Influence of the Dissolution of Al- and Zn-Based Galvanic Anodes on the Composition of Calcareous Deposits
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Samples from Seaport Installation
2.1.2. Laboratory Experiments
2.1.3. In Situ Experiments in a Seaport
2.2. Electrochemistry
2.3. Characterization of the Calcareous Deposits
2.4. Chemical Modelling
3. Results
3.1. Analysis of the Calcareous Deposits Sampled from Seaport Installation
3.2. Laboratory Experiments
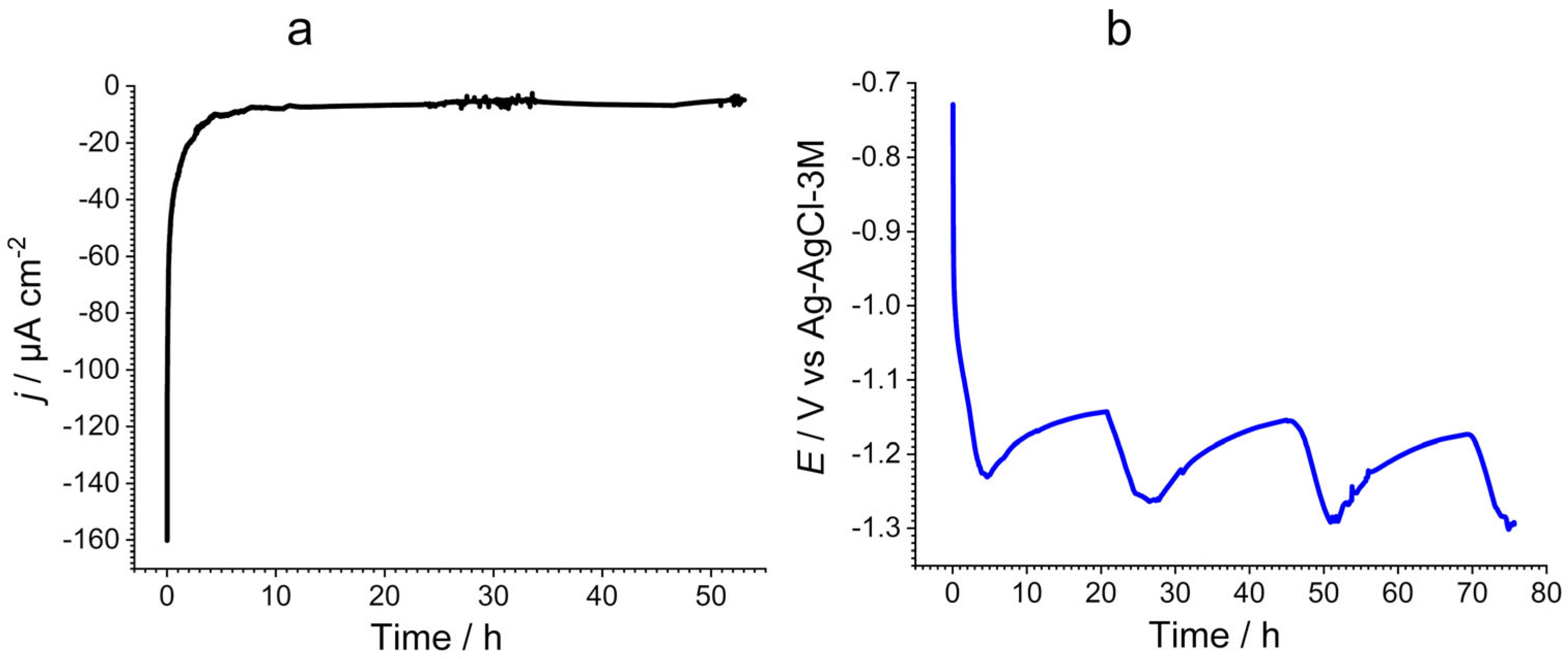
3.3. In Situ Experiment in L’Estaque Seaport
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Z.; Fu, Y.; Liu, K.; Xiao, R.; Wang, X.; Shi, H. Three-stage vertical distribution of seawater conductivity. Sci. Rep. 2018, 8, 9916. [Google Scholar] [CrossRef] [PubMed]
- Davy, H. On the corrosion of copper sheeting by sea water, and on methods of preventing this effect; and on their application to ships of war and other ships. Philos. Trans. R. Soc. Lond. 1824, 114, 151–158. [Google Scholar] [CrossRef][Green Version]
- EN ISO/DIS 9351; Galvanic Anodes for Cathodic Protection in Seawater and Saline Sediments. International Organization for Standardization (ISO): Geneva, Switzerland, 2023.
- OSPAR Commission. Riverine Inputs and Direct Discharges to Convention Waters. OSPAR Contacting Parties’ RID 2013 Data Report. 2013. Available online: https://www.ospar.org/documents?v=7373 (accessed on 19 May 2025).
- Caplat, C.; Oral, R.; Mahaut, M.-L.; Mao, A.; Barillier, D.; Guida, M.; Della Rocca, C.; Pagano, G. Comparative toxicities of aluminum and zinc from sacrificial anodes or from sulfate salt in sea urchin embryos and sperm. Ecotoxicol. Environ. Saf. 2010, 73, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Mahaut, M.-L.; Pineau, S.; Barillier, D. Assessment of sacrificial anode impact by aluminum accumulation in mussel Mytilus edulis: A large-scale laboratory test. Mar. Pollut. Bull. 2011, 62, 2707–2713. [Google Scholar] [CrossRef]
- Muttin, E.; Caplat, C.; Latire, T.; Mottier, A.; Mahaut, M.-P.; Costil, K.; Barillier, D.; Lebel, J.-M.; Serpentini, A. Effect of zinc sacrificial anode degradation on the defence system of the Pacific oyster, Crassostrea gigas: Chronic and acute exposures. Mar. Pollut. Bull. 2012, 64, 1911–1920. [Google Scholar] [CrossRef]
- Deborde, J.; Refait, P.; Bustamante, P.; Caplat, C.; Basuyaux, O.; Grolleau, A.M.; Mahaut, M.L.; Brach-Papa, C.; Gonzalez, J.L.; Pineau, S. Impact of Galvanic Anode Dissolution on Metal Trace Element Concentrations in Marine Waters. Water Air Soil Pollut. 2015, 226, 423. [Google Scholar] [CrossRef]
- Kirchgeorg, T.; Weinberg, I.; Hörnig, M.; Baier, R.; Schmid, M.J.; Brockmeyer, B. Emissions from corrosion protection systems of offshore wind farms: Evaluation of the potential impact on the marine environment. Mar. Pollut. Bull. 2018, 136, 257–268. [Google Scholar] [CrossRef]
- Leleyter, L.; Baraud, F.; Reinert, T.; Gouali, S.; Lemoine, M.; Gil, O. Fate of aluminium released by sacrificial anodes–Contamination of marine sediments by environmentally available compounds. Comptes Rendus Geosci. 2018, 350, 195–201. [Google Scholar] [CrossRef]
- Caplat, C.; Basuyaux, O.; Pineau, S.; Deborde, J.; Grolleau, A.-M.; Leglatin, S.; Mahaut, M.L. Transfer of elements released by aluminum galvanic anodes in a marine sedimentary compartment after long-term monitoring in harbor and laboratory environments. Chemosphere 2020, 239, 124720. [Google Scholar] [CrossRef]
- Bell, A.-M.; Von der Au, M.; Regnery, J.; Schmid, M.; Meermann, B.; Reifferscheid, G.; Ternes, T.; Buchinger, S. Does galvanic cathodic protection by aluminum anodes impact marine organisms? Environ. Sci. Eur. 2020, 32, 157. [Google Scholar] [CrossRef]
- Reese, A.; Voigt, N.; Zimmermann, T.; Irrgeher, J.; Pröfrock, D. Characterization of alloying components in galvanic anodes as potential environmental tracers for heavy metal emissions from offshore wind structures. Chemosphere 2020, 257, 127812. [Google Scholar] [CrossRef] [PubMed]
- Levallois, A.; Caplat, C.; Basuyaux, O.; Lebel, J.-M.; Laisney, A.; Costil, K.; Serpentini, A. Effects of chronic exposure of metals released from the dissolution of an aluminium galvanic anode on the Pacific oyster Crassostrea gigas. Aquat. Toxicol. 2022, 249, 106223. [Google Scholar] [CrossRef] [PubMed]
- Barbarin, M.; Turquois, C.; Dubillot, E.; Huet, V.; Churlaud, C.; Muttin, F.; Thomas, H. First quantitative biomonitoring study of two ports (marina, commerce) in French littoral area: Evaluation of metals released into the marine environment and resulting from galvanic anodes. Sci. Total Environ. 2023, 857, 159244. [Google Scholar] [CrossRef] [PubMed]
- Levallois, A.; Vivier, B.; Caplat, C.; Goux, D.; Orvain, F.; Lebel, J.-M.; Claquin, P.; Chasselin, L.; Basuyaux, O.; Serpentini, A. Aluminium-based galvanic anode impacts the photosynthesis of microphytobenthos and supports the bioaccumulation of metals released. Aquat. Toxicol. 2023, 258, 106501. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, A.; Wippermann, D.; Zimmermann, T.; Klein, O.; Kirchgeorg, T.; Weinberg, I.; Hasenbien, S.; Plaß, A.; Pröfrock, D. Investigation of potential metal emissions from galvanic anodes in offshore wind farms into North Sea sediments. Mar. Pollut. Bull. 2023, 194, 115396. [Google Scholar] [CrossRef]
- Imbert-Auvray, N.; Fichet, D.; Bodet, P.E.; Ory, P.; Sabot, S.; Refait, P.; Graber, M. Metabolomics-based investigation on the metabolic changes in Crassostrea gigas experimentally exposed to galvanic anodes. Metabolites 2023, 13, 869. [Google Scholar] [CrossRef]
- Humble, R.A. Cathodic protection of steel in sea water with magnesium anodes. Corrosion 1948, 4, 358–370. [Google Scholar] [CrossRef]
- Lee, R.U.; Ambrose, J.R. A hydrodynamical, morphological and chemical study of calcareous deposit. In Proceedings of the Corrosion NACE Conference 1986, Houston, TX, USA, 17–21 March 1986. [Google Scholar]
- Kunjapur, M.M.; Hartt, W.H.; Smith, S.W. Influence of temperature and exposure time upon calcareous deposits. Corrosion 1987, 43, 674–679. [Google Scholar] [CrossRef]
- Mantel, K.E.; Hartt, W.H.; Chen, T.Y. Substrate, Surface Finish and Flow Rate Influences Upon Calcareous Deposit Structure and Properties. In Proceedings of the Corrosion NACE Conference 1990, Houston, TX, USA, 9–14 March 1990. [Google Scholar]
- Barchiche, C.; Deslouis, C.; Festy, D.; Gil, O.; Refait, P.; Touzain, S.; Tribollet, B. Characterization of Calcareous Deposits in Artificial Sea Water by Impedance Techniques. 3- Deposit of CaCO3 in the presence of Mg(II). Electrochim. Acta. 2003, 48, 1645–1654. [Google Scholar] [CrossRef]
- Carré, C.; Zanibellato, A.; Jeannin, M.; Sabot, R.; Gunkel-Grillon, P.; Serres, A. Electrochemical calcareous deposition in seawater. A review. Environ. Chem. Lett. 2020, 18, 1193–1208. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, H.; Lin, Z.; Chen, Z. The influence of rust layers on calcareous deposits’ performance and protection current density in the cathodic protection process. Coatings 2024, 14, 1015. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Yang, D.; Kunte, H.G.; De Marco, R.; Wang, X. Investigation of the calcareous deposits formation controlled by interfacial pH and its effect on the hydrogen entry into AISI 4135 steel in seawater. Int. J. Hydrogen Energy 2021, 46, 5824–5841. [Google Scholar] [CrossRef]
- Nezgoda, J.; Leoni, G.B.; Delarue, S.L.; Brasil, C. Calcareous deposits formed under long-term in situ cathodic protection tests. Chem. Eng. Technol. 2021, 44, 1094–1102. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Z.; Zhao, X.; Lan, X.; Wang, J.; Lv, X.; Zhang, C.; Duan, J.; Hou, B. The Interaction of biofoulants and calcareous deposits on corrosion performance of Q235 in seawater. Materials 2020, 13, 850. [Google Scholar] [CrossRef]
- Carré, C.; Gunkel-Grillon, P.; Serres, A.; Jeannin, M.; Sabot, R.; Quiniou, T. Calcareous electrochemical precipitation, a new method to trap nickel in seawater. Environ. Chem. Lett. 2017, 15, 151–156. [Google Scholar] [CrossRef]
- Carré, C.; Gunkel-Grillon, P.; Serres, A.; Jeannin, M.; Sabot, R.; Quiniou, T. Laboratory and in-situ investigations for trapping Pb and Ni with an unusual electrochemical device, the calcareous deposit in seawater. Sci. Rep. 2019, 9, 3400. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (Version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2—A Geochemical Assessment Model for Environmental Systems—Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development U.S. Environmental Protection Agency: Athens, GA, USA, 1990. [Google Scholar]
- US Environmental Protection Agency. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems—User Manual Supplement for Version 4.0; National Exposure Research Laboratory, Ecosystems Research Division: Athens, GA, USA, 1998. [Google Scholar]
- ASTM D1141-98; Standard Practice for Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2021.
- Refait, P.; Jeannin, M.; Sabot, R.; Antony, H.; Pineau, S. Corrosion and cathodic protection of carbon steel in the tidal zone: Products, mechanisms and kinetics. Corros. Sci. 2015, 90, 375–382. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of carbon steel in marine environments: Role of the corrosion product layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Refait, P.; Jeannin, M.; Sabot, R.; Antony, H.; Pineau, S. Electrochemical formation and transformation of corrosion products on carbon steel under cathodic protection in seawater. Corros. Sci. 2013, 71, 32–36. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. Thermodynamics of aragonite-strontianite solid solutions: Results from stoichiometric solubility at 25 and 76°C. Geochim. Cosmochim. Acta 1987, 51, 1393–1411. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium carbonate formation and dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J. The influence of impurities on the growth rate of calcite. J. Cryst. Growth 1984, 66, 639–646. [Google Scholar] [CrossRef]
- Wada, N.; Yamashita, K.; Umegaki, T. Effects of divalent cations upon nucleation, growth and transformation of calcium carbonate polymorphs under conditions of double diffusion. J. Cryst. Growth 1995, 148, 297–304. [Google Scholar] [CrossRef]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the growth and dissolution kinetics of the CaCO3 polymorphs calcite and aragonite. II. The influence of divalent cation additives on the growth and dissolution rates. J. Cryst. Growth 1996, 158, 310–315. [Google Scholar] [CrossRef]
- Sarlak, M.; Shahrabi, T.; Zamanzade, M. Investigation of calcareous deposits formation on copper and 316L stainless steel under cathodic polarization in artificial seawater. Prot. Met. Phys. Chem. Surf. 2009, 45, 216–222. [Google Scholar] [CrossRef]
- Griñon-Echaniz, R.; Refait, P.; Jeaninn, M.; Sabot, R.; Paul, S.; Thornton, R. Study of cathodic reaction in defects of thermal spray aluminium coatings on steel in artificial seawater. Corros. Sci. 2021, 187, 109514. [Google Scholar] [CrossRef]





| Element | R.A. (wt.%) | R.A. Ratios | Calcareous Deposit | Seawater |
|---|---|---|---|---|
| Ca | 74.79 | Ca/Sr | 37.8 | 50 |
| Mg | 11.16 | Mg/Ca | 0.15 | 3.14 |
| Si | 0 | - | - | - |
| Fe | 11.82 | Fe/Mn | 83 | - |
| Sr | 1.98 | Sr/Zn | 86 | 0.32 |
| Al | 0 | Al/Zn | 0 | 0.56 |
| Ti | 0.064 | - | - | - |
| Zn | 0.023 | Zn/Cu | 1.1 | 0.93 |
| Mn | 0.142 | - | - | - |
| Cu | 0.021 | - | - | - |
| Elements | Experiment E1-AlZn (ECP = −950 mV/AgAgCl-3M) | Experiment I2-Al (jCP = −100 µA cm−2) |
|---|---|---|
| Ca | 29.04 | 21.58 |
| Mg | 24.19 | 76.64 |
| Fe | 16.13 | 0.23 |
| Al | 10.32 | 1.55 |
| Zn | 20.32 | - |
| Elements | R.A. (wt.%), Zone 1 | R.A. (wt.%), Zone 2 | R.A. Ratios | Zone 1 | Zone 2 | Seawater |
|---|---|---|---|---|---|---|
| Ca | 84.03 | 88.88 | Ca/Sr | 63.2 | 64.9 | 67.7 |
| Mg | 4.29 | 3.99 | Mg/Ca | 0.05 | 0.045 | 3.14 |
| Si | 5.39 | 3.40 | Si/Al | 4.4 | 4.5 | - |
| Fe | 3.52 | 1.44 | Fe/Mn | 130 | 120 | - |
| Sr | 1.33 | 1.37 | Sr/Zn | 24 | 19 | 0.5 |
| Al | 1.23 | 0.76 | Al/Zn | 22 | 11 | 0 1 |
| Ti | 0.121 | 0.064 | - | - | - | - |
| Zn | 0.056 | 0.071 | Zn/Cu | 3.5 | 5.5 | 0.99 |
| Mn | 0.027 | 0.012 | - | - | - | - |
| Cu | 0.016 | 0.013 | - | - | - | - |
| pH | 7.85 | 8.20 | 8.60 | 8.87 | 9.31 | 9.36 |
|---|---|---|---|---|---|---|
| Dissolved Al(III) conc. | 1.38 × 10−4 | 3.13 × 10−4 | 7.73 × 10−4 | 1 × 10−3 | 1 × 10−3 | 1 × 10−3 |
| Precipitated solid phase | Amorphous Al(OH)3 | Amorphous Al(OH)3 | Amorphous Al(OH)3 | None | None | Brucite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batisse, F.; Duportal, M.; Rémazeilles, C.; Edouard, A.; Meuriot, L.; Refait, P. Influence of the Dissolution of Al- and Zn-Based Galvanic Anodes on the Composition of Calcareous Deposits. J. Mar. Sci. Eng. 2025, 13, 1130. https://doi.org/10.3390/jmse13061130
Batisse F, Duportal M, Rémazeilles C, Edouard A, Meuriot L, Refait P. Influence of the Dissolution of Al- and Zn-Based Galvanic Anodes on the Composition of Calcareous Deposits. Journal of Marine Science and Engineering. 2025; 13(6):1130. https://doi.org/10.3390/jmse13061130
Chicago/Turabian StyleBatisse, Florent, Malo Duportal, Céline Rémazeilles, Alban Edouard, Ludovic Meuriot, and Philippe Refait. 2025. "Influence of the Dissolution of Al- and Zn-Based Galvanic Anodes on the Composition of Calcareous Deposits" Journal of Marine Science and Engineering 13, no. 6: 1130. https://doi.org/10.3390/jmse13061130
APA StyleBatisse, F., Duportal, M., Rémazeilles, C., Edouard, A., Meuriot, L., & Refait, P. (2025). Influence of the Dissolution of Al- and Zn-Based Galvanic Anodes on the Composition of Calcareous Deposits. Journal of Marine Science and Engineering, 13(6), 1130. https://doi.org/10.3390/jmse13061130







