Ecological Responses of Microbially Activated Water Flooding in Ultra-Low-Permeability Reservoirs: A Case Study of the B9 Reservoir in the Triassic Yanchang Formation
Abstract
1. Introduction
2. Materials and Methods
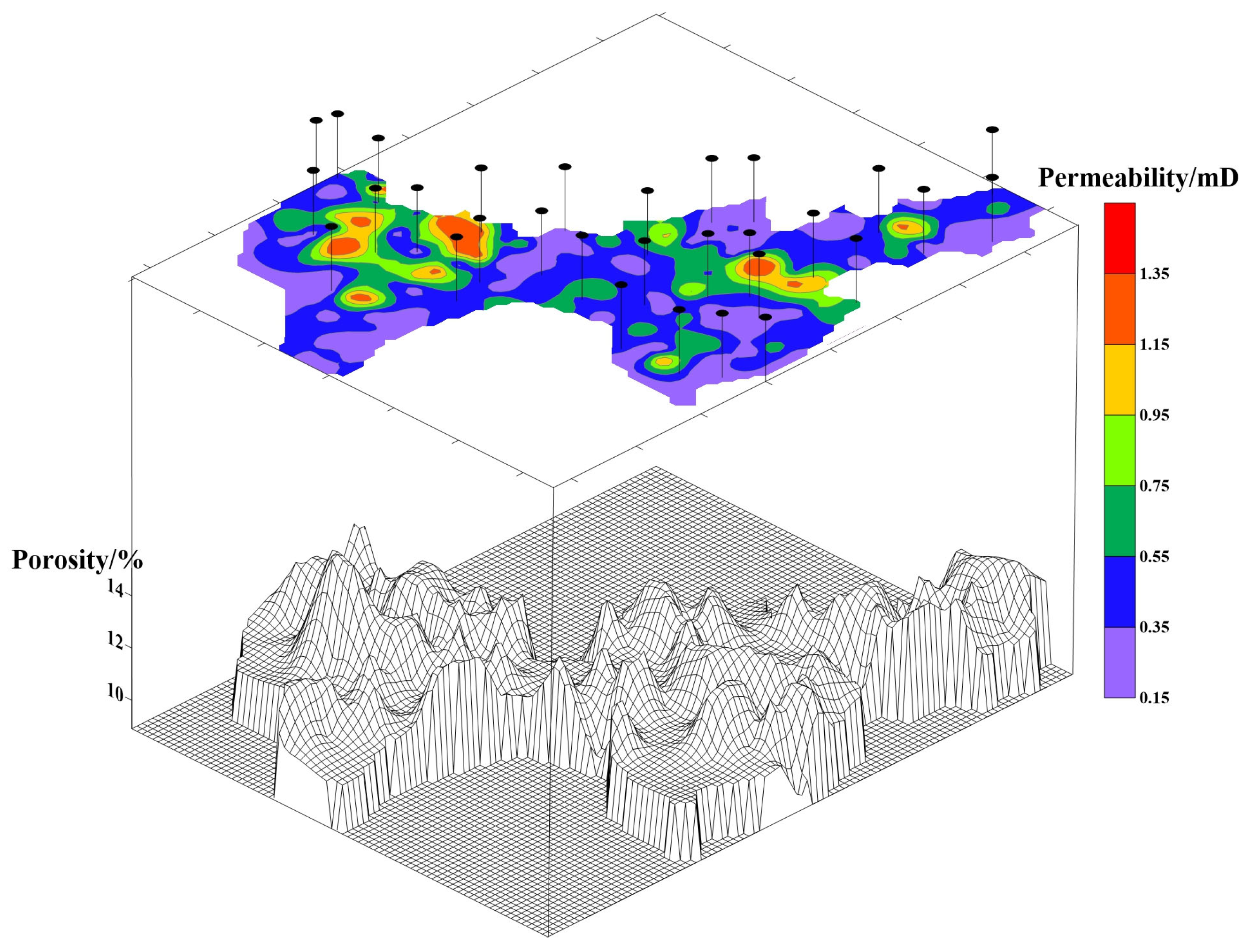
2.1. Field Test Area
2.2. Field Test Methods
- ①
- The aqueous-phase of the produced fluid first enters a buffer tank for flow regulation, quality homogenization, and preliminary oil removal.
- ②
- Subsequently, it flows into a cooling tower, where mechanical ventilation controls the inlet water temperature.
- ③
- Then, it enters an oil removal tank for further oil separation through sedimentation.
- ④
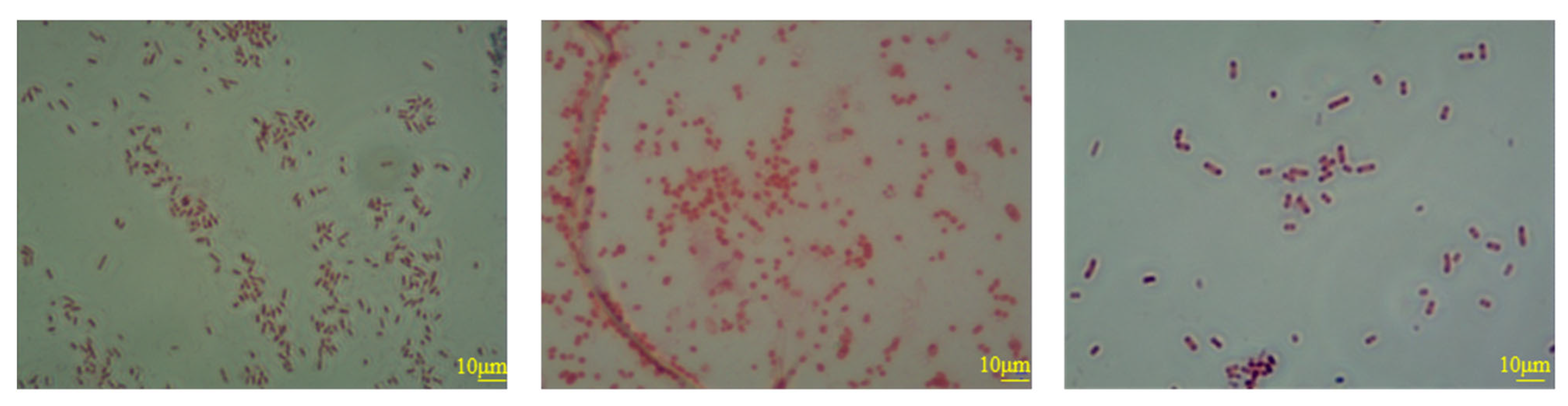
- Next, it passes through the bioreactor system, and Pseudomonas aeruginosa is introduced into the primary reaction tank, while Bacillus cereus and Acinetobacter lwoffii are added to the secondary and tertiary reaction tanks, enabling the progressive enrichment of bacterial cells and their metabolic byproducts. The strains of P. aeruginosa, B. cereus, and A. lwoffii are L1SHX-6X, J2SHX-9X, and L2SHX-11X, respectively (all aerobic strains; Figure 4). They were isolated from oil reservoirs and identified by the Research Centre for Geomicrobial Resources and Application, China University of Petroleum (Beijing), China. The culture medium of all three reaction tanks contains yeast extract (0.5 g/L), tryptone (0.25 g/L), peptone (0.75 g/L), glucose (0.5 g/L), soluble starch (0.5 g/L), dipotassium hydrogen phosphate (0.3 g/L), magnesium sulfate (0.024 g/L), and sodium pyruvate (0.3 g/L). The reaction tanks are sealed vessels where oxygen is replenished by pumping filtered air into the system using an aeration blower, and daily nutrient supplementation maintains the aforementioned concentration of the culture medium (Figure 3). Continuous monitoring for microbial contamination is implemented; when the contamination exceeds 37%, the culture medium is purged and replaced. All culture medium components used in this step were industrial-grade and purchased from Hebei Jinmaiwei Feed Technology Co., Ltd, Shijiazhuang, China.
- ⑤
- The product proceeds to a high-efficiency sedimentation tank for impurity removal via gravity settling.
- ⑥
- Finally, the microbially activated water is filtered and transferred to a water tank for injection.

2.3. Measurement of Production Parameters
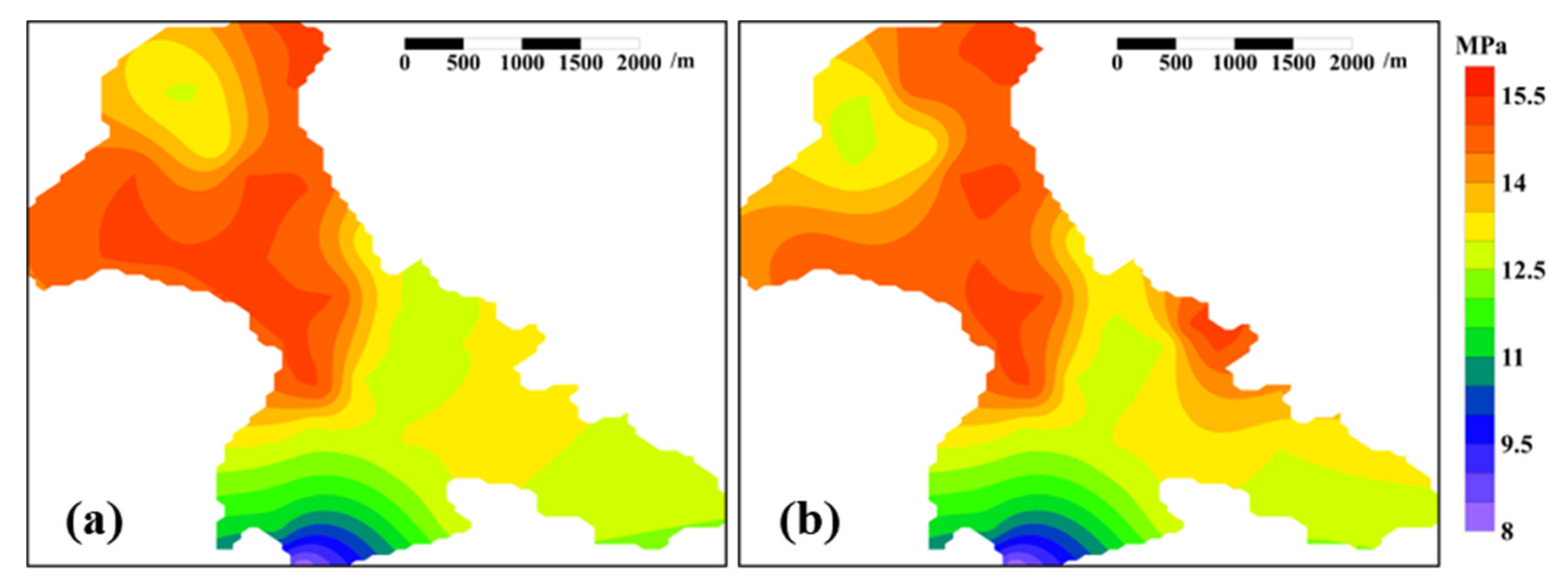
2.4. Measurements of Reservoir’s Environmental Parameters
2.5. Microbial Analysis
3. Results and Discussion
3.1. The Production Performance of Microbially Activated Water Flooding
3.2. The Impact of Microbially Activated Water Flooding on the Reservoir Environment
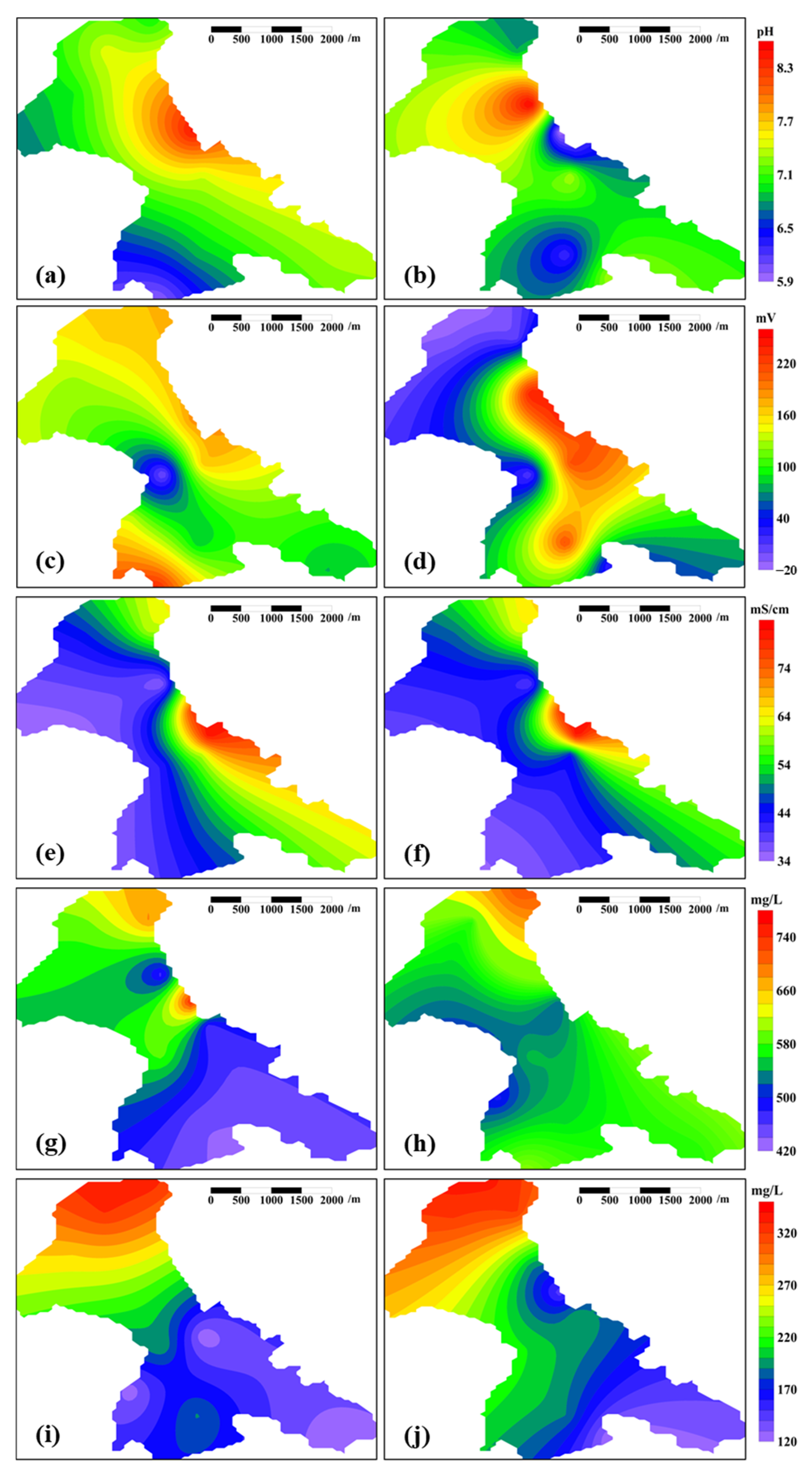
3.2.1. pH
3.2.2. Redox Potential
3.2.3. Conductivity
3.2.4. COD
3.2.5. BOD
3.3. The Impact of Microbially Activated Water Flooding on the Reservoir Microorganisms
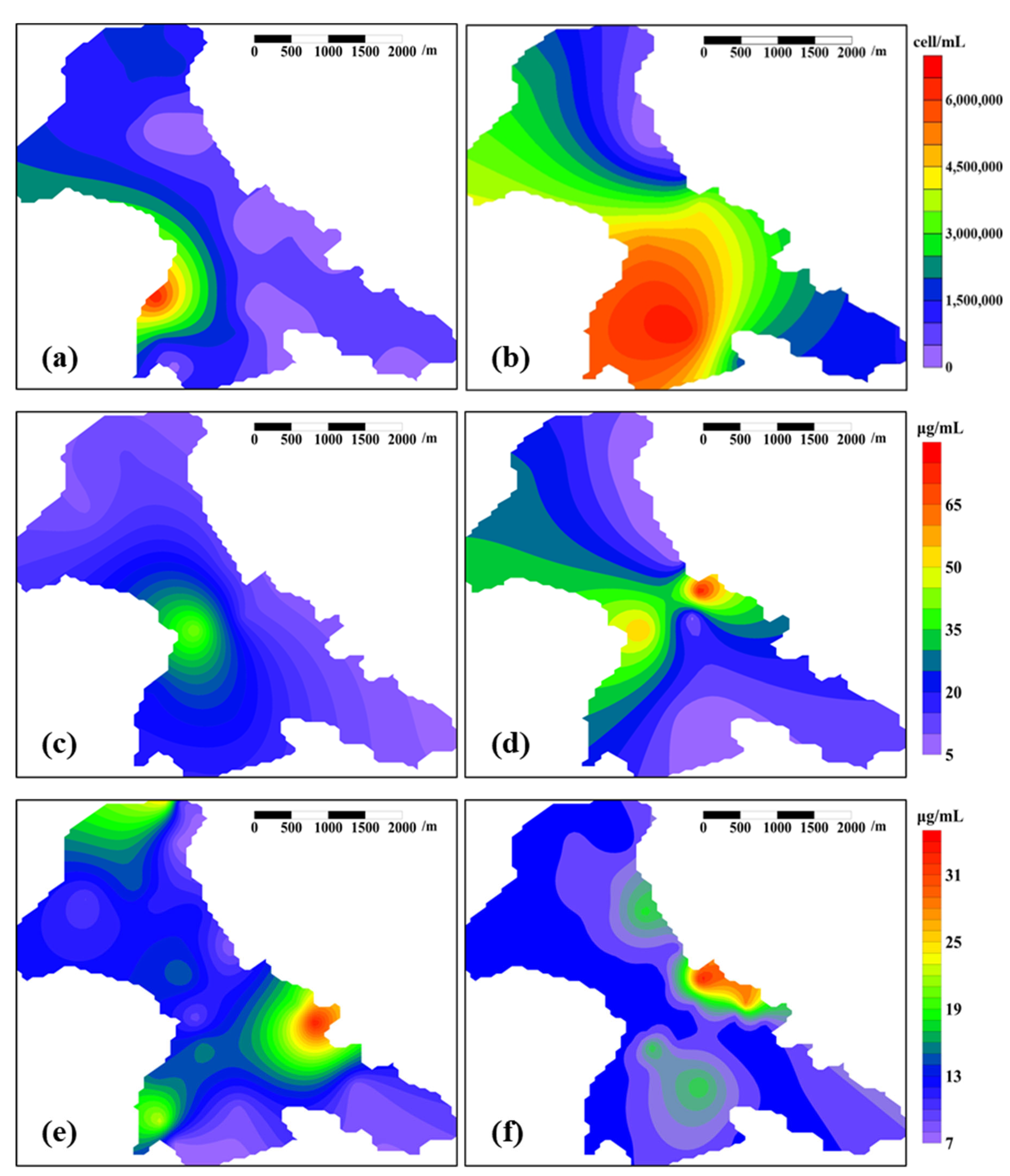
3.3.1. Microbial Biomass
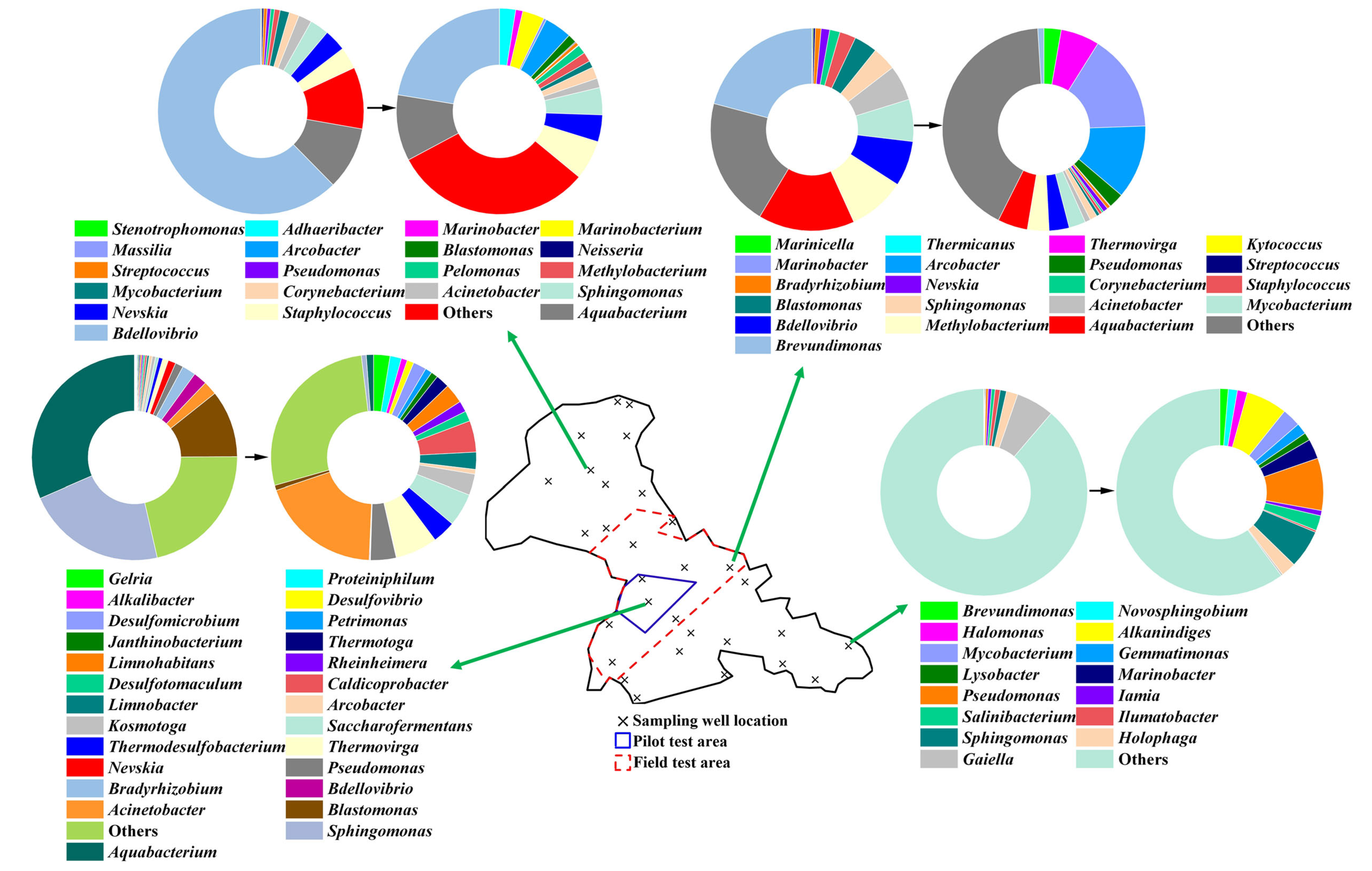
3.3.2. Microbial Species Composition
3.4. The Effects of Reservoir’s Ecological System on Oilfield Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.R. Microbial enhanced oil recovery (MEOR). Curr. Opin. Microbiol. 2010, 13, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, J.; Han, H.; Sun, G.; Song, Z. High-pressure microscopic investigation on the oil recovery mechanism by in situ biogases in petroleum reservoirs. Energy Fuels 2015, 29, 7866–7874. [Google Scholar] [CrossRef]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Cui, K.; Wang, C.J.; Li, L.; Zou, J.G.; Huang, W.H.; Zhang, Z.Z.; Wang, H.M.; Guo, K. Controlling the hydro-swelling of smectite clay minerals by Fe(III) reducing bacteria for enhanced oil recovery from low-permeability reservoirs. Energies 2022, 15, 4393. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Shi, R.; Han, S.; Ma, F.; Zhang, Y. Production of biosurfactant by a Pseudomonas aeruginosa isolate and its applicability to in situ microbial enhanced oil recovery under anoxic conditions. RSC Adv. 2015, 5, 36044–36050. [Google Scholar] [CrossRef]
- Gudina, E.J.; Pereira, J.F.B.; Rodrigues, L.R.; Coutinho, J.A.P.; Teixeira, J.A. Isolation and study of microorganisms from oil samples for application in Microbial Enhanced Oil Recovery. Int. Biodeterior. Biodegrad. 2012, 68, 56–64. [Google Scholar] [CrossRef]
- Gudina, E.J.; Pereira, J.F.B.; Costa, R.; Coutinho, J.A.P.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. J. Hazard. Mater. 2013, 261, 106–113. [Google Scholar] [CrossRef]
- Fernandes, P.L.; Rodrigues, E.M.; Paiva, F.R.; Ayupe, B.A.L.; McInerney, M.J.; Totola, M.R. Biosurfactant, solvents and polymer production by Bacillus subtilis RI4914 and their application for enhanced oil recovery. Fuel 2016, 180, 551–557. [Google Scholar] [CrossRef]
- Sivasankar, P.; Kumar, G.S. Modelling the influence of interaction between injection and formation brine salinities on in-situ microbial enhanced oil recovery processes by coupling of multiple-ion exchange transport model with multiphase fluid flow and multi-species reactive transport models. J. Pet. Sci. Eng. 2018, 163, 435–452. [Google Scholar]
- SY/T 6219-2023; China National Energy Administration Oilfield Development Level Classification. Petroleum Industry Press: Beijing, China, 2023.
- Tan, F.; Zhao, R.; Zhao, Y.; Pan, Z.; Li, H. A case study: Evaluating low-porosity and ultra-low-permeability Triassic reservoir rocks in the Ordos Basin by the integration of logs and core. Pet. Geosci. 2017, 23, 454–465. [Google Scholar] [CrossRef]
- Yao, C.; Lei, G.; Ma, J.; Zhao, F.; Cao, G. Laboratory experiment, modeling and field application of indigenous microbial flooding. J. Pet. Sci. Eng. 2012, 90–91, 39–47. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.L.; Yu, L.; Huang, L.X.; Yi, L.N.; Ma, Y.D. Biosurfactant production by Bacillus subtilis SL and its potential for enhanced oil recovery in low permeability reservoirs. Sci. Rep. 2022, 12, 7785. [Google Scholar] [CrossRef] [PubMed]
- Ghojavand, H.; Vahabzadeh, F.; Shahraki, A.K. Enhanced oil recovery from low permeability dolomite cores using biosurfactant produced by a Bacillus mojavensis (PTCC 1696) isolated from Masjed-I Soleyman field. J. Pet. Sci. Eng. 2012, 81, 24–30. [Google Scholar] [CrossRef]
- Seok, O.K. Brief review on microbial enhanced oil recovery. J. Korean Appl. Sci. Technol. 2021, 38, 1010–1019. [Google Scholar]
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Hajfarajollah, H.; Noghabi, K.A.; Manteghian, M. Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process. RSC Adv. 2019, 9, 7932–7947. [Google Scholar] [CrossRef]
- Zhu, H.; Carlson, H.K.; Coates, J.D. Applicability of anaerobic nitrate-dependent Fe(II) oxidation to Microbial Enhanced Oil Recovery (MEOR). Environ. Sci. Technol. 2013, 47, 8970–8977. [Google Scholar] [CrossRef]
- Kwon, T.; Ajo-Franklin, J.B. High-frequency seismic response during permeability reduction due to biopolymer clogging in unconsolidated porous media. Geophysics 2013, 78, 117–127. [Google Scholar] [CrossRef]
- Zekri, A.Y. Microbial enhanced oil recovery—A short review. Oil Gas Eur. Mag. 2001, 27, 22–25. [Google Scholar]
- You, J.; Wu, G.; Ren, F.; Chang, Q.; Yu, B.; Xue, Y.; Mu, B. Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl. Microbiol. Biotechnol. 2016, 100, 1469–1478. [Google Scholar] [CrossRef]
- Gao, P.; Tian, H.; Li, G.; Sun, H.; Ma, T. Microbial diversity and abundance in the Xinjiang Luliang long-term water-flooding petroleum reservoir. Microbiologyopen 2015, 4, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Nazina, T.N.; Feng, Q.; Kostryukova, N.K.; Shestakova, N.M.; Babich, T.L.; Ni, F.; Wang, J.; Min, L.; Ivanov, M.V. Microbiological and production characteristics of the Dagang high-temperature heavy oil reservoir (Block no. 1) during trials of the biotechnology for enhanced oil recovery. Microbiology 2017, 86, 653–665. [Google Scholar] [CrossRef]
- Halim, A.Y.; Pedersen, D.S.; Nielsen, S.M.; Lantz, A.E. Profiling of indigenous microbial community dynamics and metabolic activity during enrichment in molasses-supplemented crude oil-brine mixtures for improved understanding of microbial enhanced oil recovery. Appl. Biochem. Biotechnol. 2015, 176, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, T.; Zhao, L.; Lv, J.; Li, G.; Zhang, H.; Zhao, B.; Liang, F.; Liu, R. Monitoring exogenous and indigenous bacteria by PCR-DGGE technology during the process of microbial enhanced oil recovery. J. Ind. Microbiol. Biotechnol. 2008, 35, 619–628. [Google Scholar] [CrossRef]
- Xiang, T.; Liu, X.; Zhang, M.; Liu, F.; Li, B.; Fu, H.; Zhao, L.; Hu, W. Distribution of the indigenous microorganisms and mechanisms of their orientational activation in Daqing Oilfield. Sci. China Ser. D Earth Sci. 2009, 52, 128–134. [Google Scholar] [CrossRef]
- Astuti, D.I.; Purwasena, I.A.; Priharto, N.; Ariadji, T.; Afifah, L.N.; Saputro, R.B.; Aditiawati, P.; Persada, G.P.; Ananggadipa, A.A.; Abqory, M.H.; et al. Bacterial community dynamics during MEOR biostimulation of an oil reservoir in sumatera Indonesia. J. Pet. Sci. Eng. 2022, 208, 109558. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Qi, G.; Hou, Z.; Liu, Y.; Shou, L.; Zhou, L.; Yang, S.; Wu, X.; Gu, J.; et al. Microbiome changes and characteristics under nutrient injection for enhanced oil production at Daqing oilfield. Int. Biodeterior. Biodegrad. 2025, 196, 105934. [Google Scholar] [CrossRef]
- Alkan, H.; Mukherjee, S.; Kogler, F. Reservoir engineering of in-situ MEOR; impact of microbial community. J. Pet. Sci. Eng. 2020, 195, 107928. [Google Scholar] [CrossRef]
- Cheng, W.; Fan, H.; Yun, Y.; Zhao, X.; Su, Z.; Tian, X.; Liu, D.; Ma, T.; Li, G. Effects of nutrient injection on the Xinjiang oil field microbial community studied in a long core flooding simulation device. Front. Microbiol. 2023, 14, 1230274. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, L.; Mbadinga, S.M.; You, J.; Yang, H.; Liu, J.; Yang, S.; Gu, J.; Mu, B. Activation of CO2-reducing methanogens in oil reservoir after addition of nutrient. J. Biosci. Bioeng. 2016, 122, 740–747. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, B.; Cui, Q.; Wu, Y. Genetically modified indigenous Pseudomonas aeruginosa drove bacterial community to change positively toward microbial enhanced oil recovery applications. J. Appl. Microbiol. 2024, 135, lxae168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, T.; Gao, M.; Gao, P.; Cao, M.; Zhu, X.; Li, G. Characterization of microbial diversity and community in water flooding oil reservoirs in China. World J. Microbiol. Biotechnol. 2012, 28, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Bodtker, G.; Lysnes, K.; Torsvik, T.; Bjornestad, E.O.; Sunde, E. Microbial analysis of backflowed injection water from a nitrate-treated North Sea oil reservoir. J. Ind. Microbiol. Biotechnol. 2009, 36, 439–450. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, X.; Song, Z.; Rupert, W.; Gao, G.; Guo, S.; Zhao, L. Comparison of microbial community compositions of injection and production well samples in a long-term water-flooded petroleum reservoir. PLoS ONE 2011, 6, e23258. [Google Scholar] [CrossRef]
- Akhmetov, I.L.; Puntus, I.F.; Narmanova, R.A.; Appazov, N.O.; Funtikova, V.T.; Regepova, A.A.; Filonov, A.E. Recent advances in creating biopreparations to fight oil spills in soil ecosystems in sharply continental climate of Republic of Kazakhstan. Processes 2022, 10, 549. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Gabhane, L.R.; Kanidarapu, N. Environmental risk assessment using neural network in liquefied petroleum gas terminal. Toxics 2023, 11, 348. [Google Scholar] [CrossRef]
- Wang, M.; Sha, C.; Wu, J.; Su, J.; Wu, J.; Wang, Q.; Tan, J.; Huang, S. Bacterial community response to petroleum contamination in brackish tidal marsh sediments in the Yangtze River Estuary, China. J. Environ. Sci. 2021, 99, 160–167. [Google Scholar] [CrossRef]
- White, H.K.; Hsing, P.; Cho, W.; Shank, T.M.; Cordes, E.E.; Quattrini, A.M.; Nelson, R.K.; Camilli, R.; Demopoulos, A.W.J.; German, C.R.; et al. Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. Proc. Natl. Acad. Sci. USA 2012, 109, 20303–20308. [Google Scholar] [CrossRef]
- Aguilera, F.; Mendez, J.; Pasaro, E.; Laffon, B. Review on the effects of exposure to spilled oils on human health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef]
- Jerneloev, A. The threats from oil spills: Now, Then, and in the Future. Ambio 2010, 39, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.G.; Vivian, D.N.; Heintz, R.A.; Yim, U.H. Long-term ecological impacts from oil spills: Comparison of Exxon Valdez, Hebei Spirit, and Deepwater Horizon. Environ. Sci. Technol. 2020, 54, 6456–6467. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.; Ramirez-Sabag, J. Analytical model for tracer transport in reservoirs having a conductive geological fault. J. Pet. Sci. Eng. 2008, 62, 73–79. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, M.; Wang, J.; Zong, C. Performance and gas breakthrough during CO2 immiscible flooding in ultra-low permeability reservoirs. Petroleum Explor. Dev. 2014, 41, 88–95. [Google Scholar] [CrossRef]
- Pemper, R. A history of nuclear spectroscopy in well logging. Petrophysics 2020, 61, 523–548. [Google Scholar] [CrossRef]
- Ouyang, L.B. A novel flow profiling approach using permanent downhole pressure gauges. Pet. Sci. Technol. 2005, 23, 1221–1245. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Li, L.; Wan, Y.Y.; Li, Z.G.; Luo, N.; Mu, H.M.; Li, W.H.; Zhang, Y. Interaction between in-situ oil reservoir microorganisms and minerals. J. China Univ. Pet. (Ed. Nat. Sci.) 2021, 45, 121–130. [Google Scholar]
- Li, J.; Tao, T.; Li, X.; Zuo, J.; Li, T.; Lu, J.; Li, S.; Chen, L.; Xia, C.; Liu, Y.; et al. A spectrophotometric method for determination of chemical oxygen demand using home-made reagents. Desalination 2009, 239, 139–145. [Google Scholar] [CrossRef]
- HJ 828-2017; National Ministry of Environmental Protection Water Quality—Determination of the Chemical Oxygen Demand—Dichromate Method. China Environmental Science Press: Beijing, China, 2017.
- HJ 505-2009; National Ministry of Environmental Protection Water Quality—Determination of Biochemical Oxygen Demand After 5 Days (BOD5) for Dilution and Seeding Method. China Environmental Science Press: Beijing, China, 2009.
- Wan, Y.Y.; Dong, H.L. Environmental Geomicrobiology Experiments; Petroleum Industry Press: Beijing, China, 2014; pp. 100–105. [Google Scholar]
- Whitehouse, C.A.; Hottel, H.E. Comparison of five commercial DNA extraction kits for the recovery of Francisella tularensis DNA from spiked soil samples. Mol. Cell. Probes 2007, 21, 92–96. [Google Scholar] [CrossRef]
- Schofield, G.G. PicoGmeter, a custom-made fluorometer for the quantification of dsDNA by PicoGreen® fluorescence. Biotechniques 2004, 37, 778. [Google Scholar] [CrossRef]
- Yoshida, N.; Yagi, K.; Sato, D.; Watanabe, N.; Kuroishi, T.; Nishimoto, K.; Yanagida, A.; Katsuragi, T.; Kanagawa, T.; Kurane, R.; et al. Bacterial communities in petroleum oil in stockpiles. J. Biosci. Bioeng. 2005, 99, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, J.; Cottrill, M.; Yu, H.; de Lange, C.; Burton, J.; Topp, E. Evaluation of QIAamp® DNA Stool Mini Kit for ecological studies of gut microbiota. J. Microbiol. Methods 2003, 54, 13–20. [Google Scholar] [CrossRef]
- Krakova, L.; Soltys, K.; Otlewska, A.; Pietrzak, K.; Purkrtova, S.; Savicka, D.; Puskarova, A.; Buckova, M.; Szemes, T.; Budis, J.; et al. Comparison of methods for identification of microbial communities in book collections: Culture-dependent (sequencing and MALDI-TOF MS) and culture-independent (Illumina MiSeq). Int. Biodeterior. Biodegrad. 2018, 131, 51–59. [Google Scholar] [CrossRef]
- Goecks, J.; Nekrutenko, A.; Taylor, J. Galaxy Team Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010, 11, R86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shouyi, L.; Lingzhi, H.; Yaolong, C. Visualization programming for batch processing of contour maps based on VB and Surfer software. Adv. Eng. Softw. 2010, 41, 962–965. [Google Scholar]
- Brown, L.R.; Vadie, A.A.; Stephens, J.O. Sowing production decline and extending the economic life of an oil field: New MEOR technology. SPE Reserv. Eval. Eng. 2002, 5, 33–41. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Tian, C.; Shi, C.; Li, J.; Hui, G.; Hou, J.; Gao, C.; Wang, X.; Liu, P. Dynamic fractures are an emerging new development geological attribute in water-flooding development of ultra-low permeability reservoirs. Pet. Explor. Dev. 2015, 42, 247–253. [Google Scholar] [CrossRef]
- Hidalgo, K.J.; Sierra-Garcia, I.N.; Dellagnezze, B.M.; de Oliveira, V.M. Metagenomic insights into the mechanisms for biodegradation of polycyclic aromatic hydrocarbons in the oil supply chain. Front. Microbiol. 2020, 11, 561506. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wu, M.; Jiang, C.; Chen, X.; Cai, Z.; Wang, B.; Zhang, J.; Zhang, T.; Li, Z. Soil pH rather than nutrients drive changes in microbial community following long-term fertilization in acidic Ultisols of southern China. J. Soils Sediments 2018, 18, 1853–1864. [Google Scholar] [CrossRef]
- Oktyabrskii, O.N.; Smirnova, G.V. Redox potential changes in bacterial cultures under stress conditions. Microbiology 2012, 81, 131–142. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ho, M.S.; You, L.; Cao, J.; Fang, Y.; Tu, T.; Hong, Y. Industrial water pollution discharge taxes in China: A multi-sector dynamic analysis. Water 2018, 10, 1742. [Google Scholar] [CrossRef]
- Tian, W.; Yao, J.; Liu, R.; Zhu, M.; Wang, F.; Wu, X.; Liu, H. Effect of natural and synthetic surfactants on crude oil biodegradation by indigenous strains. Ecotoxicol. Environ. Saf. 2016, 129, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Koda, E.; Miszkowska, A.; Sieczka, A. Levels of organic pollution indicators in groundwater at the old landfill and waste management site. Appl. Sci. 2017, 7, 638. [Google Scholar] [CrossRef]
- Duhamel, S. The microbial phosphorus cycle in aquatic ecosystems. Nat. Rev. Microbiol. 2024, 23, 239–255. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, K.; Wang, L.; Liu, J.; Yang, S.; Gu, J.; Mu, B.Z. Different diversity and distribution o archaeal community in the aqueous and oil phases of production fluid from high-temperature petroleum reservoirs. Front. Microbiol. 2018, 9, 841. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Zhang, Y.; Han, S.; Xu, H. Sphingomonas from petroleum-contaminated soils in Shenfu, China and their PAHs degradation abilities. Braz. J. Microbiol. 2016, 47, 271–278. [Google Scholar] [CrossRef]
- Mutyala, S.; Kim, C.; Song, Y.E.; Khandelwal, H.; Baek, J.; Seol, E.; Oh, Y.; Kim, J.R. Enabling anoxic acetate assimilation by electrode-driven respiration in the obligate aerobe, Pseudomonas putida. Bioelectrochemistry 2021, 138, 107690. [Google Scholar] [CrossRef]
- Atlas, R.M.; Stoeckel, D.M.; Faith, S.A.; Minard-Smith, A.; Thorn, J.R.; Benotti, M.J. Oil biodegradation and oil-degrading microbial populations in marsh sediments impacted by oil from the Deepwater Horizon well blowout. Environ. Sci. Technol. 2015, 49, 8356–8366. [Google Scholar] [CrossRef]
- Aranda, C.P.; Valenzuela, C.; Matamala, Y.; Godoy, F.A.; Aranda, N. Sulphur-cycling bacteria and ciliated protozoans in a Beggiatoaceae mat covering organically enriched sediments beneath a salmon farm in a southern Chilean fjord. Mar. Pollut. Bull. 2015, 100, 270–278. [Google Scholar] [CrossRef]
- Rendulic, S.; Jagtap, P.; Rosinus, A.; Eppinger, M.; Baar, C.; Lanz, C.; Keller, H.; Lambert, C.; Evans, K.J.; Goesmann, A.; et al. A predator unmasked: Life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 2004, 303, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Palleroni, N.J.; Port, A.M.; Chang, H.K.; Zylstra, G.J. Hydrocarboniphaga effusa gen. nov., sp nov., a novel member of the γ-Proteobacteria active in alkane and aromatic hydrocarbon degradation. Int. J. Syst. Evol. Microbiol. 2004, 54, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Kasai, H.; Matsuo, Y.; Omura, S.; Shizuri, Y.; Takahashi, Y. Ilumatobacter fluminis gen. nov., sp nov., a novel actinobacterium isolated from the sediment of an estuary. J. Gen. Appl. Microbiol. 2009, 55, 201–205. [Google Scholar] [CrossRef]
- Han, S.K.; Nedashkovskaya, O.I.; Mikhailov, V.V.; Kim, S.B.; Bae, K.S. Salinibacterium amurskyense gen. nov., sp nov., a novel genus of the family Microbacteriaceae from the marine environment. Int. J. Syst. Evol. Microbiol. 2003, 53, 2061–2066. [Google Scholar] [CrossRef]
- Ibacache-Quiroga, C.; Ojeda, J.; Dinamarca, M.A. 16S rRNA amplicon sequencing of seawater microbiota from Quintero Bay, Chile, affected by oil spills, shows the presence of an oil-degrading marine bacterial guild structured by the bacterial genera Alcanivorax, Cobetia, Halomonas, and Oleiphilus. Microbiol. Resour. Announc. 2018, 7, 1318–1366. [Google Scholar] [CrossRef]
- Yun, S.H.; Choi, C.; Lee, S.; Lee, Y.G.; Kwon, J.; Leem, S.H.; Chung, Y.H.; Kahng, H.; Kim, S.J.; Kwon, K.K.; et al. Proteomic characterization of plasmid pLA1 for biodegradation of polycyclic aromatic hydrocarbons in the marine bacterium, Novosphingobium pentaromativorans US6-1. PLoS ONE 2014, 9, e90812. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Y. Evaluation of sedimentary characteristics of the Chang 9 Oil Layer Formation in the Yanchang Formation, Ordos Basin. Appl. Sci. 2024, 14, 4035. [Google Scholar] [CrossRef]
- Tian, H.; Gao, P.; Chen, Z.; Li, Y.; Li, Y.; Wang, Y.; Zhou, J.; Li, G.; Ma, T. Compositions and abundances of sulfate-reducing and sulfur oxidizing microorganisms in water-flooded petroleum reservoirs with different temperatures in China. Front. Microbiol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Bouanane-Darenfed, A.; Fardeau, M.; Gregoire, P.; Joseph, M.; Kebbouche-Gana, S.; Benayad, T.; Hacene, H.; Cayol, J.; Ollivier, B. Caldicoprobacter algeriensis sp nov a new thermophilic anaerobic, xylanolytic bacterium isolated from an Algerian hot spring. Curr. Microbiol. 2011, 62, 826–832. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Gao, Y.; Chen, Y.; Zhan, A. Environment-driven geographical distribution of bacterial communities and identification of indicator taxa in Songhua River. Ecol. Indic. 2019, 101, 62–70. [Google Scholar] [CrossRef]
- Yamane, K.; Hattori, Y.; Ohtagaki, H.; Fujiwara, K. Microbial diversity with dominance of 16S rRNA gene sequences with high GC contents at 74 and 98 °C subsurface crude oil deposits in Japan. FEMS Microbiol. Ecol. 2011, 76, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Mohapatra, S.C.; Guedes Soares, C. Composite breakwater of a submerged horizontal flexible porous membrane with a lower rubble mound. Appl. Ocean Res. 2020, 104, 102371. [Google Scholar] [CrossRef]
| Properties | Microbially Activated Water | Formation Water |
|---|---|---|
| Cell concentration (cell/mL) | 103~105 | 101~102 |
| DNA concentration (μg/mL) | 15.69~25.70 | 3.04~8.99 |
| COD (mg/L) | 361~650 | 59~128 |
| BOD (mg/L) | 82~139 | 17~89 |
| pH | 6.6~6.9 | 7.6~7.8 |
| Redox potential (mV) | 49.4~63.1 | 5.8~19.8 |
| Conductivity (mS/cm) | 45.7~55.3 | 39.0~43.7 |
| Silicon concentration (mg/L) | 10.02~14.77 | 8.72~11.96 |
| Iron concentration (mg/L) | - | - |
| Aluminum concentration (μg/L) | - | - |
| Manganese concentration (μg/L) | 1206.83~1773.42 | 789.44~1911.66 |
| Fluorine concentration (mg/L) | 0.34~0.47 | 0.23~0.27 |
| Cadmium concentration (μg/L) | <1 | 4.24~6.03 |
| Sulfur concentration (mg/L) | 50.90~70.93 | 0~11.07 |
| Surface tension (mN/m) | 62.7~69.3 | 72.4~75.1 |
| Production Parameters | Before | After |
|---|---|---|
| Number of producing wells/wells | 80 | 80 |
| Daily liquid production/t | 172 | 178 |
| Daily oil production/t | 100 | 102 |
| Per-well production/t | 1.3 | 1.3 |
| Comprehensive water cut/% | 31.0 | 31.9 |
| Dynamic fluid level/m | 1305 | 1265 |
| Number of water injection wells/wells | 21 | 21 |
| Daily water injection volume/m3 | 485 | 407 |
| Per-well daily water injection/m3 | 23 | 19 |
| Monthly injection–production ratio | 2.8 | 2.2 |
| Natural decline rate/% | 2.5 | 1.2 |
| Water cut rise rate/% | 1.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, C.; Su, P.; Mu, H. Ecological Responses of Microbially Activated Water Flooding in Ultra-Low-Permeability Reservoirs: A Case Study of the B9 Reservoir in the Triassic Yanchang Formation. J. Mar. Sci. Eng. 2025, 13, 836. https://doi.org/10.3390/jmse13050836
Li L, Zhang C, Su P, Mu H. Ecological Responses of Microbially Activated Water Flooding in Ultra-Low-Permeability Reservoirs: A Case Study of the B9 Reservoir in the Triassic Yanchang Formation. Journal of Marine Science and Engineering. 2025; 13(5):836. https://doi.org/10.3390/jmse13050836
Chicago/Turabian StyleLi, Lei, Chunhui Zhang, Peidong Su, and Hongmei Mu. 2025. "Ecological Responses of Microbially Activated Water Flooding in Ultra-Low-Permeability Reservoirs: A Case Study of the B9 Reservoir in the Triassic Yanchang Formation" Journal of Marine Science and Engineering 13, no. 5: 836. https://doi.org/10.3390/jmse13050836
APA StyleLi, L., Zhang, C., Su, P., & Mu, H. (2025). Ecological Responses of Microbially Activated Water Flooding in Ultra-Low-Permeability Reservoirs: A Case Study of the B9 Reservoir in the Triassic Yanchang Formation. Journal of Marine Science and Engineering, 13(5), 836. https://doi.org/10.3390/jmse13050836







