Abstract
As aquaculture expands, plant-based feeds are increasingly used, but some fish species poorly tolerate them, affecting health and growth. Probiotics can help counter these effects by improving digestion, nutrient absorption, and immunity. This study evaluated the effect of dietary incorporation of Bacillus subtilis FI99 on the intestinal health of meagre. A nutritional challenge was performed with a practical control diet and three diets higher in plant-based ingredients: one without probiotic and two with probiotic incorporated at 1 × 109 CFU g−1 and 5.5 × 1011 CFU g−1. Histomorphological analysis was used to assess intestinal health and validate previously established machine learning models in predicting fish nutritional status. No differences were observed in zootechnical performance and biometric indexes. Most effects were observed in the anterior intestinal section, where probiotics improved total area, lumen area, lumen maximum diameter, total maximum diameter, villi area, and villi + lumen area. Additionally, probiotics improved supranuclear vacuole size, eosinophilic granulocytes, and intraepithelial leukocytes presence in anterior and intermediate sections. Machine learning models could not accurately predict the nutritional status of fish. Overall, the study indicates that dietary inclusion of B. subtilis enhances the intestinal health of meagre fed plant-based diets. Machine learning models require further development for improved accuracy.
1. Introduction
Aquaculture production has increased in recent decades [1], and the sector’s growth has led to continuous innovation of feeds, moving from fishery-based to plant-based ingredients. These novel feeds aim to reduce production costs and the environmental impact of aquaculture production by minimizing the reliance on fish meal (FM) and fish oil (FO) [2]. However, reducing marine-based ingredients in diets for carnivorous fish is challenging and can impact growth performance and overall health [3]. This is mainly related to the antinutritional factors (ANFs) present in plant ingredients such as protease inhibitors, lectins, saponins, and non-starch polysaccharides (NSPs) [4]. NSPs, present in high amounts in these ingredients, play an important role as fish do not digest them as they lack the necessary enzymes to break them down [5,6]. Therefore, plant-based diets often result in intestinal inflammation and damaged enterocytes [5,7]. This can lead to lower nutrient digestibility, weakened intestinal immunity, and increased susceptibility to infectious diseases, resulting in reduced growth performance and compromised health [8,9].
Probiotics are live microbial organisms such as bacteria, fungi, and yeast that, when incorporated into feeds, positively influence the host by promoting a balanced intestinal microbiota [10,11]. This way, they have demonstrated promising results in the ability to enhance growth, boost immunity, improve disease control, and enhance nutrient digestibility [12,13,14]. One of the most extensively researched and used probiotics in aquaculture is Bacillus subtilis due to its numerous benefits for aquatic animals [15]. B. subtilis is a non-pathogenic Gram-positive, rod-shaped, aerobic, or facultatively anaerobic bacterium that can form spores [16,17]. These spores allow adaptation to various environmental conditions, as they can withstand extreme temperatures and can be dehydrated without losing viability. Moreover, they are easy to produce, easily incorporated into feeds, and have high shelf storage [15,18]. The strain used in this study is B. subtilis (FI99), which has already shown promising results for improving weight gain and feed utilization in European sea bass (Dicentrarchus labrax) fed with plant-based diets [6].
The intestine is the first organ to come into contact with feeds and is an important ‘sentinel’ of feed impact on fish’s health [19]. For this reason, studying the effects of novel feeds and dietary supplements on fish intestinal health is crucial. Histomorphology is a widely used and relevant tool in assessing the impact of diets on the fish gut, as it provides detailed insights into structural changes, cellular integrity, and tissue adaptations that may influence digestion, nutrient absorption, and overall gut health [20]. Probiotics’ effects on fish intestinal morphology have been studied in various species. For example, in Nile tilapia (Oreochromis niloticus), dietary supplementation with a probiotic blend of Bacillus sp., Pediococcus sp., Enterococcus sp., and Lactobacillus sp. resulted in increased villi height and goblet cell count in all intestinal sections [21]. Common carp fed a probiotic mixture composed of Lactobacillus plantarum + L. rhamnosus presented a posterior intestinal section with higher villi width, epithelium thickness, and goblet cell number [22]. Using Pediococcus acidilactici as a probiotic increased villi height in the intermediate intestine of European sea bass [23].
Meagre is a carnivorous fish that has the potential to become a mass-market species in the Mediterranean for several reasons: fast growth in captivity, efficient feed utilization, high tolerance to environmental changes, and excellent fillet characteristics [24,25]. However, information regarding meagre nutritional requirements and feed utilization is still limited [26]. Most importantly, no studies are available on the potential of probiotics to improve meagre performance and health, despite their growing significance in aquafeeds.
Machine learning tools may be crucial in advancing aquaculture research, particularly in diagnosing fish health and welfare. Using machine learning, researchers can develop algorithms capable of learning from data, extracting valuable insights from complex analyses, such as histological assessments, and facilitating a more accurate and efficient evaluation of fish health [27,28]. In a previous study, machine learning models were trained to predict the nutritional status of meagre using both quantitative and semi-quantitative histological data [29]. Among the models tested, three showed potential for application in the study of meagre intestinal health: Logistic Regression, Support Vector Machines, and Ensemble Stacking.
The current study evaluated the growth performance and intestinal health of meagre juveniles fed with a plant-based diet supplemented with B. subtilis as a probiotic. Furthermore, the effectiveness of previously established machine learning models [29] in diagnosing the nutritional status of the fish was also evaluated.
2. Materials and Methods
2.1. Probiotic Spore Production
Highly purified Bacillus subtilis FI99 isolate spores were prepared according to [6]. The B. subtilis FI99 strain used was previously isolated from the gut content of European seabass [6]. Briefly, sporulation occurred for 48 h at 37 °C in an orbital shaker at 200 rpm, and purified spores were obtained by centrifuging for 10 min at 10,000× g at 4 °C. Cell pellets were resuspended in 50 mM Tris-HCl (pH 7.2) containing 50 μg mL−1 lysozyme and incubated for 1 h at 37 °C. After a single wash with 1 volume of distilled water (10 min at 10,000× g, 4 °C), cell pellets were suspended in 0.05% SDS, washed three times with distilled water, suspended in 1 volume of distilled water, and lyophilized. Before their incorporation into the diets, serial dilutions of the lyophilized spores were performed in B&W salts, and the number of CFU mg−1 was determined.
2.2. Growth Trial
Four isoproteic (43%) and isolipidic (18%) diets were formulated for the growth trial. A practical diet was formulated as a positive control (CTRL) with 20% fish meal and 14% fish oil. Then, three more challenging plant-based (PB) diets, where plant-based ingredients replaced 50% of fish meal and fish oil, were prepared with no spore supplementation (PB0), with B. subtilis FI99 at 1 × 109 CFU g−1 (PB1) or 5.5 × 1011 CFU g−1 (PB2). The levels of probiotic supplementation were selected based on previously tested levels in European seabass [6], with the addition of a higher level to explore potential benefits. The ingredients for the diets were ground to a fine consistency, mixed well, and formed into dry pellets in a laboratory pellet mill (CPM, California Pellet Mill, Crawfordsville, IN, USA) through a 2 mm die. Then, the pellets were placed during 24 h in an oven at 40 °C to dry and finally saved in airtight bags until their utilization. The ingredient composition and proximate analysis of the diets are displayed in Table 1.

Table 1.
Formulation and proximate composition of experimental diets.
The trial was carried out at the Aquatic Organisms Bioterium of CIIMAR (Matosinhos, Portugal) in a recirculating aquaculture system (RAS) with 12 cylindrical tanks of 300 L capacity. Four groups of fish were randomly assigned to and distributed in 12 tanks in triplicate, each with 30 juveniles with an initial average body weight of 10.4 ± 0.05 g. Fish were then submitted to an acclimatization period of 2 weeks, during which they were fed with a commercial diet (Neo Gold Blue seabream/seabass diet (48% protein and 17% lipids; Sorgal, S.A., Aveiro, Portugal). The nutritional trial lasted 9 weeks, and fish were fed until visual satiation was achieved, two times a day, six days per week. Great care was taken to guarantee that all feed dealt was consumed. The RAS was thermoregulated to 21.9 °C, salinity averaged 36.4‰, pH was at an average of 7.09 ± 0.10, oxygen was maintained near saturation at an average of 7.57 ± 0.34 mg L−1 (Hach, HQ40D Digital multi meter kit, Loveland, CO, USA), and nitrites and ammonia were kept below 0.02 mg L−1.
2.3. Proximate Analysis of Experimental Diets
Experimental diets underwent chemical analysis through the following procedures: Dry matter was obtained after diet samples were dried at 105 °C in an oven until achieving constant weight; ash was obtained through incineration (16 h at 450 °C in a muffle furnace); protein content (N × 6.25) was measured using the Kjeldahl method with a distillation (Velp® Scientifica, Usmate Velate, Italy; model UDK 129) and a digestor unit (model 1015; Tecator Systems, Höganäs, Sweden). Lipids were quantified by petroleum ether extraction using a SoxTec extraction system (extraction unit model 1043 and service unit model 1046 Tecator Systems, Höganäs, Sweden).
2.4. Fish Sampling
Sampling was performed 4 h after the first meal of the day to ensure feed was present in the intestinal tract. Six fish from each tank were anesthetized using 0.3 mL L−1 of 2-phenoxyethanol and weighed. The fish were subsequently euthanized by decapitation, and the intestines were collected and separated into three portions: anterior (just after the pyloric caeca), intermediate (the narrowest middle portion of the intestine), and distal (the enlarged segment immediately before the anus), as illustrated in [29].
The remaining fish were fasted for one day to guarantee an empty gastrointestinal tract, anesthetized as previously, and then bulk-weighed. Within each tank, a total of three fish were selected at random and euthanized as described before, and the weights from the whole-fish, viscera, intestine, and liver were documented for the calculation of hepatosomatic (HSI), enterosomatic (ESI), and visceral (VSI) indexes. Zootechnical and organosomatic indexes were calculated as described in [29].
2.5. Histological Analysis
All collected intestinal sections were processed using the standard histological techniques [29] and stained with hematoxylin and eosin. Digital slides were used for the quantitative (QT) analyses that were performed with the NDP.view2 (HAMAMATSU photonics, Shizuoka, Japan) image viewing software. Total area (TA) and total maximum diameter (TMD), total lumen area (TLA) and maximum lumen diameter (LMD), the area covered by villi and lumen (VLA), villi area (VA), and villi number (VN) were measured as described in [29]. Villi density (VD) was calculated as follows: villi density = villi number/villi area. Semi-quantitative analyses (SQT) were carried out with a ZEISS Axio Imager A2 microscope with a ZEISS Axio Cam Mrc (Carl Zeiss Microscopy, White Plains, NY, USA) connected and with the respective software (ZEN 3.5; blue edition). For the SQT analysis, a score-based system was applied, as described by [30,31], with tissue scores ranging from 1 (normal) to 5 (highly modified) to assess the scope of the following structures: fusion of intestinal folds, widening of lamina propria and the submucosa, occurrence of goblet cells, infiltration of leukocytes in both lamina propria and submucosa, and alterations to enterocyte architecture, such as supranuclear vacuolization. Three slides per intestinal section were analyzed for each sample. The average score of each determined feature was regarded as the overall value of histomorphological changes.
2.6. Nutritional Status Classification Using Machine Learning Models
The classification models used were previously trained on histological data [29] to predict nutritional status within three levels: 1—Optimal, 2—Sub-optimal, and 3—Poor. Briefly, three classification algorithms, support vector machine (SVM), logistic regression (LR), and ensemble stacking (ES), were trained with data from each intestinal section (Anterior, Intermediate, Distal) or all together, using either quantitative or semi-quantitative data or both. The data were fed to the models, and the predicted classification was recorded.
2.7. Statistical Analysis
Data were analyzed with the IBM SPSS statistics 27.0.1 software package (SPSSR, IBM, Chicago, IL, USA). Zootechnical performance, organosomatic indexes, and histological quantitative data of PB groups (PB0, PB1, and PB2) were checked for normality with the Shapiro–Wilk test, and for variances and homogeneity, the Levene test was used to check for ANOVA assumptions. Afterwards, data were analyzed by one-way ANOVA followed by the Tukey multiple range test to find statistical differences between groups. Polynomial (linear and quadratic) contrasts were also used in the PB diets to determine data response to increasing probiotic levels. Additionally, orthogonal contrasts were used to compare the PB diets with the CTRL. Semi-quantitative data were evaluated with nonparametric Kruskal–Wallis tests succeeded by multiple comparisons.
The most frequent predictions for each diet category were determined for the variables QT, SQ, and SQ_QT using frequency analysis in SPSS. For each variable, the mode was identified along with its corresponding percentage, representing the proportion of the mode relative to the total observations. This analysis was performed separately for each tissue section (Anterior, Intermediate, Distal) and all sections together.
3. Results
No significant differences were observed in the growth and feed efficiency of fish fed the experimental diets (Table 2). Also, no significant differences were observed in the organosomatic indexes of fish fed with the PB diets (Table 3). All PB groups presented a higher ESI than the CTRL, but the VSI was similar to the CTRL. Further, the HSI was higher in fish fed the PB2 diet than the CTRL. The mortality rate was 1.1% in the CTRL and PB1 groups and 0% in the PB0 and PB2 groups, with no significant difference in mortality rates between the different groups (p > 0.05). The observed mortality had no clear correlation with diet composition.

Table 2.
Zootechnical performance of meagre under different dietary treatments.

Table 3.
Organosomatic indexes of meagre under different dietary treatments.
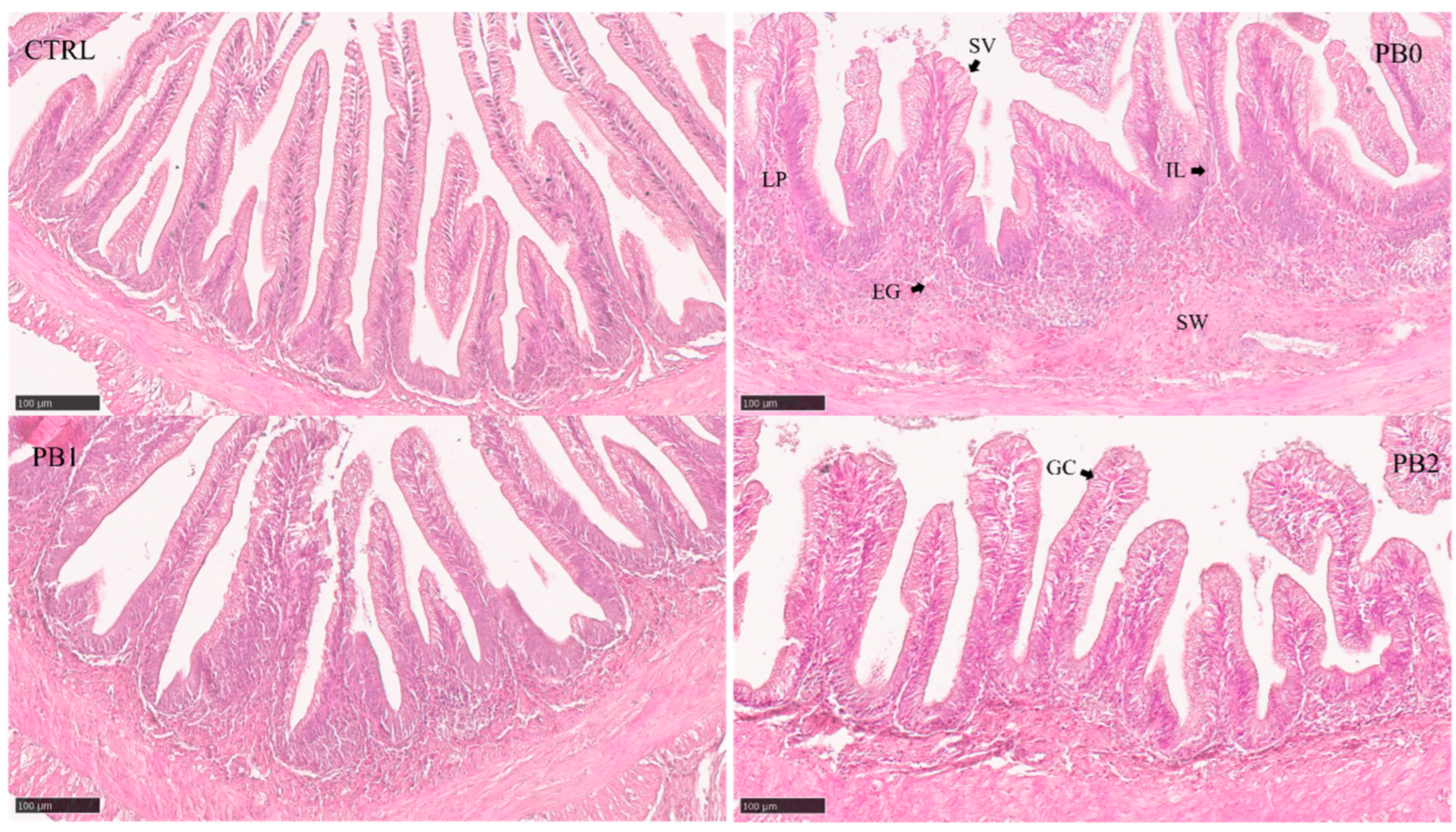
In both anterior and intermediate intestinal sections, the supranuclear vacuole size increased in PB-supplemented diets and the eosinophilic granulocytes in the anterior section were significantly higher in PB2 compared to other PB diets (Table 4). In the distal section (Figure 1), the lamina propria size, submucosa widening, and supranuclear vacuole size increased in PB-fed groups while the number of goblet cells increased in the PB2-fed fish.

Table 4.
Semi-quantitative analysis of meagre intestine sections under different dietary treatments.
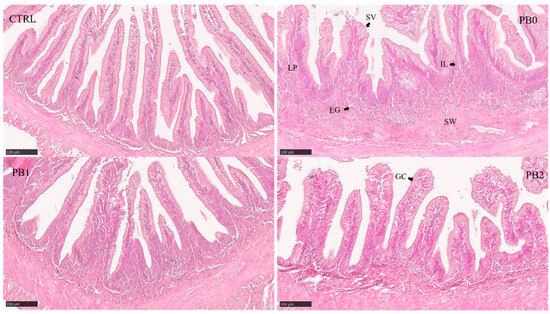
Figure 1.
Histomorphological appearance of meagre distal intestinal section when fed different experimental diets. Semi-quantitative features (Table 4) showed significant differences between CTRL and PB fed groups: SVs—Supranuclear vacuoles. ILs—Intraepithelial leukocytes. LP—Lamina propria. EGs—Eosinophilic granulocytes. SW—Submucosa widening. GC—Goblet cell.
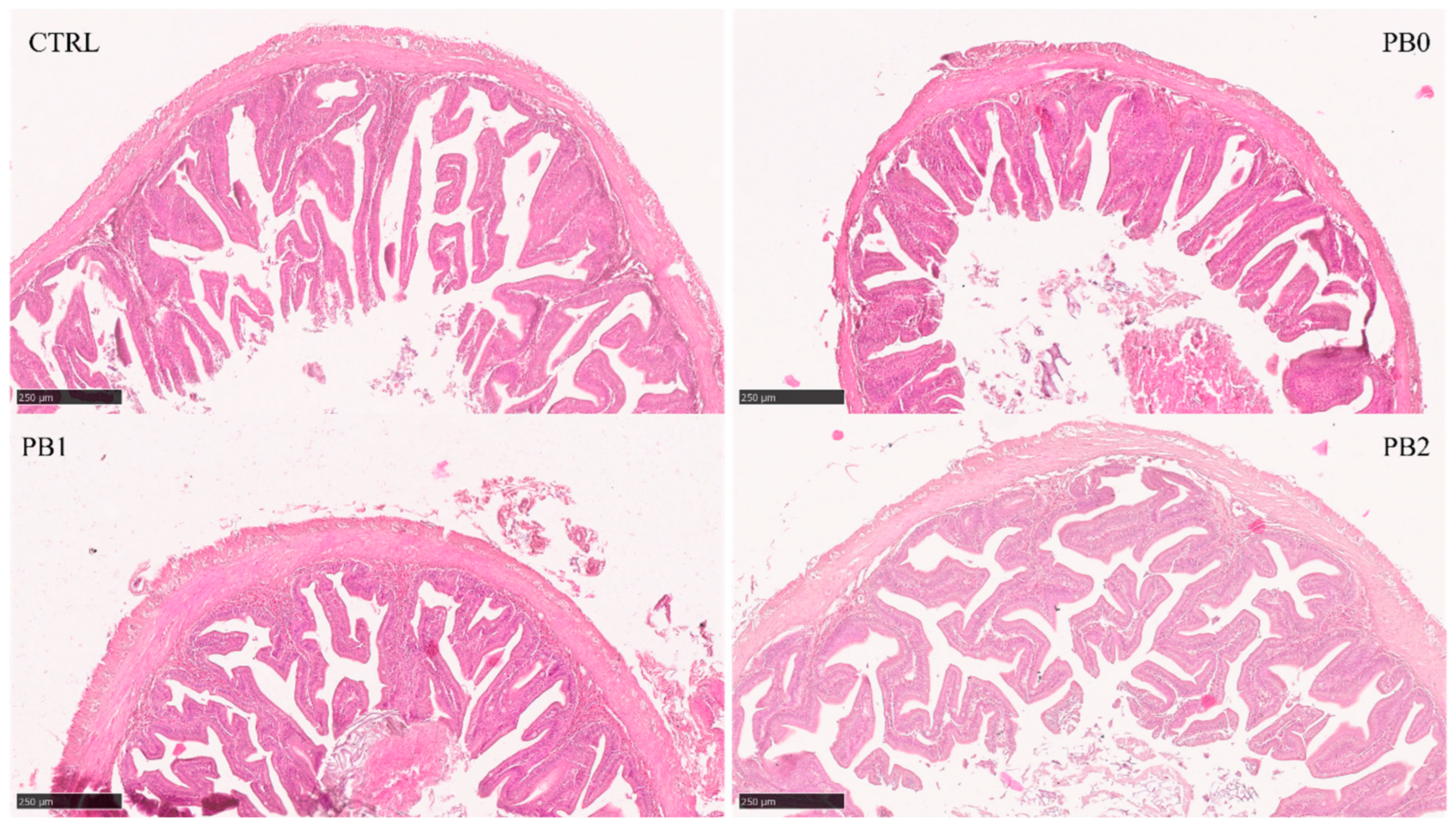
All quantitative histological parameters analyzed in the anterior intestine were lower in the PB0 than in the CTRL group (Table 5). For TA, VA, and VLA, there was an increase in PB2 when compared to PB0, while for LA and LMD, the increase was observed in both PB1 and PB2 (Figure 2). In the intermediate (Figure S1) and distal (Figure 1) intestines, VA and VN were lower, and VD was higher in the PB0 than in the CTRL group. In the intermediate intestine for TA, TMD, and VA, there was an increase and for VD a decrease in the PB1 group compared to other PB groups. No effects of the probiotic supplementation on PB fed fish were observed in the distal section.

Table 5.
Quantitative analysis of meagre intestinal sections under different dietary treatments.
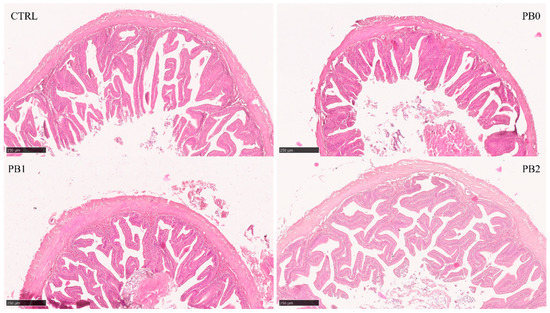
Figure 2.
Histomorphological appearance of meagre anterior intestinal section when fed different experimental diets. The absorption area in PB-fed fish improved after supplementation with B. subtilis FI99.
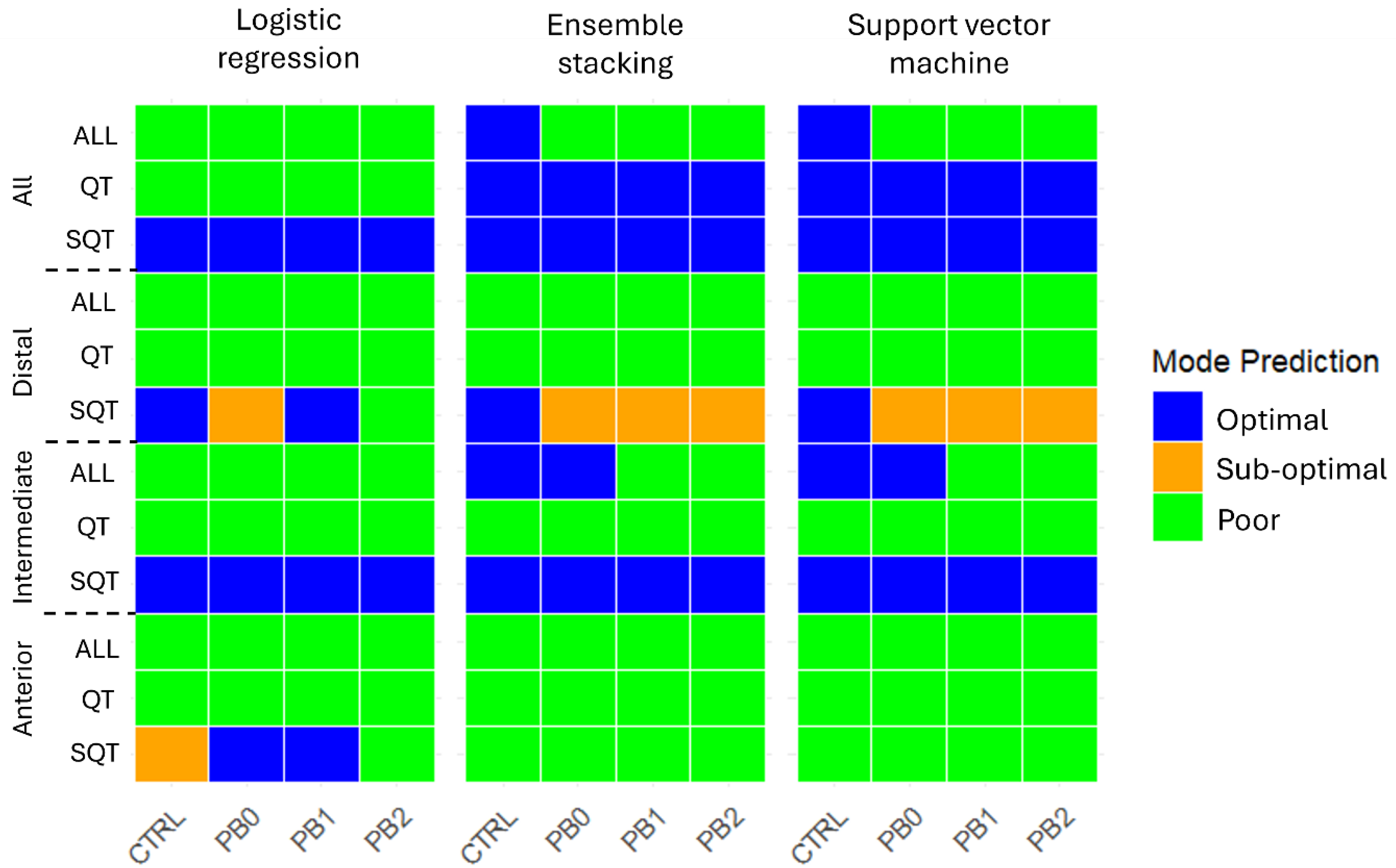
The predictions of the nutritional status (Optimal, Sub-optimal, Poor) of meagre fed with the different diets across intestinal sections and treatments are depicted in Figure 3.
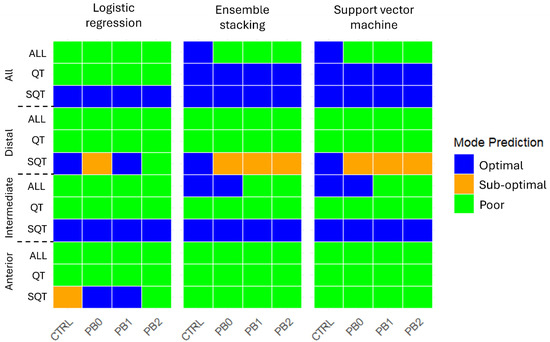
Figure 3.
Prediction of meagre nutritional status using machine learning models per intestinal section and histological features. Features: SQT—Semi-quantitative. QT—Quantitative. ALL—All features.
In the anterior intestine, all models predicted for all dietary treatments a “Poor” status except the LR model using semi-quantitative features, which predicted a “Sup-optimal” status for CTRL, “Optimal” for PB0 and PB1, and “Poor” for PB2. In the intermediate section, all models predicted a “Poor” status based on quantitative features and an “Optimal” status based on qualitative features. When considering all features together, the LR model predicted “Poor” for all diets and ES and SVM predicted “Optimal” for CTRL and PB0 and “Poor” for PB1 and PB2. In the distal intestine, all models predicted for all dietary treatments a “Poor” status based on all features or on only quantitative features. With semi-quantitative features, LR predicted “Optimal” for CTRL and PB1, “Sub-optimal” for PB0, and “Poor” for PB2, while ES and SVM predicted “Sub-Optimal” for all PB and “Optimal” for CTRL. Lastly, when considering all the sections, with semi-quantitative features, all models predicted an “Optimal” status for all groups and with quantitative features LR predicted “Poor” and ES and SVM predicted “Optimal”. Considering all features, LR predicted “Poor” for all, while ES and SVM predicted “Optimal” for CTRL and “Poor” for all PB. Mode percentages are presented in Table S1.
4. Discussion
With the rise in aquaculture production, carnivorous fish’s acceptance of feeds that include novel ingredients, namely plant ingredients, is crucial. However, these ingredients can be hard to digest, affecting animals’ health, welfare, growth, and survival. Probiotics have been shown to improve the digestion of plant-based ingredients, intestinal health, and overall fish well-being and growth [32,33,34]. One such probiotic, B. subtilis FI99, has shown promising results for improving weight gain and feed utilization in European sea bass (Dicentrarchus labrax) fed with plant-based diets [6]. The present study extended the assessment of the potential of using this probiotic in meagre fed with challenging plant-based diets.
The results of this study concur with previous studies, where meagre growth performance was not affected by replacing up to 50% [25] or 76% [26] of marine protein with plant-based protein. This study also showed that dietary supplementation with B.subtilis probiotics did not affect zootechnical performance. Differently, in European seabass juveniles, dietary supplementation with B. subtilis FI99 improved weight gain and feed efficiency of fish fed a plant-based diet (with 80% fish meal replacement) [6].
B. subtilis has shown variable effects on fish growth performance, which may be species-specific or related to the B. subtilis strain used as a probiotic. For instance, B.subtilis probiotics enhanced growth performance of red sea bream (Pagrus major) [35], large yellow croaker (Larimichthys crocea) [36], and grouper (Epinephelus coioides) [37], while in Nile tilapia (Oreochromis niloticus) [38] and Dabry’s sturgeon (Acipenser dabryanus) [39], no growth performance effects were observed.
Plant-based diets can negatively impact intestinal health by inducing morphological alterations and inflammation. This may affect nutrient absorption and metabolism, overall immunological status, and the intestine’s role as a barrier against pathogens [40]. This makes intestinal morphology evaluation an important window into the effects of nutrition on fish health [8].
In this study, ESI increased in fish fed with the PB diets compared to CTRL, likely due to antinutritional factors in the plant ingredients, such as non-starch polysaccharides, present in high amounts in such ingredients, which are indigestible and can lead to inflammation [41].
Previous studies have shown that dietary supplementation with probiotics can improve the morphological status of the host intestine [42]. In this study, dietary probiotic supplementation to plant-based diets also impacted fish intestinal health. Enterocyte vacuole size in fish fed the PB diets was increased compared to the CTRL group in all intestine sections. A similar effect was also observed in meagre fed diets with a high inclusion of plant oils [29], and it might be associated with the higher levels of n-6 and n-9 fatty acids in the diets, which can lead to inflammation [43] or to the absorption rate of fatty acids surpassing enterocytes ability to synthesize the lipoproteins required for transporting lipids to circulation [44,45]. In fish fed the probiotic-supplemented diets, enterocyte vacuole size was significantly higher than in the CTRL diet in all intestine sections. This suggests that probiotics may have contributed to further improved lipid digestion and absorption, increasing deposition in the enterocyte vacuoles. It has been shown that B. subtilis can have hypolipidemic properties that could enhance lipid metabolism [46] and increase the activity of enzymes such as amylase, protease, and lipase [35].
In the anterior portion of the intestine, eosinophilic granulocytes increased in the PB2 group compared to other PB groups. This indicates a dose-dependent effect of the probiotic on the intestinal immune response that needs further study. Similarly, in the intermediate section, the higher intraepithelial leukocytes in the PB1 group further support the potential of probiotics to enhance gut immune activity [20]. As mentioned, probiotics are well known for improving immunity in fish [47], and other probiotics such as Lactobacillus rhamnosus [48], and Pediococcus acidilactici [49] also led to an increase in intraepithelial leukocytes in Nile tilapia.
Overall, the distal intestine section presented a more significant response to the plant-based diets than the other intestinal sections. This was expected as this is the portion where the non-digestible components of the diet are concentrated, and antinutritional factors are more prone to trigger immunological responses [50]. Thus, the plant-based diet-fed fish showed signs of enteritis, such as increased lamina propria width, submucosa inflammation, and supranuclear vacuole size. Unlike the other intestinal sections, dietary probiotic supplementation did not contribute to ameliorating the adverse effects of plant ingredients in the distal section, as seen in both quantitative and semi-quantitative analysis. Previously, B. subtilis was shown to reduce goblet cell number when supplemented to gilthead seabream commercial diets [51], while in an olive flounder commercial diet, it led to an increase in mucous cells in the anterior intestine [52]. As in olive flounder, probiotics also seemed to have a higher influence in the anterior portions of the intestine in this work. This may be related to probiotic germination mostly in the anterior intestine, which is richer in nutrients, after germination is triggered in the stomach, leading to new vegetative cells that produce components that would benefit the host [6,53].
In the anterior intestinal section, the total area and villi + lumen area reached values closer to those of the CTRL in fish fed the B. subtilis-supplemented PB2 diets, indicating that the probiotic inclusion incited an increase in the absorption area of the fish. This is further supported by the lumen area and maximum lumen diameter parameters, which showed similar results for both PB1 and PB2 groups. In olive flounder (Paralichthys olivaceus), B. subtilis was also shown to increase the intestinal absorptive area with an improvement in villi density and length [52] and in largemouth bass (Micropterus salmoides), it led to higher villus height and muscular layer thickness [54].
Despite it being non-significant, the intermediate section still showed recovery in the total area, maximum diameter, and villi area of supplemented groups, particularly in the PB1 group. It was also possible to observe a significant effect of probiotics in villi density, which were significantly closer to CTRL values. This was expected, as the villi area was reduced in PB0 while the number remained unchanged, likely due to the atrophy of villi height caused by the high fibre content in the plant-based diet. This effect was mitigated by the addition of probiotics. Although villi height was not measured, previous studies have shown that villi height can increase with probiotic supplementation [52,55].
Previously developed machine learning models [29] were employed in this study, and their effectiveness in classifying the nutritional status of fish subjected to the experimental diets was evaluated. The expected classification was for the CTRL diet to be classified on the optimum level and for PB0 to be closer to a sub-optimal level. The probiotic-supplemented diets were expected to improve gut histomorphology and, therefore, to be classified between the two extremes. Predictions obtained with the three applied models were similar. However, they were inconsistent across sections and within features and often deviated from the expected classifications. These findings indicate that these models still lack the required robustness to accurately predict fish’s nutritional status based on intestine histomorphological characteristics. This constraint is strongly related to the limited amount of data the models were trained on.
5. Conclusions
This study showed that meagre could be fed with diets including high levels of plant feedstuffs without adverse effects on growth performance or feed utilization, and only limited negative effects in intestinal histomorphology. The supplementation of Bacillus subtilis FI99 as a probiotic to the diets improved intestinal morphology, particularly in the anterior section, while providing some immunological benefits to the fish. Nevertheless, further studies are required to optimize dietary probiotic inclusion levels.
Further research efforts are necessary to improve machine learning models for classifying the nutritional status of fish under diverse nutritional scenarios, as the models used here lack the required sensitivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13061013/s1, Figure S1: Histomorphological appearance of meagre intermediate intestinal section when fed different experimental diets; Table S1: Prediction of meagre nutritional status using machine learning models.
Author Contributions
Conceptualization, J.O., R.R. and A.C.; data curation, A.C.; investigation, J.O., R.R., C.S., P.E. and A.C.; methodology, J.O., R.R., G.G. and R.S.; resources, P.P.-F., A.O.-T. and A.C.; software, J.O., R.R. and A.C.; supervision, A.O.-T. and A.C.; visualization, J.O. and R.R.; writing—original draft, J.O. and R.R.; writing—review and editing, J.O., A.O.-T. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The researcher J. Oliveira was supported by a grant from the Foundation for Science and Technology (FCT), Portugal (UI/BD/150902/2021).
Institutional Review Board Statement
This study was directed by certified scientists (FELASA category C) and all procedures were conducted according to the European Union Directive 2010/63/EU recommendations on the protection of animals for scientific purposes. The animal study protocol was approved by the Ethics Committee of CIIMAR’s Animal Welfare and Ethics Body (ORBEA-CIIMAR 30-2019).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAO. The State of World Fisheries and Aquaculture 2024–Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Hodar, A.; Vasava, R.; Mahavadiya, D.; Joshi, N. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. 2020, 23, 13–21. [Google Scholar]
- Daniel, N. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018, 6, 164–179. [Google Scholar]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition–A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Colombo, S. Physiological considerations in shifting carnivorous fishes to plant-based diets. In Fish Physiology; Tillmann, J., Benfey, A.P.F., Colin, J., Brauner, Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 53–82. [Google Scholar]
- Serra, C.R.; Almeida, E.M.; Guerreiro, I.; Santos, R.; Merrifield, D.L.; Tavares, F.; Oliva-Teles, A.; Enes, P. Selection of carbohydrate-active probiotics from the gut of carnivorous fish fed plant-based diets. Sci. Rep. 2019, 9, 6384. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, A.; Kortner, T.; Jaramillo, A.; Gamil, A.; Chikwati, E.; Li, Y.; Schmidt, M.; Herman, E.; Hymowitz, T.; Teimouri, S.; et al. Removal of three proteinaceous antinutrients from soybean does not mitigate soybean-induced enteritis in Atlantic salmon (Salmo salar, L.). Aquaculture 2019, 514, 734495. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 203–233. [Google Scholar]
- Naveed, M. Functional Aquafeeds: A comprehensive approach to probiotics, amino acids and feed additives integration for sustainable production. In Complementary and Alternative Medicine: Prebiotics and Probiotics; Farooqi, S.H., Aqib, A.I., Zafar, M.A., Akhtar, T., Ghafoor, N., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 57–64. [Google Scholar]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Chattaraj, S.; Ganguly, A.; Mandal, A.; Das Mohapatra, P. A review of the role of probiotics for the control of viral diseases in aquaculture. Aquac. Int. 2022, 30, 2513–2539. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2020, 13, 862–906. [Google Scholar] [CrossRef]
- Soares, M.B.; Almada, C.N.; Pereira, E.P.R.; Ferreira, B.M.; Balthazar, C.F.; Khorshidian, N.; Rocha, R.S.; Xavier-Santos, D.; Cruz, A.G.; Ranadheera, C.S.; et al. Review-Sporeforming probiotic bacteria: Characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023, 138, 453–469. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, V.; Oliveira, B.; Canadas-Sousa, A.; Abreu, H.; Dias, J.; Kiron, V.; Valente, L.M.P. An in-depth characterisation of European seabass intestinal segments for assessing the impact of an algae-based functional diet on intestinal health. Sci. Rep. 2023, 13, 11686. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, B.; Stankovic, M.; Markovic, Z.; Poleksic, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. Belgrade 2011, 56, 87–100. [Google Scholar] [CrossRef]
- Ramos, M.A.; Batista, S.; Pires, M.A.; Silva, A.P.; Pereira, L.F.; Saavedra, M.J.; Ozório, R.O.A.; Rema, P. Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 2017, 11, 1259–1269. [Google Scholar] [CrossRef]
- Mohammadian, T.; Monjezi, N.; Peyghan, R.; Mohammadian, B. Effects of dietary probiotic supplements on growth, digestive enzymes activity, intestinal histomorphology and innate immunity of common carp (Cyprinus carpio): A field study. Aquaculture 2022, 549, 737787. [Google Scholar] [CrossRef]
- Eissa, E.-S.H.; Baghdady, E.S.; Gaafar, A.Y.; El-Badawi, A.A.; Bazina, W.K.; Abd Al-Kareem, O.M.; Abd El-Hamed, N.N.B. Assessing the Influence of Dietary Pediococcus acidilactici Probiotic Supplementation in the Feed of European Sea Bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm Water Quality, Growth, Feed Utilization, Survival Rate, Body Composition, Blood Biochemical Parameters, and Intestinal Histology. Aquac. Nutr. 2022, 2022, 5841220. [Google Scholar] [CrossRef]
- Monfort, M.C. Present Market Situation and Prospects of Meagre (Argyrosomus regius), as an Emerging Species in Mediterranean Aquaculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010. [Google Scholar]
- Ribeiro, L.; Moura, J.; Santos, M.; Colen, R.; Rodrigues, V.; Bandarra, N.; Soares, F.; Ramalho, P.; Barata, M.; Moura, P.; et al. Effect of vegetable based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture 2015, 447, 116–128. [Google Scholar] [CrossRef]
- Estévez, A.; Treviño, L.; Kotzamanis, Y.; Karacostas, I.; Tort, L.; Gisbert, E. Effects of different levels of plant proteins on the ongrowing of meagre (Argyrosomus regius) juveniles at low temperatures. Aquac. Nutr. 2011, 17, e572–e582. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2019, 9, 381–386. [Google Scholar] [CrossRef]
- Mandal, A.; Ghosh, A.R. Role of artificial intelligence (AI) in fish growth and health status monitoring: A review on sustainable aquaculture. Aquac. Int. 2024, 32, 2791–2820. [Google Scholar] [CrossRef]
- Oliveira, J.; Barata, M.; Soares, F.; Pousão-Ferreira, P.; Oliva-Teles, A.; Couto, A. Machine Learning-Based Classification of Malnutrition Using Histological Biomarkers of Fish Intestine: Preliminary Data. J. Mar. Sci. Eng. 2024, 12, 2177. [Google Scholar] [CrossRef]
- Couto, A.; Barroso, C.; Guerreiro, I.; Pousão-Ferreira, P.; Matos, E.; Peres, H.; Oliva-Teles, A.; Enes, P. Carob seed germ meal in diets for meagre (Argyrosomus regius) juveniles: Growth, digestive enzymes, intermediary metabolism, liver and gut histology. Aquaculture 2016, 451, 396–404. [Google Scholar] [CrossRef]
- Urán, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Obach, A.; Jensen, L.; Koppe, W.; Verreth, J.A.J. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac. Nutr. 2008, 14, 324–330. [Google Scholar] [CrossRef]
- Nimalan, N.; Sørensen, S.L.; Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Gancarčíková, S.; Vatsos, I.N.; Bisa, S.; Kiron, V.; Sørensen, M. Mucosal barrier status in Atlantic salmon fed marine or plant-based diets supplemented with probiotics. Aquaculture 2022, 547, 737516. [Google Scholar] [CrossRef]
- Jang, W.J.; Lee, J.M.; Hasan, M.T.; Lee, B.-J.; Lim, S.G.; Kong, I.-S. Effects of probiotic supplementation of a plant-based protein diet on intestinal microbial diversity, digestive enzyme activity, intestinal structure, and immunity in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2019, 92, 719–727. [Google Scholar] [CrossRef]
- Bilal, S.; Ishtiaq, A.; Ghaffar, A.; Ishtiaq, T.; Naeem, M. Impact of in-feed Multispecies Probiotic Mixtures on Growth Patterns and Length-weight Relationships of Pangasianodon hypophthalmus. TSF J. Biol. 2025, 3, 27–39. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Bakr, A.; El-Keredy, A.M.S.; Dawood, M.A.O.; Dossou, S.; Wang, W.; Yukun, Z. Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol. 2018, 79, 303–312. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, C.-H.; Wang, S.-W.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Addo, S.; Carrias, A.A.; Williams, M.A.; Liles, M.R.; Terhune, J.S.; Davis, D.A. Effects of Bacillus subtilis Strains on Growth, Immune Parameters, and Streptococcus iniae Susceptibility in Nile Tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2017, 48, 257–267. [Google Scholar] [CrossRef]
- Di, J.; Chu, Z.; Zhang, S.; Huang, J.; Du, H.; Wei, Q. Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immunol. 2019, 93, 711–719. [Google Scholar] [CrossRef]
- Grosell, M.; Farrell, A.; Brauner, C. Fish Physiology: The Multifunctional Gut of Fish; Academic Press: Cambridge, MA, USA, 2010; Volume 30. [Google Scholar]
- Deng, J.; Zhang, X.; Sun, Y.; Mi, H.; Zhang, L. Effects of different types of non-starch polysaccharides on growth, digestive enzyme activity, intestinal barrier function and antioxidant activity of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2021, 21, 100864. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological Changes in Fish Gut in Response to Prebiotics and Probiotics Treatment to Improve Their Health Status: A Review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Olsen, R.E.; Myklebust, R.; Ringø, E.; Mayhew, T.M. The influences of dietary linseed oil and saturated fatty acids on caecal enterocytes in Arctic char (Salvelinus alpinus L.): A quantitative ultrastructural study. Fish Physiol. Biochem. 2000, 22, 207–216. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr.; Stryer, L. The Biosynthesis of Membrane Lipids and Steroids. In Biochemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2015. [Google Scholar]
- El-Son, M.A.M.; Elshopakey, G.E.; Rezk, S.; Eldessouki, E.A.A.; Elbahnaswy, S. Dietary mixed Bacillus strains promoted the growth indices, enzymatic profile, intestinal immunity, and liver and intestinal histomorphology of Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2022, 27, 101385. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Standen, B.; Rawling, M.; Davies, S.; Castex, M.; Foey, A.; Gioacchini, G.; Carnevali, O.; Merrifield, D. Probiotic Pediococcus acidilactici modulates both localised intestinal-and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2013, 35, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Aidos, L.; Mirra, G.; Pallaoro, M.; Herrera Millar, V.R.; Radaelli, G.; Bazzocchi, C.; Modina, S.C.; Di Giancamillo, A. How Do Alternative Protein Resources Affect the Intestine Morphology and Microbiota of Atlantic Salmon? Animals 2023, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M.Á. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 2013, 34, 1063–1070. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, R.; Wang, Z.; Qiang, L.; Yao, H. The effects of Bacillus subtilis on the immunity, mucosal tissue morphology, immune-related gene transcriptions, and intestinal microbiota in flounder (Paralichthys olivaceus) with two feeding methods: Continuous versus discontinuous feeding. Vet. Immunol. Immunopathol. 2024, 271, 110742. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-Y.; Zhang, H.-Q.; Chen, J.-X.; Zhu, J.; He, J.-Y.; Luo, L.; Lin, S.-M.; Chen, Y.-J. Effects of dietary Bacillus subtilis DSM 32315 supplementation on the growth, immunity and intestinal morphology, microbiota and inflammatory response of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 2119–2131. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Zhao, R.; Lu, T.; Li, S.; Wang, D. Effects of Dietary Supplementation with Endogenous Probiotics Bacillus subtilis on Growth Performance, Immune Response and Intestinal Histomorphology of Juvenile Rainbow Trout (Oncorhynchus mykiss). Fishes 2024, 9, 229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).