Spatial Dynamics and Ecological Risk Assessment of Microplastics in Littoral Sediments of the Sea of Marmara, Türkiye
Abstract
1. Introduction
2. Material and Methods
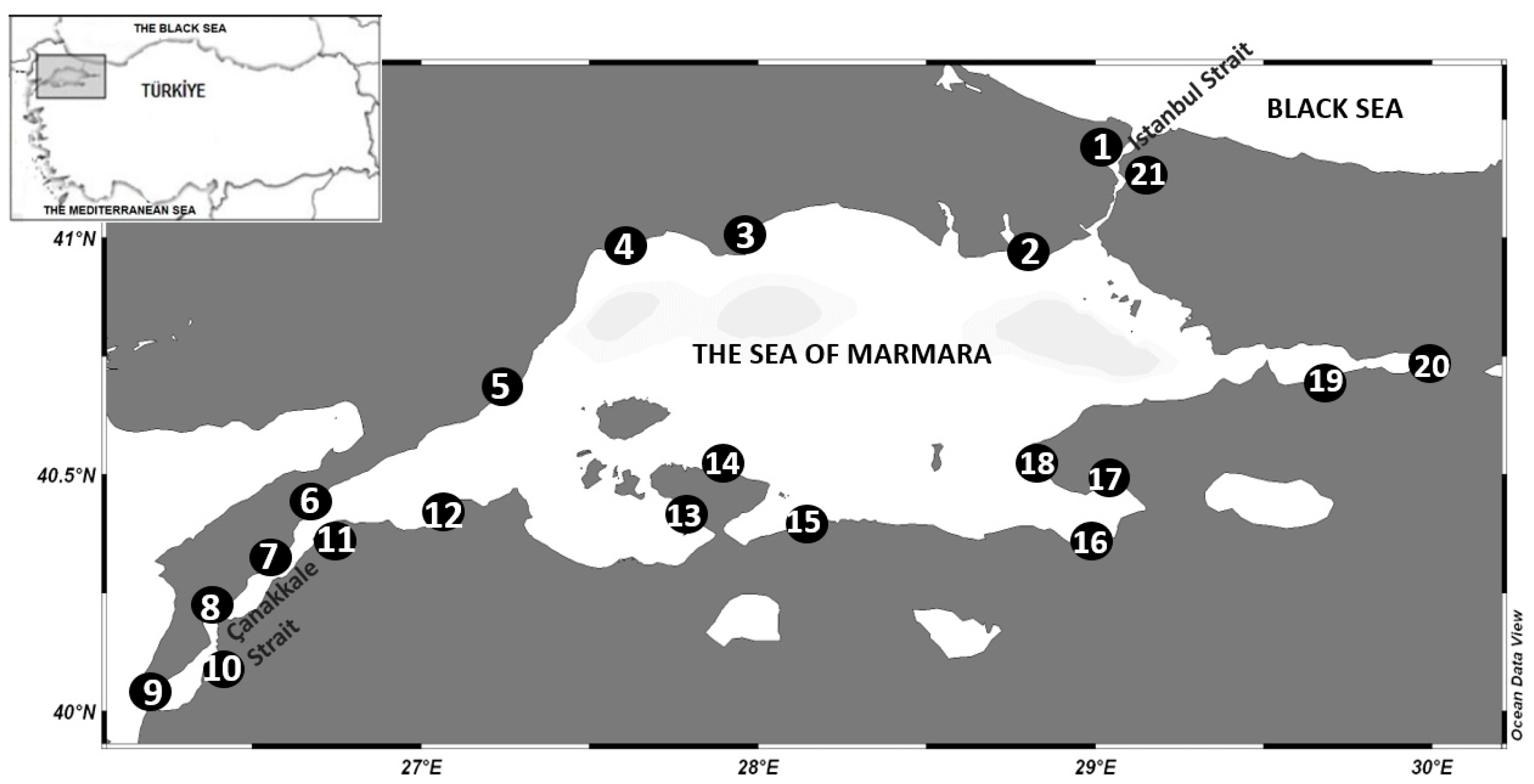
2.1. Research Area
2.2. Sampling
2.3. Microplastic Extraction
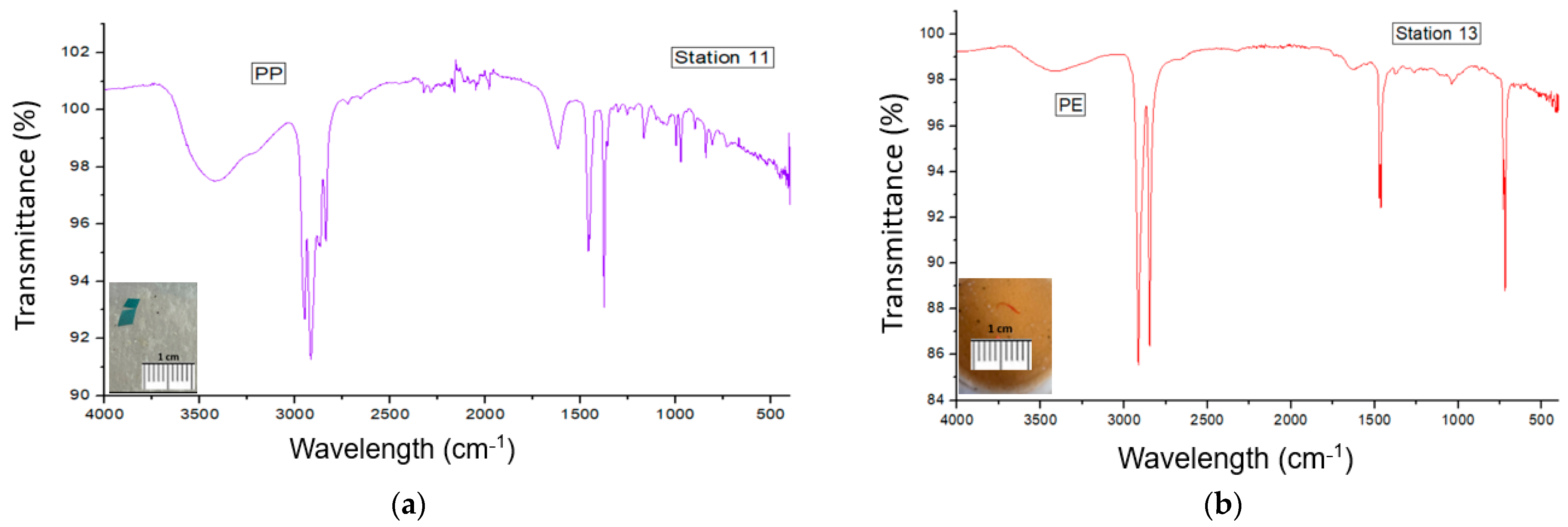
2.4. Physical and Chemical Characterization
2.5. QA/QC
2.6. Microplastics Contamination Factor and Pollution Risk Index
2.7. Hazard Index and Pollution Risk Index
2.8. Statistical Analysis
3. Results
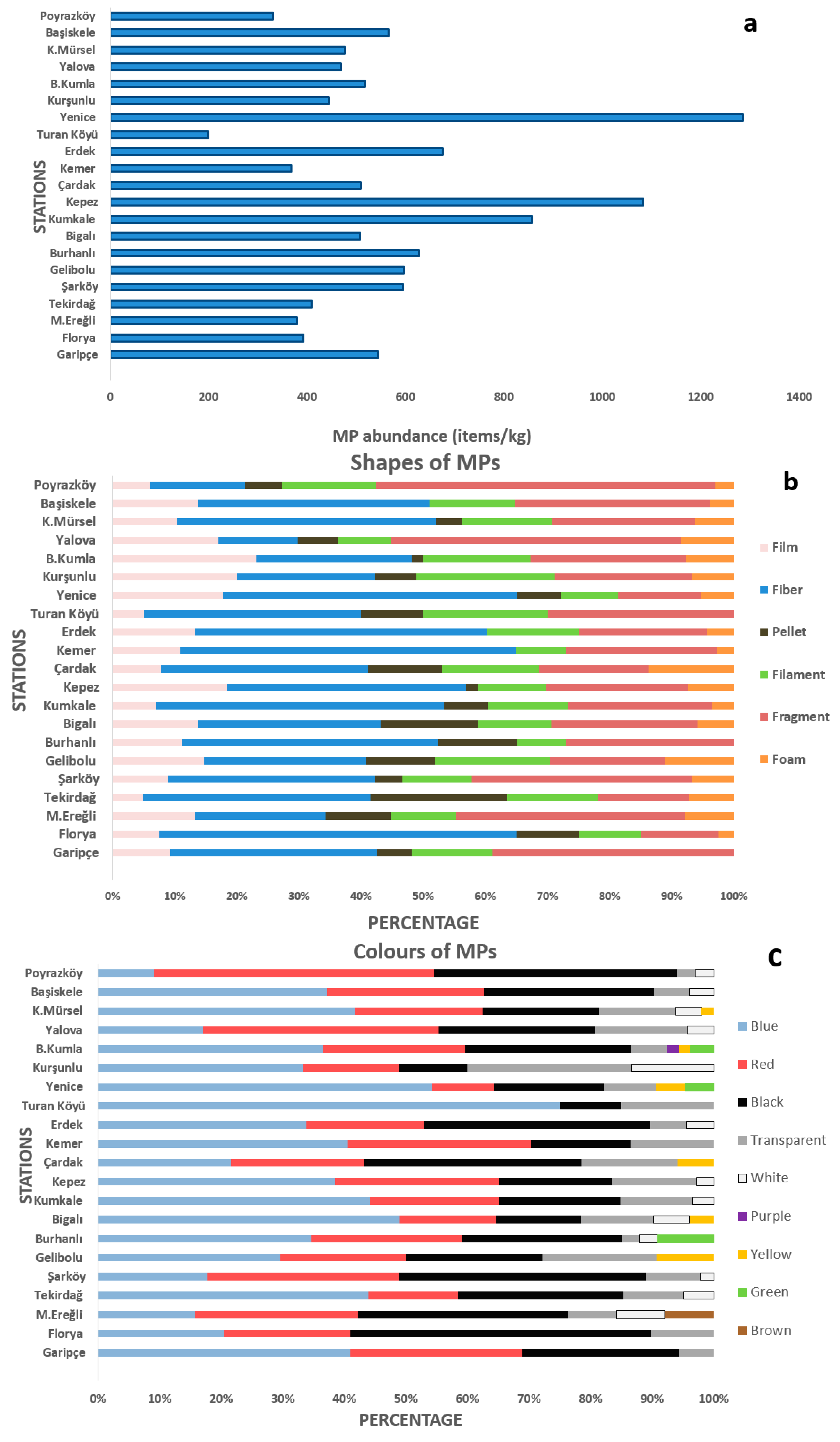
3.1. Abundance and Distribution of MPs in Sediments
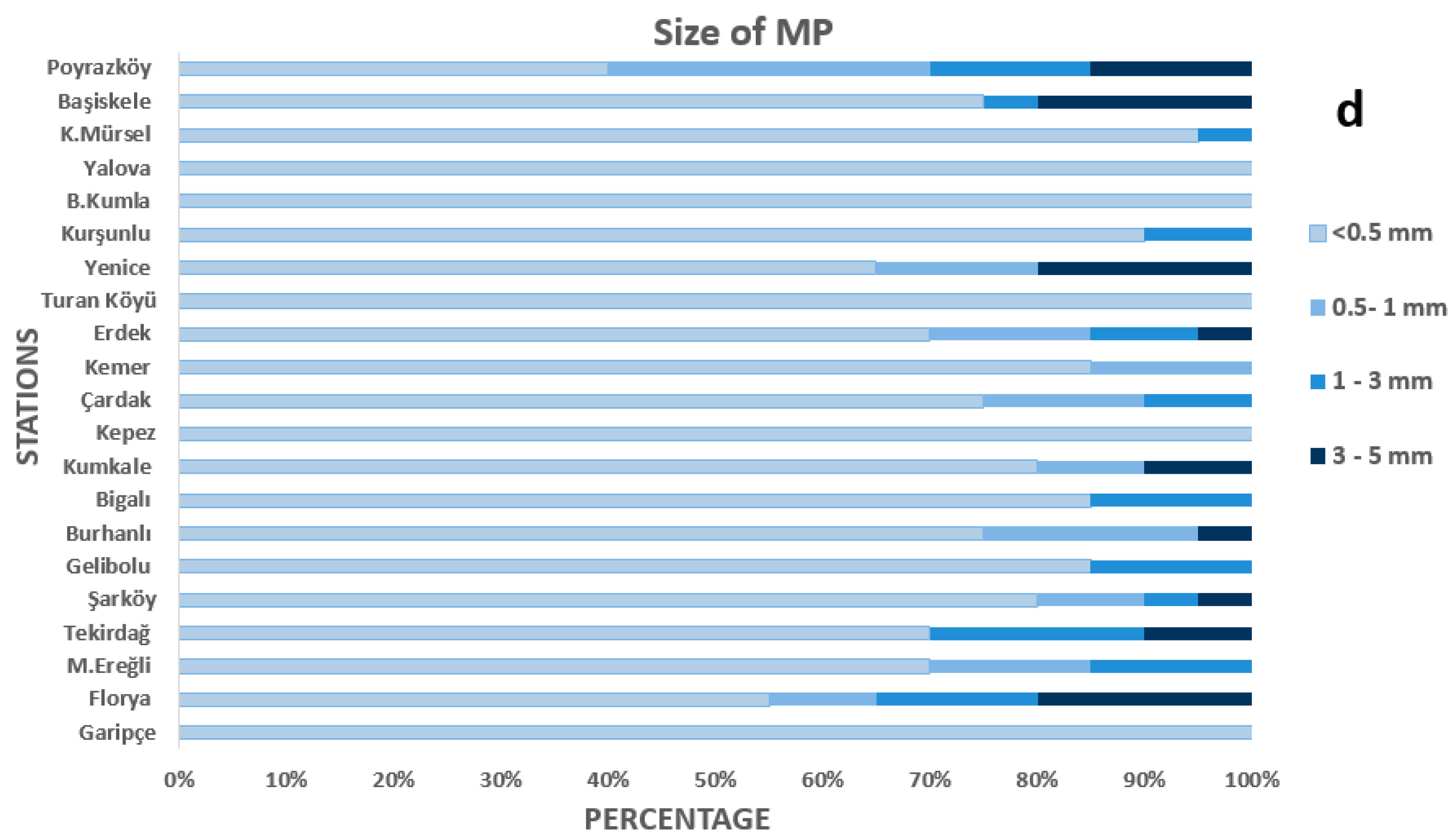
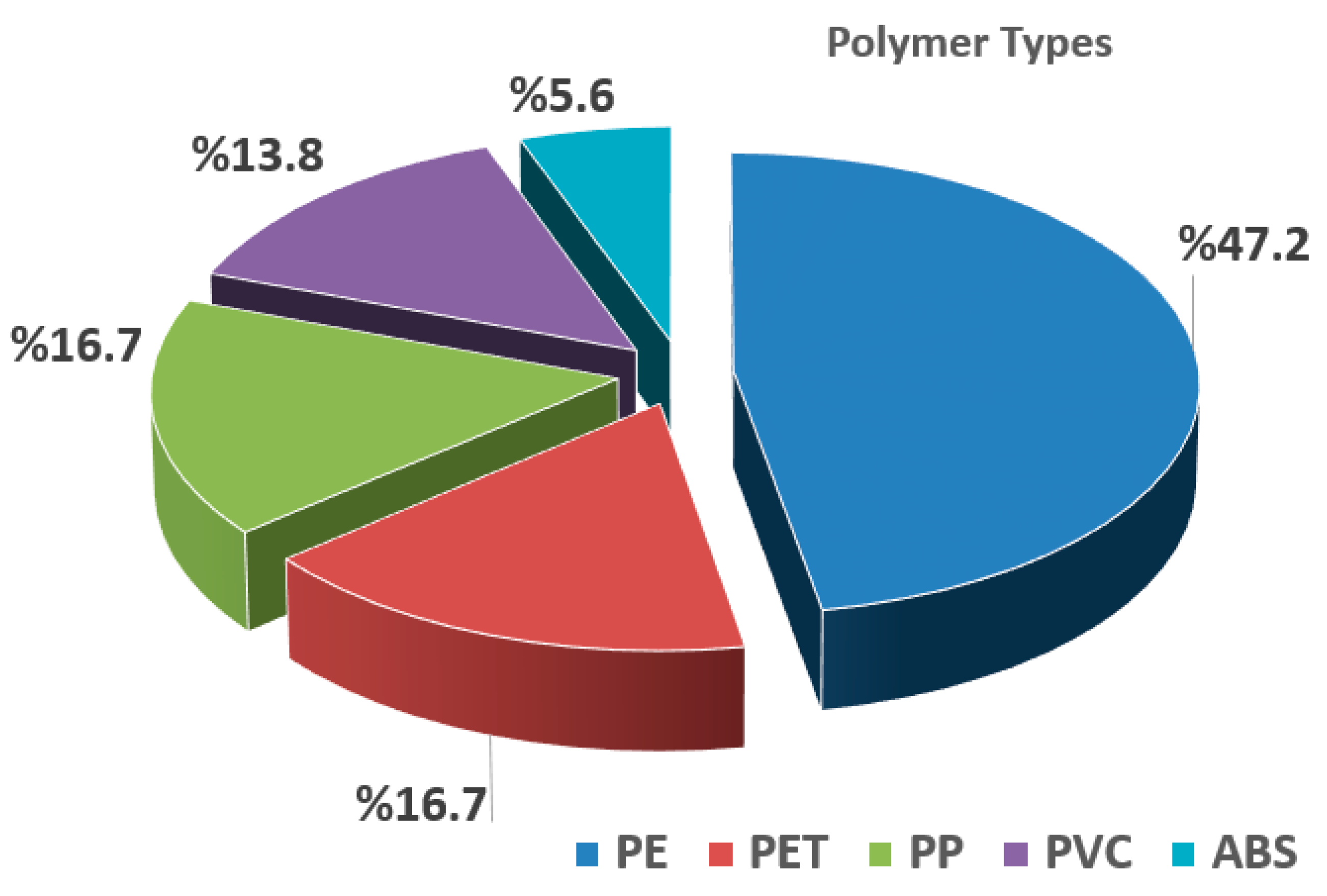
3.2. Physical and Chemical Profiling of MPs
3.3. Contamination Factor and Pollution Load Index
3.4. Pollution Risk Index and Polymer Hazard Index
4. Discussion
| Regions/ Locations | Amount of MPs (items/kg) | Solution Density | Dominant Color | Dominant Polymer | Dominant Shape | Reference |
|---|---|---|---|---|---|---|
| Guangdong Coastal Areas, South China | 433.3–4166.3 | ZnCl2 (1.5 g/cm3) | Transparent | Rayon | Fiber | [7] |
| Pendik-Tuzla (SoM), Türkiye | 0.3–85.6 | ZnCl2 (1.4 g/cm3) | Black | ABS | Fragment | [27] |
| Istanbul Strait (SoM), Türkiye | 1957–4079.96 | NaCl (1.20 g/cm3) | Blue, white | - | Filament, fragment | [28] |
| Istanbul Strait (SoM), Türkiye | 9500 ± 20,300 | NaCl (1.2 g/cm3) | Blue, White/transparent | PE, PP | Fiber, fragment | [29] |
| Istanbul Strait (SoM), Türkiye | 144.4–177.6 | NaCl (1.20 g/cm3) | Black, transparent | PPS | Fiber | [30] |
| Southern Coast of Istanbul (SoM), Türkiye | 1364 ± 600 | NaCl (6.14 M) | Transparent | PE, PET | Fiber | [31] |
| Golden Horn (SoM), Türkiye | 140 | NaCl (140 g/L) | Yellow, transparent | - | Film, fiber | [32] |
| Golden Horn (SoM), Türkiye | 700–4100 | NaCl (35 g/100 mL) | - | - | Fragment, fiber | [33] |
| Gemlik Bay (SoM), Türkiye | 3333–9733 | NaCl (1.2 g/cm3) | Blue, black | PVC, PP | Fiber | [35] |
| Gulf of Bandırma (SoM), Türkiye | 195–226 | ZnCl2 (1.65 g/cm3) | Blue, black | PET | Fiber | [36] |
| Persian Gulf, Iran | 1346 ± 601 | NaI (1.6 g/cm3) | Black | PE, PP | Fiber | [70] |
| Black Sea | 106.7 | NaCl (1.2 g/cm3) | Black, blue, | PE, PP | Fragment | [84] |
| Southeast Black Sea, Türkiye | 108 | ZnCl2 (1.65 g/cm3) | - | PE | Fragment, fiber | [85] |
| Jiaochou Bay (China) | 25 | ZnCl2 (1.5 g/cm3) | Black, blue | PET, PP, PE | Fiber | [96] |
| South Baltic Sea (Poland) | 0–27 | NaCl (1.2 g/cm3) | Transparent | PVA | Fiber | [101] |
| Tunisian Coast (Mediterranean Sea) | 141–461 | NaCl (140 g/L) | Black | PE, PP, PS | Fiber, fragment | [102] |
| Venice Lagoon, Italy | 672–2175 | NaCl (120 g/L) | Blue, red | PE, PP | Fragment | [135] |
| Southern Black Sea, Türkiye | 64.06 ± 895 | ZnCl2 (1.65 g/cm3) | - | SAC, PET, PE | Fiber, fragment | [136] |
| Cape town South Africa | 38 ± 2 | NaCl (360 g/L) | White, blue/green | Nylon | Filament | [137] |
| Coasts of SoM, Türkiye | 199–1286 | ZnCl2 (1.7 g/cm3) | Blue, black | PE, PET | Fiber, fragment | Present Study |
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| SoM | Sea of Marmara |
| IS | Istanbul Strait |
| ÇS | Çanakkale Strait |
| SS | South Shelf |
| NS | North Shelf |
| GB | Gemlik Bay |
| GI | Gulf of Izmit |
| TSS | Turkish Straits System |
| St | Station |
| Cf | Contamination Factor |
| PLI | Pollution Load Index |
| HI | Hazard Index |
| PRI | Pollution Risk Index |
References
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 2); IMO: London, UK, 2016; 221p. [Google Scholar]
- Dharmadasa, S.W.S.; Andrady, A.L.; Kumara, P.T.P.; Maes, T.; Gangabadage, C.S. Microplastics pollution in Marine Protected Areas of Southern Sri Lanka. Mar. Pollut. Bull. 2021, 168, 112462. [Google Scholar] [CrossRef] [PubMed]
- Bozdaş, K.; Üstün, G.E.; Aygün, A. Micro Plastics and Removal Methods in Wastewater Treatment Plants. Uludağ Univ. J. Fac. Eng. 2020, 25, 1577–1592. (In Turkish) [Google Scholar] [CrossRef]
- Marcus, I.M.; Barriquand, T.B.; Thompson, R.M.; Hosselkus, B.C.; Hutson, C.T.; Jacobs, M.; McNeil, C.; Newton, L.; Olivarez, S.M.; Abell, J. Spatial and Temporal Variations of Microplastics within Humboldt Bay, California. CSU J. Sustain. Clim. Change 2023, 3, 3. [Google Scholar] [CrossRef]
- GESAMP. Guidelines for the Monitoring & Assessment of Plastic Litter in the Ocean; United Nations Environment Programme: Nairobi, Kenya; IMO: London, UK, 2019. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chen, G.; Xu, K.; Gong, H.; Huang, K.; Yan, M.; Wang, J. Microplastics in Surface Waters and Sediments from Guangdong Coastal Areas, South China. Sustainability 2021, 13, 2691. [Google Scholar] [CrossRef]
- Marques Mendes, A.; Golden, N.; Bermejo, R.; Morrison, L. Distribution and abundance of microplastics in coastal sediments depends on grain size and distance from sources. Mar. Pollut. Bull. 2021, 172, 112802. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, P.; Wang, D.; Wang, L.; Zhao, H.; Wang, X.; Zeng, Z.; Li, P.; Wang, T.; Liu, W.; et al. Microplastics in Marine Sediments in Eastern Guangdong in the South China Sea: Factors Influencing the Seasonal and Spatial Variations. Water 2023, 15, 1160. [Google Scholar] [CrossRef]
- Godoy, V.; Prata, J.C.; Pérez, A.; da Costa, J.P.; Rocha-Santos, T.; Duarte, A.C. Microplastics in Sediments from a Sandy Beach in Costa Nova (Aveiro, Portugal). Sustainability 2023, 15, 6186. [Google Scholar] [CrossRef]
- Weerasekara, P.G.Y.W.; De Silva, D.S.M.; De Silva, R.C.L.; Amarathunga, A.A.D.; Bakir, A.; McGoran, A.R.; Sivyer, D.B.; Reeve, C. Sampling and Analysis of Microplastics in the Coastal Environments of Sri Lanka: Estuaries of the Kelani River to Mahaoya. Water 2024, 16, 1932. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A. Microplastics in the Marine Environment: Distribution, Interactions and Effects. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef]
- Tanhua, T.; Gutekunst, S.B.; Biastoch, A. A near-synoptic survey of ocean microplastic concentration along an around-the-world sailing race. PLoS ONE 2020, 15, e0243203. [Google Scholar] [CrossRef] [PubMed]
- Lear, G.; Kingsbury, J.M.; Franchini, S.; Gambarini, V.; Maday, S.D.M.; Wallbank, J.A.; Weaver, L.; Pantos, O. Plastics and the microbiome: Impacts and solutions. Environ. Microbiome 2021, 16, 2. [Google Scholar] [CrossRef]
- Rodríguez, C.; Fossatti, M.; Carrizo, D.; Sánchez-García, L.; Teixeira De Mello, F.; Weinstein, F.; Lozoya, J.P. Mesoplastics and large microplastics along a use gradient on the Uruguay Atlantic coast: Types, sources, fates, and chemical loads. Sci. Total Environ. 2020, 721, 137734. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Sözen, S.; Orhon, D.; Kirca, V.S.O.; Sumer, B.M. Impact of mixing on water quality in the Bosphorus—Implications on sustainable management of wastewater marine discharges. Mar. Pollut. Bull. 2023, 189, 114799. [Google Scholar] [CrossRef] [PubMed]
- Yümün, Z.Ü.; Ekici, G. Investigation of Pollution Loads of Marmara Sea Discharge Points Between Suleymanpaşa (Tekirdag) and Kuçukçekmece Lake (Istanbul). Eur. J. Sci. Technol. 2023, 52, 294–301. [Google Scholar] [CrossRef]
- Öztürk, I.; Seker, M. Ecology of the Marmara Sea: Formation and Interactions of Marine Mucilage, and Recommendations for Solutions; Turkish Academy of Sciences: Ankara, Türkiye, 2021. [Google Scholar] [CrossRef]
- Baysal, A.; Saygin, H.; Ustabasi, G.S. Microplastic occurrences in sediments collected from Marmara Sea-Istanbul, Turkey. Bull. Environ. Contam. Toxicol. 2020, 105, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Erkan, H.S.; Turan, N.B.; Albay, M.; Engin, G.Ö. Microplastic pollution in seabed sediments at different sites on the shores of Istanbul-Turkey: Preliminary results. J. Clean. Prod. 2021, 328, 129539. [Google Scholar] [CrossRef]
- Erkan, H.S.; Takatas, B.; Ozturk, A.; Gündoğdu, S.; Aydın, F.; Koker, L.; Ozdemir, O.K.; Albay, M.; Engin, G.Ö. Spatio-temporal distribution of microplastic pollution in surface sediments along the coastal areas of Istanbul, Turkey. Mar. Pollut. Bull. 2023, 195, 115461. [Google Scholar] [CrossRef] [PubMed]
- Olguner, B.; Mülayim, A.; Gündüz, S.K. Microplastic concentration in the sediment of the Istanbul Strait (the Sea of Marmara, Turkey). J. Soils Sediments 2023, 23, 2892–2904. [Google Scholar] [CrossRef]
- Akarsu, C.; Sönmez, V.Z.; Sivri, N. Seasonal Distribution, Characterization, Indexing and Risk Assessment of Micro- and Nanoplastics in Coastal Sediments: A Case Study from Istanbul. Int. J. Environ. Res. 2024, 18, 95. [Google Scholar] [CrossRef]
- Doğruyol, P.; Şener, M.; Balkaya, N. Determination of microplastics and large plastics in the sediments of the Golden Horn Estuary (Haliç), Istanbul, Turkey. Desalination Water Treat. 2019, 172, 344–350. [Google Scholar] [CrossRef]
- Belivermiş, M.; Kılıç, Ö.; Sezer, N.; Sıkdokur, E.; Güngör, N.D.; Altuğ, G. Microplastic inventory in sediment profile: A case study of Golden Horn estuary, Sea of Marmara. Mar. Pollut. Bull. 2021, 173, 113117. [Google Scholar] [CrossRef]
- Işlek, Ş.; Bostan, Z.; Güney, E.; Sönmez, V.Z. Occurrence and spatial distribution of microplastics in coastal lagoon sediments: The case from Küçükçekmece Lagoon. Commagene J. Biol. 2023, 7, 1–11. [Google Scholar] [CrossRef]
- Yücedağ, E.; Mülayim, A.; Kecel Gündüz, S. Concentration and Characterisation with Spectroscopic Technique of Microplastics in the Surface Sediment and Commercial Fish Species of Gemlik Bay (Marmara Sea). Turk. J. Fish. Aquat. Sci. 2024, 24, TRJFAS25858. [Google Scholar] [CrossRef]
- Mutlu, T.; Eryaşar, A.R.; Karaoğlu, K.; Veske, E.; Gedik, K. Microplastics pollution in Gulf of Bandırma, Sea of Marmara: Biota and sediment. Mar. Pollut. Bull. 2025, 213, 117667. [Google Scholar] [CrossRef]
- Beşiktepe, Ş.T.; Sur, H.I.; Özsoy, E.; Latif, M.A.; Oǧuz, T.; Ünlüata, Ü. The Circulation and Hydrography of the Marmara Sea. Prog. Oceanogr. 1994, 34, 285–334. [Google Scholar] [CrossRef]
- Beşiktepe, Ş.T. Density Currents in the Two-Layer Flow: An Example of Dardanelles Outflow. Oceanol. Acta 2003, 26, 243–253. [Google Scholar] [CrossRef]
- Özsoy, E.; Tuğrul, S.; Delfanti, R.; Sannino, G. Dynamics of High Energy Environment: Processes at the Turkish Straits System (DEEP), TUBITAK1001. 2015. Available online: https://open.metu.edu.tr/bitstream/handle/11511/49585/TVRVeU16UTA.pdf (accessed on 6 March 2025).
- Enders, K.; Lenz, R.; Ivar do Sul, J.A.; Tagg, A.S.; Labrenz, M. When every particle matters: A QuEChERS approach to extract microplastics from environmental samples. MethodsX 2020, 7, 100784. [Google Scholar] [CrossRef]
- Pritzker, P.; Sullivan, K.; Callender, R. Laboratory Methods for the Analysis of Microplastics in the Marine Environment. 2015; pp. 1–39. Available online: https://repository.library.noaa.gov/view/noaa/10296 (accessed on 13 April 2025).
- Lares, M.; Ncibi, M.C.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, C.; Miller, R.Z. Are We Contaminating Our Samples? A Preliminary Study to Investigate Procedural Contamination During Field Sampling and Processing for Microplastic and Anthropogenic Microparticles. Mar. Pollut. Bull. 2021, 173, 113095. [Google Scholar] [CrossRef]
- Kabir, A.H.M.E.; Sekine, M.; Imai, T.; Yamamoto, K.; Kanno, A.; Higuchi, T. Assessing small-scale freshwater microplastics pollution, land-use, source-to-sink conduits, and pollution risks: Perspectives from Japanese rivers polluted with microplastics. Sci. Total Environ. 2021, 768, 144655. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Jiang, J.; He, L.; Zheng, S.; Liu, J.; Gong, L. A review of microplastic transport in coastal zones. Mar. Environ. Res. 2024, 196, 106397. [Google Scholar] [CrossRef]
- Sarafraz, J.; Rajabizadeh, M.; Kamrani, E. The preliminary assessment of abundance and composition of marine beach debris in the northern Persian Gulf, Bandar Abbas City, Iran. J. Mar. Biol. Assoc. UK 2016, 96, 131–135. [Google Scholar] [CrossRef]
- Kim, I.S.; Chae, D.H.; Kim, S.K.; Choi, S.; Woo, S.B. Factors influencing the spatial variation of microplastics on high-tidal coastal beaches in Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S.; Naik, B.G. Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere 2016, 159, 496–505. [Google Scholar] [CrossRef]
- Imhof, H.K.; Sigl, R.; Brauer, E.; Feyl, S.; Giesemann, P.; Klink, S.; Leupolz, K.; Löder, M.G.; Löschel, L.A.; Missun, J.; et al. Spatial and temporal variation of macro-, meso- and microplastic abundance on a remote coral island of the Maldives, Indian Ocean. Mar. Pollut. Bull. 2017, 116, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Asensio, M.; Martínez, I.; Santana, A.; Packard, T.; Gómez, M. Microplastic and tar pollution on three Canary Islands beaches: An annual study. Mar. Pollut. Bull. 2018, 129, 494–502. [Google Scholar] [CrossRef]
- Yabanlı, M.; Yozukmaz, A.; Şener, İ.; Ölmez, Ö.T. Microplastic pollution at the intersection of the Aegean and Mediterranean Seas: A study of the Datça Peninsula (Turkey). Mar. Pollut. Bull. 2019, 145, 47–55. [Google Scholar] [CrossRef]
- de Haan, W.P.; Sanchez-Vidal, A.; Canals, M.; Party, N.S.S. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 523–535. [Google Scholar] [CrossRef]
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Nel, H.A.; Hean, J.W.; Noundou, X.S.; Froneman, P.W. Do microplastic loads reflect the population demographics along the southern African coastline? Mar. Pollut. Bull. 2017, 115, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, Ü.; Tekin Özan, S. Helminth Fauna of Chub (Squalius cephalus L.) in Susurluk Creek (Bursa-Balıkesir). Süleyman Demirel Univ. Fac. Arts Sci. J. Sci. 2012, 7, 77–85. (In Turkish) [Google Scholar]
- Kudun, K. Hydrogeochemistry of the Simav Brook. Master’s Thesis, Istanbul Technical University, Graduate School of Natural and Applied Sciences, Istanbul, Türkiye, 1994. [Google Scholar]
- Çiçek, N.L.; Güçlü, S.S.; Erdoğan, Ö.; Küçük, F. Water Quality Assessment of Simav River (Susurluk Basin/Turkey) According to Seasons and Stations. Int. J. Comput. Exp. Sci. Eng. 2023, 9, 68–80. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Trevisan, R.; Ranasinghe, P.; Greg, B.M.; Santos, J.; Hong, A.; Edward, W.C.; Jayasundara, N.; Somarelli, J.A. A growing crisis for One Health: Impacts of plastic pollution across layers of biological function. Front. Mar. Sci. 2022, 9, 980705. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Gimenez, B.C.G. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Li, Y.; Huang, R.; Hu, L.; Zhang, C.; Xu, X.; Song, L.; Wang, Z.; Pan, X.; Christakos, G.; Wu, J. Microplastics distribution in different habitats of Ximen Island and the trapping effect of blue carbon habitats on microplastics. Mar. Pollut. Bull. 2022, 181, 113912. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.A.; Adyel, T.M.; Macreadie, P.I.; Trevathan-Tackett, S.M. Prioritising plastic pollution research in blue carbon ecosystems: A scientometric overview. Sci. Total Environ. 2024, 914, 169868. [Google Scholar] [CrossRef]
- Azoulay, D.; Villa, P.; Arellano, Y.; Gordon, M.; Moon, D.; Miller, K.; Thompson, K. Plastic and Health: The Hidden Costs of a Plastic Planet; Center for International Environmental Law (CIEL): Washington, DC, USA, 2019. [Google Scholar]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Islam, T.; Cheng, H. Existence and fate of microplastics in terrestrial environment: A global fretfulness and abatement strategies. Sci. Total Environ. 2024, 953, 176163. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Shadi, A.; Abadi, A.; Nemati, M.; Senapathi, V.; Karthikeyan, S.; Kulandaisamy, P. Exploring the microplastic pollution: Unveiling origins and varieties in coastal sediments and waters of the Bushehr Province, Persian Gulf, Iran. Mar. Pollut. Bull. 2024, 198, 115939. [Google Scholar] [CrossRef]
- Yahaya, T.; Abdulazeez, A.; Oladele, E.O.; Williams, E.F.; Obadiah, C.D.; Umar, J.; Salisu, N. Microplastics Abundance, Characteristics, and Risk in Badagry Lagoon in Lagos State, Nigeria. Pollution 2022, 8, 1325–1337. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Ju, H.; Tang, D.W.S.; Xue, S.; Geissen, V.; Yang, X. Chlorpyrifos degradation and its impacts on phosphorus bioavailability in microplastic-contaminated soil. Ecotoxicol. Environ. Saf. 2024, 277, 116378. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242, 1557–1565. [Google Scholar] [CrossRef]
- Hossain, M.B.; Banik, P.; Nur, A.A.U.; Rahman, T. Abundance and characteristics of microplastics in sediments from the world’s longest natural beach, Cox’s Bazar, Bangladesh. Mar. Pollut. Bull. 2021, 163, 111956. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Zalewski, M.; Jakubowska, A.; Wodzinowski, T.; Malinga, M.; Pałys, B.; Dąbrowska, A. Microplastics on sandy beaches of the southern Baltic Sea. Mar. Pollut. Bull. 2020, 155, 111170. [Google Scholar] [CrossRef]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Terzi, Y.; Gedik, K.; Eryaşar, A.R.; Oztürk, R.Ç.; Şahin, A.; Yılmaz, F. Microplastic contamination and characteristics spatially vary in the southern Black Sea beach sediment and sea surface water. Mar. Pollut. Bull. 2022, 174, 113228. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Ayat, B.; Aydoğan, B.; Çevik, C.; Karaca, S. Hydrometeorological assessments of the transport of microplastic pellets in the Eastern Mediterranean. Sci. Total Environ. 2022, 823, 153676. [Google Scholar] [CrossRef] [PubMed]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Akkan, T.; Gedik, K.; Mutlu, T. Protracted dynamicity of microplastics in the coastal sediment of the Southeast Black Sea. Mar. Pollut. Bull. 2023, 188, 114722. [Google Scholar] [CrossRef]
- Al-Nahian, S.; Rakib, M.R.J.; Haider, S.M.B.; Kumar, R.; Mohsen, M.; Sharma, P.; Khandaker, M.U. Occurrence, spatial distribution, and risk assessment of microplastics in surface water and sediments of Saint Martin Island in the Bay of Bengal. Mar. Pollut. Bull. 2022, 179, 113720. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface water and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Sunitha, T.G.; Monisha, V.; Sivanesan, S.; Vasanthy, M.; Prabhakaran, M.; Omine, K.; Sivasankar, V.; Darchen, A. Micro-plastic pollution along the Bay of Bengal coastal stretch of Tamil Nadu, South India. Sci. Total Environ. 2021, 756, 144073. [Google Scholar] [CrossRef] [PubMed]
- Yıbar, A.; Nur Genc, M.; Ceylan, A.; Suzer, B.; Duman, M. Determination of Microplastic and Mold Species in Mussels from the Marmara Sea, Turkey. Acta Vet. Eurasia 2024, 50, 196–209. [Google Scholar]
- Frias, J.; Pagter, E.; Nash, R.; O’Connor, I.; Carretero, O.; Filgueiras, A.; Viñas, L.; Gago, J.; Antunes, J.; Bessa, F.; et al. Standardised Protocol for Monitoring Microplastics in Sediments; JPI-Oceans BASEMAN Project; JPI Oceans: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Ramavandi, B.; Ghasemi, A.; Sorial, G.A. Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 2021, 264, 128543. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms in mangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastics in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Cao, W.; Liu, X.; Jiang, F.; Ding, J.; Yin, X.; Sun, C. Distribution characteristics of microplastics in the seawater and sediment: A case study in Jiaozhou Bay, China. Sci. Total Environ. 2019, 674, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020, 259, 113948. [Google Scholar] [CrossRef]
- Hosseini, R.; Sayadi, M.H.; Aazami, J.; Savabieasfehani, M. Accumulation and distribution of microplastics in the sediment and coastal water samples of Chabahar Bay in the Oman Sea, Iran. Mar. Pollut. Bull. 2020, 160, 111682. [Google Scholar] [CrossRef]
- Rasta, M.; Sattari, M.; Taleshi, M.S.; Namin, J.I. Identification and distribution of microplastics in the sediments and surface waters of Anzali Wetland in the Southwest Caspian Sea, Northern Iran. Mar. Pollut. Bull. 2020, 160, 111541. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; El Menif, N.T. Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef] [PubMed]
- John, K.I.; Omorogie, M.O.; Bayode, A.A.; Adeleye, A.T.; Helmreich, B. Environmental microplastics and their additives—A critical review on advanced oxidative techniques for their removal. Chem. Pap. 2023, 77, 657–676. [Google Scholar] [CrossRef]
- Abidli, S.; Toumi, H.; Lahbib, Y.; Trigui El Menif, N. The first evaluation of microplastics in sediments from the complex Lagoon-Channel of Bizerte (northern Tunisia). Water Air Soil Pollut. 2017, 228, 262. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Bagheri, T.; Gholizadeh, M.; Abarghouei, S.; Zakeri, M.; Hedayati, A.; Rabaniha, M.; Aghaeimoghadam, A.; Hafezieh, M. Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay, Caspian Sea. Chemosphere 2020, 253, 127201. [Google Scholar] [CrossRef]
- Shaw, D.G.; Day, R.H. Colour- and form-dependent loss of plastic micro-debris from the North Pacific Ocean. Mar. Pollut. Bull. 1994, 28, 39–43. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Osorio, E.D.; Tanchuling, M.A.N.; Diola, M.B.L.D. Microplastics occurrence in surface waters and sediments in five river mouths of Manila Bay. Front. Environ. Sci. 2021, 9, 719274. [Google Scholar] [CrossRef]
- MSFD Technical Group on Marine Litter. Guidance on the Monitoring of Marine Litter in European Seas: An Update to Improve the Harmonised Monitoring of Marine Litter Under the Marine Strategy Framework Directive; EUR 31539 EN; JRC133594; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Phuong, N.N.; Fauvelle, V.; Grenz, C.; Ourgaud, M.; Schmidt, N.; Strady, E.; Semépré, R. Highlights from a review of microplastics in marine sediments. Sci. Total Environ. 2021, 777, 146225. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Haave, M.; Lorenz, C.; Primpke, S.; Gerdts, G. Different stories told by small and large microplastics in sediment—First report of microplastic concentrations in an urban recipient in Norway. Mar. Pollut. Bull. 2019, 141, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, X.; Peng, C.; Qiao, S.; Wang, T.; Yu, W.; Khokiattiwong, S.; Kornkanitnan, N. Occurrence and distribution of microplastics in surface sediments from the Gulf of Thailand. Mar. Pollut. Bull. 2020, 152, 110916. [Google Scholar] [CrossRef]
- Molina, N.; López Pérez, T.M.; Puerta Cerpa, Y.D. Molecular mechanisms of microplastic toxicity in coastal sediments of La Guajira, Colombia and emerging ecological risks. Case Stud. Chem. Environ. Eng. 2025, 11, 101108. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment: A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Jualaong, S.; Pransilpa, M.; Pradit, S.; Towatana, P. Type and Distribution of Microplastics in Beach Sediment Along the Coast of the Eastern Gulf of Thailand. J. Mar. Sci. Eng. 2021, 9, 1405. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Tripathi, D.; Ebrary, I. Practical Guide to Polypropylene; Rapra Technology Limited: Shrewsbury, UK, 2002. [Google Scholar]
- Chae, D.-H.; Kim, I.-S.; Kim, S.-K.; Song, Y.K.; Shim, W.J. Abundance and distribution characteristics of microplastics in surface seawaters of the Incheon/Kyeonggi coastal region. Arch. Environ. Contam. Toxicol. 2015, 69, 269–278. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Li, Y.; Tan, S.; Kang, Z.; Yu, X.; Lan, W.; Cai, L.; Wang, J.; Shi, H. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China. Sci. Total Environ. 2018, 658, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastic pollution in freshwater and its impact on environmental health. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Murphy, F.; Ewins, C. Analytical methods for the detection of microplastics in marine environments. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A Method for Extracting Soil Microplastics through Circulation of Sodium Bromide Solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Asadi, M.A.; Hertika, A.M.S.; Feni, I.; Aisyah, Y.Y. Microplastics in the sediment of intertidal areas of Lamongan, Indonesia. Aquac. Aquar. Conserv. Legis. 2019, 12, 1065–1073. Available online: https://www.researchgate.net/publication/335365727_Microplastics_in_the_sediment_of_intertidal_areas_of_Lamongan_Indonesia (accessed on 17 March 2025).
- Béraud, E.; Bednarz, V.; Otto, I.; Golbuu, Y.; Ferrier-Pagès, C. Plastics are a new threat to Palau’s coral reefs. PLoS ONE 2022, 17, e0270237. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, L.; Cizdziel, J.; Huang, Y. Research progress on microplastics in wastewater treatment plants: A holistic review. J. Environ. Manag. 2023, 325, 116411. [Google Scholar] [CrossRef]
- Rabari, V.; Patel, K.; Patel, H.; Trivedi, J. Quantitative assessment of microplastic in sandy beaches of Gujarat state, India. Mar. Pollut. Bull. 2022, 181, 113925. [Google Scholar] [CrossRef]
- Soursou, V.; Campo, J.; Picó, Y. A critical review of the novel analytical methods for the determination of microplastics in sand and sediment samples. TrAC Trends Anal. Chem. 2023, 166, 117190. [Google Scholar] [CrossRef]
- Pinho, I.; Amezcua, F.; Rivera, J.M.; Green-Ruiz, C.; Piñón-Colin, T.J.; Wakida, F. First report of plastic contamination in batoids: Plastic ingestion by Haller’s Round Ray (Urobatis halleri) in the Gulf of California. Environ. Res. 2022, 211, 113077. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Terzi, Y.; Oztürk, R.Ç.; Eryaşar, A.R.; Yandı, İ.; Şahin, A.; Yılmaz, F.; Gündoğdu, S. Riverine microplastic discharge along the southern Black Sea coast of Turkey. Environ. Res. Lett. 2025, 20, 024061. [Google Scholar] [CrossRef]
- Ferguson, L.; Awe, A.; Sparks, C. Microplastic concentrations and risk assessment in water, sediment and invertebrates from Simon’s Town, South Africa. Heliyon 2024, 10, e28514. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Underwood, A.J.; Chapman, M.G.; Williams, R.; Thompson, R.C.; van Franeker, J.A. Linking effects of anthropogenic debris to ecological impacts. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142929. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Jiang, R.; Li, W.; Sun, X.; Lin, H.; Jiang, S.; Huang, H. Microplastic pollution and ecological risk assessment in an estuarine environment: The Dongshan Bay of China. Chemosphere 2021, 262, 127876. [Google Scholar] [CrossRef]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Vianello, A.; Kirstein, I.V.; Molazadeh, M.S.; Lorenz, C.; Vollertsen, J. Assessment of microplastic pollution and polymer risk in the sediment compartment of the Limfjord, Denmark. Sci. Total Environ. 2024, 950, 175017. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar]
- Bucci, K.; Rochman, C.M. Microplastics: A multidimensional contaminant requires a multidimensional framework for assessing risk. Microplast. Nanoplast. 2022, 2, 7. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo Hasselerharm, P.E.; Mohamed Nor, N.H.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Takeshita, K.M.; Ueda, K.; Naito, W. Estimating species sensitivity distributions for microplastics by quantitatively considering particle characteristics using a recently created ecotoxicity database. Microplast. Nanoplast. 2023, 3, 21. [Google Scholar] [CrossRef]
- Avio, C.G.; Cardelli, L.R.; Gorbi, S.; Pellegrini, D.; Regoli, F. Microplastics Pollution after the Removal of the Costa Concordia Wreck: First Evidences from a Biomonitoring Case Study. Environ. Pollut. 2017, 227, 207–214. [Google Scholar] [CrossRef]
| Station No | Stations | HI | PRI | Regions | PLI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CF | PE | PET | PP | PE | PET | PP | ||||
| 21 | Poyrazköy | 1.66 | 1100 | - | - | 1826 | - | - | IS | 1.66 |
| 1 | Garipçe | 2.73 | - | - | - | - | - | - | ||
| 2 | Florya | 1.97 | 275 | 200 | 25 | 602 | 438 | 55 | NS | 2.19 |
| 3 | M. Ereğlisi | 1.91 | 550 | - | - | 1205 | - | - | ||
| 4 | Tekirdağ | 2.06 | - | - | - | - | - | - | ||
| 5 | Şarköy | 2.99 | - | - | - | - | - | - | ||
| 6 | Gelibolu | 3.00 | - | - | 100 | - | - | 336 | ÇS | 3.36 |
| 7 | Burhanlı | 3.16 | 550 | - | - | 1848 | - | - | ||
| 8 | Bigalı | 2.55 | - | 200 | 50 | - | 672 | 168 | ||
| 9 | Kumkale | 4.31 | 550 | 200 | - | 1848 | 672 | - | ||
| 10 | Kepez | 5.44 | - | - | - | - | - | - | ||
| 11 | Çardak | 2.56 | 550 | - | 50 | 1848 | - | 168 | ||
| 12 | Kemer | 1.85 | 1100 | - | - | 3784 | - | - | SS | 3.44 |
| 13 | Erdek | 3.40 | 1100 | - | - | 3784 | - | - | ||
| 15 | Yenice | 6.46 | 319 | 116 | 14 | 1097 | 399 | 48 | ||
| 16 | Kurşunlu | 2.24 | 1100 | - | - | 2651 | - | - | GB | 2.41 |
| 17 | Büyükkumla | 2.60 | - | - | - | - | - | - | ||
| 18 | Yalova | 2.36 | - | - | - | - | - | - | ||
| 19 | Karamürsel | 2.40 | 1100 | - | - | 2772 | - | - | GI | 2.52 |
| 20 | Başiskele | 2.84 | 550 | - | - | 1386 | - | - | ||
| Risk Category [44] | Low (I) | Moderate (II) | High (III) | Very High (IV) | Dangerous (V) | |||||
| Contamination Factor (CF) | <1 | 1–3 | 3–6 | >6 | ||||||
| Pollution Load Index (PLI) | <1 | 1–3 | 3–4 | 4–5 | >5 | |||||
| Polymer Risk Index (HI) | <10 | 10–100 | 101–1000 | 1000–10,000 | >10,000 | |||||
| Pollution Risk Index (PRI) | <150 | 150–300 | 300–600 | 600–1200 | >1200 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcıoğlu İlhan, E.B. Spatial Dynamics and Ecological Risk Assessment of Microplastics in Littoral Sediments of the Sea of Marmara, Türkiye. J. Mar. Sci. Eng. 2025, 13, 1159. https://doi.org/10.3390/jmse13061159
Balcıoğlu İlhan EB. Spatial Dynamics and Ecological Risk Assessment of Microplastics in Littoral Sediments of the Sea of Marmara, Türkiye. Journal of Marine Science and Engineering. 2025; 13(6):1159. https://doi.org/10.3390/jmse13061159
Chicago/Turabian StyleBalcıoğlu İlhan, Esra Billur. 2025. "Spatial Dynamics and Ecological Risk Assessment of Microplastics in Littoral Sediments of the Sea of Marmara, Türkiye" Journal of Marine Science and Engineering 13, no. 6: 1159. https://doi.org/10.3390/jmse13061159
APA StyleBalcıoğlu İlhan, E. B. (2025). Spatial Dynamics and Ecological Risk Assessment of Microplastics in Littoral Sediments of the Sea of Marmara, Türkiye. Journal of Marine Science and Engineering, 13(6), 1159. https://doi.org/10.3390/jmse13061159







