Dietary Bacillus subtilis Supplementation Improves Intestinal Health of Meagre (Argyrosomus regius) Juveniles Fed Plant-Based Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Spore Production
2.2. Growth Trial
2.3. Proximate Analysis of Experimental Diets
2.4. Fish Sampling
2.5. Histological Analysis
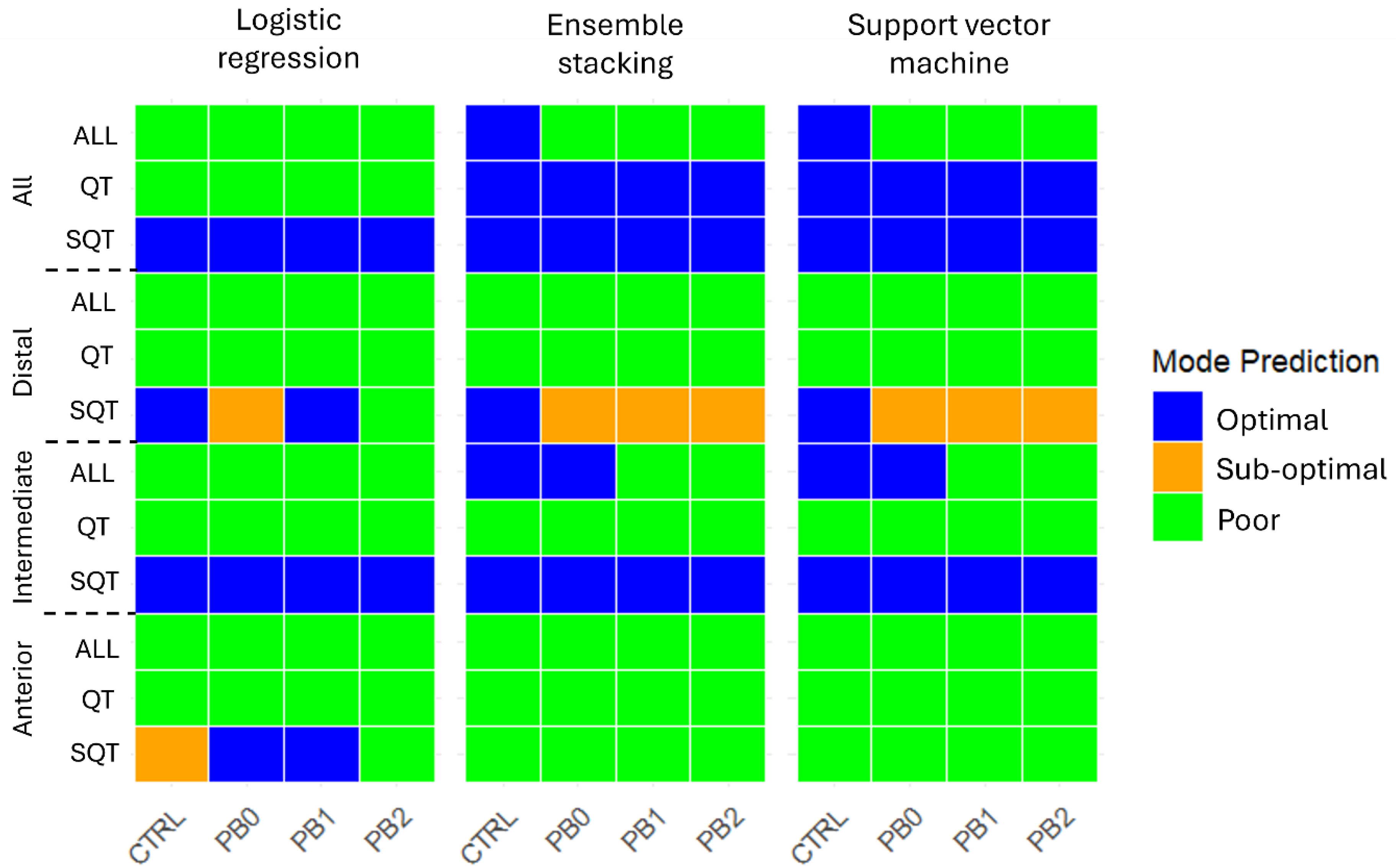
2.6. Nutritional Status Classification Using Machine Learning Models
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024–Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Hodar, A.; Vasava, R.; Mahavadiya, D.; Joshi, N. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. 2020, 23, 13–21. [Google Scholar]
- Daniel, N. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018, 6, 164–179. [Google Scholar]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition–A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Colombo, S. Physiological considerations in shifting carnivorous fishes to plant-based diets. In Fish Physiology; Tillmann, J., Benfey, A.P.F., Colin, J., Brauner, Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 53–82. [Google Scholar]
- Serra, C.R.; Almeida, E.M.; Guerreiro, I.; Santos, R.; Merrifield, D.L.; Tavares, F.; Oliva-Teles, A.; Enes, P. Selection of carbohydrate-active probiotics from the gut of carnivorous fish fed plant-based diets. Sci. Rep. 2019, 9, 6384. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, A.; Kortner, T.; Jaramillo, A.; Gamil, A.; Chikwati, E.; Li, Y.; Schmidt, M.; Herman, E.; Hymowitz, T.; Teimouri, S.; et al. Removal of three proteinaceous antinutrients from soybean does not mitigate soybean-induced enteritis in Atlantic salmon (Salmo salar, L.). Aquaculture 2019, 514, 734495. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 203–233. [Google Scholar]
- Naveed, M. Functional Aquafeeds: A comprehensive approach to probiotics, amino acids and feed additives integration for sustainable production. In Complementary and Alternative Medicine: Prebiotics and Probiotics; Farooqi, S.H., Aqib, A.I., Zafar, M.A., Akhtar, T., Ghafoor, N., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 57–64. [Google Scholar]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Chattaraj, S.; Ganguly, A.; Mandal, A.; Das Mohapatra, P. A review of the role of probiotics for the control of viral diseases in aquaculture. Aquac. Int. 2022, 30, 2513–2539. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2020, 13, 862–906. [Google Scholar] [CrossRef]
- Soares, M.B.; Almada, C.N.; Pereira, E.P.R.; Ferreira, B.M.; Balthazar, C.F.; Khorshidian, N.; Rocha, R.S.; Xavier-Santos, D.; Cruz, A.G.; Ranadheera, C.S.; et al. Review-Sporeforming probiotic bacteria: Characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023, 138, 453–469. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, V.; Oliveira, B.; Canadas-Sousa, A.; Abreu, H.; Dias, J.; Kiron, V.; Valente, L.M.P. An in-depth characterisation of European seabass intestinal segments for assessing the impact of an algae-based functional diet on intestinal health. Sci. Rep. 2023, 13, 11686. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, B.; Stankovic, M.; Markovic, Z.; Poleksic, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. Belgrade 2011, 56, 87–100. [Google Scholar] [CrossRef]
- Ramos, M.A.; Batista, S.; Pires, M.A.; Silva, A.P.; Pereira, L.F.; Saavedra, M.J.; Ozório, R.O.A.; Rema, P. Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 2017, 11, 1259–1269. [Google Scholar] [CrossRef]
- Mohammadian, T.; Monjezi, N.; Peyghan, R.; Mohammadian, B. Effects of dietary probiotic supplements on growth, digestive enzymes activity, intestinal histomorphology and innate immunity of common carp (Cyprinus carpio): A field study. Aquaculture 2022, 549, 737787. [Google Scholar] [CrossRef]
- Eissa, E.-S.H.; Baghdady, E.S.; Gaafar, A.Y.; El-Badawi, A.A.; Bazina, W.K.; Abd Al-Kareem, O.M.; Abd El-Hamed, N.N.B. Assessing the Influence of Dietary Pediococcus acidilactici Probiotic Supplementation in the Feed of European Sea Bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm Water Quality, Growth, Feed Utilization, Survival Rate, Body Composition, Blood Biochemical Parameters, and Intestinal Histology. Aquac. Nutr. 2022, 2022, 5841220. [Google Scholar] [CrossRef]
- Monfort, M.C. Present Market Situation and Prospects of Meagre (Argyrosomus regius), as an Emerging Species in Mediterranean Aquaculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010. [Google Scholar]
- Ribeiro, L.; Moura, J.; Santos, M.; Colen, R.; Rodrigues, V.; Bandarra, N.; Soares, F.; Ramalho, P.; Barata, M.; Moura, P.; et al. Effect of vegetable based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture 2015, 447, 116–128. [Google Scholar] [CrossRef]
- Estévez, A.; Treviño, L.; Kotzamanis, Y.; Karacostas, I.; Tort, L.; Gisbert, E. Effects of different levels of plant proteins on the ongrowing of meagre (Argyrosomus regius) juveniles at low temperatures. Aquac. Nutr. 2011, 17, e572–e582. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2019, 9, 381–386. [Google Scholar] [CrossRef]
- Mandal, A.; Ghosh, A.R. Role of artificial intelligence (AI) in fish growth and health status monitoring: A review on sustainable aquaculture. Aquac. Int. 2024, 32, 2791–2820. [Google Scholar] [CrossRef]
- Oliveira, J.; Barata, M.; Soares, F.; Pousão-Ferreira, P.; Oliva-Teles, A.; Couto, A. Machine Learning-Based Classification of Malnutrition Using Histological Biomarkers of Fish Intestine: Preliminary Data. J. Mar. Sci. Eng. 2024, 12, 2177. [Google Scholar] [CrossRef]
- Couto, A.; Barroso, C.; Guerreiro, I.; Pousão-Ferreira, P.; Matos, E.; Peres, H.; Oliva-Teles, A.; Enes, P. Carob seed germ meal in diets for meagre (Argyrosomus regius) juveniles: Growth, digestive enzymes, intermediary metabolism, liver and gut histology. Aquaculture 2016, 451, 396–404. [Google Scholar] [CrossRef]
- Urán, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Obach, A.; Jensen, L.; Koppe, W.; Verreth, J.A.J. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac. Nutr. 2008, 14, 324–330. [Google Scholar] [CrossRef]
- Nimalan, N.; Sørensen, S.L.; Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Gancarčíková, S.; Vatsos, I.N.; Bisa, S.; Kiron, V.; Sørensen, M. Mucosal barrier status in Atlantic salmon fed marine or plant-based diets supplemented with probiotics. Aquaculture 2022, 547, 737516. [Google Scholar] [CrossRef]
- Jang, W.J.; Lee, J.M.; Hasan, M.T.; Lee, B.-J.; Lim, S.G.; Kong, I.-S. Effects of probiotic supplementation of a plant-based protein diet on intestinal microbial diversity, digestive enzyme activity, intestinal structure, and immunity in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2019, 92, 719–727. [Google Scholar] [CrossRef]
- Bilal, S.; Ishtiaq, A.; Ghaffar, A.; Ishtiaq, T.; Naeem, M. Impact of in-feed Multispecies Probiotic Mixtures on Growth Patterns and Length-weight Relationships of Pangasianodon hypophthalmus. TSF J. Biol. 2025, 3, 27–39. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Bakr, A.; El-Keredy, A.M.S.; Dawood, M.A.O.; Dossou, S.; Wang, W.; Yukun, Z. Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol. 2018, 79, 303–312. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, C.-H.; Wang, S.-W.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Addo, S.; Carrias, A.A.; Williams, M.A.; Liles, M.R.; Terhune, J.S.; Davis, D.A. Effects of Bacillus subtilis Strains on Growth, Immune Parameters, and Streptococcus iniae Susceptibility in Nile Tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2017, 48, 257–267. [Google Scholar] [CrossRef]
- Di, J.; Chu, Z.; Zhang, S.; Huang, J.; Du, H.; Wei, Q. Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immunol. 2019, 93, 711–719. [Google Scholar] [CrossRef]
- Grosell, M.; Farrell, A.; Brauner, C. Fish Physiology: The Multifunctional Gut of Fish; Academic Press: Cambridge, MA, USA, 2010; Volume 30. [Google Scholar]
- Deng, J.; Zhang, X.; Sun, Y.; Mi, H.; Zhang, L. Effects of different types of non-starch polysaccharides on growth, digestive enzyme activity, intestinal barrier function and antioxidant activity of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2021, 21, 100864. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological Changes in Fish Gut in Response to Prebiotics and Probiotics Treatment to Improve Their Health Status: A Review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Olsen, R.E.; Myklebust, R.; Ringø, E.; Mayhew, T.M. The influences of dietary linseed oil and saturated fatty acids on caecal enterocytes in Arctic char (Salvelinus alpinus L.): A quantitative ultrastructural study. Fish Physiol. Biochem. 2000, 22, 207–216. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr.; Stryer, L. The Biosynthesis of Membrane Lipids and Steroids. In Biochemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2015. [Google Scholar]
- El-Son, M.A.M.; Elshopakey, G.E.; Rezk, S.; Eldessouki, E.A.A.; Elbahnaswy, S. Dietary mixed Bacillus strains promoted the growth indices, enzymatic profile, intestinal immunity, and liver and intestinal histomorphology of Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2022, 27, 101385. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Standen, B.; Rawling, M.; Davies, S.; Castex, M.; Foey, A.; Gioacchini, G.; Carnevali, O.; Merrifield, D. Probiotic Pediococcus acidilactici modulates both localised intestinal-and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2013, 35, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Aidos, L.; Mirra, G.; Pallaoro, M.; Herrera Millar, V.R.; Radaelli, G.; Bazzocchi, C.; Modina, S.C.; Di Giancamillo, A. How Do Alternative Protein Resources Affect the Intestine Morphology and Microbiota of Atlantic Salmon? Animals 2023, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M.Á. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 2013, 34, 1063–1070. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, R.; Wang, Z.; Qiang, L.; Yao, H. The effects of Bacillus subtilis on the immunity, mucosal tissue morphology, immune-related gene transcriptions, and intestinal microbiota in flounder (Paralichthys olivaceus) with two feeding methods: Continuous versus discontinuous feeding. Vet. Immunol. Immunopathol. 2024, 271, 110742. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-Y.; Zhang, H.-Q.; Chen, J.-X.; Zhu, J.; He, J.-Y.; Luo, L.; Lin, S.-M.; Chen, Y.-J. Effects of dietary Bacillus subtilis DSM 32315 supplementation on the growth, immunity and intestinal morphology, microbiota and inflammatory response of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 2119–2131. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Zhao, R.; Lu, T.; Li, S.; Wang, D. Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss). Fishes 2024, 9, 229. [Google Scholar] [CrossRef]
| Diets: | CTRL | PB0 | PB1 | PB2 |
|---|---|---|---|---|
| Ingredients (% dry weight basis) | ||||
| Fish meal a | 20 | 10 | 10 | 10 |
| CPSP b | 5 | 5 | 5 | 5 |
| Pea protein concentrate c | 20 | 0 | 0 | 0 |
| Wheat gluten d | 10 | 15 | 15 | 15 |
| Corn gluten e | 10.3 | 8 | 8 | 8 |
| Soybean meal f | 0 | 20 | 20 | 20 |
| Rapeseed meal g | 0 | 10 | 10 | 10 |
| Sunflower meal h | 0 | 10 | 10 | 10 |
| Wheat meal i | 16.4 | 2.7 | 2.7 | 2.7 |
| Fish oil j | 14.4 | 7.5 | 7.5 | 7.5 |
| Soy oil k | 0 | 4 | 4 | 4 |
| Rapeseed oil l | 0 | 4 | 4 | 4 |
| Vitamin m | 1 | 1 | 1 | 1 |
| Mineral n | 1 | 1 | 1 | 1 |
| Choline | 0.5 | 0.5 | 0.5 | 0.5 |
| Binder o | 1 | 1 | 1 | 1 |
| Taurine | 0.3 | 0.3 | 0.3 | 0.3 |
| B. subtilis FI99 spores (CFU g−1 feed) | 0 | 0 | 1 × 109 | 5.5 × 1011 |
| Proximate analysis (% dry matter basis) | ||||
| Dry matter | 96.9 | 97.4 | 99.0 | 99.0 |
| Crude protein | 42.3 | 42.7 | 43.5 | 42.9 |
| Crude fat | 17.9 | 17.4 | 16.8 | 17.9 |
| Ash | 7.0 | 6.6 | 6.8 | 6.8 |
| Diets: | CTRL | PB0 | PB1 | PB2 | ANOVA |
|---|---|---|---|---|---|
| Final weight (g) | 32.9 ± 0.9 | 35.1 ± 4.9 | 36.6 ± 7.8 | 38.4 ± 9.4 | 0.871 |
| Weight gain (g) | 22.6 ± 0.9 | 24.7 ± 5.0 | 26.3 ± 7.8 | 28.0 ± 9.4 | 0.885 |
| Feed intake (g kg ABW−1 day−1) | 24.9 ± 1.2 | 24.3 ± 3.2 | 22.9 ± 4.7 | 21.4 ± 3.9 | 0.697 |
| Feed efficiency | 0.63 ± 0.04 | 0.68 ± 0.16 | 0.76 ± 0.27 | 0.82 ± 0.28 | 0.779 |
| Daily growth index | 1.53 ± 0.04 | 1.62 ± 0.23 | 1.69 ± 0.34 | 1.76 ± 0.40 | 0.879 |
| Specific growth rate | 1.73 ± 0.04 | 1.81 ± 0.21 | 1.87 ± 0.30 | 1.93 ± 0.35 | 0.805 |
| Diets: | CTRL | PB0 | PB1 | PB2 | ANOVA |
|---|---|---|---|---|---|
| HSI | 3.77 ± 0.74 | 4.01 ± 0.84 | 3.94 ± 0.58 | 4.80 ± 0.92 | 0.053 |
| ESI | 1.11 ± 0.35 | 1.65 ± 0.49 | 1.54 ± 0.28 | 1.66 ± 0.47 | 0.813 |
| VSI | 1.51 ± 0.44 | 1.38 ± 0.34 | 1.38 ± 0.24 | 1.47 ± 0.29 | 0.743 |
| Polynomial contrast | Orthogonal contrast | ||||
| Linear | Quadratic | CTRL vs. PB0 | CTRL vs. PB1 | CTRL vs. PB2 | |
| HSI | 0.043 | 0.158 | 0.543 | 0.617 | 0.019 |
| ESI | 0.942 | 0.527 | 0.017 | 0.011 | 0.014 |
| VSI | 0.495 | 0.730 | 0.494 | 0.474 | 0.851 |
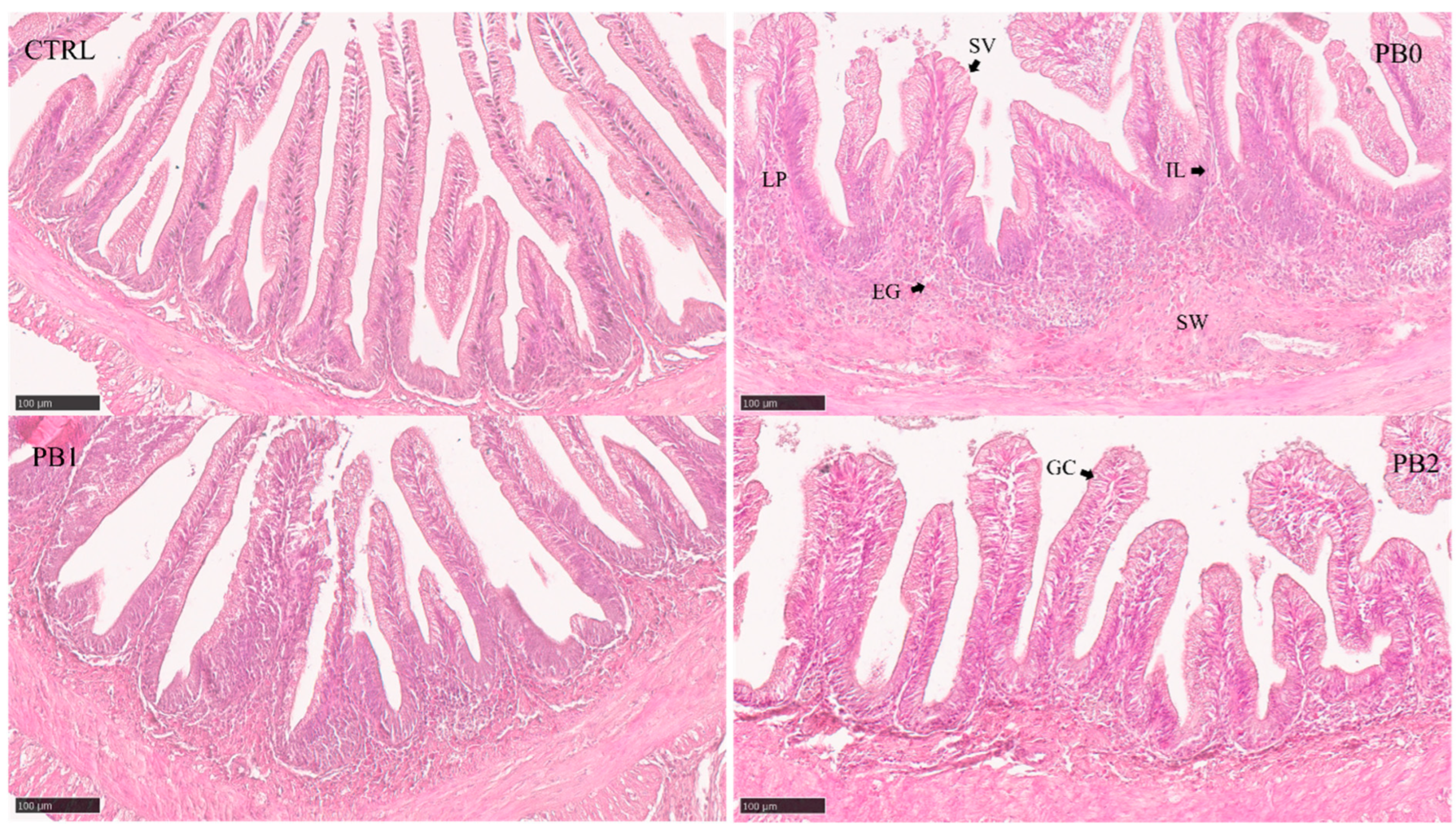
| Intestine Section | Semi-Quantitative Feature | CTRL | Diets PB0 | PB1 | PB2 | Kruskal–Wallis |
|---|---|---|---|---|---|---|
| Villi fusion | 1.53 ± 0.64 | 1.19 ±0.54 | 1.20 ± 0.41 | 1.20 ± 0.41 | 0.086 | |
| Lamina propria size | 2.07 ± 0.26 | 1.91 ± 0.33 | 2.27 ± 0.50 | 2.13 ± 0.64 | 0.170 | |
| Submucosa widening | 1.57 ± 0.50 | 1.63 ± 0.39 | 1.67 ± 0.41 | 1.70 ± 0.53 | 0.583 | |
| Anterior | Supranuclear vacuole size | 2.03 ± 0.67 a | 2.56 ± 0.48 ab | 2.57 ± 0.73 b | 2.80 ± 1.00 b | 0.013 |
| Goblet cells | 1.43 ± 0.62 | 1.47 ± 0.62 | 1.77 ±1.08 | 1.80 ± 0.96 | 0.634 | |
| Eosinophilic granulocytes | 2.47 ± 0.83 ab | 2.06 ± 0.63 a | 2.00 ± 0.50 a | 2.83 ± 0.84 b | 0.013 | |
| Intraepithelial leukocytes | 2.47 ± 0.72 | 2.03 ± 0.67 | 2.33 ± 0.96 | 2.10 ± 1.06 | 0.720 | |
| Villi fusion | 1.61 ± 0.92 | 1.20 ± 0.41 | 1.29 ± 0.47 | 1.20 ± 0.41 | 0.543 | |
| Lamina propria size | 2.22 ± 0.65 | 2.07 ± 0.56 | 2.50 ± 0.44 | 2.20 ± 0.53 | 0.225 | |
| Submucosa widening | 1.72 ± 0.39 | 1.73 ± 0.32 | 1.75 ± 0.47 | 1.60 ± 0.47 | 0.792 | |
| Intermediate | Supranuclear vacuole size | 1.33 ± 0.66 a | 1.37 ± 0.55 ab | 1.68 ± 0.42 b | 1.77 ± 0.59 b | 0.005 |
| Goblet cells | 2.86 ± 1.06 | 2.97 ± 1.06 | 2.79 ± 0.96 | 2.87 ± 0.92 | 0.984 | |
| Eosinophilic granulocytes | 1.92 ± 0.86 | 1.33 ± 0.45 | 1.79 ± 0.64 | 1.47 ± 0.44 | 0.076 | |
| Intraepithelial leukocytes | 2.28 ± 0.99 ab | 1.83 ± 0.59 a | 2.64 ± 0.72 b | 2.43 ± 0.56 ab | 0.035 | |
| Villi fusion | 1.64 ± 0.93 | 1.76 ± 0.90 | 1.81 ± 0.83 | 2.00 ± 0.97 | 0.802 | |
| Lamina propria size | 1.79 ± 0.47 a | 2.44 ± 0.43 b | 2.47 ± 0.43 b | 2.44 ± 0.44 b | <0.001 | |
| Distal | Submucosa widening | 1.36 ± 0.31 a | 2.71 ± 0.90 b | 2.78 ± 0.58 b | 2.53 ± 0.56 b | <0.001 |
| Supranuclear vacuole size | 1.93 ± 0.39 a | 2.71 ± 0.83 b | 2.59 ± 0.78 b | 3.06 ± 0.83 b | <0.001 | |
| Goblet cells | 1.43 ± 0.47 a | 2.53 ± 1.41 ab | 2.44 ± 1.20 ab | 2.88 ± 1.02 b | 0.006 | |
| Eosinophilic granulocytes | 1.96 ± 0.72 | 1.88 ± 0.78 | 1.75 ± 0.88 | 2.31 ± 0.91 | 0.160 | |
| Intraepithelial leukocytes | 1.46 ± 0.54 | 1.91 ± 0.67 | 1.81 ± 0.57 | 1.91 ± 0.42 | 0.077 |
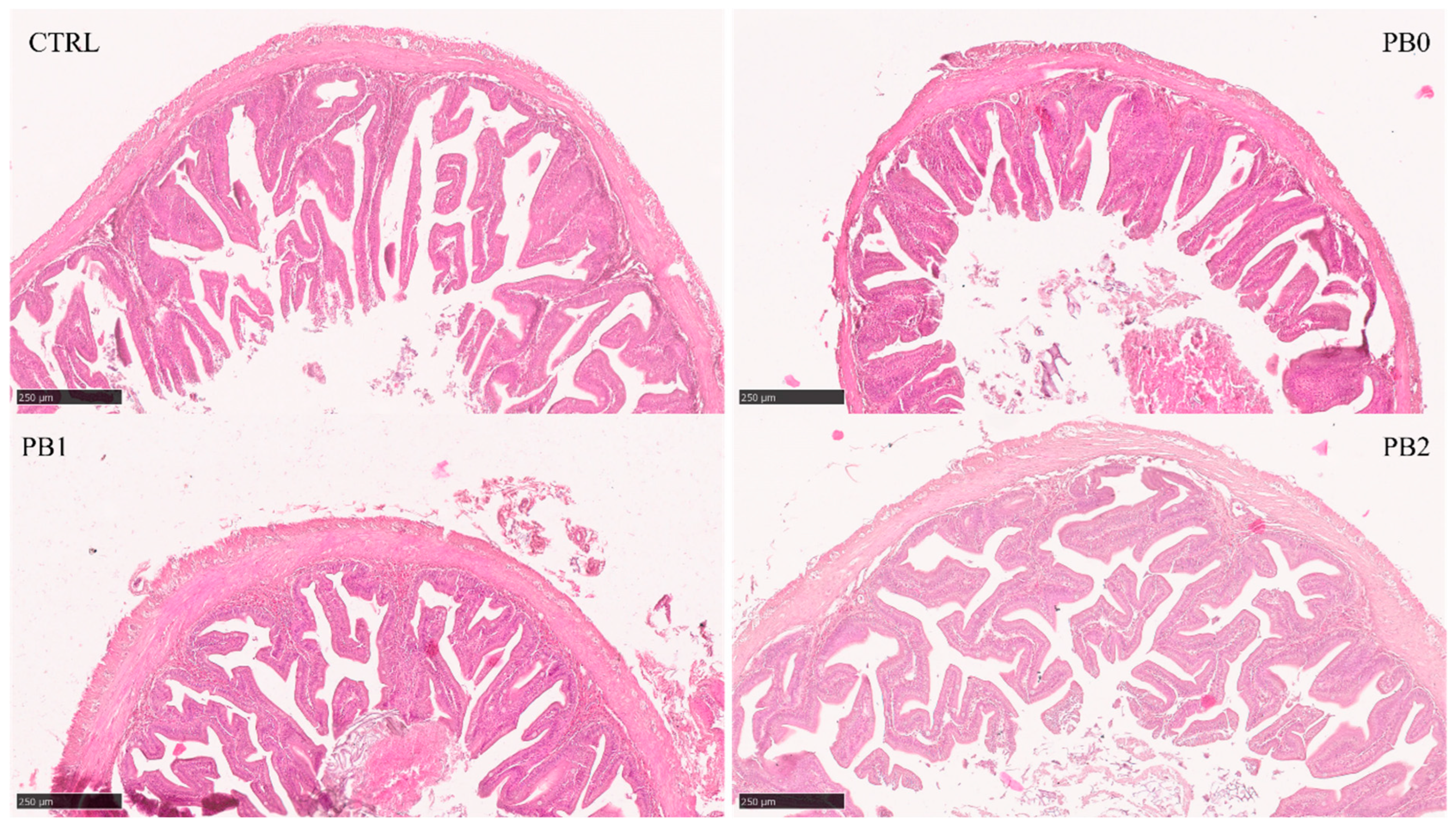
| Quantitative Feature | CTRL | PB0 | PB1 | PB2 | ANOVA | Polynomial Contrast | Orthogonal Contrast | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Anterior | Linear | Quadratic | CTRL vs. PB0 | CTRL vs. PB1 | CTRL vs. PB2 | |||||
| TA (mm2) | 2.97 ± 0.79 | 1.53 ± 0.39 a | 2.04 ± 0.63 ab | 2.40 ± 1.12 b | 0.007 | 0.002 | 0.763 | <0.001 | <0.001 | 0.118 |
| TMD (mm) | 2.19 ± 0.26 | 1.57 ± 0.28 | 1.76 ± 0.26 | 1.86 ± 0.46 | 0.053 | 0.018 | 0.653 | <0.001 | <0.001 | 0.019 |
| LA (mm2) | 0.62 ± 0.44 | 0.26 ± 0.19 a | 0.60 ± 0.34 b | 0.64 ± 0.43 b | 0.003 | 0.001 | 0.165 | 0.005 | 0.858 | 0.887 |
| LMD (mm) | 1.07 ± 361 | 0.69 ± 0.26 a | 1.02 ± 0.28 b | 1.03 ± 0.43 b | 0.005 | 0.004 | 0.114 | 0.001 | 0.717 | 0.795 |
| VA (mm2) | 1.92 ± 0.33 | 1.0 ± 0.24 a | 1.14 ± 0.35 ab | 1.44 ± 0.58 b | 0.017 | 0.006 | 0.477 | <0.001 | <0.001 | 0.010 |
| VLA (mm2) | 2.54 ± 0.69 | 1.28 ± 0.34 a | 1.74 ± 0.59 ab | 2.08 ± 0.98 b | 0.005 | 0.001 | 0.794 | <0.001 | 0.001 | 0.143 |
| VN | 23.7 ± 5.7 | 31.8 ± 3.9 | 30.9 ± 6.0 | 33.5 ± 5.8 | 0.382 | 0.391 | 0.276 | <0.001 | 0.002 | <0.001 |
| VD | 12.6 ± 3.1 | 32.5 ± 7.9 | 29.4 ± 10.4 | 26.5 ± 10.4 | 0.207 | 0.078 | 0.952 | <0.001 | <0.001 | <0.001 |
| Intermediate | ||||||||||
| TA (mm2) | 2.54 ± 0.83 | 2.09 ± 0.70 a | 2.87 ± 0.95 b | 2.57 ± 1.05 ab | 0.046 | 0.122 | 0.048 | 0.093 | 0.274 | 0.947 |
| TMD (mm) | 1.95 ± 0.36 | 1.79 ± 0.32 a | 2.09 ± 0.33 b | 1.97 ± 0.39 ab | 0.044 | 0.113 | 0.049 | 0.169 | 0.240 | 0.856 |
| LA (mm2) | 0.93 ± 0.47 | 1.06 ± 0.40 | 1.25 ± 0.61 | 1.18 ± 0.70 | 0.613 | 0.517 | 0.457 | 0.411 | 0.093 | 0.263 |
| LMD (mm) | 1.23 ± 0.33 | 1.35 ± 0.24 | 1.43 ± 0.34 | 1.99 ± 2.18 | 0.286 | 0.153 | 0.501 | 0.253 | 0.092 | 0.222 |
| VA (mm2) | 1.33 ± 0.46 | 0.81 ± 0.33 a | 1.28 ± 0.48 b | 1.08 ± 0.38 ab | 0.005 | 0.044 | 0.008 | <0.001 | 0.756 | 0.111 |
| VLA (mm2) | 2.26 ± 0.76 | 1.86 ± 0.64 | 2.53 ± 0.88 | 2.27 ± 0.98 | 0.073 | 0.155 | 0.069 | 0.102 | 0.339 | 0.988 |
| VN | 35.8 ± 10.7 | 28.8 ± 8.6 | 31.1 ± 3.9 | 31.1 ± 6.0 | 0.516 | 0.321 | 0.565 | 0.040 | 0.089 | 0.122 |
| VD | 28.9 ± 9.3 | 39.5 ± 12.9 b | 27.9 ± 11.7 a | 31.7 ± 10.5 ab | 0.019 | 0.056 | 0.033 | 0.009 | 0.782 | 0.434 |
| Distal | ||||||||||
| TA (mm2) | 6.46 ± 2.24 | 5.37 ± 1.82 | 4.71 ± 1.52 | 5.22 ± 2.22 | 0.587 | 0.841 | 0.314 | 0.150 | 0.018 | 0.129 |
| TMD (mm) | 3.12 ± 0.53 | 2.75 ± 0.47 | 2.63 ± 0.38 | 2.86 ± 0.61 | 0.426 | 0.537 | 0.251 | 0.050 | 0.006 | 0.202 |
| LA (mm2) | 2.43 ± 1.46 | 2.11 ± 1.05 | 1.73 ± 0.87 | 2.07 ± 1.32 | 0.571 | 0.930 | 0.294 | 0.503 | 0.128 | 0.482 |
| LMD (mm) | 1.99 ± 0.62 | 1.77 ± 0.46 | 1.67 ± 0.40 | 1.87 ± 0.54 | 0.470 | 0.533 | 0.291 | 0.279 | 0.102 | 0.573 |
| VA (mm2) | 3.09 ± 0.78 | 2.18 ± 0.61 | 1.91 ± 0.54 | 2.13 ± 0.80 | 0.460 | 0.842 | 0.221 | 0.001 | <0.001 | 0.002 |
| VLA (mm2) | 5.52 ± 2.07 | 4.30 ± 1.56 | 3.64 ± 1.31 | 4.20 ± 2.00 | 0.487 | 0.890 | 0.236 | 0.076 | 0.006 | 0.079 |
| VN | 51.2 ± 6.9 | 45.4 ± 5.4 | 47.2 ± 6.0 | 48.2 ± 10.1 | 0.562 | 0.292 | 0.858 | 0.015 | 0.096 | 0.326 |
| VD | 17.7 ± 5.4 | 22.0 ± 5.2 | 26.2 ± 6.1 | 24.2 ± 5.8 | 0.123 | 0.282 | 0.080 | 0.030 | <0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.; Ribeiro, R.; Gonçalves, G.; Santos, R.; Serra, C.; Enes, P.; Pousão-Ferreira, P.; Oliva-Teles, A.; Couto, A. Dietary Bacillus subtilis Supplementation Improves Intestinal Health of Meagre (Argyrosomus regius) Juveniles Fed Plant-Based Diets. J. Mar. Sci. Eng. 2025, 13, 1013. https://doi.org/10.3390/jmse13061013
Oliveira J, Ribeiro R, Gonçalves G, Santos R, Serra C, Enes P, Pousão-Ferreira P, Oliva-Teles A, Couto A. Dietary Bacillus subtilis Supplementation Improves Intestinal Health of Meagre (Argyrosomus regius) Juveniles Fed Plant-Based Diets. Journal of Marine Science and Engineering. 2025; 13(6):1013. https://doi.org/10.3390/jmse13061013
Chicago/Turabian StyleOliveira, Joana, Raquel Ribeiro, Gabriela Gonçalves, Rafaela Santos, Cláudia Serra, Paula Enes, Pedro Pousão-Ferreira, Aires Oliva-Teles, and Ana Couto. 2025. "Dietary Bacillus subtilis Supplementation Improves Intestinal Health of Meagre (Argyrosomus regius) Juveniles Fed Plant-Based Diets" Journal of Marine Science and Engineering 13, no. 6: 1013. https://doi.org/10.3390/jmse13061013
APA StyleOliveira, J., Ribeiro, R., Gonçalves, G., Santos, R., Serra, C., Enes, P., Pousão-Ferreira, P., Oliva-Teles, A., & Couto, A. (2025). Dietary Bacillus subtilis Supplementation Improves Intestinal Health of Meagre (Argyrosomus regius) Juveniles Fed Plant-Based Diets. Journal of Marine Science and Engineering, 13(6), 1013. https://doi.org/10.3390/jmse13061013






