Locally Freezing Control via Superhydrophobic Patterns on Hydrophilic Substrates
Abstract
1. Introduction
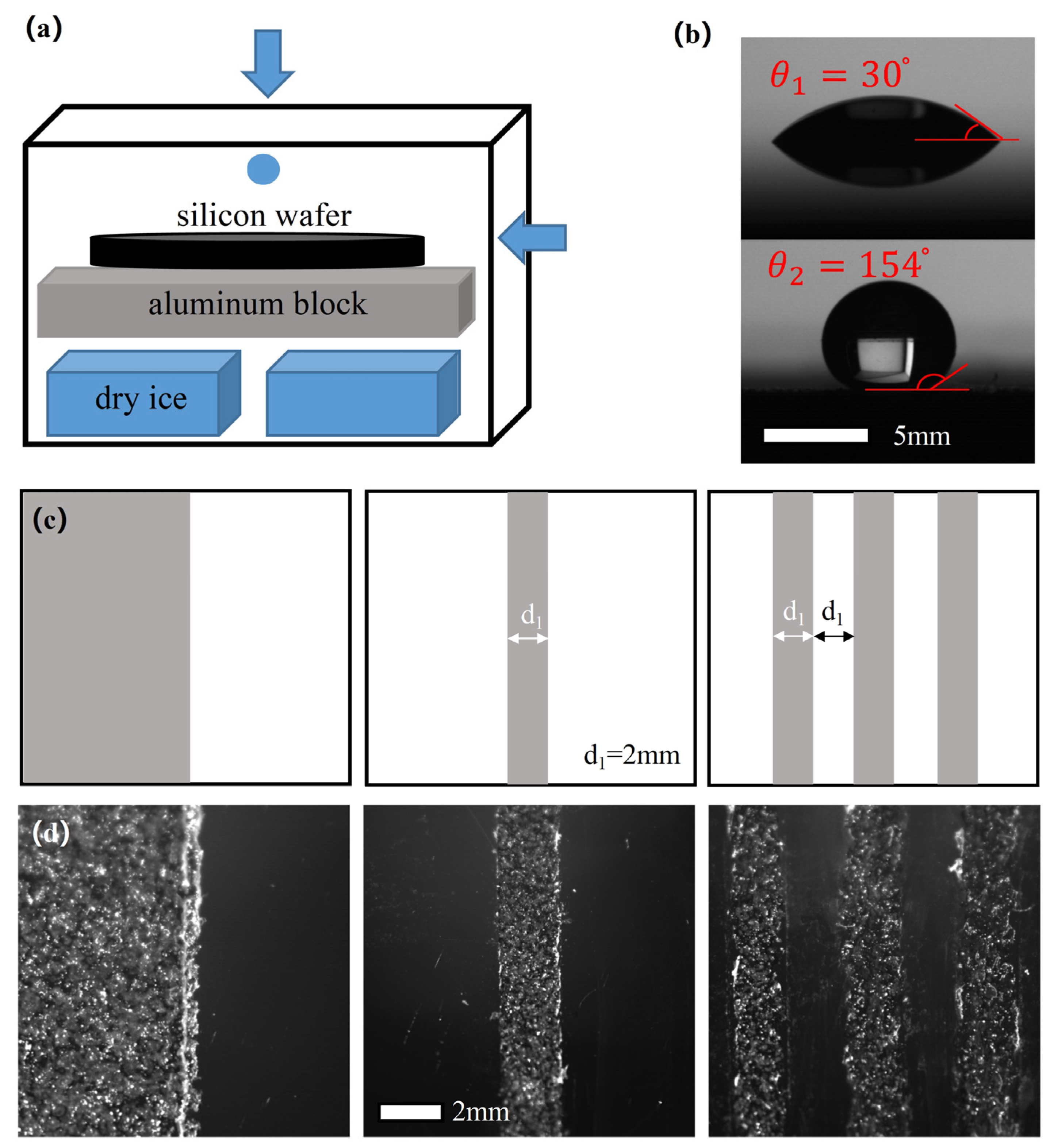
2. Materials and Methods
3. Results and Discussion
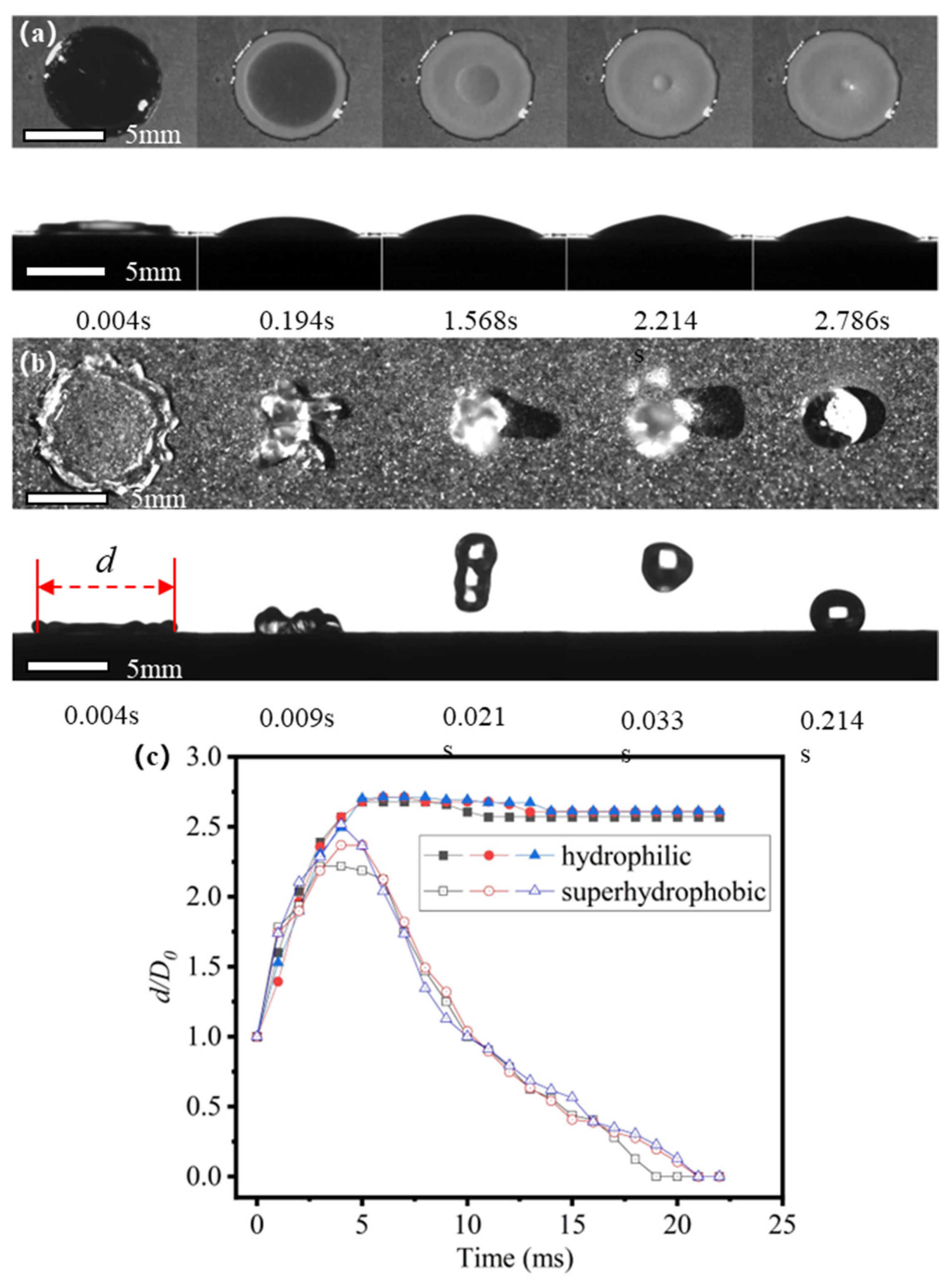
3.1. Freezing Process of an Impacting Droplet on Substrate with Uniform Wettability
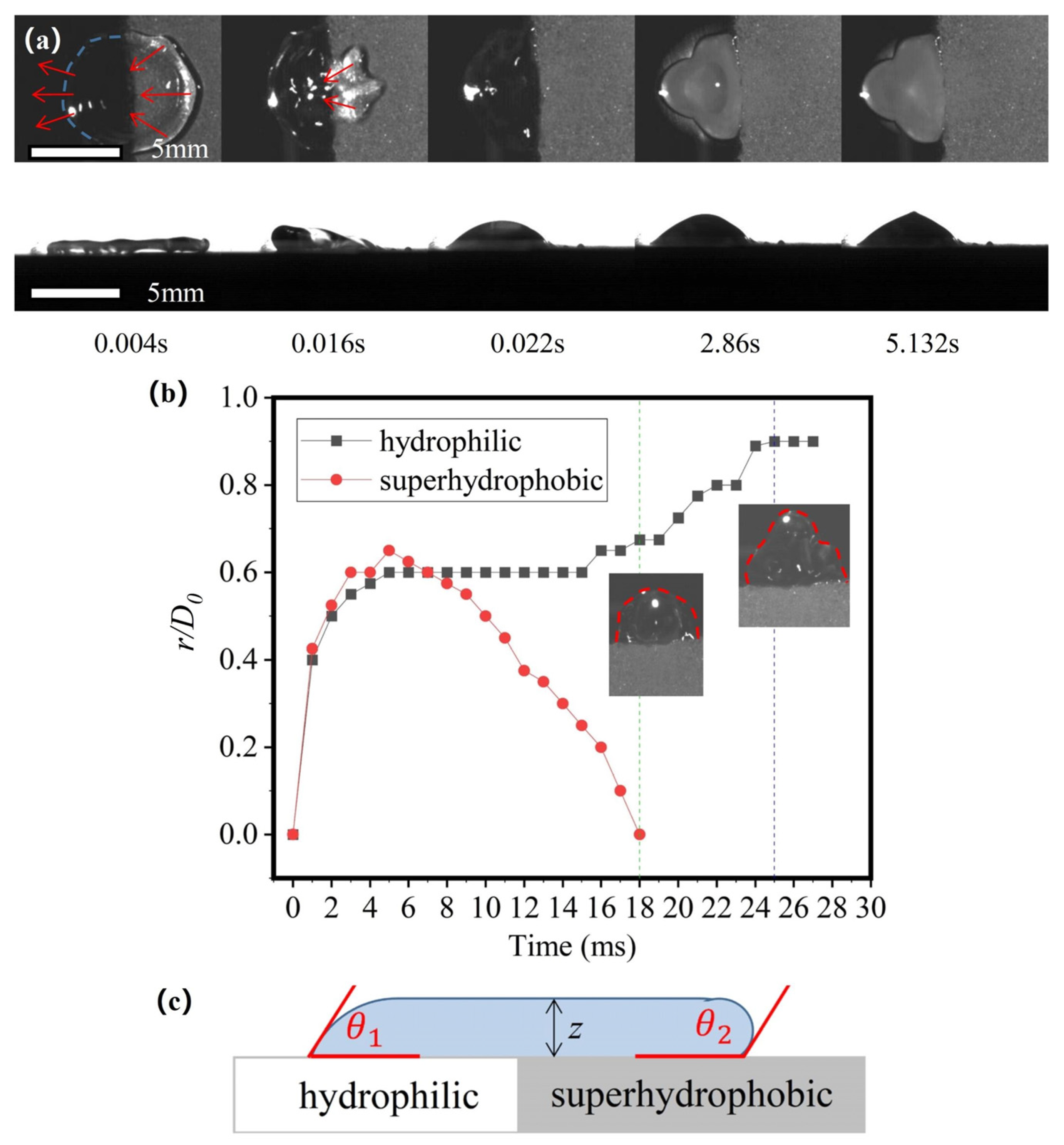
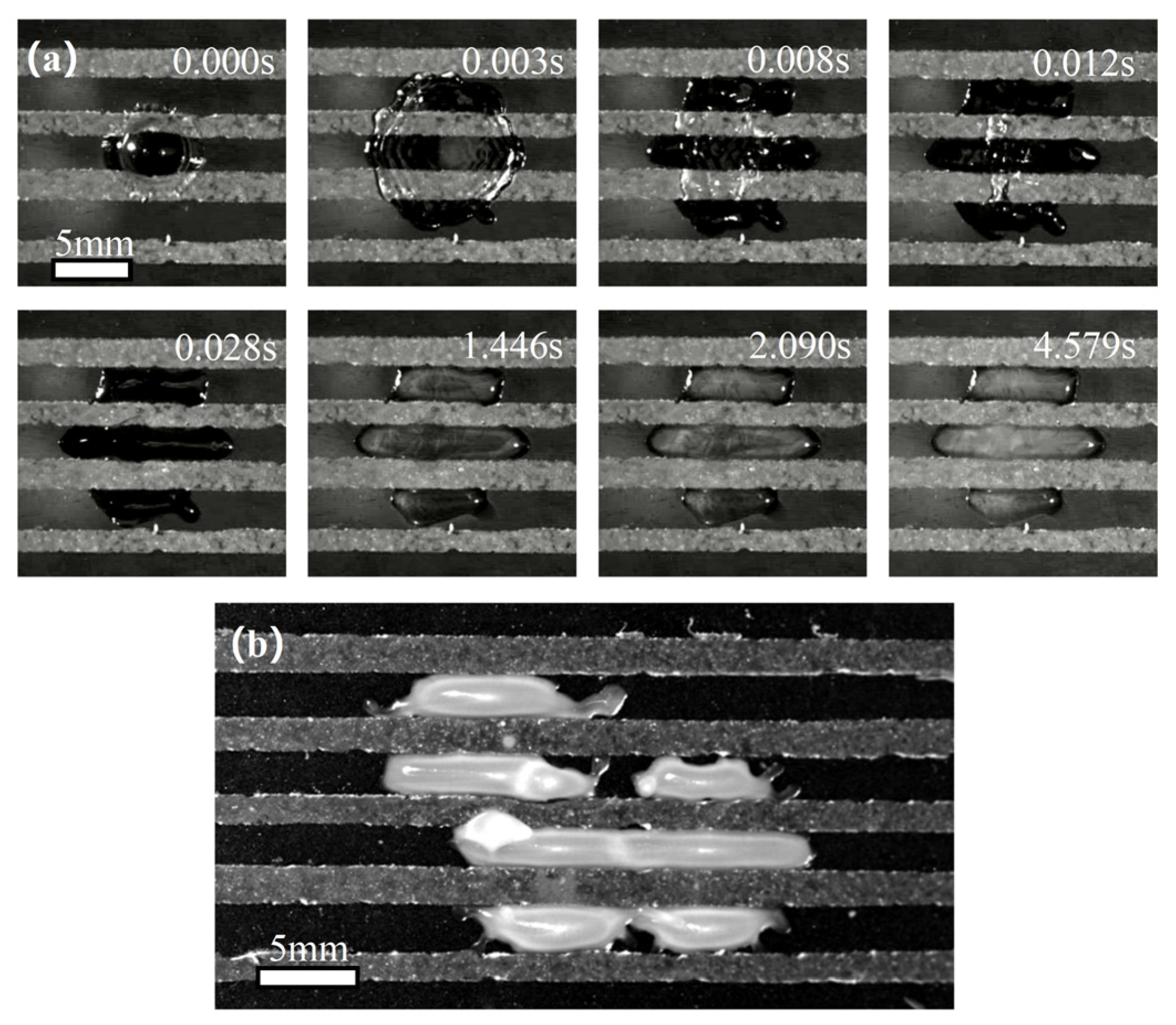
3.2. Using Superhydrophobic Patterns on a Hydrophilic Substrate to Control the Freezing Behavior
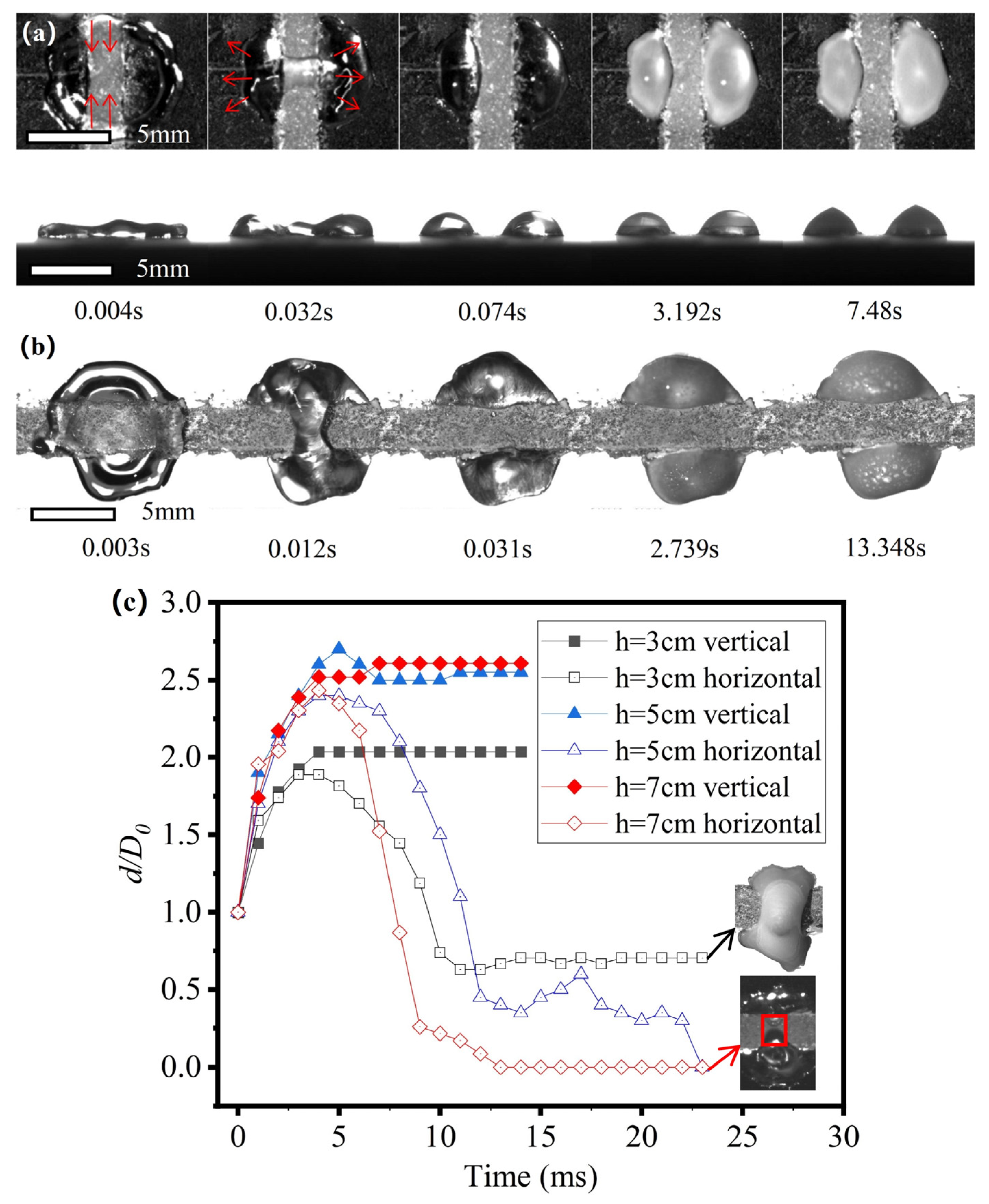
3.3. Failure of the Effect of the Hybrid Patterns on the Manipulation of the Freezing Process
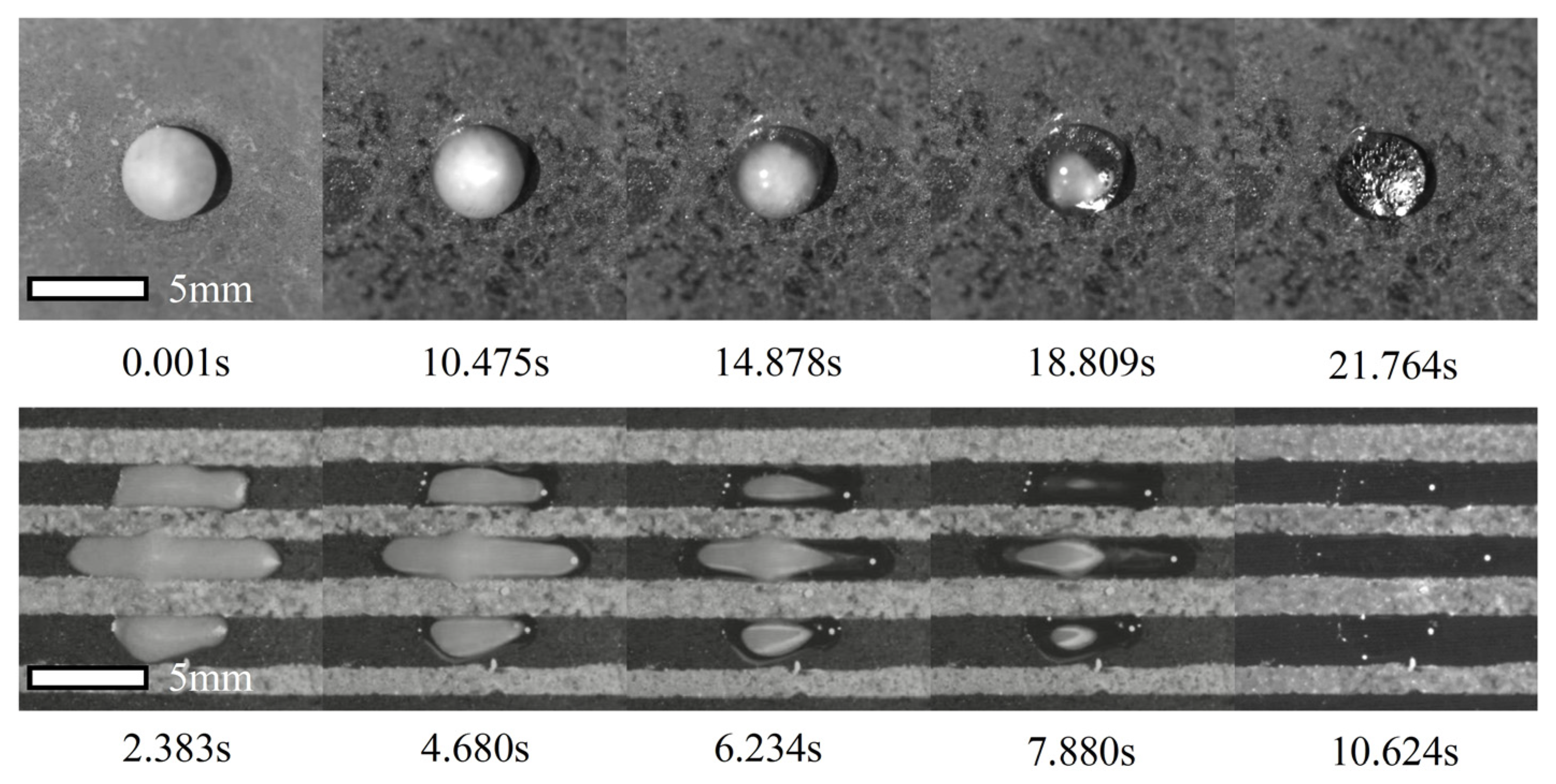
3.4. Enhancement of Deicing During the Melting Process on the Hybrid Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, D.; Xu, C.; Song, B.; Pan, G.; Hu, H.; Choi, C.-H. Splitting an Impacting Droplet by a Superhydrophobic Wire. Coatings 2022, 12, 1866. [Google Scholar] [CrossRef]
- Gent, R.W.; Dart, N.P.; Cansdale, J.T. Aircraft icing. Philos. Trans. R. Soc. Lond. Ser. A-Math. Phys. Eng. Sci. 2000, 358, 2873–2911. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Luo, G. Study on de-icing criterion of anti-icing coating and simulation analysis method of mechanical de-icing process for polar ship superstructure. Ocean Eng. 2023, 288, 115811. [Google Scholar] [CrossRef]
- Mintu, S.; Molyneux, D. Ice accretion for ships and offshore structures. Part 1-State of the art review. Ocean Eng. 2022, 258, 111501. [Google Scholar] [CrossRef]
- Shi, K.W.; Duan, X.L. A Review of Ice Protection Techniques for Structures in the Arctic and Offshore Harsh Environments. J. Offshore Mech. Arct. Eng.-Trans. ASME 2021, 143, 064502. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, R.; Yi, X. Research and development of anti-icing/deicing techniques for vessels: Review. Ocean Eng. 2022, 260, 112008. [Google Scholar] [CrossRef]
- Fu, J.G.; Liao, X.G.; Ji, Y.L.; Mo, Y.Q.; Zhang, J.F. Research Progress on Preparation of Superhydrophobic Surface and Its Application in the Field of Marine Engineering. J. Mar. Sci. Eng. 2024, 12, 1741. [Google Scholar] [CrossRef]
- Guo, C.; Liu, L.; Yang, R.; Lu, J.; Liu, S. Bouncing Regimes of Supercooled Water Droplets Impacting Superhydrophobic Surfaces with Controlled Temperature and Humidity. Langmuir 2023, 39, 10199–10208. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, K.; Duan, X.; Naterer, G.F. Experimental investigation of water droplet impact and freezing on micropatterned stainless steel surfaces with varying wettabilities. Int. J. Heat Mass Transf. 2019, 129, 953–964. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.D.; Pham, H.T.; Lam, S.S.; Vo, D.-V.N.; Shokouhimehr, M.; Vu, T.H.H.; Nguyen, T.-B.; Kim, S.Y.; Le, Q.V. Anti-icing performance on aluminum surfaces and proposed model for freezing time calculation. Sci. Rep. 2021, 11, 3641. [Google Scholar] [CrossRef]
- Fang, W.-Z.; Zhu, F.; Tao, W.-Q.; Yang, C. How different freezing morphologies of impacting droplets form. J. Colloid Interface Sci. 2021, 584, 403–410. [Google Scholar] [CrossRef]
- Merlen, A.; Brunet, P. Impact of Drops on Non-wetting Biomimetic Surfaces. J. Bionic Eng. 2009, 6, 330–334. [Google Scholar] [CrossRef]
- McDonald, B.; Patel, P.; Zhao, B. Droplet freezing and ice adhesion strength measurement on super-cooled hydrophobic surfaces. J. Adhes. 2015, 93, 375–388. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Srijanto, B.R.; Nguyen, T.D.; Vega, C.; Fuentes-Cabrera, M.; Collier, C.P. Dynamic Defrosting on Nanostructured Superhydrophobic Surfaces. Langmuir 2013, 29, 9516–9524. [Google Scholar] [CrossRef]
- Gurumukhi, Y.; Chavan, S.; Sett, S.; Boyina, K.; Ramesh, S.; Sokalski, P.; Fortelka, K.; Lira, M.; Park, D.; Chen, J.-Y.; et al. Dynamic Defrosting on Superhydrophobic and Biphilic Surfaces. Matter 2020, 3, 1178–1195. [Google Scholar] [CrossRef]
- Adanur, B.; Cremaschi, L.; Harges, E. Effect of mixed hydrophilic and hydrophobic surface coatings on droplets freezing and subsequent frost growth during air forced convection channel flows. Sci. Technol. Built Environ. 2019, 25, 1302–1312. [Google Scholar] [CrossRef]
- Van Dyke, A.S.; Collard, D.; Derby, M.M.; Betz, A.R. Droplet coalescence and freezing on hydrophilic, hydrophobic, and biphilic surfaces. Appl. Phys. Lett. 2015, 107, 141602. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Zhan, B.; Li, S.; Han, Z.; Liu, Y. A Novel Icephobic Strategy: The Fabrication of Biomimetic Coupling Micropatterns of Superwetting Surface. Adv. Mater. Interfaces 2019, 6, 1900864. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Zhu, X.; Ding, Y.D.; Chen, R.; Liao, Q. Droplet splitting on chemically striped surface. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 537, 139–148. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Zhu, X.; Ding, Y.D.; Liao, Q. Chemically striped surface accelerate the droplet evaporation. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 612, 125994. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Qin, X.Z.; Chen, T.K.; Jin, J.F.; Liu, C.Z.; Wang, M.Q. Research Progress of Superhydrophobic Materials in the Field of Anti-/De-Icing and Their Preparation: A Review. Materials 2023, 16, 5151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Moevius, L.; Xu, X.P.; Qian, T.Z.; Yeomans, J.M.; Wang, Z.K. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.A.; Song, D.; Swarctz, C.; Lee, J.; Choi, C.H. Anti-Icing or Deicing: Icephobicities of Superhydrophobic Surfaces with Hierarchical Structures. Langmuir 2018, 34, 13821–13827. [Google Scholar] [CrossRef]
- Song, D.; Liu, X.; Wang, X.; Du, X.X.; Hu, H.B. Experimental Investigation on the Droplet Stability of Superhydrophobic Mesh. Coatings 2023, 13, 756. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Zhang, J.; Xu, C.; Wang, X.; Huang, S.; Ye, P. Locally Freezing Control via Superhydrophobic Patterns on Hydrophilic Substrates. J. Mar. Sci. Eng. 2025, 13, 1009. https://doi.org/10.3390/jmse13061009
Song D, Zhang J, Xu C, Wang X, Huang S, Ye P. Locally Freezing Control via Superhydrophobic Patterns on Hydrophilic Substrates. Journal of Marine Science and Engineering. 2025; 13(6):1009. https://doi.org/10.3390/jmse13061009
Chicago/Turabian StyleSong, Dong, Jiacheng Zhang, Changsheng Xu, Xiang Wang, Sihan Huang, and Pengcheng Ye. 2025. "Locally Freezing Control via Superhydrophobic Patterns on Hydrophilic Substrates" Journal of Marine Science and Engineering 13, no. 6: 1009. https://doi.org/10.3390/jmse13061009
APA StyleSong, D., Zhang, J., Xu, C., Wang, X., Huang, S., & Ye, P. (2025). Locally Freezing Control via Superhydrophobic Patterns on Hydrophilic Substrates. Journal of Marine Science and Engineering, 13(6), 1009. https://doi.org/10.3390/jmse13061009




