Abstract
Understanding the dynamics of phytoplankton size classes (PSCs), highly sensitive to environmental conditions in marine ecosystems, is crucial for comprehending variations in primary production and biogeochemical processes. Over the past decades, the littoral seas of Korea have undergone significant environmental shifts, yet long-term studies on PSC distribution remain limited. Employing a regionally developed deep neural network model and 20 years (2003–2022) of satellite ocean color data, we assessed spatiotemporal variability in dominant PSCs in the Yellow Sea (YS), South Sea of Korea (SS), and East/Japan Sea (EJS). Micro-size phytoplankton dominated turbid nearshore waters of the YS and western SS year-round, while nano-size phytoplankton were seasonally prevalent in the central YS and EJS. Pico-size phytoplankton exhibited strong summer dominance under warm, stratified, nutrient-depleted conditions, showing a sustained long-term expansion across all regions, particularly in the southwestern EJS. This expansion was closely linked to rising sea surface temperatures and changes in nutrient stoichiometry. The increasing dominance of smaller phytoplankton may reduce primary production, alter food web structure, and ultimately diminish fishery productivity. These findings provide new insight into climate-driven ecological shifts in marginal seas and underscore the need for integrated long-term monitoring to anticipate future ecosystem responses in a rapidly warming ocean.
1. Introduction
Phytoplankton, the foundational producers at the base of the marine food web, account for approximately 40 to 50% of global primary production [1,2] and significantly contribute to the food web as well as biogeochemical cycling dynamics in marine ecosystems [1,3,4]. The community and size structures of phytoplankton are intricately linked to the physical and chemical properties in the ocean [5,6]. Their varying sizes and community compositions influence primary production and biogeochemical processes by regulating photosynthetic efficiency, transferring organic matter to higher trophic levels, and exporting carbon to the deep ocean [2,6,7,8]. Small phytoplankton cells (<2 µm) typically dominate in nutrient-poor, stratified oligotrophic waters and have traditionally been considered minor contributors to primary production and carbon export to the deep ocean. In contrast, large phytoplankton cells (>2 µm) are generally regarded as the major drivers of primary production, especially under conditions with sufficient light and nutrient availability [8,9,10,11]. Environmental changes, such as ocean warming and enhanced stratification, have been shown to shift phytoplankton community composition towards smaller-size cells [12,13,14], suggesting that small phytoplankton can play increasingly important roles and contribute substantially to the total biomass and production of the phytoplankton community under warming scenarios [13,14,15,16]. Therefore, acquiring accurate knowledge of the functional and structural classes of phytoplankton and their distributions is essential for understanding the dynamics of marine ecosystems under different environmental conditions and their potential responses to climate change.
According to the classification proposed by Sieburth et al. [17], the dominant size of phytoplankton is traditionally categorized into three classes: micro- (>20 µm), nano- (2–20 µm), and pico-phytoplankton (<2 µm). Investigations on phytoplankton size classes (PSCs) are generally conducted through shipboard observation using microscopy and pigment analysis. However, shipboard measurements alone often cannot capture the strong spatial and temporal variations in PSCs due to their limitations (laborious and expensive). Satellite remote sensing data, with high temporal and spatial resolution, provide an opportunity to obtain a synoptic view of the optical properties of the upper waters [12,18,19]. Several algorithms for identifying PSCs, including abundance- and spectral-based methods, have been developed using satellite-based data [20,21,22,23]. However, since these algorithms were designed for the global ocean scale, they are not well suited for application in regional seas where physicochemical properties vary rapidly [24,25,26]. Accordingly, Kang et al. [27] introduced a new algorithm tailored for the littoral seas of Korea using deep learning techniques, distinguishing it from traditional algorithms. Although the new algorithm detected only the dominant PSCs at the satellite pixel level, its accuracy (67%) for field observations in the littoral seas of Korea was higher than the Aph (54%; Hirata et al. [21]) and three-component (13%; Ye and Tang [28]) models [27].
The littoral seas of Korea, situated in the northwestern part of the Pacific Ocean, are composed of three marginal seas: Yellow Sea (YS), northern East China Sea (hereafter referred to as the South Sea of Korea, SS), and East/Japan Sea (EJS) (Figure 1a). These seas exhibit distinct oceanographic characteristics, including various seasonal currents, but they feature high phytoplankton productivity that potentially provides abundant fishery yields to neighboring countries [29,30,31]. In recent years, however, these regions have experienced rapid changes in physicochemical conditions associated with rising sea surface temperature and altered nutrient availability [31,32,33,34]. Consequently, several recent studies have focused on the responses of phytoplankton to these environmental changes [35,36,37,38,39,40]. In particular, changes in the community composition and size classes of phytoplankton are likely to have a significant impact on primary production and the structure of the entire ecosystem’s food web within the region. Nonetheless, while Kang et al. [27] focused on developing and validating a region-specific DNN algorithm for Korean waters, their study was limited to short-term model performance. In contrast, this study leverages that algorithm to analyze two decades of satellite data, providing the first long-term, basin-wide assessment of phytoplankton size structure in the region.
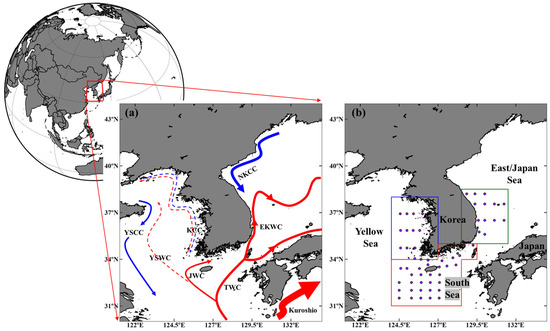
Figure 1.
Study area and oceanographic features of the littoral seas of Korea. (a) A representative schematic map of the major currents in the study area, illustrating typical circulation patterns irrespective of season (redrawn by the authors based on general schematic features from Park et al. [41]). (b) Sampling stations for surface nutrient and phytoplankton size class data obtained from the Korea Oceanographic Data Center (KODC). Colored boxes indicate the subregions used for statistical analyses: Yellow Sea (Blue), South Sea of Korea (red), and East/Japan Sea (green). Abbreviations: JWC, Jeju Warm Current; KCC, Korea Coastal Current; YSCC, Yellow Sea Coastal Current; YSWC, Yellow Sea Warm Current; NKCC, North Korea Cold Current; EKWC, East Korea Warm Current; TWC, Tsushima Warm Current.
In this study, we apply a regionally trained deep neural network (DNN) model, developed by Kang et al. [27], to 20 years of satellite ocean color data (2003–2022) from the littoral seas of Korea—encompassing the YS (34–40° N, 121–127° E), SS (31–34° N, 123–130° E; 34–35° N, 127–130° E), and EJS (35–44° N, 127–134° E) (Figure 1). Specifically, we aim to (1) characterize the spatiotemporal distributions of dominant size of phytoplankton, (2) scrutinize their long-term trends and interannual variations, (3) assess the major controlling factors influencing these variations in each sea using physicochemical data from sub-regions in each sea, and (4) discuss the ecological implications of increasing pico-phytoplankton dominance for marine ecosystems and fisheries under climate change scenarios.
2. Materials and Methods
2.1. DNN-Based Classification for Dominant PSCs in the Littoral Seas of Korea
To derive monthly data for dominant PSCs from satellite observations between 2003 and 2022, we employed a DNN model developed and validated by Kang et al. [27]. This model used sea surface temperature (SST), total suspended solids (TSSs), and total chlorophyll-a (chl-a) concentration—seasonally measured from field observations in the littoral seas of Korea during 2018–2020 (Figure 1b)—as input parameters to classify dominant PSCs in the marine environment. In addition, the DNN was trained using field-observed, size-fractionated chl-a (micro-, nano-, and pico-size phytoplankton) as ground truth data, ensuring accurate PSC classification.
The DNN architecture consisted of ten layers: one input layer, eight hidden layers, and one output layer. The input layer normalized the variables (SST, TSS, and chl-a) using a sigmoid activation function, allowing each feature to contribute proportionally to the classification task (Figure 2 in Kang et al. [27]). The eight hidden layers employed hyperbolic tangent activation functions, chosen for their balance between convergence speed and accuracy in modeling non-linear relationships among the input variables and PSC categories. To prevent overfitting, dropout regularization was applied between the hidden layers. Finally, the output layer used a softmax function to classify sample into micro-, nano-, or pico-size phytoplankton dominance.
The model was trained and validated using 531 in situ samples, which were collected during seasonal field surveys from 2018 to 2020 at stations distributed across the littoral seas of Korea [27]. The model demonstrated an overall classification accuracy of 70.5%. The model training and application were conducted using Python (version 3.9). The DNN architecture was implemented using TensorFlow, and the processing of satellite and field data was carried out using GDAL, NetCDF4, Pandas, and Numpy libraries.
However, the model has several limitations. It provides only the dominant size class as output and does not quantify the relative contributions of micro-, nano-, and pico-size phytoplankton. In addition, the training dataset is spatially and temporally limited and shows class imbalance, with a higher frequency of pico-dominant samples that might introduce prediction bias. Nonetheless, as described above, the model demonstrated higher classification accuracy (test accuracy = 70.5%; field agreement = 69.5%) than traditional approaches—particularly for pico-dominant conditions (precision = 80.1%, recall = 85.8%, F1-score = 82.8)—and was therefore considered suitable for detecting long-term spatiotemporal trends in phytoplankton size structure [27].
2.2. Satellite Data Collection
We obtained satellite-based ocean color data from MODIS-Aqua level-3 products (4 km × 4 km resolution) provided by NASA Goddard Space Flight Center (https://oceandata.sci.gsfc.nasa.gov/, accessed on 20 October 2024). Monthly composite data from 2003 to 2022 were used in this study to match the temporal scope of the PSC analysis and to minimize noise from clouds and short-term variability. MODIS-Aqua was selected because it provided the only continuous and consistent ocean color dataset that spanned the full 20-year study period, making it suitable for long-term trend analysis in the littoral seas of Korea. We estimated TSSs using an empirical algorithm developed by Moon et al. [42], which was specifically designed and validated for Korean waters. Rrs555 was applied in this study due to its strong sensitivity and its widespread use in regional optical water quality assessment [42]. Assuming that ocean color data closely approximated in situ measurements, we applied the trained DNN model to generate monthly distributions of the dominant PSCs in the littoral seas of Korea over the study period.
2.3. In-Situ Nutrients Data
Major dissolved inorganic nutrients (nitrite + nitrate, NO2− + NO3−; dissolved inorganic phosphate, DIP; silicate, Si(OH)4) at the surface layer in each sea were obtained from the Korea Oceanographic Data Center (KODC; https://www.nifs.go.kr/kodc/index.kodc, accessed on 15 November 2024), managed by the National Institute of Fisheries Science (NIFS). These in situ datasets, spanning 2003 to 2022, were collected bimonthly from stations in each sea (Figure 1b). Based on the ocean data standards endorsed by UNESCO and the International Oceanographic Commission (IOC), the KODC assigns quality control (QC) flags to observational data in accordance with the IOC Oceanographic Data Exchange Policy. The QC process involves two stages: the first stage includes tests for position accuracy, data range checks, national and regional climatological ranges, spike detection (both layer-wise and depth-specific), and gradient tests. The second stage consists of visual inspections and statistical analyses. Only data that passed both QC stages were used in this study to ensure the reliability of nutrient measurements.
2.4. Statistical Analyses
To explore correlations between physical (SST and TSSs), chemical (surface nutrients), and biological (chl-a) factors and the annual mean proportions of dominant PSC areas in the YS, SS, and EJS, we conducted Spearman’s rank correlation analysis, as several variables did not meet the assumption of normality based on a Shapiro–Wilk test (p < 0.05). Given its non-parametric nature, Spearman’s method was considered more suitable for identifying monotonic relationships under these conditions. While monthly data might provide finer temporal resolution, our objective was to examine persistent environmental drivers that operate across the timescales. In temperate regions like our study areas, environmental variables exhibit strong seasonal fluctuations that can obscure long-term relationships when analyzed at a monthly scale [43]. Annual means, therefore, provide a more stable basis for identifying monotonic trends related to dominant PSC areas across years. For this purpose, annual mean SST, TSSs, and chl-a in each region were derived from monthly ocean color products, while bimonthly nutrient measurements were averaged on an annual basis. Because nutrient data did not cover the entire study area uniformly, we defined subregions for each sea—YS (34–38° N, 124–127° E), SS (31–34° N, 124–128.5° E and 34–35° N, 127–129.5° E), and EJS (35–38.5° N, 128.5–131.5° E)—to maintain consistency in the statistical analysis (Figure 1b). A correlation was deemed significant at p < 0.05, and any parameters displaying a significant relationship with each PSC-dominant area percentage were noted.
To examine non-linear relationship between response (the proportion of pico-phytoplankton-dominant areas) and explanatory variables (SST) in the YS, SS, and EJS, generalized additive models (GAMs) were employed based on the monthly datasets in each sea. GAMs utilize smoothed functions of predictor variables to model the response variable in a flexible, non-parametric framework [44]. The model was structured as follows: Y = α + s(X), where Y represents the monthly proportion of pico-phytoplankton-dominant areas, X indicates monthly mean SST, s is a smooth function of each parameter, and α denotes the overall mean of the response variable. To minimize overfitting, a random factor-smooth (fs) term and a basic dimension (k) set below 5 were applied to each smooth function [44,45].
All statistical analyses were conducted in R software (version 4.3.3; R core Team, 2024). The “shapiro.test()” function from base R was used to assess the normality of environmental variables. Spearman’s rank correlations were calculated using the “rcorr()” function from the “Hmisc” package, and GAMs were implemented using the “gam()” function from the “mgcv” package. The “tidyverse” suit (including “dplyr” and “ggplot2”) was used for data preprocessing, spatial aggregation, and visualization.
3. Results
3.1. Spatial–Temporal Distributions of the Dominant PSCs in the Littoral Seas of Korea
We generated monthly climatological images of dominant PSCs in the littoral seas of Korea from January 2003 to December 2022 (Figure 2), based on a developed algorithm and satellite ocean color data. The spatial distributions of dominant PSCs in these seas exhibited distinct seasonal characteristics for each region. In the YS, micro-size phytoplankton generally prevailed from January to April, whereas nano-size phytoplankton predominated in the central region for May. From June onward, pico-size phytoplankton expanded rapidly northward from the south, covering the entire central area until September. Between October and December, nano-size phytoplankton began to dominate along the periphery of the central region, as micro-size phytoplankton areas gradually expanded. Unlike the dynamic shifts observed offshore, the coastal zone of the YS consistently showed a stable dominance in micro-size phytoplankton throughout the year. According to the observations of PSCs, the EJS from January to April was characterized by the dominant areas of nano-size phytoplankton. Following a similar seasonal pattern to the YS, pico-dominated regions in the EJS gradually extended northward from May to June, becoming prevalent across the area by July and persisting until September. In October, however, nano-size phytoplankton expanded southward again, continuing through December. In the SS, although nano-size phytoplankton occasionally dominated in localized regions, particularly to the west and north of Jeju Island from March to May, micro- and pico-size phytoplankton generally maintained a clear spatial separation. Micro-size phytoplankton were predominantly found in the western SS, while pico-size phytoplankton were prevalent in the eastern SS. Their boundary shifted seasonally, indicating dynamic changes in phytoplankton community structure throughout the year.
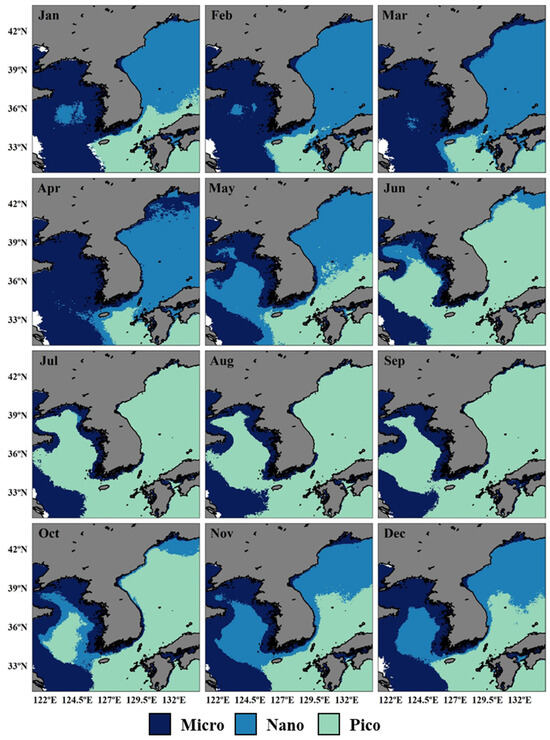
Figure 2.
Monthly climatology of dominant phytoplankton size class (PSC) areas in the littoral seas of Korea from 2003 to 2022. Each panel shows the dominant PSC area for a given month: indigo for micro-size, blue for nano-size, and green for pico-size phytoplankton.
Figure 3 illustrates the monthly percentage of areas dominated by each PSC in the three seas over the entire study period. Although some interannual differences arose in each region, the general pattern remained consistent year to year. In the YS, the proportion of micro-, nano-, and pico-phytoplankton-dominant areas ranged from 30.2 to 100%, 0.0 to 53.3%, and 0.0 to 69.7%, respectively (Figure 3a). Overall, micro-size phytoplankton dominated in winter (84.2 ± 11.2%), spring (80.4 ± 17.9%), and autumn (55.7 ± 7.0%), whereas pico-size phytoplankton were prevalent in summer (52.6 ± 9.5%) (Table 1). In the SS, the proportion of each PSC varied between 7.2–53.7% for micro-phytoplankton, 0.0–54.0% for nano-phytoplankton, and 5.9–90.1% for pico-phytoplankton (Figure 3b). While the dominant areas of micro- (35.8 ± 9.0%) and pico-size (38.4 ± 13.9%) phytoplankton were similar in spring, pico-size phytoplankton remained consistently dominant throughout the four seasons (38.3–74.9%) (Table 1). In the EJS, micro-size phytoplankton accounted for 0.0–51.3%, nano-size phytoplankton for 0.0–97.6%, and pico-size phytoplankton for 0.0–98.8%, respectively (Figure 3c). Nano-size phytoplankton were more extensive in winter (79.9 ± 15.0%) and spring (75.3 ± 13.0%), transitioning to pico-size dominance in summer (93.5 ± 4.9%) and autumn (70.5 ± 24.8%) (Table 1). Micro-phytoplankton, however, consistently remained a minor component (3.0–15.4%) across seasons.
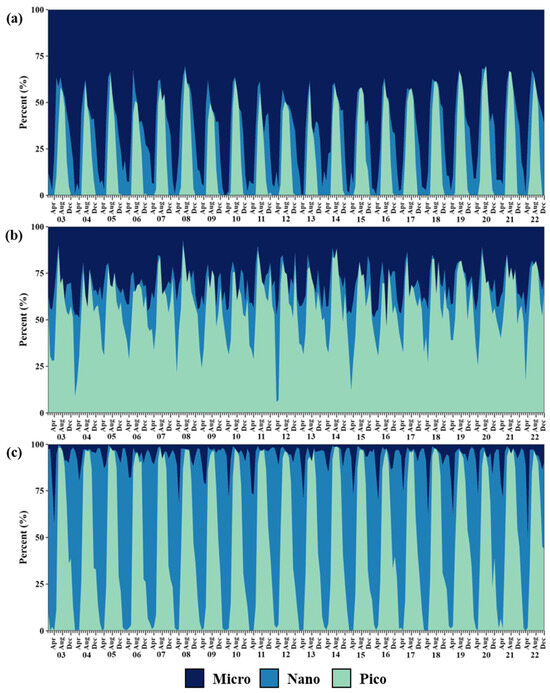
Figure 3.
Monthly time series of the percent of dominant phytoplankton size class areas relative to the total area of each sea in the Yellow Sea (a), South Sea of Korea (b), and East/Japan Sea (c) from 2003 to 2022. Indigo, blue, and green represent the proportion of dominant areas for micro-size, nano-size, and pico-size phytoplankton, respectively.

Table 1.
Seasonal mean proportion (%) of dominant phytoplankton size class areas—micro-, nano-, and pico-size—in the Yellow Sea (YS), South Sea of Korea (SS), and East/Japan Sea (EJS) from 2003 to 2022. Values in parentheses indicate standard deviations.
3.2. Long-Term Trends of the PSCs in the Littoral Seas of Korea
Using the monthly percentages of PSC-dominant areas in each region, we calculated annual mean values from 2003 to 2022 (Figure 4). Among the three seas, micro-phytoplankton were generally the predominant group (65.3 ± 3.4%) in the YS throughout the study period, whereas pico-phytoplankton (57.2 ± 3.0%) typically prevailed in the SS (Figure 4a,b). In the EJS, both nano- and pico-size phytoplankton (45.6 ± 3.0 and 47.5 ± 2.5%) were major contributors (Figure 4c). Despite regional differences in dominant PSCs, pico-size phytoplankton exhibited clear increasing trends in all regions during the observation period. In the EJS, in particular, pico-size phytoplankton increased alongside nano-size cells, eventually becoming the overall predominant group from 2018 to 2022 (Figure 4c).
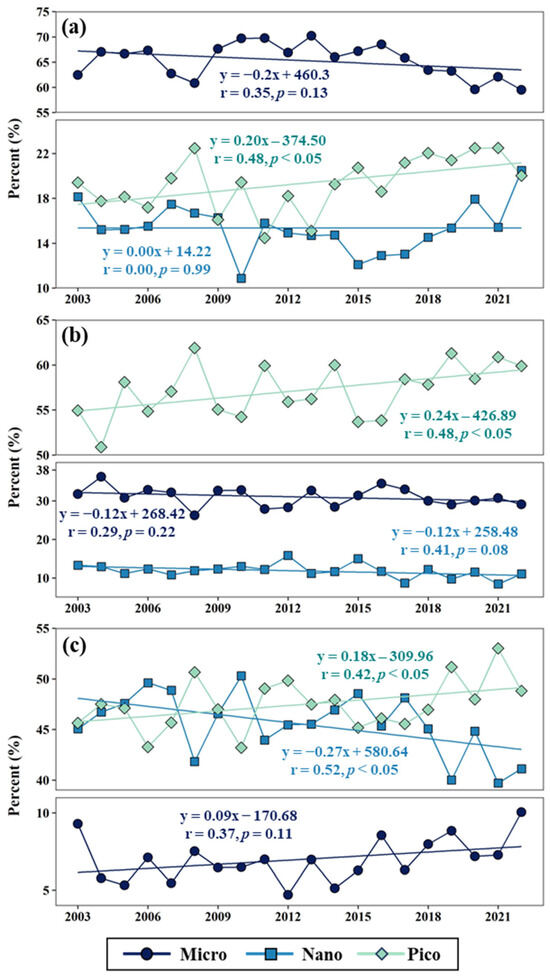
Figure 4.
Annual mean percent of dominant phytoplankton size class (PSC) areas in the Yellow Sea (a), South Sea of Korea (b), and East/Japan Sea (c) from 2003 to 2022. Each linear regression line represents the long-term trend in the proportion of dominant areas for micro-size (indigo), nano-size (blue), pico-size (green) phytoplankton, relative to the total area of each region.
To identify areas where pico-size phytoplankton dominance expanded, we compared the most frequent pico-dominant regions in early (2003–2006), mid (2011–2014), and late (2019–2022) periods (Figure 5). The results showed varying expansion patterns among the three seas. In the YS, pico-phytoplankton were initially dominant in central offshore waters during the early period. During the mid period, no clear spatial pattern of dominance was observed. In the late period, although dominance slightly decreased in the northern YS, pico-phytoplankton-dominant areas expanded along the western and eastern coastal regions in the central and southern YS. In the SS, there were no striking variations in pico-phytoplankton-dominant areas between the early and mid-periods; however, in the late period, these areas broadened northwestward toward the central waters of the YS. Meanwhile, for the EJS, regions of pico-phytoplankton dominance that were primarily located in the south during the early period exhibited a gradual expansion toward the northwest.
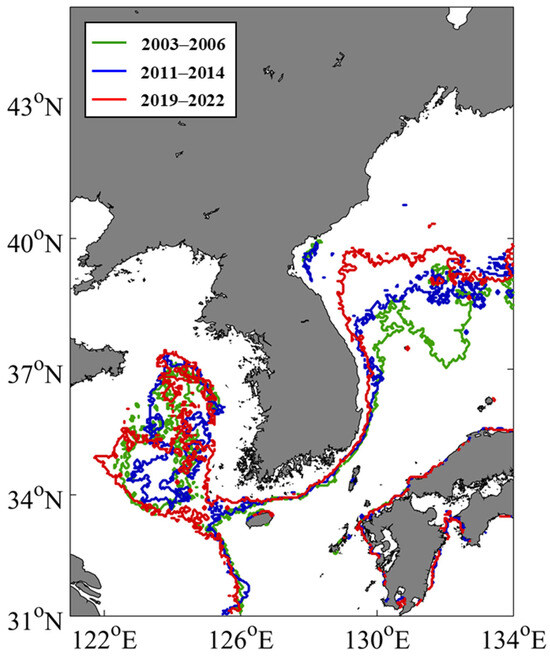
Figure 5.
Spatial changes in the distributions of pico-phytoplankton-dominant areas in the littoral seas of Korea for three representative periods: early (2003–2006, green), mid (2011–2014, blue), late (2019–2022, red). The contour lines depict the outer boundaries of regions dominated by pico-sized phytoplankton during each period.
3.3. Relationships Between Environmental Parameters and the PSCs in the Littoral Seas of Korea
Spearman’s correlation analysis was conducted on subregions with available nutrient data in each sea to determine environmental parameters influencing annual variations in areas dominated by different PSCs (Figure 6). In the YS, micro-size phytoplankton demonstrated positive correlations with turbidity (r = 0.63, p < 0.01) and chl-a (r = 0.68, p < 0.01), while displaying negative correlations with SST (r = −0.46, p < 0.05) and silicate (r = −0.54, p < 0.05) (Figure 6a). Nano-size phytoplankton showed a positive correlation with silicate (r = 0.60, p < 0.01) but a negative correlation with turbidity (r = −0.71, p < 0.01). Pico-size phytoplankton were positively correlated with SST (r = 0.51, p < 0.05), while negatively correlated with DIP (r = −0.40, p < 0.05) and chl-a (r = −0.73, p < 0.01). Micro-size phytoplankton in the SS were positively associated with turbidity (r = 0.61, p < 0.01) and chl-a (r = −0.85, p < 0.01), whereas nano- and pico-size phytoplankton negatively correlated with SST (r = −0.64, p < 0.01) and chl-a (r = −0.85, p < 0.01), respectively (Figure 6b). For the EJS, micro-size phytoplankton were positively related only with chl-a (r = 0.81, p < 0.01) (Figure 6c). In contrast, nano- and pico-size phytoplankton were influenced by the identical environmental factors (SST: r = −0.70 and 0.64, p < 0.01; nitrite + nitrate: r = 0.67 and −0.60, p < 0.01; phosphate: r = 0.53 and −0.49, p < 0.05; chl-a: r = 0.47 and −0.65, p < 0.01 and 0.05) but exhibited opposite correlations.
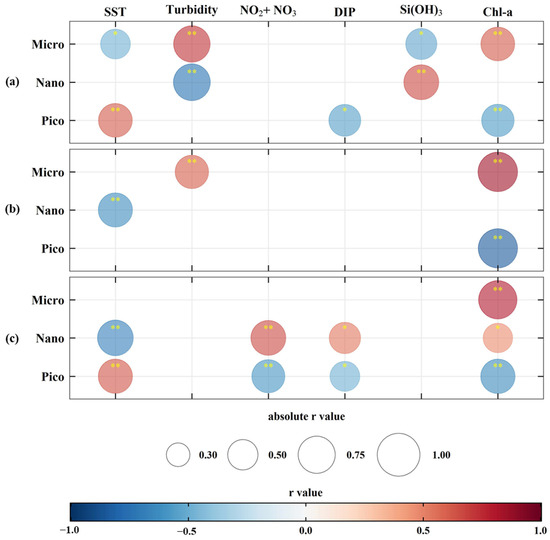
Figure 6.
Spearman’s correlation matrix between environmental variables and the annual mean percent of dominant phytoplankton size class areas in subregion of the Yellow Sea (a), South Sea of Korea (b), and East/Japan Sea (c). Asterisks indicate statistical significance: p < 0.05 (*), p < 0.01 (**).
The results of GAMs revealed variations in the proportion of pico-phytoplankton-dominant areas in response to SST across all study regions (Figure 7). All three seas generally showed an increase in pico-phytoplankton-dominant areas with rising SST, although the specific patterns differed by each region. In the YS, pico-size phytoplankton tended to rapidly increase as SST rose from 15 to 25 °C, displaying a constant fraction (approximately 60%) above 25 °C (Figure 7a). In the SS, pico-phytoplankton increased linearly with SST up to 20 °C, after which the increase became more gradual (Figure 7b). Compared to the YS and SS, pico-size phytoplankton in the EJS displayed exponentially an increasing trend with temperature rising up to 23–24 °C, followed by a slight decrease thereafter (Figure 7c).
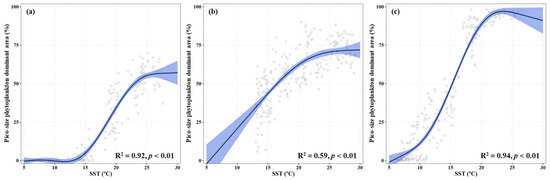
Figure 7.
Results of generalized additive models (GAMs) describing the relationship between monthly mean sea surface temperature and the monthly proportion of pico-phytoplankton-dominant areas in the Yellow Sea (a), South Sea of Korea (b), and East/Japan Sea (c) from 2003 to 2022. Solid lines represent significant GAM smoothing function (p < 0.01), and shaded areas indicate 95% confidence intervals. The distribution of plotted points represents the data distribution.
4. Discussion
4.1. Spatial and Seasonal Variability of Dominant PSCs in the Littoral Seas of Korea
The 2003–2022 satellite record provides a synoptic, two-decade perspective that captures persistent yet evolving spatial and temporal mosaics of dominant PSCs across the littoral seas of Korea. Their distributions were shaped by distinct hydrographic features, vertical-mixing regimes, and nutrient availability. Micro-size phytoplankton prevailed year-round in nearshore regions like the coastal YS and western part of the SS, characterized by high turbidity, frequent mixing, and regular riverine nutrient inputs (Figure 2). Although turbid waters limit light and thus phytoplankton growth, multiple studies have observed a persistent dominance of micro-size phytoplankton under these conditions [26,46,47]. One possible explanation is that high turbidity often coincides with the frequent resuspension of particles and continuous nutrient input from river discharge or coastal mixing, which can favor larger phytoplankton groups despite lower light levels [48]. Additionally, their higher pigment content per cell or thicker chloroplast layer could enhance photosynthetic performance under low irradiance [46,47]. Our Spearman correlation further supported this, showing strong positive links between turbidity and micro-phytoplankton-dominant areas in the YS and SS (Figure 6a,b).
Nano-size phytoplankton displayed transitional dominance during late spring and autumn, particularly in the central YS (Figure 2). These periods coincide with moderate stratification and episodic nutrient pulses that favor intermediate-sized taxa [49,50,51]. For example, following spring blooms dominated by micro-size diatoms (e.g., Thalassiosira pacifica, Chaetoceros cinctus, and Skeletonema spp.) in the YS [49,51], nutrient exhaustion and increasing column stability often lead to a shift in community composition toward nano-size groups, including chlorophytes and cryptophytes [52]. In the EJS, nano-size phytoplankton expanded southward from late autumn, maintained a widespread dominance through winter and spring, and retreated northward by late spring (Figure 2). As in the YS, previous field-based studies have reported micro-size diatom dominance in the EJS during spring (March–April) [53,54]; however, this pattern was not clearly detected in our satellite-derived estimates. This discrepancy might be partly attributed to differences in observational approaches, as satellite data reflect temporally averaged surface conditions and may fail to capture short-lived or subsurface micro-phytoplankton blooms. Furthermore, past studies have shown that dominant phytoplankton composition in the EJS varied with survey timing, location, and depths [55,56]. It is also worth noting that the classification model used in this study assigned the dominant PSCs at each pixel only when its proportion exceeded that of other size classes. Thus, even if micro-size phytoplankton were abundant during the spring bloom, they would not be classified as dominant unless they surpassed other groups in relative contribution.
Pico-size phytoplankton showed distinct seasonal dominance in the central YS and entire EJS, thriving during summer (June–September) when surface waters are warm and strongly stratified (Figure 2). These patterns align with previous studies showing that pico-phytoplankton dominate during summer in Korean waters, contributing substantially to total biomass and primary production, especially in the YS and EJS [26,36,38]. Furthermore, our statistical analyses (Spearman correlation and GAMs) supported these findings, revealing strong positive relationships between SST and pico-dominant areas, and negative correlations with DIP and nitrite + nitrate, particularly in the YS and EJS (Figure 6 and Figure 7).
Taken together, these region-specific patterns suggest that the underlying mechanisms shaping PSC distributions are not only seasonally distinct but also exhibit signs of long-term intensification. Although these seasonal and spatial patterns are broadly consistent with earlier findings, our decadal-scale satellite-based approach enables a clearer assessment of how such patterns have intensified and shifted in response to recent environmental change. The observed persistence and gradual intensification of pico-size phytoplankton dominance under warming and nutrient-depleted conditions may reflect an incipient ecological regime shift in the structure of phytoplankton communities. Detailed quantitative trends and their statistical significance are provided in Section 4.2 (Figure 5, Figure 6 and Figure 7). The broader ecosystem implications of this transition—including potential impacts on primary production and trophic energy flow—are discussed in detail in Section 4.2.3.
4.2. Long-Term Expansion in Pico-Size Phytoplankton Dominance and Their Ecological Implications
4.2.1. Temporal Patterns of Pico-Size Phytoplankton Dominance
One of the most salient long-term findings of this study was the consistent expansion of pico-dominant areas in all regions from 2003 to 2022 (Figure 4). Although the annual increase rates were similar among the YS (0.20% y−1), SS (0.24% y−1), and EJS (0.18% y−1), the shift was pronounced in the EJS, where dominance transitioned from nano-size to pico-size phytoplankton in the mid-2010s (Figure 4c). This expansion could be driven by environmental changes, especially sea surface warming. Rising SST frequently enhances vertical stratification, reducing vertical mixing and limiting nutrient supply to the euphotic zone [39,40,57]. Under increasingly nutrient-limited conditions, smaller phytoplankton generally outcompete larger cells because of their higher surface-area-to-volume ratios, facilitating more efficient nutrient uptake [58,59]. Consistently, Agawin et al. [60] documented that pico-size phytoplankton contribution to total biomass increased with higher temperature and lower nitrate concentration in the Mediterranean Sea. Morán et al. [16] also found an elevated pico-phytoplankton abundance in the North Atlantic under comparable conditions. In line with these findings, our analyses revealed strong positive correlations between SST and pico-dominant areas in the YS and EJS, and negative correlation with nutrients (Figure 6 and Figure 7). Although no significant nutrient–pico- phytoplankton relationship was detected in the SS (Figure 6b), both satellite-derived SST measurements and in situ nutrient observations indicated a pronounced warming trend and declining nutrient availability over the past two decades (Supplementary Figure S1). Given that the littoral seas of Korea have warmed faster than the global mean [32,34], our results suggest that pico-size phytoplankton could expand even more rapidly here under warming scenarios. Notably, our GAMs showed a steeper SST response in pico-dominant areas of the EJS compared to the other regions, suggesting that continued warming might further accelerate the pico-phytoplankton expansion in this region (Figure 7).
Changes in nutrient stoichiometry, particularly the increasing N/P ratio, could also promote pico-size phytoplankton expansion in the YS and SS. Many studies have reported that anthropogenic nitrogen inputs, especially via atmospheric deposition and river discharge (e.g., the Changjiang River), have gradually raised N/P ratios in these seas, while phosphorus input remains relatively low [61,62,63]. As a result, phosphorus limitation has become more common in these ecosystems [63,64], disadvantaging large phytoplankton (>2 µm), such as diatoms, while favoring small flagellates (<2 µm) [65]. Kim et al. [38] reported that diatom contributions in the YS declined from over 80% in earlier years to ~55% in 2019, while smaller groups (e.g., cryptophytes) became more prevalent. Similarly, Jang et al. [35] found that pico-size phytoplankton made up ~50% of total biomass and primary production in the YS and SS during summer, marking a notable increase from earlier observations. Other studies have also highlighted phosphorus limitation as a key driver of non-diatom flagellates’ dominance in the YS and SS [33,66]. Consistent with these findings, our analysis revealed a significant negative correlation between DIP and pico-dominant areas in the YS (Figure 6a), supporting the role of phosphorus in shaping phytoplankton size structure. Moreover, our nutrient data—primarily from the eastern YS and northern East China Sea (Figure 1b)—showed consistent declines in both NO2− + NO3− and DIP over the study period (Supplementary Figure S1). Although spatial coverage is limited, a clearer long-term trend in the N/P ratio emerged after excluding values in the YS (2006) and SS (2003–2004) that were notably higher than those in adjacent years. In the YS, NO2− + NO3− and DIP decreased by −0.2%y−1 and −2.3% y−1, respectively, while in the SS, the rates were −2.3% and −3.8% y−1. As a result, the N/P ratio increased at a rate of +0.3 y−1 in the YS (p < 0.05) and +0.3 y−1 in the SS (p = 0.13) (Supplementary Figure S2). While the increasing trend in the SS was not statistically significant at the 0.05 level, the direction of change was consistent with that in the YS, suggesting a possible, albeit weaker, shift toward phosphorus limitation. These findings imply that pico-size phytoplankton might be increasingly favored over larger competitors under evolving nutrient regimes, thereby contributing to the long-term changes in phytoplankton community composition observed in both regions. Nonetheless, the precise role of nutrient limitation remains unclear, partly due to the spatial constraints of our dataset, which was concentrated in the eastern YS and adjacent shelf waters. This highlights the need for more comprehensive spatiotemporal data on nutrient fluxes and phytoplankton structure across broader regions.
4.2.2. Spatial Expansion of Pico-Size Phytoplankton Dominance
We identified the spatial expansion of pico-dominant phytoplankton dominance across all regions over three distinct periods (early, mid, and late), with notable regional variability patterns (Figure 5). In the central part of the southwestern YS, pico-dominant areas extended from offshore to the coastal zone, while in the SS, they expanded northwestward. The most striking change occurred in the southwestern EJS, where pico-phytoplankton rapidly advanced into northern waters. This northward expansion might be partially associated with the intensification of the Tsushima Warm Current (TWC), which brings warm, nutrient-poor Kuroshio waters into the EJS. The strength of the TWC is known to be modulated by large-scale climate oscillations such as the Pacific Decadal Oscillation (PDO); negative PDO phases weaken the Kuroshio path while enhancing the TWC inflow [67,68,69]. In line with this climatological relationship, our analysis showed that negative PDO phases predominated across the three examined periods (Figure 8a). In addition, our analysis indicated a long-term intensification of TWC and a gradual shift toward more negative PDO phases over the study period, consistent with previous findings. These decadal-scale changes—including increased TWC inflow through the western channel via the East Korea Warm Current (Figure 1a)—likely contributed to circulation-driven nutrient limitation and stratification, thereby facilitating the observed northward expansion of pico-dominant regions in the southwestern EJS (Figure 5 and Figure 8b). Furthermore, a northward displacement of the subpolar front [70] and weakened influence of the colder North Korea Cold Current have reinforced stratification in the southwestern EJS [71,72], creating optimal conditions for pico-size phytoplankton. This oceanographic shift likely contributed to the northward expansion of pico-dominant areas in the southwestern EJS. Moreover, increased inflow of oligotrophic waters and enhanced stratification due to surface warming could have acted synergistically, further favoring pico-phytoplankton dominance. In contrast, the specific mechanisms behind pico-dominant expansion remain unclear. These regions experience complex interactions involving seasonal freshwater inflows, coastal mixing, and multiple water masses (e.g., Kuroshio Water, Changjiang Diluted Water, Yellow Sea Warm Water, Yellow Sea Cold Water), making it difficult to attribute pico-expansion to any single factor [73,74,75]. Future studies should incorporate high-resolution, long-term observations and coupled biogeochemical models to better elucidate the spatial dynamics of pico-phytoplankton in these marginal seas.
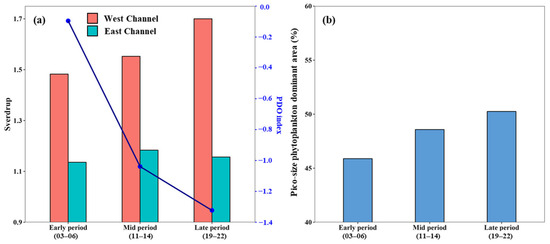
Figure 8.
(a) Bar plots showing the averaged volume transport (in Sverdrup) of the Tsushima Warm Current (TWC) through the west and east channels along with the Pacific Decadal Oscillation (PDO) index across three periods: early (2003–2006), mid (2011–2014), and late (2019–2022). TWC transport data were obtained from the National Institute of Fisheries Science (NIFS), and the PDO index was retrieved from the National Center for Environmental Information (NCEI) under NOAA. (b) Temporal changes in the proportion of pico-phytoplankton-dominant areas in the East/Japan Sea during the same period.
4.2.3. Potential Ecological Effects of Pico-Size Phytoplankton Expansion
The observed expansion of pico-phytoplankton-dominant areas in the Korean waters could have far-reaching ecological implications. First, while pico-size cells thrive in warm, nutrient-poor environments, they generally have lower biomass-specific photosynthetic efficiency than larger phytoplankton [60,76]. Their small cell volume limits their ability to increase photosynthetic components (e.g., chloroplasts) [58]. Consistent with this, multiple studies have reported an inverse relationship between total primary production and the proportion of small (<2 µm) phytoplankton [18,76,77]. Our findings also suggest that the long-term increase in pico-size phytoplankton dominance may be linked to declining primary production. This trend aligns with findings from Jang et al. [36], who reported that recent primary productions in the littoral seas of Korea have been lower than historical values, raising concerns about potential ecosystem impacts. As primary production underpins food availability for higher trophic levels, its decline could have contributed to the long-term decrease in fishery yields in Korean waters. Globally, annual fish landings are closely linked to phytoplankton-driven primary production [78]. Based on Jang et al. [36], the annual primary production in 2018 was 108 g C m−1 y−1. Applying the yield equation proposed by Nixon and Thomas [78], this corresponds to an estimated fishery yield of 1.54 g m−2 y−1. Multiplying by the fishing area (6.85 × 1011 m2) [79] gives a projected annual yield of ~1.06 million tons, closely matching the actual 2018 catch (1.01 million tons; Korean Statistical Information Service (KOSIS), https://kosis.kr, accessed on 10 October 2024). This agreement supports the importance of primary production in shaping fishery yield in Korean waters. Given the continued decline of fishery yields since the 1980s [80], our findings suggest that bottom-up control via primary production driven by rising pico-phytoplankton dominance might be a key contributing factor [81]. However, as multiple factors regulate fishery production, including overfishing, climate variability, and habitat changes [82], attributing fishery declines solely to reductions in primary production remains challenging. Moreover, our analysis is based on a single-year estimate of primary production; thus, it requires validation with multi-year data to clarify its relationship with fishery productivity.
Second, a shift toward smaller phytoplankton also alters food web structure, beyond reducing primary production. Since many marine predators feed selectively by size, changes in phytoplankton size structure can reshape herbivore communities and impact higher trophic levels, including key fishery species [39,83,84]. With increasing pico-phytoplankton dominance, microzooplankton (e.g., ciliates, flagellates) are expected to proliferate, while mesozooplankton, especially copepods, may decline due to reduced prey availability [85,86]. This shift reduces energy transfer efficiency, increasing reliance on microbial pathway and gelatinous zooplankton, such as salps and doliolids [86]. These changes can benefit fish species that feed on micro- or gelatinous zooplankton but disadvantage species dependent on mesozooplankton, including many of commercial value [85,86]. Moreover, as pico-phytoplankton replace larger cells like diatoms, microbial food webs become more prominent, adding trophic steps and intensifying metabolic losses before energy reaches higher consumers [87]. These inefficiencies in trophic transfer can lower fishery yields in affected regions [82,88]. In summary, increasing pico-dominance, coupled with reduced primary production and altered food web structures, may significantly affect fishery resources in the littoral seas of Korea. Anticipating long-term ecosystem shifts, multidisciplinary research and adaptive management strategies will be essential for ensuring sustainable fisheries under future climate change.
5. Summary and Conclusions
This study examined the spatiotemporal distribution and long-term variability of PSCs in the littoral seas of Korea by applying a DNN-based model, developed by Kang et al. [27], to 20 years (2003–2022) of satellite ocean color data. Our findings revealed pronounced spatial and seasonal variability in dominant PSCs. Micro-size phytoplankton consistently dominated nearshore turbid waters throughout the year, particularly in the coastal YS and western SS. Nano-size groups exhibited transitional dominance in the central YS during late spring and autumn, while in the EJS, they prevailed from late autumn through winter and spring. In contrast, pico-size phytoplankton displayed a strong seasonal peak, dominating during summer under warm, stratified and nutrient-depleted conditions—particularly in the central YS and throughout EJS.
Importantly, long-term analyses showed a consistent expansion of pico-dominant areas across all regions, with the most notable increase observed in the EJS since the mid-2010s. This trend appears to be driven by a combination of rising SST, which enhances stratification and limits nutrient mixing, and shifts in nutrient stoichiometry (e.g., increased N/P ratio) that collectively favor smaller phytoplankton. Spatially, pico-dominant areas extended toward coastal zones in the YS and advanced northward in the EJS—possibly influenced by the warm, nutrient-poor TWC entering via the west channel, under the continued negative phase of the PDO during the study period. The sustained expansion of pico-size phytoplankton carries significant ecological implications. First, smaller phytoplankton are generally less efficient in photosynthetic processes, potentially contributing to reduced primary production and, in turn, to lower fishery yields in Korean waters. Second, the shift toward smaller phytoplankton may alter trophic transfer efficiency and food web structure, favoring microzooplankton and gelatinous species, disadvantaging fish that rely on mesozooplankton. These changes raise concerns about long-term ecosystem productivity and the sustainability of regional fisheries.
However, some limitations should be noted. The DNN-based classification model used in this study identified only the dominant PSC at each satellite pixel, which overlooked the full composition and the coexistence of multiple phytoplankton groups within a given region. As such, it could not fully capture the dynamic shifts in community structure. Furthermore, the spatial resolution (4 km × 4 km) of MODIS-Aqua data could not fully resolve sub-pixel variability and fine-scale oceanographic processes such as coastal fronts, small eddies, or localized mixing. These small-scale dynamics can influence phytoplankton distribution and community composition at spatial scales smaller than a satellite pixel, particularly in coastal and estuarine areas where water mass interactions are highly dynamic. Nonetheless, the use of monthly composites and long-term averaging in this study was designed to minimize short-term spatial variability and emphasize regional-scale and decadal trends in PSC distributions, which are appropriately captured at this resolution. To better understand the finer-scale dynamics of phytoplankton communities in response to ongoing environmental changes, future research should incorporate high-frequency in situ observations, detailed size-fractionated composition data, and integrated bio-optical and biogeochemical analyses.
In conclusion, this study presents the first large-scale, long-term assessment of PSC dynamics in the littoral seas of Korea, underscoring the growing significance of pico-size phytoplankton under warming scenarios. Our results highlight the need for continued in situ and satellite-based monitoring, improved ecosystem models, and integrated assessments to better predict the future of marine ecosystems and fishery resources under global climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13061064/s1, Figure S1: Annual variations in surface environmental variables in the Yellow Sea (YS; blue), South Sea of Korea (SS; red), and East/Japan Sea (EJS; green): sea surface temperature (a), turbidity (b), nitrite + nitrate (c), dissolved inorganic phosphate (d), silicate (e), chlorophyll-a concentration (f). Each linear regression line represents the long-term trend.; Figure S2: Annual trends in the molar N/P ratio (DIN/DIP; where DIN = nitrite + nitrate and DIP = dissolved inorganic phosphate) in the Yellow Sea (YS; blue circles) and South Sea of Korea (SS; red squares) from 2003 to 2022. Values from 2006 (YS) and 2003–2004 (SS), which were notably higher than those in adjacent years, were excluded to better capture the underlying trend. Solid lines represent linear regression fits.
Author Contributions
Conceptualization, H.-K.J., C.K. and H.J. (Huitae Joo); methodology, C.K., J.-J.K. and H.J. (Huitae Joo); validation, J.-J.K., S.-H.Y. and H.J. (Huitae Joo); formal analysis, H.-K.J. and H.J. (Huitae Joo); data curation, H.-K.J., C.K. and H.J. (Hwaeun Jung); writing—original draft preparation, H.-K.J., C.K. and H.J. (Huitae Joo); writing—review and editing, H.-K.J., C.K., S.-H.Y. and H.J. (Huitae Joo); visualization, H.-K.J. and H.J. (Hwaeun Jung); supervision, S.-H.Y.; project administration, S.-H.Y.; funding acquisition, H.J. (Huitae Joo). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Fisheries and Science (‘Development of marine ecological forecasting system for Korean waters’; R2025058).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely acknowledge the National Oceanic and Atmospheric Administration (NOAA) for providing satellite-derived data, and the Korea Oceanographic Data Center (KODC) for supplying nutrient data. We are also grateful to Jae-Joong Kang for developing the regional model used in this study. Finally, we would like to thank the anonymous reviewers for their constructive and valuable comments, which contributed significantly to improving this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24, GB3016. [Google Scholar] [CrossRef]
- Litchman, E.; Edwards, K.F.; Klausmeier, C.A.; Thomas, M.K. Phytoplankton niches, traits and eco-evolutionary responses to global environmental change. Mar. Ecol. Prog. Ser. 2012, 470, 235–248. [Google Scholar] [CrossRef]
- Henson, S.A.; Cael, B.B.; Allen, S.R.; Dutkiewicz, S. Future phytoplankton diversity in a changing climate. Nat. Commun. 2021, 12, 5372. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Agustí, S.; González-Gordillo, J.I.; Vaqué, D.; Estrada, M.; Cerezo, M.I.; Salazar, G.; Gasol, J.M.; Duarte, C.M. Ubiquitous healthy diatoms in the deep sea confirm deep carbon injection by the biological pump. Nat. Commun. 2015, 6, 7608. [Google Scholar] [CrossRef]
- Cavan, E.L.; Laurenceau-Cornec, E.C.; Bressac, M.; Boyd, P.W. Exploring the ecology of the mesopelagic biological pump. Prog. Oceanogr. 2019, 176, 102125. [Google Scholar] [CrossRef]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.C.; Finkel, Z.; Iudicone, D.; et al. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Huete-Ortega, M.; Calvo-Díaz, A.; Graña, R.; Mouriño-Carballido, B.; Marañón, E. Effect of environmental forcing on the biomass, production and growth rate of size-fractionated phytoplankton in the central Atlantic Ocean. J. Mar. Syst. 2011, 88, 203–213. [Google Scholar] [CrossRef]
- Morán, X.A.G.; Scharek, R. Photosynthetic parameters and primary production, with focus on large phytoplankton, in a temperate mid-shelf ecosystem. Estuar. Coast. Shelf Sci. 2015, 154, 255–263. [Google Scholar] [CrossRef]
- Uitz, J.; Huot, Y.; Bruyant, F.; Babin, M.; Claustre, H. Relating phytoplankton photophysiological properties to community structure on large scales. Limnol. Oceanogr. 2008, 53, 614–630. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, J.; Lee, D.; Park, J.-W.; Kwon, J.-I.; Zhao, J.; Son, S. Spatial variations of small phytoplankton contributions in the Northern Bering Sea and the Southern Chukchi Sea. GISci. Remote Sens. 2019, 56, 794–810. [Google Scholar] [CrossRef]
- Mena, C.; Reglero, P.; Hidalgo, M.; Sintes, E.; Santiago, R.; Martín, M.; Moyà, G.; Balbín, R. Phytoplankton community structure is driven by stratification in the oligotrophic Mediterranean Sea. Front. Microbiol. 2019, 10, 1698. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Feng, M.; Zhang, Y.; Shen, X.; Zhan, H.; He, Q. Reduced and smaller phytoplankton during marine heatwaves in eastern boundary upwelling systems. Commun. Earth Environ. 2024, 5, 629. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Joo, H.; Kang, S.H.; Kang, C.K.; Whitledge, T.E. Contribution of small phytoplankton to total primary production in the Chukchi Sea. Cont. Shelf Res. 2013, 68, 43–50. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; Li, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Change Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Sieburth, J.M.N.; Smetacek, V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Joo, H.; Son, S.; Park, J.W.; Kang, J.J.; Jeong, J.Y.; Kwon, J.I.; Kim, C.-K.; Lee, S.H. Small phytoplankton contribution to the total primary production in the highly productive Ulleung Basin in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 54–61. [Google Scholar] [CrossRef]
- Son, S.; Kim, Y.; Kwon, J.; Kim, H.; Park, K. Characterization of spatial and temporal variation of suspended sediments in the Yellow and East China Seas using satellite ocean color data. GISci. Remote Sens. 2014, 51, 212–226. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Model. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Hirata, T.; Aiken, J.; Hardman-Mountford, N.; Smyth, T.; Barlow, R. An absorption model to determine phytoplankton size classes from satellite ocean colour. Remote Sens. Environ. 2008, 112, 3153–3169. [Google Scholar] [CrossRef]
- Mouw, C.B.; Hardman-Mountford, N.J.; Alvain, S.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Ciotti, A.M.; Devred, E.; Fujiwara, A.; Hirata, T.; et al. A consumer’s guide to satellite remote sensing of multiple phytoplankton groups in the global ocean. Front. Mar. Sci. 2017, 4, 41. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Oceans 2006, 111, C08005. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Xiao, W.; Laws, E.A.; Huang, B. Spatial and temporal variations of satellite-derived phytoplankton size classes using a three-component model bridged with temperature in marginal seas of the western Pacific Ocean. Prog. Oceanogr. 2021, 191, 102511. [Google Scholar] [CrossRef]
- Sun, D.; Huan, Y.; Wang, S.; Qiu, Z.; Ling, Z.; Mao, Z.; He, Y. Remote sensing of spatial and temporal patterns of phytoplankton assemblages in the Bohai Sea, Yellow Sea, and East China Sea. Water Res. 2019, 157, 119–133. [Google Scholar] [CrossRef]
- Kang, J.J.; Oh, H.J.; Youn, S.H.; Park, Y.; Kim, E.; Joo, H.T.; Hwang, J.D. Estimation of phytoplankton size classes in the littoral sea of Korea using a new algorithm based on deep learning. J. Mar. Sci. Eng. 2022, 10, 1450. [Google Scholar] [CrossRef]
- Ye, H.; Tang, D. A three-component model of phytoplankton size classes for the South China Sea. Malays. J. Sci. 2013, 32, 325–332. [Google Scholar]
- Fei, X.; Zhang, Y.; Qiao, X.; Song, Z.; Wang, H.; Hu, J.; Liu, X.; Guo, C.; Liu, S.M. Changjiang River–sourced nutrients support massive biological carbon fixation in the East China Sea. Nat. Commun. 2024, 15, 1991. [Google Scholar]
- Joo, H.T.; Park, J.W.; Son, S.; Noh, J.-H.; Jeong, J.-Y.; Kwak, J.H.; Saux-Picart, S.; Choi, J.H.; Kang, C.-K.; Lee, S.H. Long-term annual primary production in the Ulleung Basin as a biological hot spot in the East/Japan Sea. J. Geophys. Res. Oceans 2014, 119, 3002–3011. [Google Scholar] [CrossRef]
- Lin, C.; Ning, X.; Su, J.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Mar. Syst. 2005, 55, 223–234. [Google Scholar] [CrossRef]
- Han, I.-S.; Lee, J.-S.; Jung, H.-K. Long-term pattern changes of sea surface temperature during summer and winter due to climate change in the Korea Waters. Fish. Aquat. Sci. 2023, 26, 639–648. [Google Scholar] [CrossRef]
- Jin, J.; Liu, S.M.; Ren, J.L.; Liu, C.G.; Zhang, J.; Zhang, G.L.; Huang, D.J. Nutrient dynamics and coupling with phytoplankton species composition during the spring blooms in the Yellow Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 97, 16–32. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Park, K.-A. Change in the recent warming trend of sea surface temperature in the East Sea (Sea of Japan) over decades (1982–2018). Remote Sens. 2019, 11, 2613. [Google Scholar] [CrossRef]
- Jang, H.K.; Kang, J.J.; Lee, J.H.; Kim, M.; Ahn, S.H.; Jeong, J.Y.; Yun, M.S.; Han, I.S.; Lee, S.H. Recent primary production and small phytoplankton contribution in the Yellow Sea during the summer in 2016. Ocean Sci. J. 2018, 53, 509–519. [Google Scholar] [CrossRef]
- Jang, H.-K.; Youn, S.-H.; Joo, H.; Kim, Y.; Kang, J.-J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.-J.; Kim, S.; et al. First concurrent measurement of primary production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.H.; Lee, D.; Lee, J.H.; Bae, H.; Lee, C.H.; Kang, C.K.; Lee, S.H. Characteristics of different size phytoplankton for primary production and biochemical compositions in the Western East/Japan Sea. Front. Microbiol. 2020, 11, 560102. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, S.-H.; Oh, H.-J.; Joo, H.; Jang, H.-K.; Kang, J.-J.; Lee, D.; Jo, N.; Kim, K.; Park, S.; et al. Seasonal compositions of size-fractionated surface phytoplankton communities in the Yellow Sea. J. Mar. Sci. Eng. 2022, 10, 1087. [Google Scholar] [CrossRef]
- Lee, D.; Kang, J.J.; Jo, N.; Kim, K.; Jang, H.K.; Kim, M.J.; Kim, Y.; Park, S.; Son, S.; Kwon, J.I.; et al. Variations in phytoplankton primary production driven by the Pacific Decadal Oscillation in the East/Japan Sea. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG007094. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.H.; Joo, H.; Jang, H.K.; Park, S.; Kim, Y.; Kim, S.; Kim, J.; Kim, M.; Kwon, J.I.; et al. Long-term variability of phytoplankton primary production in the Ulleung Basin, East Sea/Japan Sea using ocean color remote sensing. J. Geophys. Res. Oceans 2024, 129, e2024JC020898. [Google Scholar] [CrossRef]
- Park, K.A.; Park, J.J.; Park, J.E.; Choi, B.J.; Lee, S.H.; Byun, D.S.; Lee, E.L.; Kang, B.S.; Shin, H.R.; Lee, S.R. Interdisciplinary mathematics and sciences in schematic ocean current maps in the seas around Korea. In Handbook of the Mathematics of the Arts and Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 2359–2388. [Google Scholar]
- Moon, J.-E.; Ahn, Y.-H.; Ryu, J.-H.; Palanisamy, S. Development of ocean environmental algorithms for Geostationary Ocean Color Imager. Korean J. Remote Sens. 2010, 26, 198–207. [Google Scholar]
- Dunstan, P.K.; Foster, S.D.; King, E.; Risbey, J.; O’Kane, T.J.; Monselesan, D.; Hobday, A.J.; Hartog, J.R.; Thompson, P.A. Global patterns of change and variation in sea surface temperature and chlorophyll a. Sci. Rep. 2018, 8, 14624. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Youn, S.-H.; Oh, H.J.; Joo, H.-T.; Kim, Y.; Kang, J.J.; Lee, D.; Kim, K.; Jang, H.K.; Jo, N.; et al. Spatial and temporal distribution of phytoplankton community in relation to environmental factors in the southern coastal waters of Korea. Front. Mar. Sci. 2022, 9, 950234. [Google Scholar] [CrossRef]
- Wood, S.; Wood, M.S. Package ‘mgcv’; R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2015; Volume 1, p. 729. [Google Scholar]
- Domingues, R.B.; Anselmo, T.P.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Light as a driver of phytoplankton growth and production in the freshwater tidal zone of a turbid estuary. Estuar. Coast. Shelf Sci. 2011, 91, 526–535. [Google Scholar] [CrossRef]
- Riegman, R.; Noordeloos, A.A.M. Size-fractionated uptake of nitrogenous nutrients and carbon by phytoplankton in the North Sea during summer 1994. Mar. Ecol. Prog. Ser. 1998, 173, 85–96. [Google Scholar] [CrossRef][Green Version]
- Ramakrishnan, R.; Thayapurath, S.; Manguesh, U.G.; Dias, A.B. Low light phytoplankton genera observed in the coastal and estuarine waters of Goa, India. Appl. Ecol. Environ. Res. 2018, 16, 1783–1796. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, Z.; Liu, J.; Chen, Q.; Zeng, J.; Huang, W. Seasonal variations of net-phytoplankton community structure in the southern Yellow Sea. J. Ocean Univ. China 2013, 12, 557–567. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Gao, Y.; Zhai, H.; Jin, H.; Zhou, F.; Yan, X.; Chen, Q. Regulation of spatial changes in phytoplankton community by water column stability and nutrients in the southern Yellow Sea. J. Geophys. Res. Biogeosci. 2019, 124, 2610–2627. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, C.; Lu, X.; Zhu, L.; Sun, Q.; Wang, S. Phytoplankton blooms and its influencing environmental factors in the southern Yellow Sea. Reg. Stud. Mar. Sci. 2021, 47, 101916. [Google Scholar] [CrossRef]
- Hyun, M.J.; Choi, D.H.; Lee, H.; Won, J.; Kim, G.-U.; Lee, Y.; Jeong, J.-Y.; Ra, K.; Yang, W.; Lee, J.; et al. Phytoplankton spring succession pattern in the Yellow Sea surveyed at Socheongcho Ocean Research Station. Front. Mar. Sci. 2023, 10, 1280612. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Park, H.J.; Choy, E.J.; Jeong, H.D.; Kim, K.R.; Kang, C.K. Monthly measured primary and new productivities in the Ulleung Basin as a biological “hot spot” in the East/Japan Sea. Biogeosciences 2013, 10, 4405–4417. [Google Scholar] [CrossRef]
- Yamada, K.; Ishizaka, J.; Yoo, S.; Kim, H.-C.; Chiba, S. Seasonal and interannual variability of sea surface chlorophyll a concentration in the Japan/East Sea (JES). Prog. Oceanogr. 2004, 61, 193–211. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Hwang, J.; Suh, Y.S.; Park, H.J.; Chang, K.I.; Kim, K.-R.; Kang, C.K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/Japan Sea and the Western Subarctic Pacific. J. Geophys. Res. Ocean. 2014, 119, 4505–4519. [Google Scholar] [CrossRef]
- Kwak, J.H.; Han, E.; Lee, S.H.; Park, H.J.; Kim, K.R.; Kang, C.K. A consistent structure of phytoplankton communities across the warm–cold regions of the water mass on a meridional transect in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef]
- Raven, J.A. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Agustí, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Dai, Z.; Du, J.; Zhang, X.; Su, N.; Li, J. Variation of riverine material loads and environmental consequences on the Changjiang (Yangtze) Estuary in recent decades (1955−2008). Environ. Sci. Technol. 2011, 45, 223–227. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, K.; Najjar, R.G.; Jeong, H.D.; Jeong, H.J. Increasing N abundance in the northwestern Pacific Ocean due to atmospheric nitrogen deposition. Science 2011, 334, 505–509. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, K.; Lim, W.A.; Lee, E.; Dai, M.; Choi, Y.H.; Han, I.S.; Shin, K.; Kim, J.M.; Chae, J. Anthropogenic nitrogen is changing the East China and Yellow Seas from being N deficient to being P deficient. Limnol. Oceanogr. 2021, 66, 914–924. [Google Scholar] [CrossRef]
- Park, S.; Kim, G.; Kwon, H.K.; Han, I.-S. Long-term changes in the concentrations of nutrients in the marginal seas (Yellow Sea, East China Sea, and East/Japan Sea) neighboring the Korean Peninsula. Mar. Pollut. Bull. 2023, 192, 115012. [Google Scholar] [CrossRef] [PubMed]
- Egge, J.K. Are diatoms poor competitors at low phosphate concentrations? J. Mar. Syst. 1998, 16, 191–198. [Google Scholar] [CrossRef]
- Guo, S.; Sun, X.; Zhang, J.; Yao, Q.; Wei, C.; Wang, F. Unveiling the evolution of phytoplankton communities: Decades-long insights into the southern Yellow Sea, China (1959–2023). Mar. Pollut. Bull. 2024, 201, 116179. [Google Scholar] [CrossRef]
- Andres, M.; Park, J.H.; Wimbush, M.; Zhu, X.H.; Nakamura, H.; Kim, K.; Chang, K.I. Manifestation of the Pacific Decadal Oscillation in the Kuroshio. Geophys. Res. Lett. 2009, 36, L16602. [Google Scholar] [CrossRef]
- Shin, H.R.; Lee, J.H.; Kim, C.H.; Yoon, J.H.; Hirose, N.; Takikawa, T.; Cho, K. Long-term variation in volume transport of the Tsushima Warm Current estimated from ADCP current measurement and sea level differences in the Korea/Tsushima Strait. J. Mar. Syst. 2022, 232, 103750. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wu, C.-R.; Chao, S.-Y. Warming and weakening trends of the Kuroshio during 1993–2013. Geophys. Res. Lett. 2016, 43, 9200–9207. [Google Scholar] [CrossRef]
- Kim, D.; Ji, R.; Park, H.J.; Feng, Z.; Jang, J.; Lee, C.L.; Kang, Y.H.; Kang, C.K. Impact of shifting subpolar front on phytoplankton dynamics in the western margin of East/Japan Sea. Front. Mar. Sci. 2021, 8, 790703. [Google Scholar] [CrossRef]
- Pak, J.; Park, J.-H.; Moon, J.-H.; Lee, J.-H.; Min, H.-S.; Kim, S.-Y. Quantification of the Extremely Intensified East Korea Warm Current in the Summer of 2021: Offshore and Coastal Variabilities. Front. Mar. Sci. 2023, 10, 1252302. [Google Scholar] [CrossRef]
- Lee, E.A.; Kim, S.Y.; Min, H.S. Climatological Descriptions on Regional Circulation around the Korean Peninsula. Tellus A Dyn. Meteorol. Oceanogr. 2019, 71, 1604058. [Google Scholar] [CrossRef]
- Gong, G.C.; Chen, Y.L.L.; Liu, K.K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Yoon, S.C.; Youn, S.H.; Whang, J.D.; Suh, Y.S.; Yoon, Y.Y. Long-term variation in ocean environmental conditions of the Northern East China Sea. J. Korean Soc. Mar. Environ. Energy 2015, 18, 189–206. [Google Scholar] [CrossRef]
- Zhang, S.W.; Wang, Q.Y.; Lü, Y.; Cui, H.; Yuan, Y.L. Observation of the seasonal evolution of the Yellow Sea Cold Water Mass in 1996–1998. Cont. Shelf Res. 2008, 28, 442–457. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Jang, H.K.; Kang, J.J.; Kim, K.; Lee, D.; Jo, N.; Park, S.H.; Lee, J.H.; Ahn, S.H.; et al. Size-differential photosynthetic traits of phytoplankton in the Chukchi Sea. Cont. Shelf Res. 2023, 255, 104933. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.W.; Lee, S.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.S.; Lee, S.H. Seasonal variations in the small phytoplankton contribution to the total primary production in the Amundsen Sea, Antarctica. J. Geophys. Res. Oceans 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Nixon, S.W.; Thomas, A.C. On the size of the Peru upwelling ecosystem. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 2521–2528. [Google Scholar] [CrossRef]
- Korea Customs Service. FTA Trade Report; Korea Customs Service: Daejeon, Republic of Korea, 2022; Volume 2, pp. 44–61. Available online: https://www.customs.go.kr/upload/ftaportalkor/ebook/FTA-report-40/index.html#page=1 (accessed on 10 October 2024).
- KOSIS: Korea Statistical Information Service, Fishery Production Trend Survey. 2024. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1EW0005&conn_path=I2 (accessed on 10 October 2024).
- Hunter, M.D.; Price, P.W. Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 1992, 73, 724–732. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Jennings, S.; Holmes, R.; Harle, J.; Merino, G.; Allen, J.I.; Holt, J.; Dulvy, N.K.; Barange, M. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2979–2989. [Google Scholar] [CrossRef]
- Finkel, Z.V. Does phytoplankton cell size matter? The evolution of modern marine food webs. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 333–350. [Google Scholar] [CrossRef]
- Hansen, B.; Bjornsen, P.K.; Hansen, P.J. The size ratio between planktonic predators and their prey. Limnol. Oceanogr. 1994, 39, 395–403. [Google Scholar] [CrossRef]
- López-Abbate, M.C. Microzooplankton communities in a changing ocean: A risk assessment. Diversity 2021, 13, 82. [Google Scholar] [CrossRef]
- Richardson, A.J. In hot water: Zooplankton and climate change. ICES J. Mar. Sci. 2008, 65, 279–295. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Chassot, E.; Bonhommeau, S.; Dulvy, N.K.; Mélin, F.; Watson, R.; Gascuel, D.; Le Pape, O. Global marine primary production constrains fisheries catches. Ecol. Lett. 2010, 13, 495–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).