition and Distribution Characteristics and Geochemical Significance of n-Alkanes in Core Sediments in the Northern Part of the South Yellow Sea. J. Chem. 2016, 2016, 4741939. [Google Scholar] [CrossRef]
- Li, S.L.; Zhang, S.Y.; Zhao, Q.F.; Dong, H.P. The geochemical characteristics and its significance of saturated hydrocarbon in Surface Sediments from the South Yellow Sea. Mar. Geol. Lett. 2009, 25, 1–7. [Google Scholar]
- Zhang, S.Y.; Li, S.L.; Dong, H.P.; Shi, J.A. Characteristics of biomarkers composition and geochemical significance of surface sediments in the northern part of south Yellow Sea. Mar. Sci. Bull. 2012, 31, 198–206. [Google Scholar]
- Zhang, S.Y.; Li, S.L.; Dong, H.P.; Zhao, Q.; Lu, X.; Shi, J.A. An analysis of organic matter sources for surface sediments in the central South Yellow Sea, China: Evidence based on macroelements and n-alkanes. Mar. Pollut. Bull. 2014, 88, 389–397. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Pei, L.; Chang, Q. Organic carbon provenance and its response to the East Asian winter monsoon in the northern offshore mud area of Shandong Peninsula over the past 3000 years. Reg. Stud. Mar. Sci. 2025, 81, 104008. [Google Scholar] [CrossRef]
- Duan, Y.; Wen, Q.B.; Zheng, G.D.; Luo, B.; Ma, L. Isotopic composition and probable origins of individual fatty acids in modern sediments from Ruoergai Marsh and Nansha Sea, China. Org. Geochem. 1997, 27, 583–589. [Google Scholar] [CrossRef]
- Duan, Y.; Song, J.M.; Cui, M.Z.; Luo, B.J. Organic geochemical studies of sinking particulate material in China sea area-Ⅰ. Organic matter fluxes and distributional features of hydrocarbon compounds and fatty acids. Sci. China (Ser. D) 1998, 41, 208–214. [Google Scholar] [CrossRef]
- Hayashi, H.; Takii, S. Vertical distribution and some properties of bacteria in a stored core sample from Lake Biwa. In Paleolimnology of Lake Biwa and the Japanese Pleistocene; Hone, S., Ed.; Kyoto University Press: Kyoto, Japan, 1977; pp. 235–243. [Google Scholar]
- Heras, X.D.L.; Grimalt, J.O.; Albaigés, J.; Julia, R.; Anadon, P. Origin and diagenesis of the organic matter in Miocene freshwater lacustrine phosphates (Cerdanya Basin, Eastern Pyrenees). Org. Geochem. 1989, 14, 667–677. [Google Scholar] [CrossRef]
- Matsuda, H.; Koyama, T. Early diagenesis of fatty acids in lacustrine sediments—I. Identification and distribution of fatty acids in recent sediment from a freshwater lake. Geochim. Cosmochim. Acta 1977, 41, 777–783. [Google Scholar] [CrossRef]
- Matsuda, H.; Koyama, T. Early diagenesis of fatty acids in lacustrine sediments—II. A statistical approach to changes in fatty acid composition from recent sediments and some source materials. Geochim. Cosmochim. Acta 1977, 41, 1825–1834. [Google Scholar] [CrossRef]
- Erwin, J.A. Comparative biochemistry of fatty acids in eukary microorganisms. In Lipids and Biomembranes of Eukaryotic Microorganisms; Erwin, J.A., Ed.; Academic Press: New York, NY, USA, 1973; pp. 91–143. [Google Scholar]
- Venkatesan, M.I. Organic geochemistry of marine sediments in Antarctic region: Marine lipids in McMurdo Sound. Org. Geochem. 1988, 12, 13–27. [Google Scholar] [CrossRef]
- Schleder, A.; Froehner, S.; Sanez, J.; Parron, L.; Hansel, F.; Guerreiro, R.L.; Bahniuk, A. Insights into the organic matter composition of soda lakes in the Pantanal, Brazil, through fatty acids analysis in sediments. Environ. Sci. Pollut. Res. Int. 2023, 30, 103932–103946. [Google Scholar] [CrossRef]
- Wang, X.Q.; Chen, S.; Xu, L.F.; Peng, D. Geochemical Characteristics of Acid Compounds in Columnar Sediments in the Southwest Subsea Basin of the South China Sea. Mar. Geol. Front. 2020, 36, 17–24. [Google Scholar]
- Kaneda, T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J. Bacteriol. 1967, 93, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.J.; Volkman, J.K.; Johns, R.B.; Bavor, H.J., Jr. Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta 1979, 43, 1715–1725. [Google Scholar] [CrossRef]
- Taylor, J.; Parkes, R.J. The Cellular Fatty Acids of the Sulphate-reducing Bacteria, Desulfobacter sp., Desulfobulbus sp. and Desulfovibrio desulfuricans. Microbiology 1983, 129, 3303–3309. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Canuel, E.A. Fatty acids and sterols of particulate matter in a brackish and seasonally anoxic coastal salt pond. Org. Geochem. 1990, 16, 703–713. [Google Scholar] [CrossRef]
- Lebreton, B.; Richard, P.; Galois, R.; Radenac, G.; Pfléger, C.; Guillou, G.; Mornet, F.; Blanchard, G.F. Trophic importance of diatoms in an intertidal Zostera noltii seagrassbed: Evidence from stable isotope and fatty acid analyses. Estuar. Coast. Shelf Sci. 2011, 92, 140–153. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Xing, L.; Liu, Y.; Tao, S.; Zhang, H. The composition and distribution of n-alkanes in surface sediments from the South Yellow Sea and their potential as organic matter source indicators. Period. Ocean. Univ. China 2011, 41, 90–96. [Google Scholar]
- Gu, Y.; Chang, X.; Kong, F.; Lan, K.; Zhuang, G.C.; Liu, X.T. Holocene sediment source-to-sink processes and their controlling factors in the central South Yellow Sea mud area. Mar. Geol. Quat. Geol. 2024, 44, 140–150. [Google Scholar]
- Li, H.; Han, Z.; Yan, T.; Zhao, K.X.; Yang, Y.P. Similarity measurement algorithm of REE distribution curve and its application for provenance discrimination of sediments in the Yellow Sea. Mar. Geol. Front. 2023, 39, 88–97. [Google Scholar]
- Hao, X.; Song, J.; Li, X. A review of the major progress on carbon cycle researches in the Chinese marginal seas and the analysis of the key influence factors. Mar. Sci. 2008, 32, 83–90. [Google Scholar]
- Qin, Y.S.; Zhao, Y.Y.; Chen, L.R. Geology of the Yellow Sea; China Ocean Press: Beijing, China, 1989. [Google Scholar]
- Sholkovitz, E.R. Flocculation of dissolved organic and inorganic matter during mixing of river water and seawater. Geochim. Cosmochim. Acta 1976, 40, 831–845. [Google Scholar] [CrossRef]
- Liang, Y.; Mai, K.S.; Sun, S.C.; Huang, G.X.; Hu, H.Y. Effects of salinity on the growth and fatty acid composition of six strains of marine diatoms. Trans. Oceanol. Limnol. 2000, 4, 53–62. [Google Scholar]
- Schleder, A.A.; Froehner, S.; Guerreiro, R.L.; Parron, L.; Hansel, F.; Sánez, J.; de Castro Martins, C.; Maltempi, E.; Balsanelli, E. Disentangling sources and variation of organic matter in soda lakes from Nhecolândia (Pantanal, Brazil) based on hydrocarbons and bacterial composition. J. S. Am. Earth Sci. 2022, 114, 103718. [Google Scholar] [CrossRef]
- Cai, J.G.; Xu, J.L.; Yang, S.Y.; Bao, Y.J.; Lu, L.F. The Fractionation of an Argillaceous Sediment and Difference in Organic Matter Enrichment in Different Fractions. Geol. J. China Univ. 2006, 12, 234–241. [Google Scholar]
- Shi, W.; Sun, M.Y.; Molina, M.; Hodson, R.E. Variability in the distribution of lipid biomarkers and their molecular isotopic composition in Altamaha estuarine sediments implications for the relative contribution of organic matter from various sources. Org. Geochem. 2001, 32, 453–467. [Google Scholar] [CrossRef]
- Disnar, J.R.; Stefanova, M.; Bourdon, S.; Laggoun-Défarge, F. Sequential fatty acid analysis of a peat core covering the last two millennia (Tritrivakely lake, Madagascar): Diagenesis appraisal and consequences for palaeoenvironmental reconstruction. Org. Geochem. 2005, 36, 1391–1404. [Google Scholar] [CrossRef][Green Version]
- Niggemann, J.; Schubert, C.J. Fatty acid biogeochemistry of sediments from the Chilean coastal upwelling region: Sources and diagenetic changes. Org. Geochem. 2006, 37, 626–647. [Google Scholar] [CrossRef]
- Venturini, N.; Salhi, M.; Bessonar, M.; Piresvanin, A.M.S. Fatty acid biomarkers of organic matter sources and early diagenetic signatures in sediments from a coastal upwelling area (south-eastern Brazil). Chem. Ecol. 2012, 28, 221–238. [Google Scholar] [CrossRef]
- Johnson, R.W.; Calder, J.A. Early diagenesis of fatty acids and hydrocarbons in a salt marsh environment. Geochim. Cosmochim. Acta 1973, 37, 1943–1955. [Google Scholar] [CrossRef]
- Zhang, H.S.; Pan, J.M.; Chen, J.F.; Chen, R.H.; Lu, B.; Xue, B. Biomarkers in sediments in the Arctic areas and Ecologtcai envtronmental response. Mar. Geol. Quat. Geol. 2007, 27, 41–49. [Google Scholar]
- Chen, J.L.; Li, F.; He, X.P.; He, X.; Wang, J. Lipid biomarker as indicator for assessing the input of organic matters into sediments and evaluating phytoplankton evolution in upper water of the East China Sea. Ecol. Indic. 2019, 101, 380–387. [Google Scholar] [CrossRef]
- Lajat, M.; Saliot, A.; Schimmelmann, A. Free and bound lipids in recent (1835–1987) sediments from Santa Barbara Basin. Org. Geochem. 1990, 16, 793–803. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wan, Z.F.; Chen, C.M.; Chen, S. Geochemical Characteristics of Hydrocarbons in Core Sediments from the Southwest Sub-Basin of the South China Sea and Its Implications for the Sedimentary Environment. Front. Earth Sci. 2021, 9, 664959. [Google Scholar] [CrossRef]


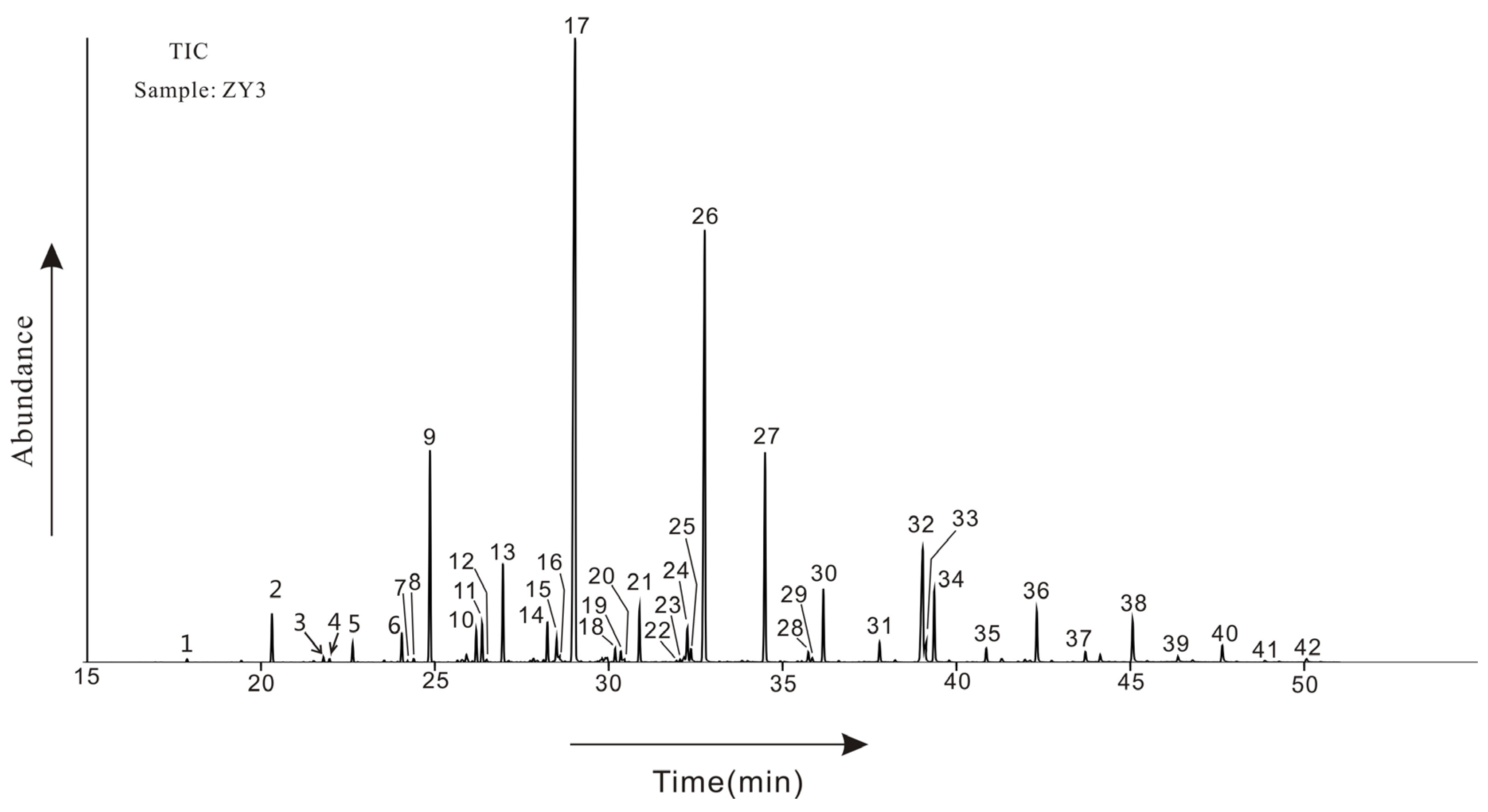
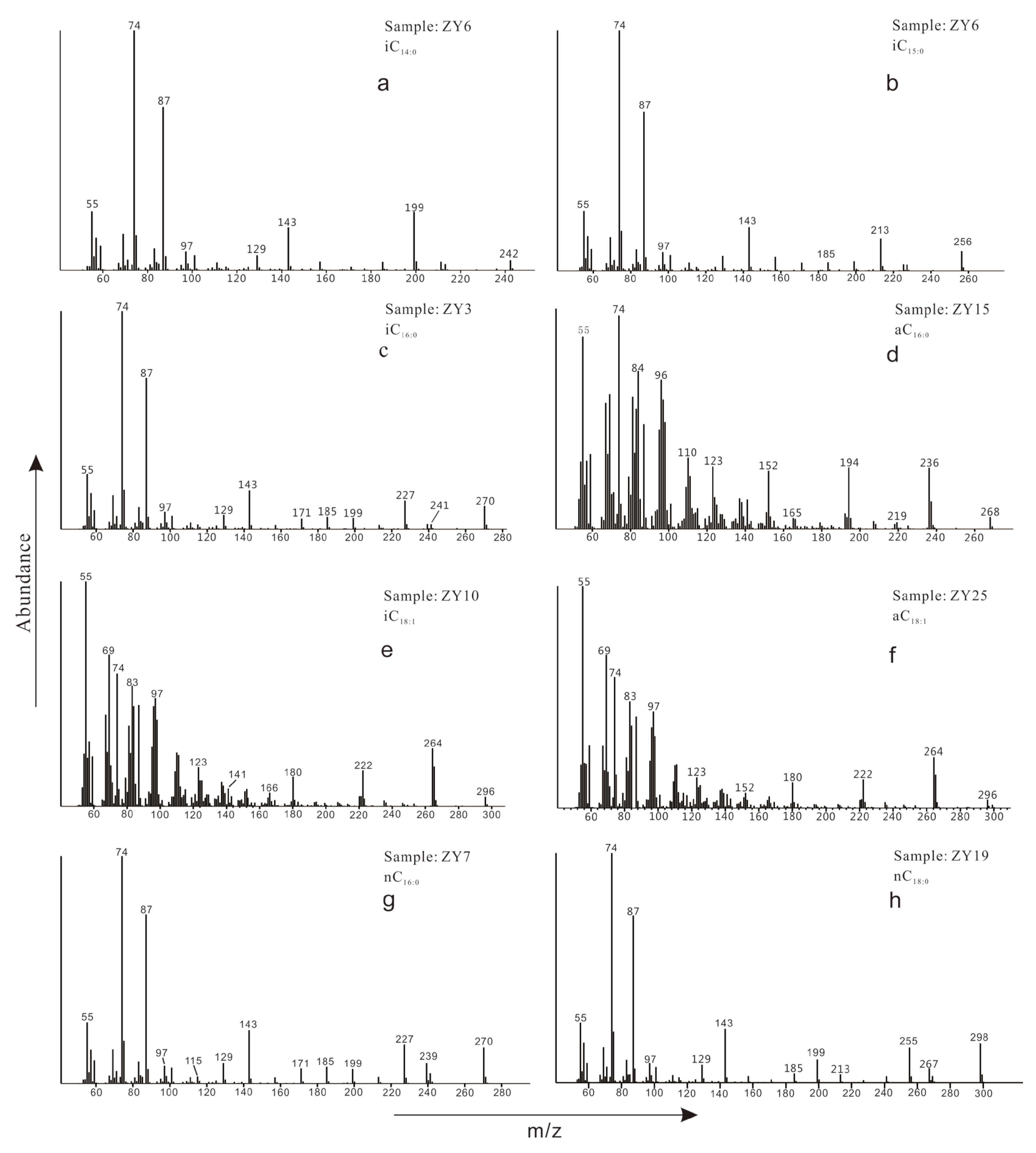
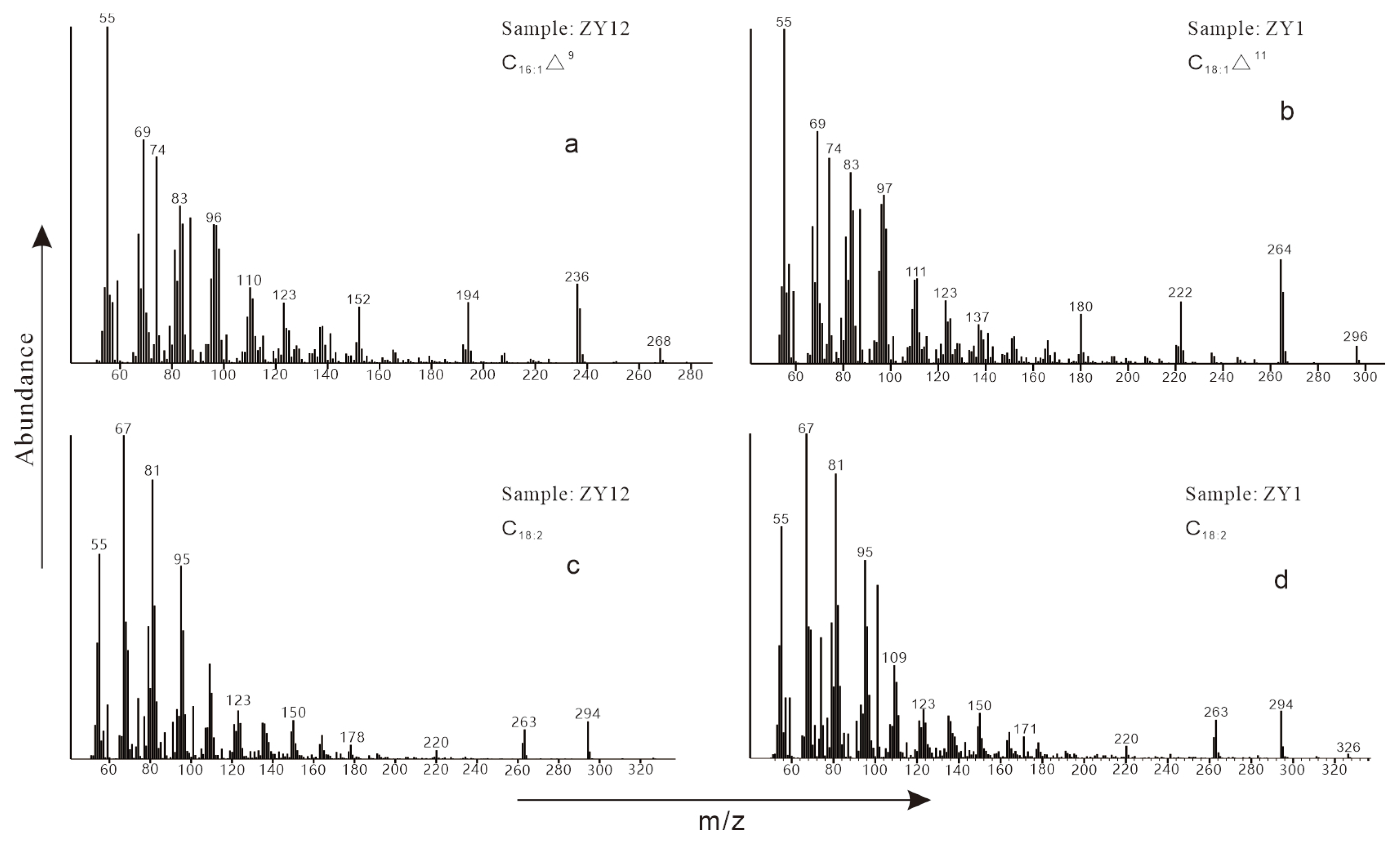
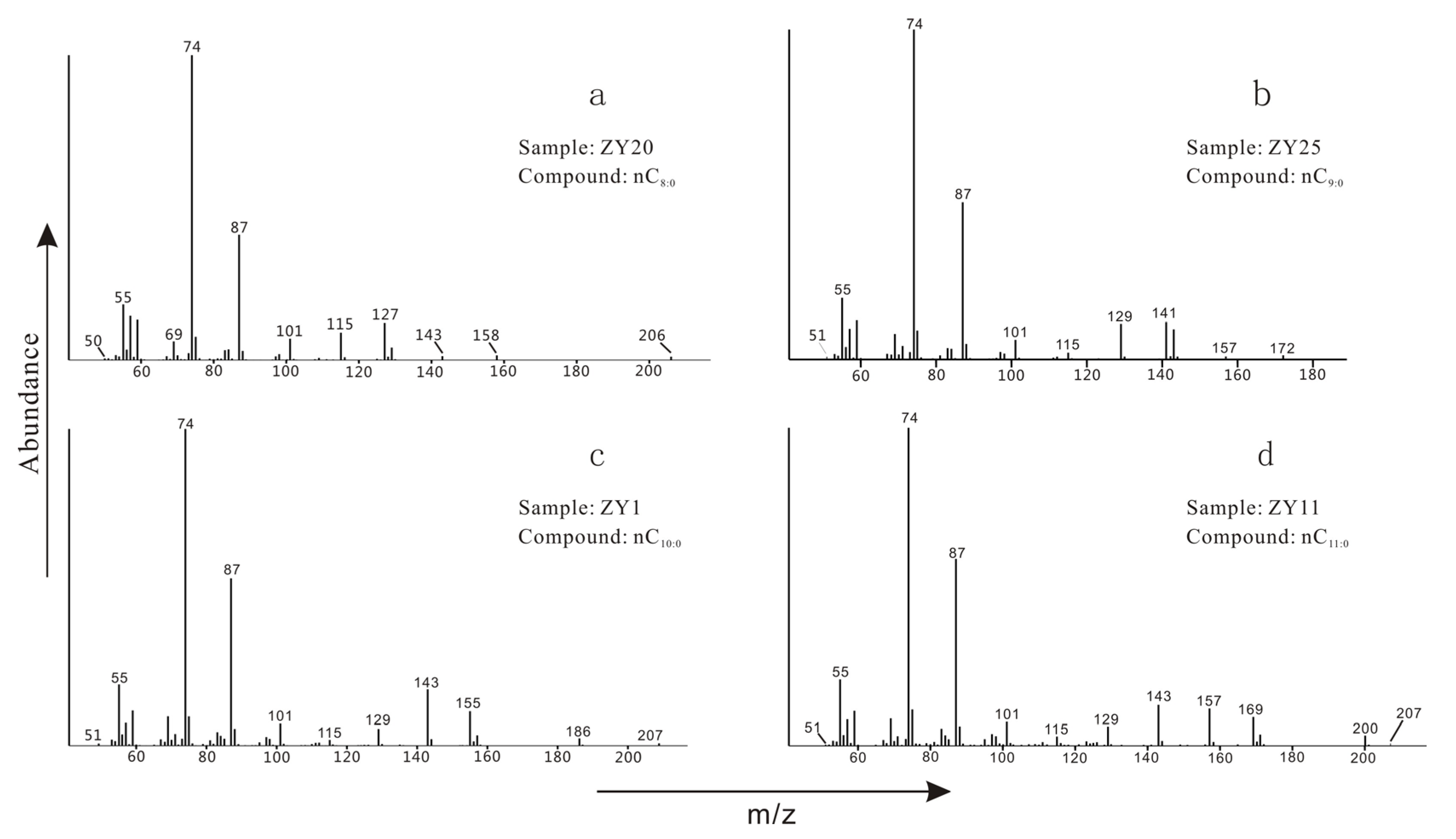
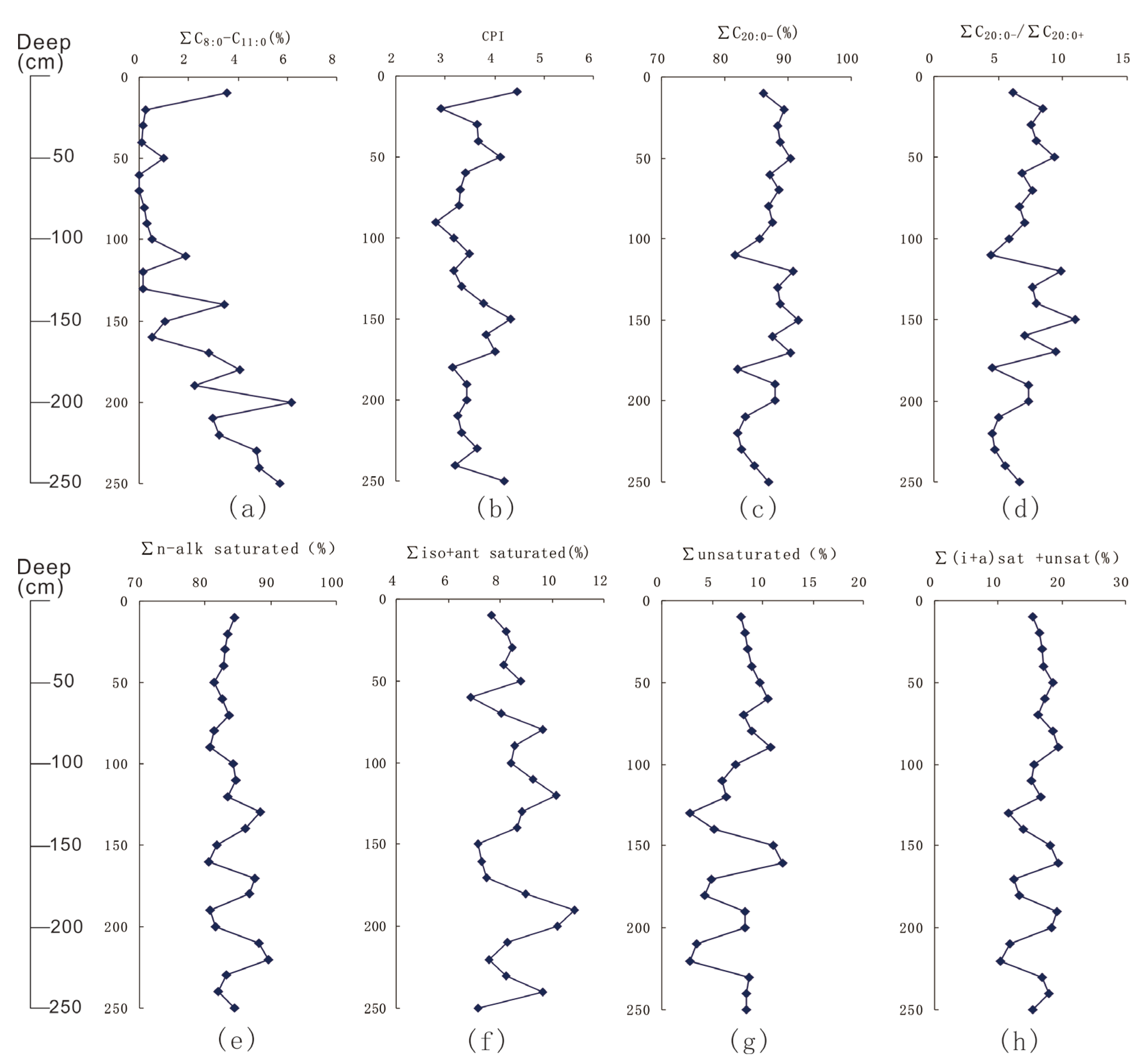
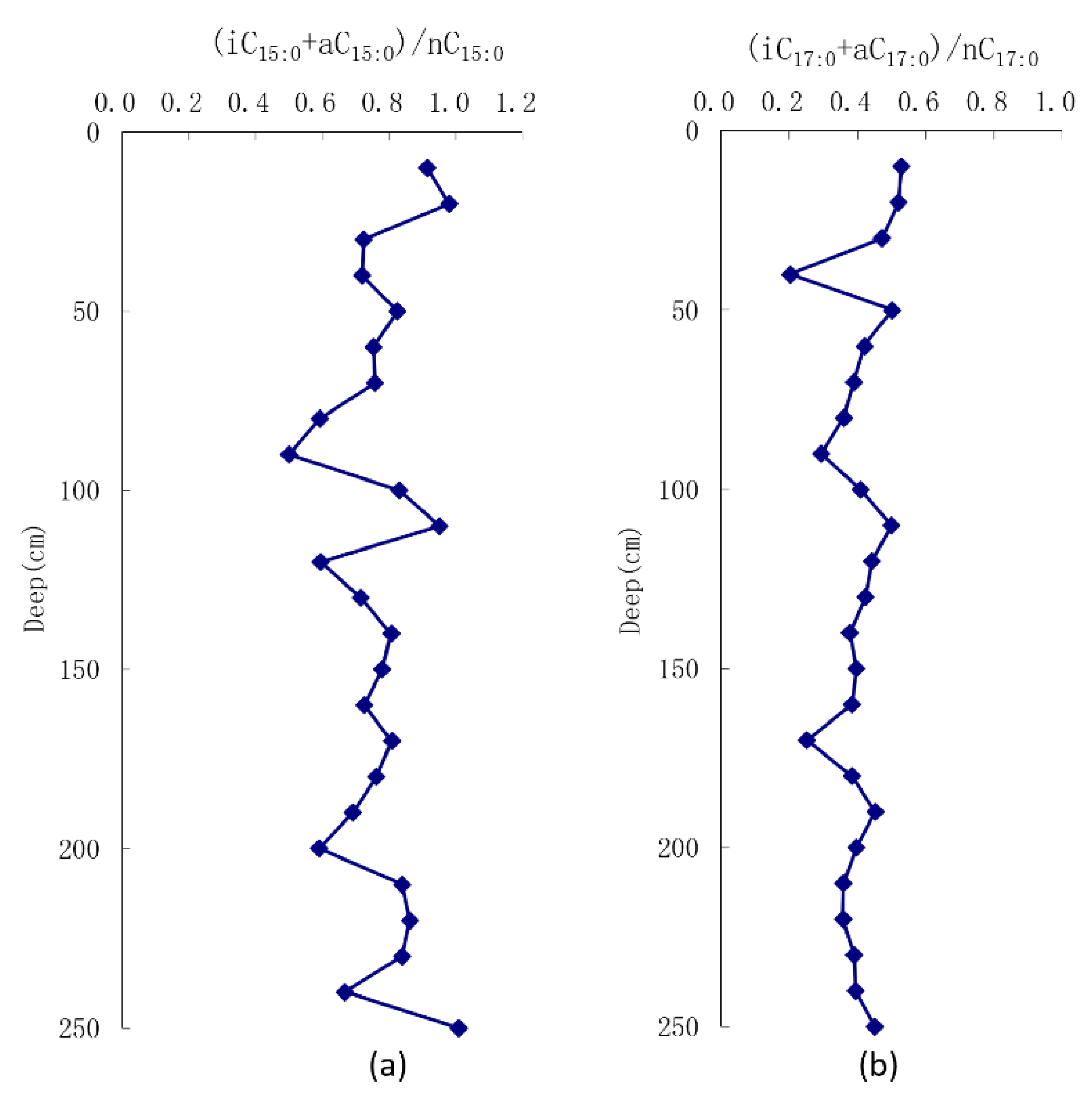
| Sample No. | Depth /cm | Range of Carbon Numbers | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|---|
| ZY1 | 10 | 8–30 | 6.18 | 4.45 | 0.17 | 0.91 | 3.54 | 82.54 | 13.92 | 30.84 |
| ZY2 | 20 | 11–30 | 8.42 | 2.92 | 0.10 | 0.98 | 0.26 | 89.13 | 10.61 | 28.49 |
| ZY3 | 30 | 11–30 | 7.58 | 3.65 | 0.12 | 0.72 | 0.16 | 88.19 | 11.65 | 31.15 |
| ZY4 | 40 | 11–30 | 7.97 | 3.68 | 0.10 | 0.72 | 0.09 | 88.77 | 11.15 | 34.41 |
| ZY5 | 50 | 9–30 | 9.39 | 4.11 | 0.09 | 0.82 | 0.98 | 89.39 | 9.63 | 33.88 |
| ZY6 | 60 | 12–30 | 6.81 | 3.42 | 0.14 | 0.75 | 0.00 | 87.20 | 12.80 | 32.61 |
| ZY7 | 70 | 12–30 | 7.68 | 3.31 | 0.12 | 0.76 | 0.00 | 88.48 | 11.52 | 32.39 |
| ZY8 | 80 | 11–30 | 6.66 | 3.28 | 0.15 | 0.59 | 0.19 | 86.76 | 13.05 | 29.68 |
| ZY9 | 90 | 10–30 | 7.07 | 2.80 | 0.13 | 0.50 | 0.29 | 87.33 | 12.38 | 25.82 |
| ZY10 | 100 | 10–30 | 5.86 | 3.16 | 0.19 | 0.83 | 0.53 | 84.89 | 14.57 | 29.59 |
| ZY11 | 110 | 9–30 | 4.43 | 3.50 | 0.24 | 0.95 | 1.86 | 79.71 | 18.42 | 29.16 |
| ZY12 | 120 | 11–30 | 9.85 | 3.17 | 0.08 | 0.59 | 0.14 | 90.64 | 9.22 | 33.17 |
| ZY13 | 130 | 11–30 | 7.66 | 3.34 | 0.14 | 0.72 | 0.14 | 88.31 | 11.55 | 30.02 |
| ZY14 | 140 | 8–30 | 7.93 | 3.79 | 0.11 | 0.81 | 3.45 | 85.35 | 11.20 | 31.97 |
| ZY15 | 150 | 9–30 | 10.96 | 4.34 | 0.07 | 0.78 | 1.06 | 90.58 | 8.36 | 35.05 |
| ZY16 | 160 | 10–30 | 7.09 | 3.84 | 0.12 | 0.73 | 0.51 | 87.13 | 12.36 | 33.23 |
| ZY17 | 170 | 8–30 | 9.46 | 4.02 | 0.09 | 0.81 | 2.80 | 87.64 | 9.56 | 35.65 |
| ZY18 | 180 | 8–30 | 4.56 | 3.15 | 0.24 | 0.76 | 4.06 | 77.95 | 17.98 | 26.65 |
| ZY19 | 190 | 9–30 | 7.35 | 3.43 | 0.13 | 0.69 | 2.25 | 85.77 | 11.98 | 30.04 |
| ZY20 | 200 | 8–30 | 7.36 | 3.43 | 0.13 | 0.59 | 6.15 | 81.89 | 11.96 | 26.93 |
| ZY21 | 210 | 8–30 | 5.00 | 3.24 | 0.21 | 0.84 | 2.99 | 80.34 | 16.67 | 28.76 |
| ZY22 | 220 | 8–30 | 4.58 | 3.34 | 0.23 | 0.86 | 3.23 | 78.84 | 17.93 | 28.01 |
| ZY23 | 230 | 8–30 | 4.74 | 3.64 | 0.23 | 0.84 | 4.77 | 77.81 | 17.42 | 29.09 |
| ZY24 | 240 | 8–30 | 5.52 | 3.21 | 0.20 | 0.67 | 4.85 | 79.81 | 15.34 | 25.72 |
| ZY25 | 250 | 8–30 | 6.62 | 4.19 | 0.16 | 1.01 | 5.69 | 81.19 | 13.12 | 29.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhang, X.; Ma, R.; Huang, Z.; Li, J.; Sun, P.; Song, J. The Geochemical Characteristics of the Fatty Acids in the Core Sediments in the Northern South Yellow Sea. J. Mar. Sci. Eng. 2025, 13, 1511. https://doi.org/10.3390/jmse13081511
He J, Zhang X, Ma R, Huang Z, Li J, Sun P, Song J. The Geochemical Characteristics of the Fatty Acids in the Core Sediments in the Northern South Yellow Sea. Journal of Marine Science and Engineering. 2025; 13(8):1511. https://doi.org/10.3390/jmse13081511
Chicago/Turabian StyleHe, Jinxian, Xiaoli Zhang, Ruihua Ma, Zhengxin Huang, Juhao Li, Peilin Sun, and Jiayao Song. 2025. "The Geochemical Characteristics of the Fatty Acids in the Core Sediments in the Northern South Yellow Sea" Journal of Marine Science and Engineering 13, no. 8: 1511. https://doi.org/10.3390/jmse13081511
APA StyleHe, J., Zhang, X., Ma, R., Huang, Z., Li, J., Sun, P., & Song, J. (2025). The Geochemical Characteristics of the Fatty Acids in the Core Sediments in the Northern South Yellow Sea. Journal of Marine Science and Engineering, 13(8), 1511. https://doi.org/10.3390/jmse13081511








