Ecological Geography of the Phytoplankton Associated to Bio-Optical Variability and HPLC-Pigments in the Central Southwestern Gulf of Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Collection
2.2.1. Chlorophyll and Carotenoids
2.2.2. Phytoplankton Absorption Coefficients
2.2.3. Phytoplankton Taxonomic
2.2.4. Inorganic Nutrients
2.2.5. Statistical Analysis and Concept Definition
3. Results
3.1. Hydrographic and Chemical-Biological Conditions
3.1.1. Temperature, Salinity, Sigma-t, Chlorophyll a, and Nano-Microphytoplankton
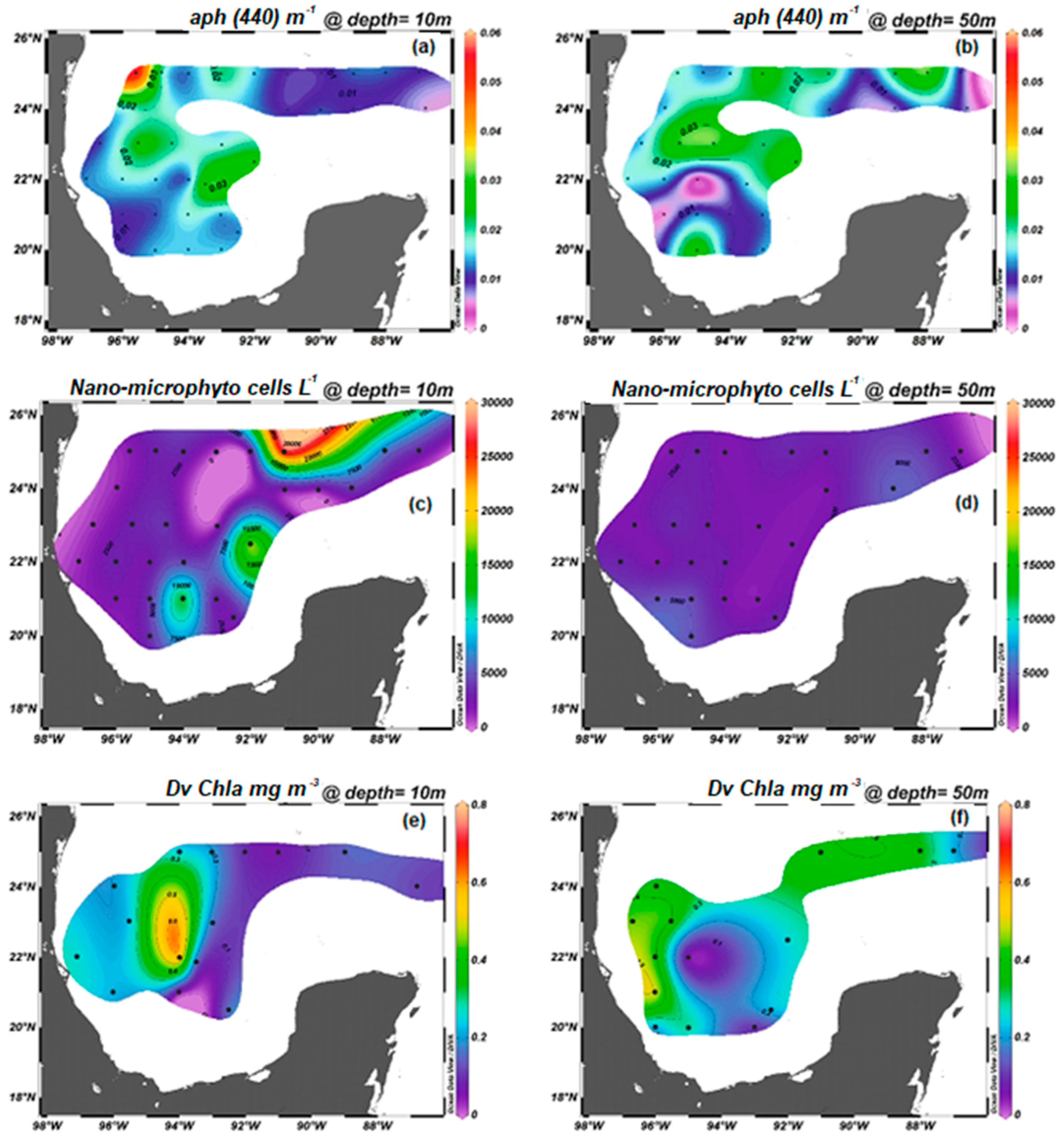
3.1.2. Bio-Optical Variability and Biogeochemical Conditions
3.1.3. Spatial Distribution of Pigments
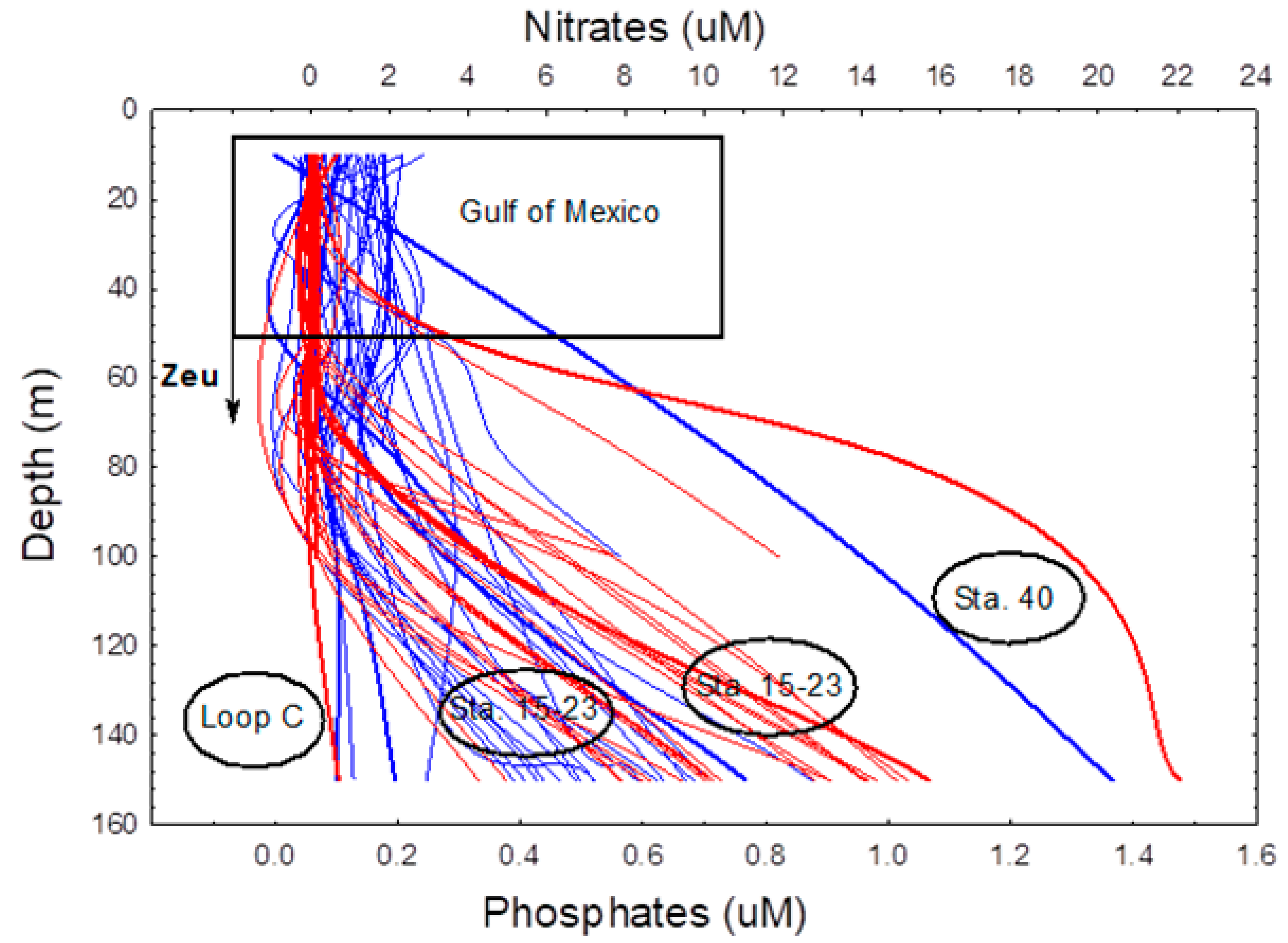
3.1.4. Spatial Distribution of Inorganic Nutrients
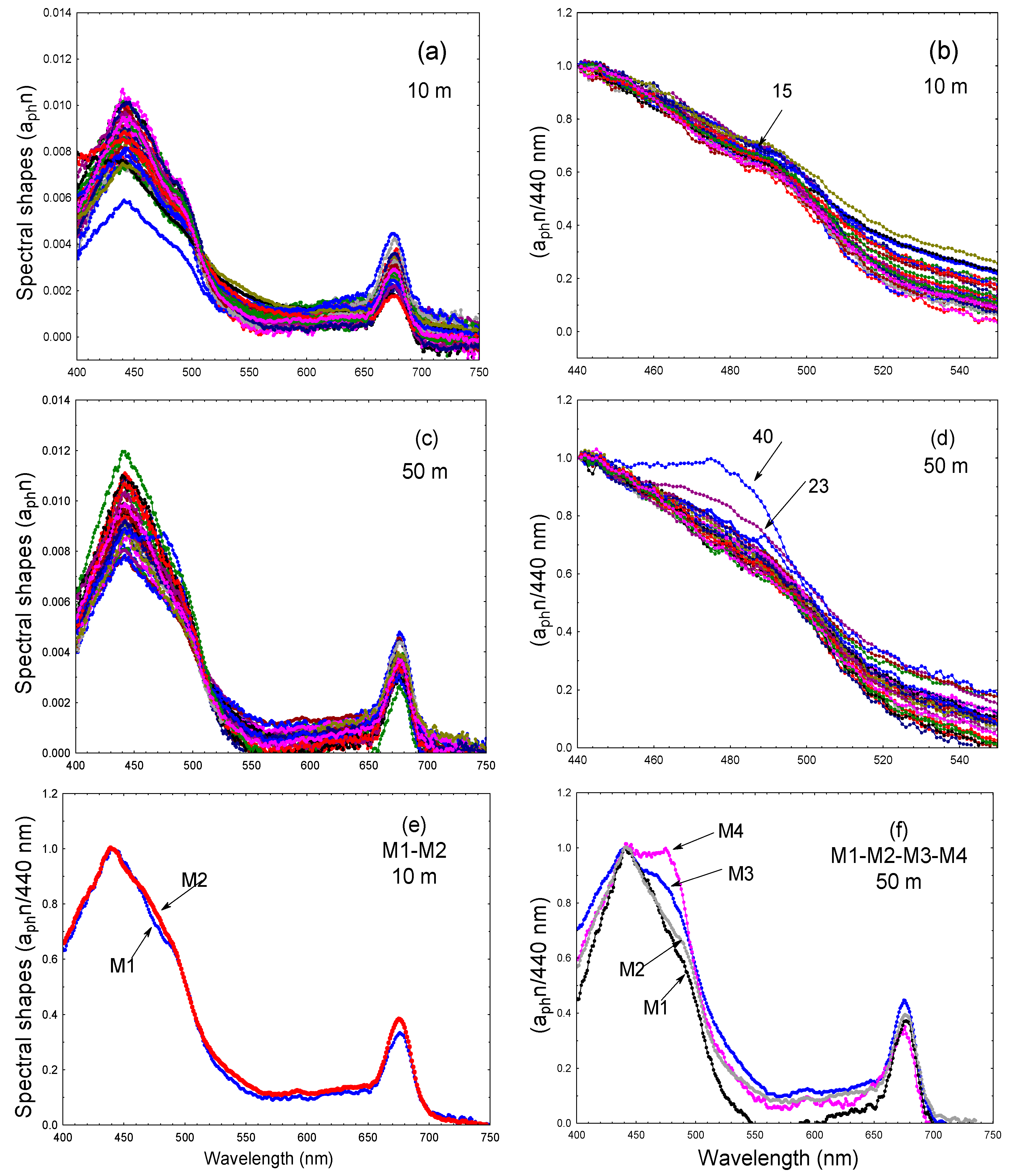
3.1.5. Phytoplankton Spectral Shapes
4. Discussion
4.1. Oceanographic Conditions in the Gulf of Mexico
4.1.1. Phytoplankton and Absorption Properties
4.1.2. Optical Properties and Phytoplankton Pigments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parr, A.E. Report on hydrographic observations in the Gulf of Mexico and the adyacent straits made during the Yale Oceanography Expedition on the Mabel Taylor in 1932. Bull. Bingham Ocean. 1935, 5, 1–93. [Google Scholar]
- Leipper, D.F. Physical oceanography of the Gulf of Mexico. Fish. Bull. Fish Wildl. Serv. 1954, 55, 119–137. [Google Scholar]
- Androulidakis, Y.; Kourafalou, V.; Olascoaga, M.J.; Berón-Vera, F.J.; Le Hénaff, M.; Kang, H.; Ntaganou, N. Impact of Caribbeans anticyclones on Loop Current variability. Ocean. Dyn. 2021, 71, 935–956. [Google Scholar] [CrossRef]
- Weisberg, R.H.; Liu, Y. On the Loop Current penetration into the Gulf of Mexico. J. Geophys. Res.-Oceans 2017, 122, 9679–9694. [Google Scholar] [CrossRef]
- Buenfil, J. Executive summary. In Adaptación a los Impactos del Cambio Climático en los Humedales Costeros del Golfo de México; Buenfil, J., Ed.; SEMARNAT: Mexico City, Mexico, 2009; pp. 25–34. [Google Scholar]
- Pasqueron de Fommervault, O.; Pérez-Brunius, P.; Damien, P.; Camacho-Ibar, V.F.; Sheinbaum, J. Temporal variability of chlorophyll distribution in the Gulf of Mexico: Bio-optical data from profiling flots. Biogeosciences 2017, 14, 5647–5662. [Google Scholar] [CrossRef]
- Yingling, N.; Thomas, K.B.; Shropshire, T.A.; Landry, M.R.; Selph, K.E.; Knapp, A.N.; Kranz, S.A.; Stukel, M.R. Taxon-specific phytoplankton growth, nutrient utilization and light limitation in the oligotrophic Gulf of Mexico. J. Plankton Res. 2022, 44, 656–676. [Google Scholar] [CrossRef]
- Yentsch, C.S.; Phinney, D.A. A bridge between ocean optics and microbial ecology. Limnol. Oceanogr. 1989, 34, 1694–1705. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994; p. 509. [Google Scholar]
- Gieskes, W.W.C.; Kraay, G.W.; Nontji, A.; Setiapermana, D.; Sutomo, D. Monsoonal alteration of a mixed and a layered structure in the euphotic zone of the Banda Sea (Indonesia): A mathematical analysis of algal pigment fingerprints. Neth. J. Sea Res. 1988, 22, 123–137. [Google Scholar] [CrossRef]
- Goericke, R.; Repeta, D.J. The pigments of Prochlorococcus marinus: The presence of divinyl chlorophyll a and b in a marine prokaryote. Limnol. Oceanogr. 1992, 37, 425–433. [Google Scholar] [CrossRef]
- Sánchez-Pérez, E.D.; Millán-Núñez, E. Ecological zonation of phytoplankton and biomass based on bio-optical parameters off Baja California during three summer seasons. Open J. Mar. Sci. 2019, 9, 121–136. [Google Scholar] [CrossRef]
- Nababan, B. Bio-Optical Variability of Surface Waters in the Northeastern Gulf of Mexico. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2005. Available online: http://scholarcommons.usf.edu/etd/784 (accessed on 3 May 2005).
- Damien, P.; Sheinbaum, J.; Pasqueron de Fommervault, O.; Jouanno, J.; Linacre, L.; Duteil, O. Do Loop Current Eddies stimulate productivity in the Gulf of Mexico. Biogeosciences 2021, 18, 4281–4303. [Google Scholar] [CrossRef]
- Throndsen, J. Preservation and storage. In Phytoplankton Manual. Monographs on Oceanographic Methodology; Sournia, A., Ed.; UNESCO: Paris, France, 1978; Volume 6, pp. 69–74. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.; Holmes, R.; Strickland, J. Fluorimetric determination of chlorophyll. J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C. Computer assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Cleveland, J.S.; Weidemann, A.D. Quantifying absorption by aquatic particles: A multiple scattering correction for glass- fiber filters. Limnol. Oceanogr. 1993, 38, 1321–1327. [Google Scholar] [CrossRef]
- Millán-Núñez, E.; Macías-Carballo, M. Phytogeography associated at spectral absorption shapes in the southern region of the California Current. CalCOFI Rep. 2014, 55, 183–196. [Google Scholar]
- Hasle, G.R. The inverted-microscope method. In Phytoplankton Manual; Sournia, A., Ed.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1978; Volume 5.2.1, p. 337. [Google Scholar]
- Tomas, C.S. Identifying Marine Phytoplankton; Academic Press, Inc.: New York, NY, USA, 1997; p. 345. [Google Scholar]
- Gordon, L.I.; Jennings, J.C., Jr.; Ross, A.A.; Krest, J.M. A suggested protocol for continuous flow automated analysis of seawater nutrients (phosphate, nitrate, nitrite and silicie acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study. In Methods Manual WHPO: Oregon State University College of Oceanography; WOCE Hydrographic Program Office, 1993; pp. 1–55. Available online: https://www2.whoi.edu/site/wp-content/uploads/sites/84/2019/07/gordnut_133885.pdf (accessed on 3 May 2025).
- García-Córdova, J.; Sheinbaum, J. Hidrografía y Condiciones Físicas, CTD (Temperatura, Salinidad, O2, Fluorescencia, Clorofila-a). In: Monitoreo Ambiental en Aguas Profundas del Golfo de México en Respuesta al Derrame Petrolero Asociado a la Plataforma Deepwater Horizon. Informe Final. 2014, p. 447. Available online: https://www.gob.mx/cms/uploads/attachment/file/344074/CGACC_2014_XIXIMI_3_CICESE_PARTE1.pdf (accessed on 3 May 2025).
- Quintanilla, J.G.; Herguera, J.C.; Sheinbaum, J. Oxygenation of the Gulf of Mexico thermocline linked to the detachment of Loop Current eddies. Front. Mar. Sci. 2024, 11, 1479837. [Google Scholar] [CrossRef]
- Millán-Núñez, E.; Delgadillo-Hinojosa, F.; Hakspiel-Segura, C.; Torres-Delgado, E.V.; Félix-Bermudez, A.; Segovia-Zavala, J.A.; Camacho-Ibar, V.F.; Muñoz-Barbosa, A. Phytoplankton composition and biomass under oligotrophic conditions in the Guaymas Basin (Gulf of California). Cienc. Mar. 2023, 49, 1–15. [Google Scholar] [CrossRef]
- Wu, J.; Hong, H.; Shang, S.; Dai, M.; Jee, Z. Variation of phytoplankton absorption coefficients in the northern South China Sea during spring and autumn. Biogeosci. Discuss. 2007, 4, 1555–1584. [Google Scholar]
- Millán-Núñez, E.; Millán-Núñez, R. Specific Absorption Coefficient and Phytoplankton Community Structure in the Southern Region of the California Current during January 2002. J. Oceanogr. 2010, 66, 719–730. [Google Scholar] [CrossRef]
- Mann, K.H.; Lazier, J.R. Dinamics of Marine Ecosystems: Biological-Physical Interactions in the Oceans; Blackwell: Oxford, UK, 1991; p. 466. [Google Scholar]
- Aldeco, J.; Monreal-Gómez, M.A.; Signoret, M.; Salas-de León, D.A.; Hernández-Becerril, D.U. Ocurrence of a subsurface anticyclonic eddy, fronts, and Trichodesmium spp. over the Campeche Canyon region, Gulf of México. Cienc. Mar. 2009, 35, 333–344. [Google Scholar] [CrossRef]
- Linacre, L.; Lara-Lara, R.; Camacho-Ibar, V.; Herguera, J.C.; Bazán-Guzmán, C.; Ferreira-Bartrina, V. Distribution pattern of picoplankton carbon biomass linked to mesoscale dynamics in the southern gulf of Mexico during winter conditions. Deep.-Sea Res. I 2015, 106, 55–67. [Google Scholar] [CrossRef]




| Survey Stations Latitude (° N) | Zeu (m) | Temperature (°C) 10–50 m | Salinity (psu) 10–50 m | Sigma-t (kg m−3) 10–50 m | aph440 (m−1) 10–50 m | ad350 (m−1) 10–50 m | Nano (cells L−1) 10–50 m | Micro (cells L−1) (µM) | NO3 10–50 m |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 46 | 22.54-22.30 | 36.42-36.53 | 25.14-25.30 | 0.055-0.018 | 0.101-0.012 | 1653-1652 | 0-414 | 0.04-0.03 |
| 16 | 90 | 23.94-23.84 | 36.48-36.48 | 24.78-24.81 | 0.019-0.013 | 0.040-0.012 | 1790-2616 | 828-276 | 0-0 |
| 17 | --- | 23.73-23.65 | 36.49-36.48 | 24.85-24.87 | 0.012-0.015 | 0.057-0.006 | 3301-1652 | 0-276 | 0.14-0 |
| 18 | 74 | 22.95-22.60 | 36.54-36.54 | 25.12-25.22 | 0.021-0.025 | 0.078-0.006 | 690- | 138- | 0-0 |
| 19 | --- | 23.05-22.68 | 36.55-36.54 | 25.09-25.20 | 0.015-0.020 | 0.049-0.007 | 1375-3027 | 756-206 | 0-0 |
| 20 | --- | 23.06-22.30 | 36.52-36.50 | 25.07-25.27 | 0.009-0.021 | 0.045-0.009 | 27,778-2063 | 276-138 | 0-0 |
| 21 | 63 | 23.28-22.66 | 36.53-36.51 | 25.01-25.18 | 0.009-0.009 | 0.048-0.010 | - | - | 0-0 |
| 22 | --- | 23.87-23.07 | 36.52-36.49 | 24.83-25.04 | 0.012-0.022 | 0.056-0.009 | - | - | 0-0 |
| 23 | --- | 25.38-23.00 | 36.07-36.54 | 24.04-25.10 | 0.011-0.031 | 0.024-0.016 | 4951-6327 | 1377-414 | 0-0.57 |
| 24 | 71 | 26.59-25.80 | 35.90-36.00 | 23.53-23.85 | 0.009-0.009 | 0.023-0.009 | 3577-1790 | 138-138 | 0.02-0 |
| 12 | --- | 23.89-23.91 | 36.49-36.49 | 24.80-24.79 | 0.017-0.014 | 0.034-0.008 | 828- | 414- | 0.07-0 |
| 32 | 68 | 23.03-23.02 | 36.56-36.56 | 25.11-25.12 | 0.009-0.011 | 0.020-0.007 | 550-1380 | 412-550 | 0-0 |
| 31 | --- | 23.77-23.57 | 36.55-36.55 | 24.88-24.99 | 0.011-0.009 | 0.082-0.008 | 1101- | 0- | 0.10- |
| 30 | 71 | 24.36-23.68 | 36.43-36.52 | 24.61-24.89 | 0.009-0.007 | 0.016-0.010 | 2753-3715 | 1515-828 | 0.20-0.53 |
| 27 | --- | 26.87-25.92 | 35.81-36.07 | 23.37-23.86 | 0.006-0.007 | 0.064-0.007 | - | - | 0-0 |
| 2 | 54 | 24.13-24.06 | 36.49-36.48 | 24.73-24.75 | 0.011-0.014 | 0.011-0.046 | 964-1652 | 550-0 | 0.10-0.05 |
| 3B | --- | 23.56-23.58 | 36.53-36.53 | 24.93-24.92 | 0.024-0.030 | 0.007-0.040 | 2888-3303 | 138-138 | 0.15-0.04 |
| 3 | 60 | 23.72-23.69 | 36.53-36.53 | 24.88-24.89 | 0.021-0.031 | 0.007-0.042 | 3097-2066 | 276-552 | 0.19-0.12 |
| 44 | --- | 22.93-22.76 | 36.55-36.55 | 25.13-25.18 | 0.017-0.021 | 0.022-0.022 | 688-1652 | 138-412 | 0.27-0.04 |
| 47 | 74 | 23.77-23.70 | 36.59-36.58 | 24.91-24.92 | 0.026-0.029 | 0.006-0.023 | 19,389-1789 | 552-690 | 0.08-0.03 |
| 1 | --- | 24.06-24.07 | 36.50-36.50 | 24.76-24.76 | 0.012-0.016 | 0.114-0.007 | 1102-3028 | 0-0 | 0.66-0 |
| 4 | --- | 23.61-23.61 | 36.54-36.54 | 24.92-24.92 | 0.016-0.015 | 0.040-0.006 | 2201-1790 | 828-138 | 0-0.07 |
| 5 | --- | 23.78-23.74 | 36.53-36.53 | 24.87-24.87 | 0.015-0.002 | 0.023-0.001 | 2064-2205 | 550-414 | 0.05-0 |
| 7 | 82 | 23.79-23.59 | 36.52-36.51 | 24.85-24.90 | 0.012-0.010 | 0.051-0.002 | -3441 | -826 | 0.0 |
| 43 | --- | 23.56-23.48 | 36.52-36.52 | 24.92-24.95 | 0.029-0.015 | 0.047-0.006 | - | - | 0.08-0 |
| 39 | 92 | 23.90-23.84 | 36.53-36.53 | 24.83-24.85 | 0.010-0.004 | 0.006-0.009 | 1789-4127 | 414-826 | 0-0 |
| 38 | 66 | 23.67-23.60 | 36.51-36.51 | 24.88-24.90 | 0.011-0.013 | 0.017-0.006 | 3165-3441 | 414-1378 | 0-0 |
| 37 | --- | 23.95-23.88 | 36.47-36.51 | 24.77-24.82 | 0.017-0.009 | 0.036-0.009 | 12,526-1376 | 384-552 | 0-0 |
| 36 | --- | 24.00-23.97 | 36.50-36.48 | 24.77-24.77 | 0.013-0.012 | 0.054-0.008 | 1651-1378 | 690-276 | 0.04-0 |
| 35 | 63 | 24.20-24.16 | 36.42-36.42 | 24.66-24.67 | 0.015-0.013 | 0.019-0.010 | 2202-2368 | 414-412 | 0.03-0.11 |
| 46 | --- | 23.93-23.70 | 36.50-36.53 | 24.80-24.89 | 0.010-0.010 | 0.014-0.009 | - | - | 0.04-0 |
| 40 | --- | 23.72-21.93 | 36.42-36.42 | 24.80-25.32 | 0.014-0.027 | 0.023-0.008 | 2203-3027 | 1650-9602 | -3.37 |
| 41 | --- | 24.29-22.82 | 36.36-36.46 | 24.59-25.09 | 0.015-0.010 | 0.061-0.007 | - | - | 0.09- |
| 42 | 92 | 24.41-24.36 | 36.32-36.55 | 24.51-24.71 | 0.014-0.013 | 0.031-0.011 | - | - | 0.04-0.27 |
| Stations | Stations | ||||||
|---|---|---|---|---|---|---|---|
| 10 m | 50 m | ||||||
| Sta.-1 | (mg m−3) | Sta.-22 | (mg m−3) | Sta.-2 | (mg m−3) | Sta.-35 | (mg m−3) |
| DV chla | 0.262 | DV chla | 0.145 | DV chla | 0.446 | DV chla | 0.232 |
| Chlb | 0.081 | Zea | 0.076 | Chlb | 0.079 | Zea | 0.060 |
| Zea | 0.064 | Hex-fuco | 0.013 | Zea | 0.069 | Hex-fuco | 0.024 |
| Sta.-3b | Sta.-27 | Sta.-3b | Sta.-39 | ||||
| DV chla | 0.231 | DV chla | 0.118 | DV chla | 0.408 | DV chla | 0.504 |
| Chlb | 0.160 | Zea | 0.065 | Chlb | 0.231 | Chlb | 0.110 |
| Hex-fuco | 0.048 | Hex-fuco | 0.009 | Hex-fuco | 0.074 | Zea | 0.058 |
| Sta.-7 | Sta.-35 | Sta.-4 | Sta.-40 | ||||
| Chlb | 0.761 | DV chla | 0.158 | DV chla | 0.410 | Chlb | 0.360 |
| DV chla | 0.606 | Zea | 0.040 | Zea | 0.063 | DV chla | 0.299 |
| Hex-fuco | 0.081 | Chlb | 0.040 | Hex-fuco | 0.050 | Hex-fuco | 0.046 |
| Sta.-12 | Sta.-37 | Sta.-5 | Sta.-42 | ||||
| Dvchla | 0.202 | DV chla | 0.039 | DV chla | 0.039 | DV chla | 0.095 |
| Chlb | 0.087 | Hex-fuco | 0.009 | Hex-fuco | 0.009 | Zea | 0.058 |
| Hex-fuco | 0.006 | Zea | 0.006 | Zea | 0.006 | Hex-fuco | 0.018 |
| Sta.-17 | Sta.-39 | Sta.-12 | Sta.-46 | ||||
| DV chla | 0.250 | DV chla | 0.225 | DV chla | 0.358 | DV chla | 0.218 |
| Chlb | 0.086 | Zea | 0.051 | Chlb | 0.208 | Chlb | 0.077 |
| Zea | 0.067 | Chlb | 0.051 | Zea | 0.062 | Zea | 0.028 |
| Sta.-18 | Sta.-43 | Sta.-20 | Sta.-47 | ||||
| DV chla | 0.208 | DV | 0.153 | DV chla | 0.388 | Chlb | 0.268 |
| Chlb | 0.164 | Zea | 0.098 | Chlb | 0.146 | DV chla | 0.258 |
| Zea | 0.153 | Chlb | 0.092 | Hex-fuco | 0.050 | Hex-fuco | 0.024 |
| Sta.-19 | Sta.-44 | Sta.-23 | |||||
| DVchla | 0.091 | DV chla | 0.168 | Chlb | 0.475 | ||
| Zea | 0.058 | Chlb | 0.13 | DV chla | 0.357 | ||
| Hex-fuco | 0.008 | Zea | 0.009 | Hex-fuco | 0.168 | ||
| Sta.-20 | Sta.-24 | ||||||
| DV chla | 0.063 | DV chla | 0.213 | ||||
| Zea | 0.049 | Chlb | 0.066 | ||||
| Hex-fuco | 0.008 | Hex-fuco | 0.005 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Núñez, E.; De la Cruz-Orozco, M.E. Ecological Geography of the Phytoplankton Associated to Bio-Optical Variability and HPLC-Pigments in the Central Southwestern Gulf of Mexico. J. Mar. Sci. Eng. 2025, 13, 1128. https://doi.org/10.3390/jmse13061128
Millán-Núñez E, De la Cruz-Orozco ME. Ecological Geography of the Phytoplankton Associated to Bio-Optical Variability and HPLC-Pigments in the Central Southwestern Gulf of Mexico. Journal of Marine Science and Engineering. 2025; 13(6):1128. https://doi.org/10.3390/jmse13061128
Chicago/Turabian StyleMillán-Núñez, Eduardo, and Martín Efraìn De la Cruz-Orozco. 2025. "Ecological Geography of the Phytoplankton Associated to Bio-Optical Variability and HPLC-Pigments in the Central Southwestern Gulf of Mexico" Journal of Marine Science and Engineering 13, no. 6: 1128. https://doi.org/10.3390/jmse13061128
APA StyleMillán-Núñez, E., & De la Cruz-Orozco, M. E. (2025). Ecological Geography of the Phytoplankton Associated to Bio-Optical Variability and HPLC-Pigments in the Central Southwestern Gulf of Mexico. Journal of Marine Science and Engineering, 13(6), 1128. https://doi.org/10.3390/jmse13061128







