Paleobiodiversity, Paleobiogeography, and Paleoenvironments of the Middle–Upper Eocene Benthic Foraminifera in the Fayum Area, Western Desert, Egypt
Abstract
1. Introduction
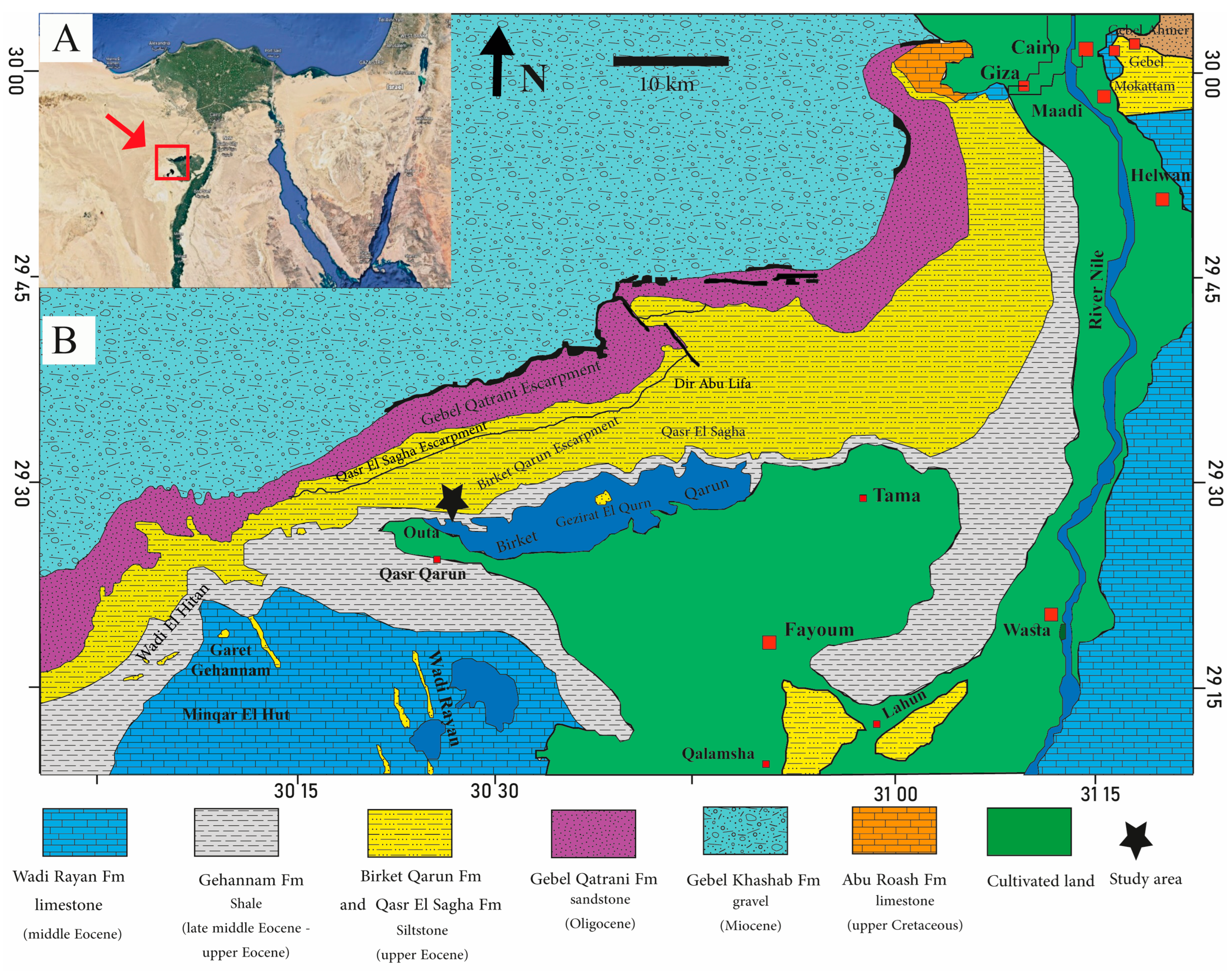
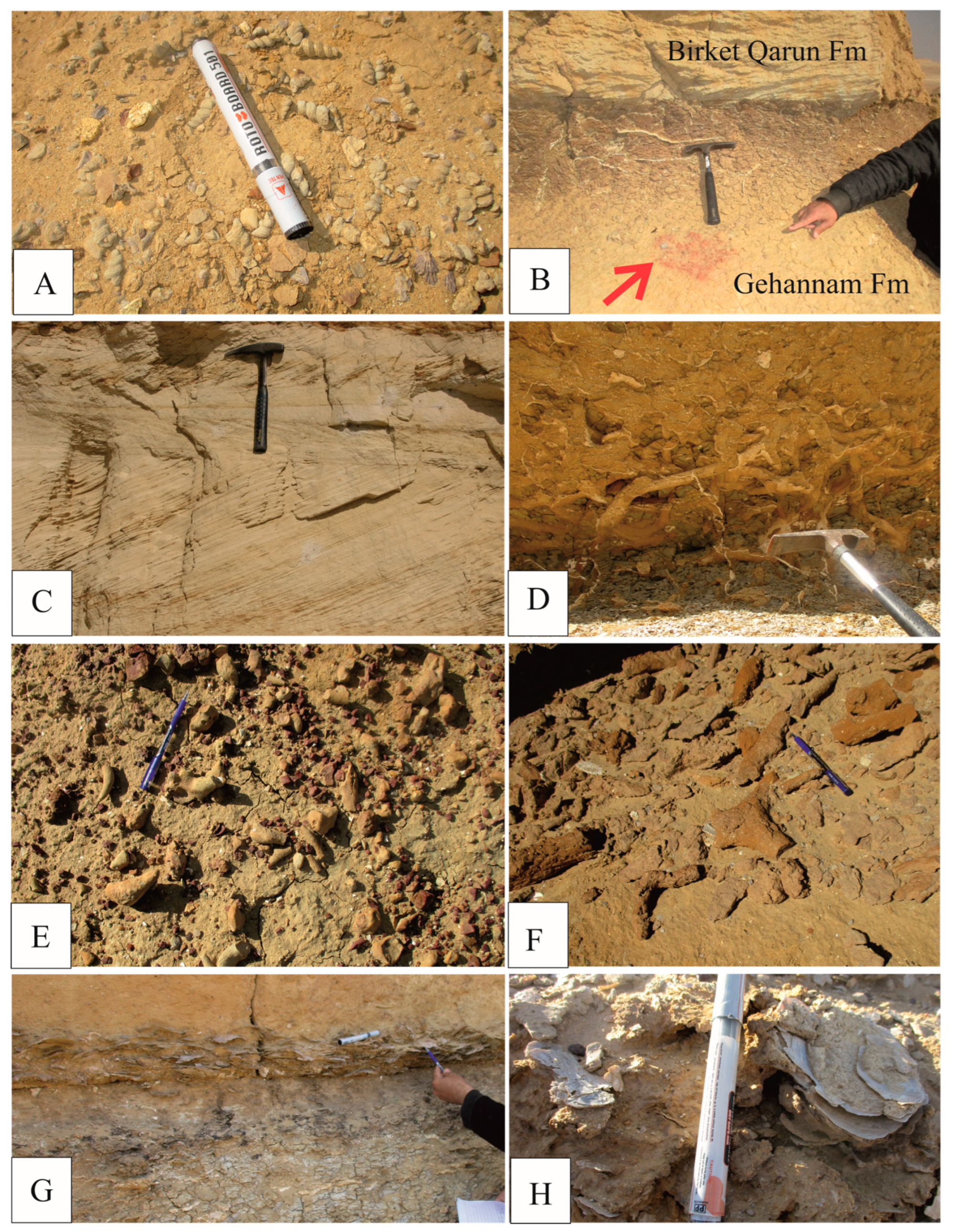
2. Geologic Setting and Stratigraphy
2.1. The Gehannam Formation (Bartonian-Priabonian)
2.2. The Birket Qarun Formation (Priabonian)
2.3. The Qasr El Sagha Formation (Priabonian)
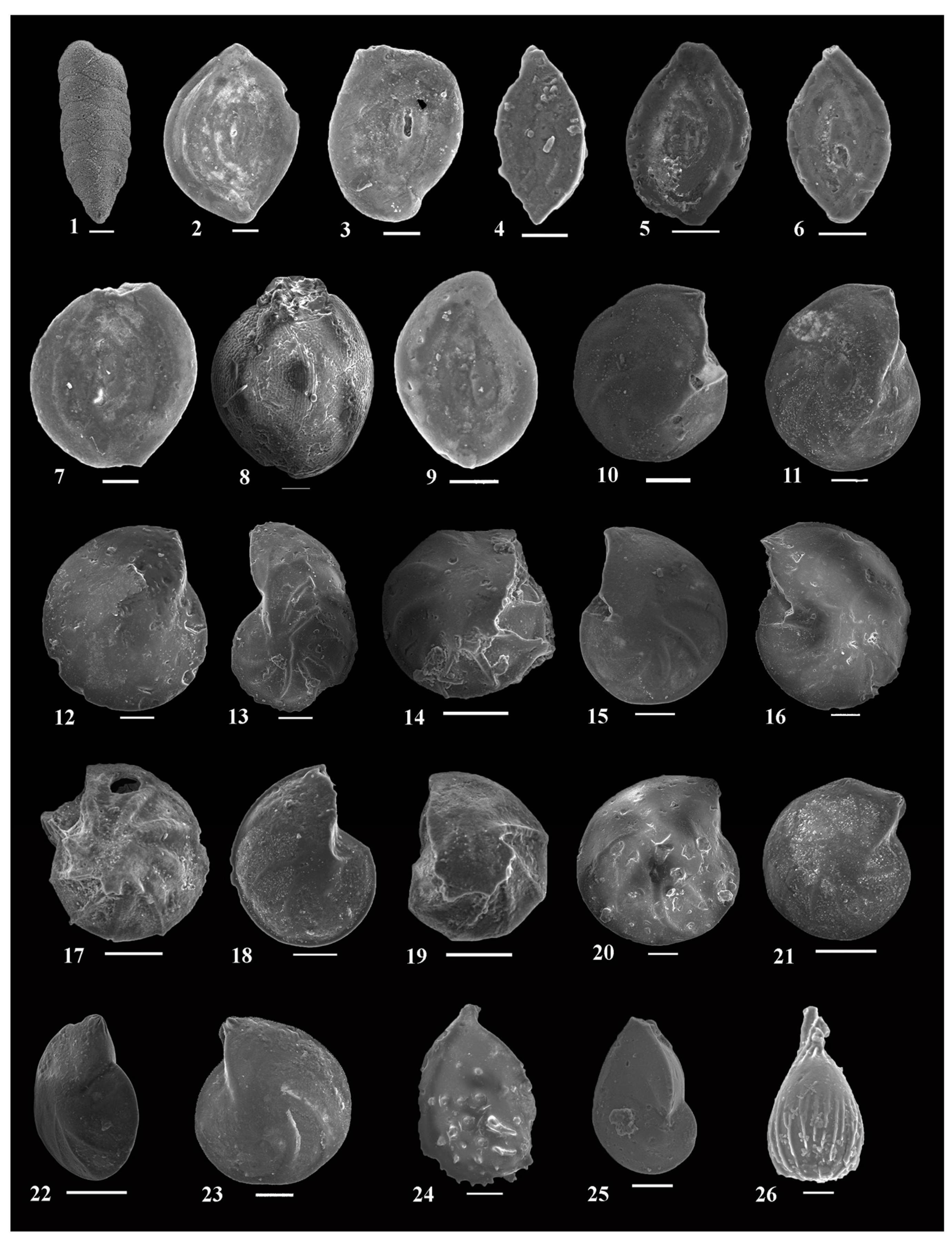
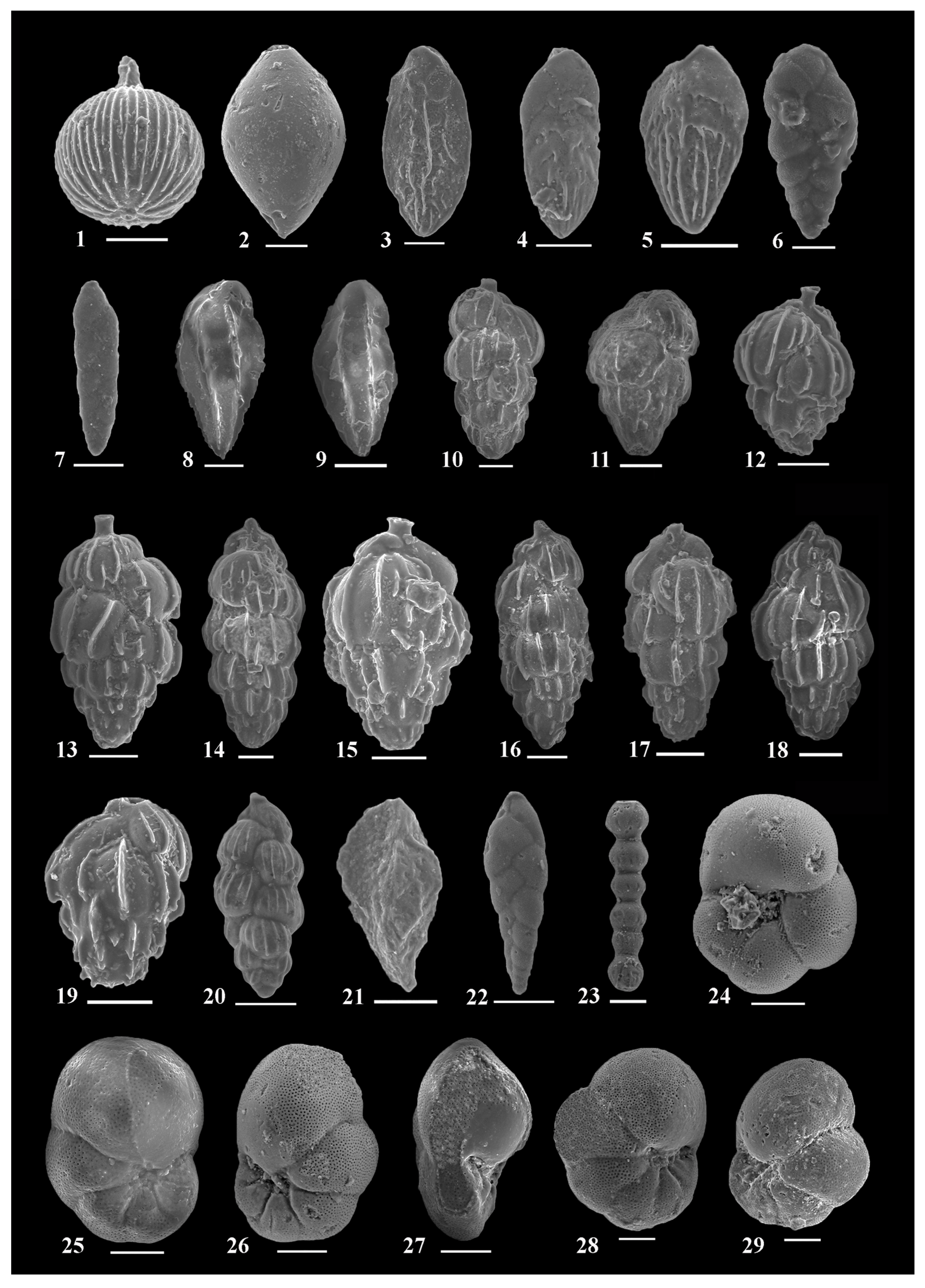
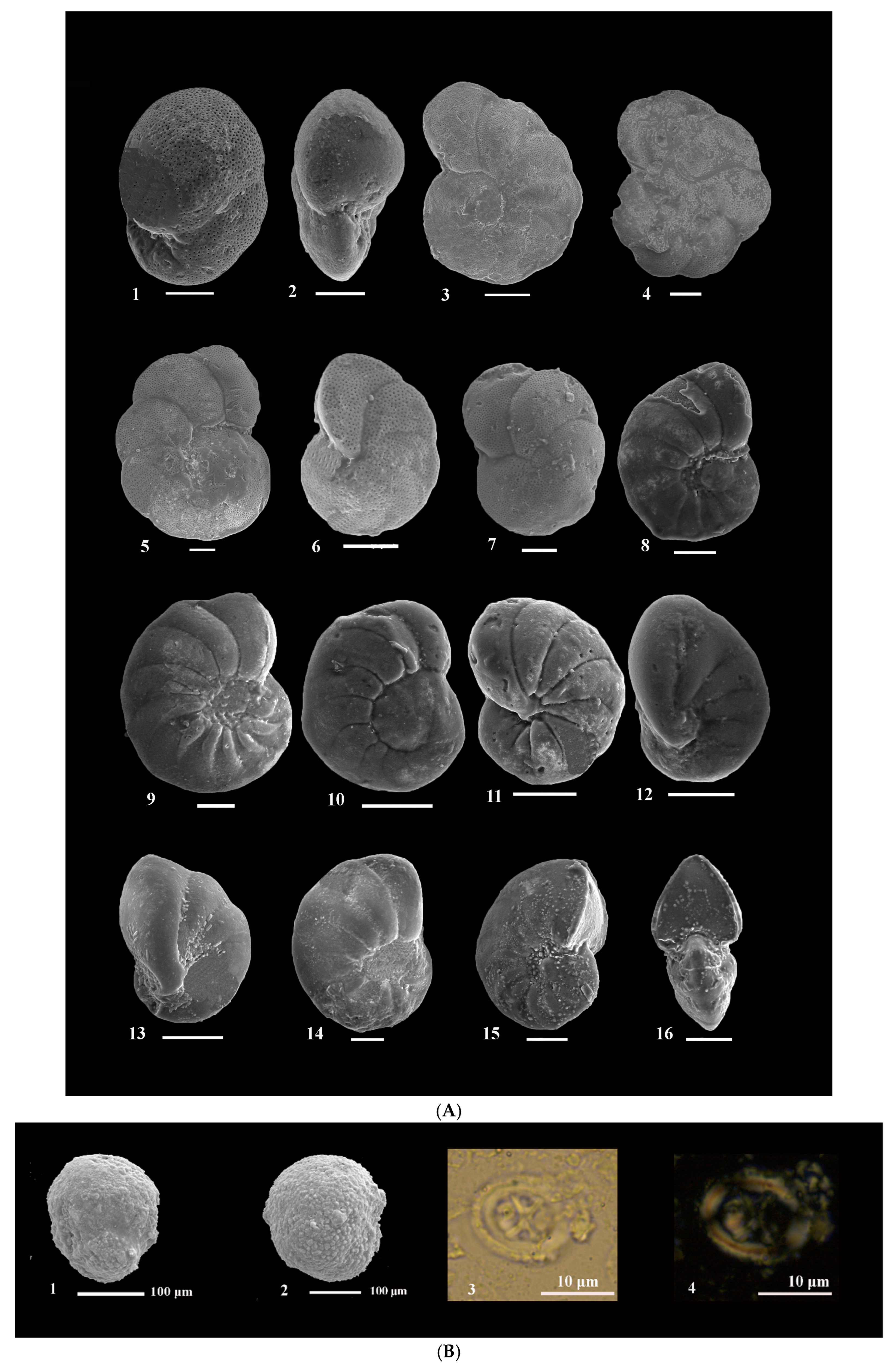
3. Materials and Methods
4. Results
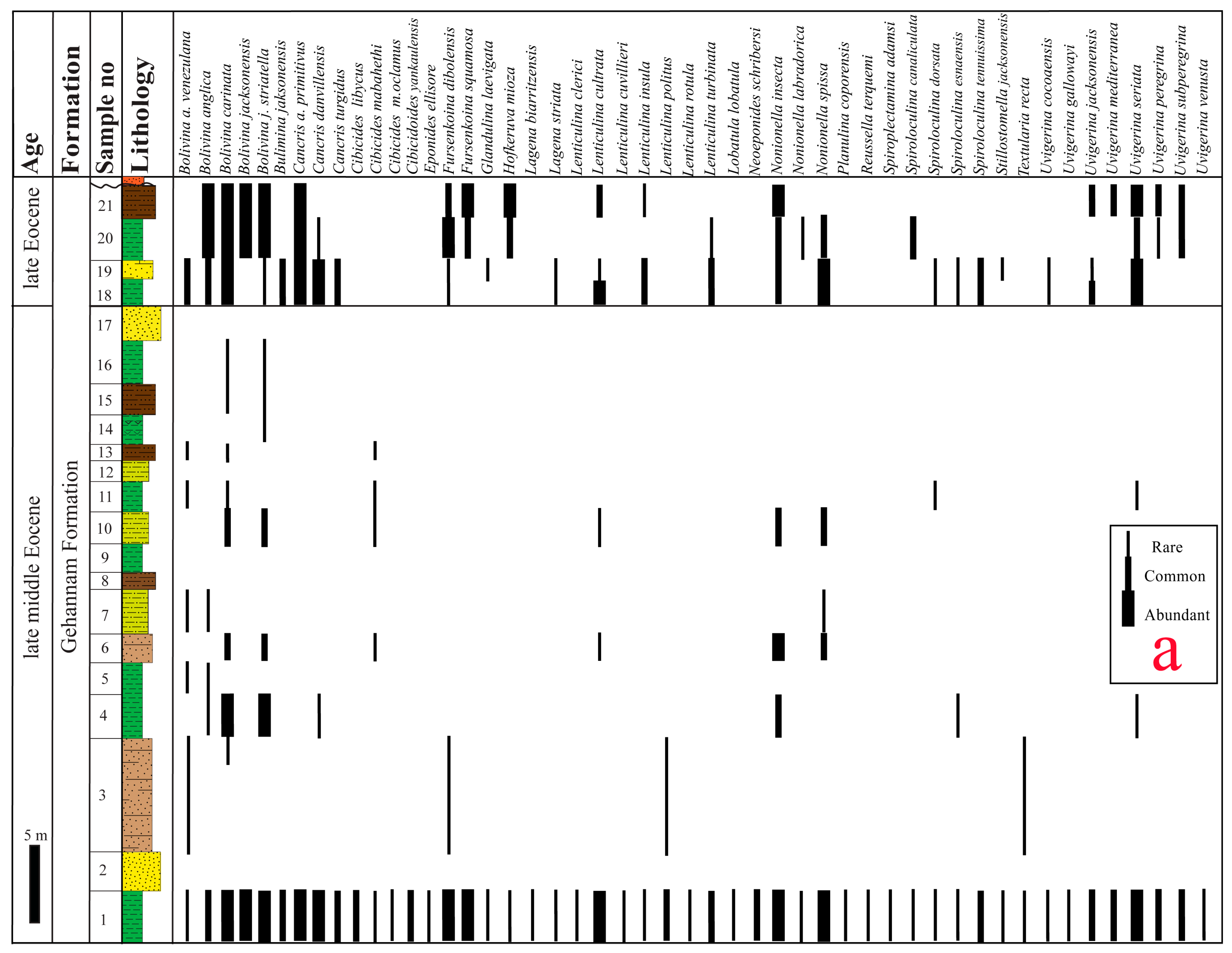
4.1. Biostratigraphy
4.1.1. Bolivina carinata Zone
- Category: Lowest Occurrence Zone.
- Age: late middle Eocene (Bartonian).
- Definition: Interval from the lowest occurrence (LO) of Bulimina jacksonensis to the lowest occurrence of Lenticulina costata.
- Assemblages: Fifty-five species and subspecies are recorded from this zone. The lower part of this zone is characterized by the occurrence of planktonic foraminifera and high dominance and diversity of the recorded benthic foraminifera (49 species), whereas the middle and upper parts are marked by low representation compared to the lower part (23 species). The most common species are Bolivina anglica, B. jacksonensis, B. jacksonensis striatella, Bulimina jacksonensis, Cancris auriculus primitivus, C. danvillensis, Cibicidoides yankaulensis, Fursenkoina dibolensis, F. squamosa, Lenticulina cultrata, L. insula, Nonionella insecta, N. spissa, Uvigerina peregrina, U. subperegrina, and U. seriata.
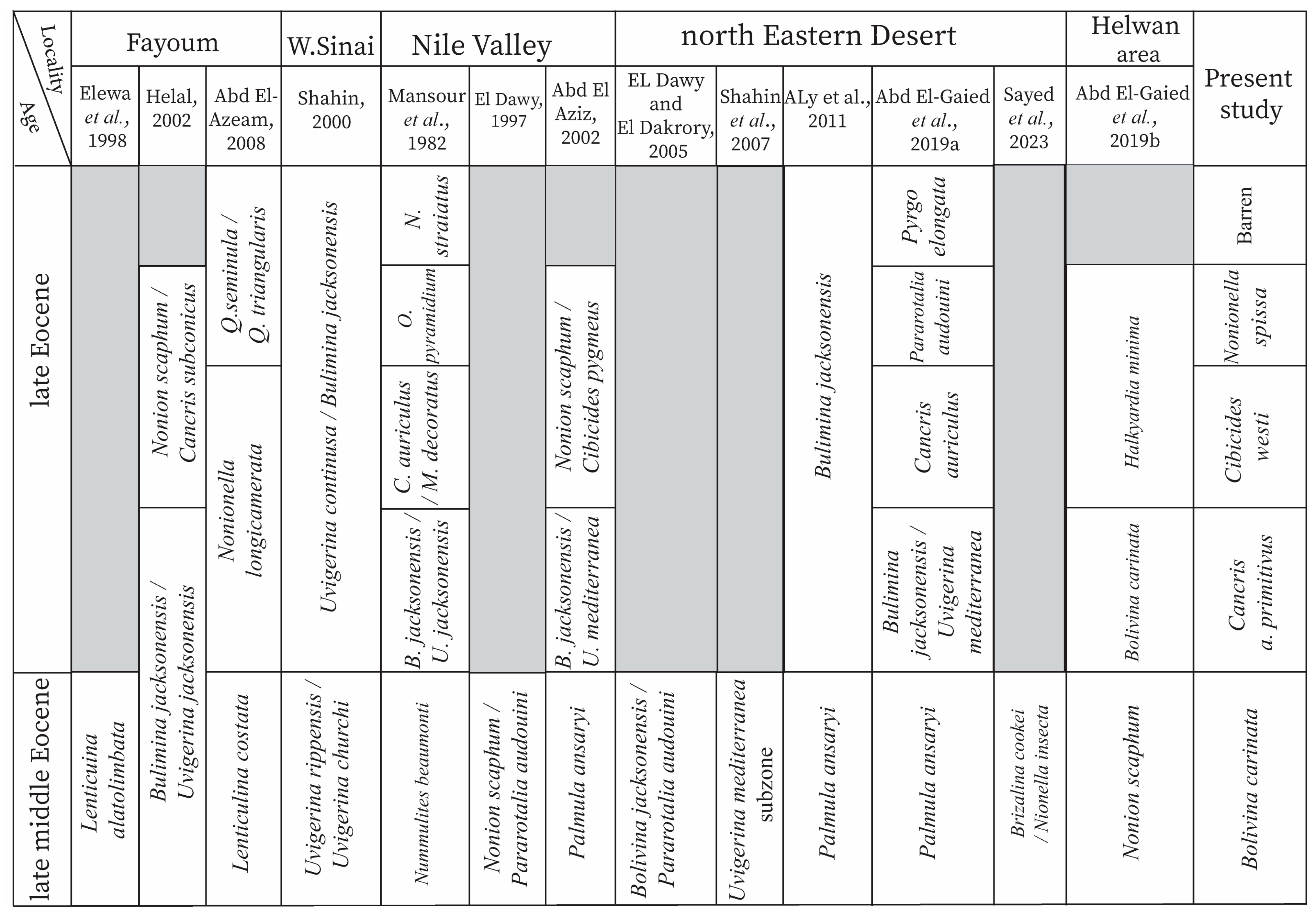
- Correlation: The present zone matches with the standard middle Eocene planktonic foraminifera E13 Morozovella crassatus Zone and the lower part of the late Eocene E14 Globigerinatheka semiinvoluta Zone [62]. Globally, this zone corresponds to the top part of the following shallow benthic zones (SBZs): Biozone 51 (Nummulites-Alveolina assemblage subzone) of Wynd [63] from south Iran, SBZ 17–18 of Serra-Kiel et al. [35], Assemblage Zone B of Babazadeh and Cluzel [64], and Assemblage Zone of Changaei et al. [65]. It is completely equivalent to the Brizalina cookie/Nonionella insecta Zone and the Nonion scaphum Zone that were previously recorded from north Eastern Desert and Helwan area [4,32]. It is also matched with the top part of the Palmula ansaryi Zone from the middle Eocene of north Eastern Desert [30,66] and the Nile Valley [39], as shown in Figure 10. In addition, it correlates with the Bolivina jacksonensis/Pararotalia audouini Zone and the Uvigerina mediterranea Subzone from the north Eastern Desert [67,68]. It is equated to the top part of the following zones: the Uvigerina rippensis/Uvigerina churchi Zone from Western Sinai [69], the Nonion scaphum/Pararotalia audouini Zone from the Nile Valley [70], and Lenticulina alatolimbata [71], Bulimina jacksonensis/Uvigerina jacksonensis [36], and the Lenticulina costata [42] zones that were defined from the Fayum area (Figure 10). Moreover, it is coinciding with the large benthic foraminifera Nummulites beaumonti Zone recorded from the middle Eocene of the Nile Valley [72].
- Age: Based on the occurrence of the spinose planktonic species Acarinina rohri, Morozovelloides spp., and Globigerinatheka semiinvoluta, this zone corresponds to the planktonic Morozovelloides crassatus Zone (E13) and the lower part of the Globigerinatheka semiinvoluta Zone (E14), assigning a late middle Eocene (Bartonian) age [32,62,73]. Previously in Egypt, the Bartonian/Priabonian boundary was placed at the first appearance of Globigerinatheka semiinvoluta [28,29]. However, Strougo [74] stated that the first appearance of the Globigerinatheka semiinvoluta, in conjunction with the disappearance of large Acarininids, Truncorotaloides, and Morozovelloides, marks the middle/upper Eocene boundary. Additionally, some authors located the boundary at the top of a new local biozone named the Turborotalia pseudoampliapertura Zone [51,54]. In the Mediterranean and tropical areas, the Bartonian/Priabonian boundary is placed at the last occurrence of the middle Eocene planktonic spinose forms [52,75,76], either at the P14/P15 zonal boundary or in the lower part of zone P15 [76,77]. However, several studies mentioned that Globigerinatheka semiinvoluta appeared below the last appearance of the spinose planktonic forms [62,73,78]. Therefore, the middle/upper Eocene (Bartonian/Priabonian) boundary in the present study is placed within the Globigerinatheka semiinvoluta Zone, at the top of the Bolivina carinata Zone (at the base of sample 18), according to Wade et al. [62]. Furthermore, this boundary is marked at the NP17/NP18 boundary by the lowest occurrence of Chiasmolithus oamaruensis [61], showing a good correlation with Subchron C17n.1n and Zone SBZ18A documented by Özcan et al. [79].
4.1.2. Cancris auriculus primitivus Zone
- Category: Concurrent-Range Zone.
- Age: late Eocene (Priabonian).
- Definition: Interval from the lowest occurrence (LO) of Lenticulina costata to the highest occurrence (HO) of Cancris auriculus primitivus.
- Assemblages: Fifty-nine species and subspecies are identified from this zone. This zone is characterized by the highest dominance and diversity of the recorded benthic foraminifera. The most abundant species are Bolivina anglica, B. jacksonensis, B. j. striatella, B. kuriani, Bulimina jacksonensis, Cancris auriculus primitivus, C. danvillensis, C. turgidus, Fursenkoina dibolensis, F. squamosa, Hofkeruva mioza, Lenticulina cultrata, Nonionella africana, N. insecta, N. spissa, Uvigerina peregrina, U. subperegrina, U. rippensis, U. seriata, and U. yazoensis.
- Correlation: The present zone matches with the standard late Eocene planktonic foraminifera Globigerinatheka semiinvoluta Zone [62]. It correlates with the Bolivina carinata Zone from the Helwan area [32], the Bulimina jacksonensis/Uvigerina mediterranea Zone, and the lower part of the Bulimina jacksonensis Zone recorded from the north Eastern Desert [30,66]. It is equated to the Bulimina jacksonensis/Uvigerina jacksonensis Zone [72] and the Bulimina jacksonensis/Uvigerina mediterranea Zone [39] in the Nile Valley (Figure 10). It is also correlated with the lower part of the Uvigerina continusa/Bulimina jacksonensis Zone from Western Sinai [69]. Moreover, this zone is coeval with the topmost part of the Bulimina jacksonensis/Uvigerina jacksonensis Zone [36] and the lower part of the Nonionella longicamerata Zone [42] from the Fayum area (Figure 10).
- Age: This zone is attributed to the late Eocene (Priabonian) age and correlated to the planktonic Globigerinatheka semiinvoluta Zone (E14), based on the occurrence of the index planktonic foraminifera Globigerinatheka semiinvoluta [28,62,80,81], along with the rare base occurrence of the marker nannofossil Chiasmolithus oamaruensis, which defines the base of Zone NP18 [82] and the base of CNE17 [83]. In addition, the recorded benthic assemblages within the present zone show strong similarity with those identified in Fayum, the Nile Valley, and the north Eastern Desert [30,32,36,39,42,66,72].
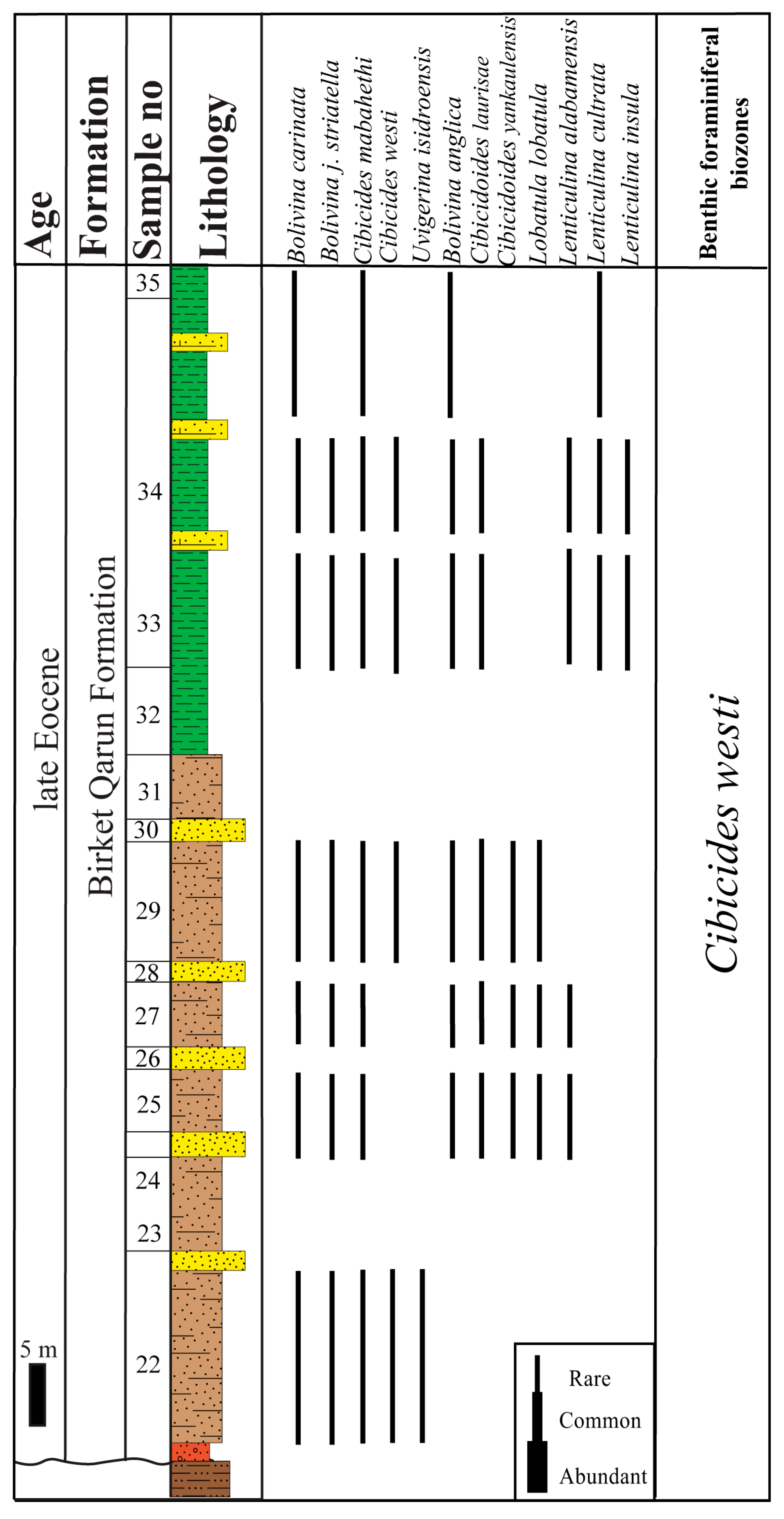
4.1.3. Cibicides westi Zone
- Category: Concurrent-Range Zone.
- Age: late Eocene (Priabonian).
- Definition: Interval from the lowest occurrence (LO) of Cibicides mabahethi to the highest occurrence (HO) of Cibicides westi.
- Assemblages: Twelve species and subspecies are recorded from this zone. This zone is marked by the lowest dominance and diversity compared with the other zones. The identified species are represented by Bolivina anglica, B. carinata, B. j. striatella, Cibicides mabahethi, C. westi, Cibicidoides laurisae, C. yankaulensis, Lobatula lobatula, Lenticulina alabamensis, L. cultrata, L. insula, and Uvigerina isidroensis.
- Correlation: In the present study, this zone is well correlated with the Cancris auriculus Zone [30] and the middle part of the Bulimina jacksonensis Zone [66] from the late Eocene of north Eastern Desert (Figure 10). It coincides with the lower and middle parts of the Halkyardia minima Zone from the upper Eocene succession in the Helwan area [32]. Also, it completely matches the Cancris cocoaensis/Massilina decoratus Zone [72] and is coeval to the lower and middle parts of the Nonion scaphum/Cibicides pygmous Zone [39] in the Nile Valley (Figure 10). It is correlated to the middle part of the Uvigerina continusa/Bulimina jacksonensis Zone recorded from Western Sinai [69]. Additionally, this zone correlates with the following zones in the Fayum area: the lower part of the late Eocene Nonion scaphum/Cancris subconicus Zone [36] and the middle and top parts of the Nonionella longicamerata Zone [42]; see Figure 10.
- Age: This zone witnessed a complete absence of planktonic foraminifera and nannofossil assemblages. Based on the abovementioned data, stratigraphic position, as well as the occurrence of the index Carolia placunoides, and previous studies that have been performed on the studied section, this zone is assigned to a late Eocene (Priabonian) age [26,38,84].
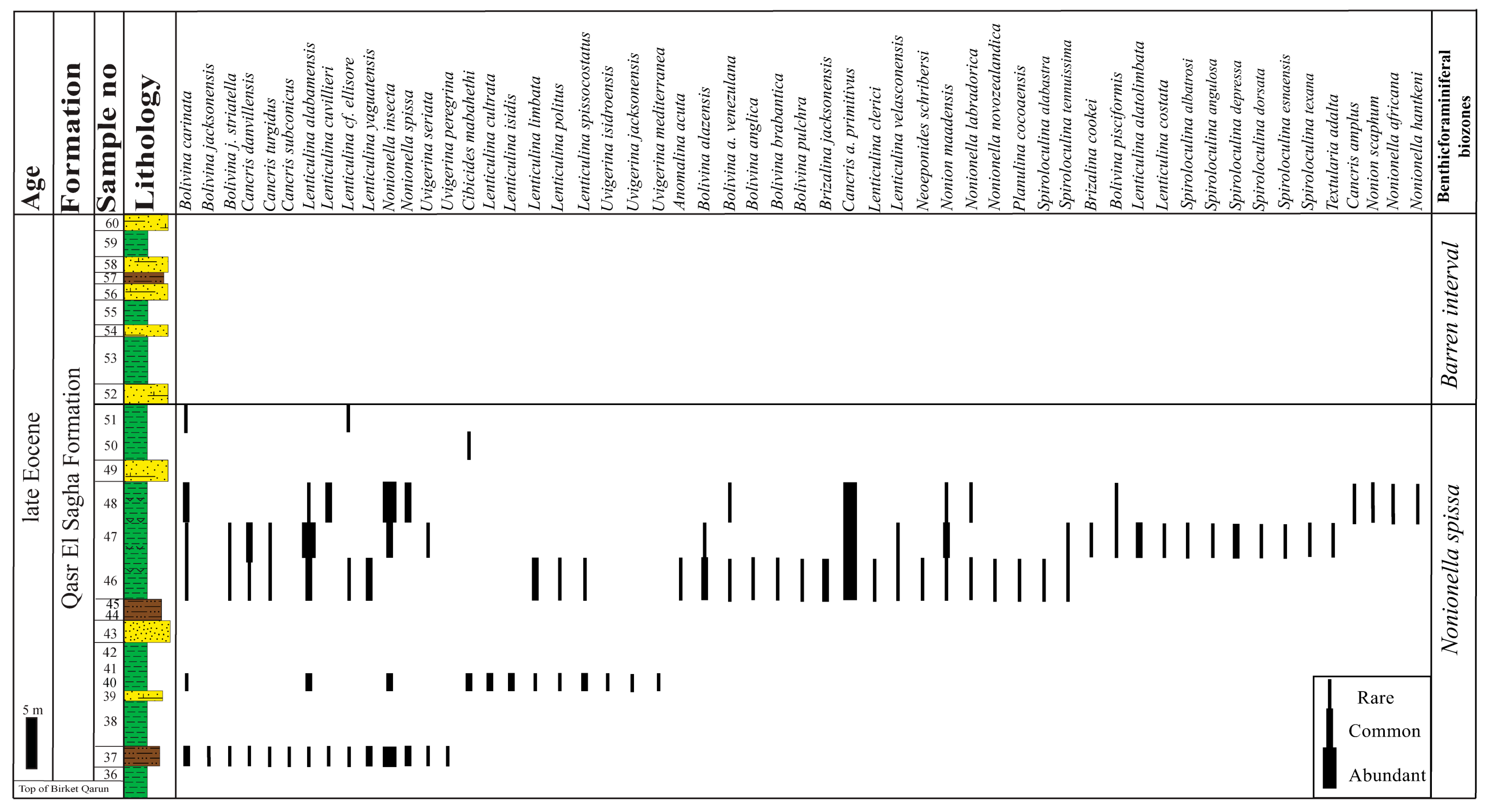
4.1.4. Nonionella spissa Zone
- Category: Concurrent-Range Zone.
- Age: late Eocene (Priabonian).
- Definition: Interval from the lowest occurrence (LO) of Lenticulina yaguatensis to the highest occurrence (HO) of Lenticulina ellisorae.
- Assemblages: Fifty-five species and subspecies are recorded from this zone, which is characterized by high diversity and moderate dominance. The most common species are Bolivina carinata, Cancris auriculus primitivus, C. danvillensis, Lenticulina alabamensis, L. alatolimbata, L. cuvillieri, L. limbata, L. yaguatensis, Nonionella insecta, N. spissa, and Spiroloculina depressa.
- Correlation: In the present study, this zone is equivalent to the late Eocene Pararotalia audouini Zone [30] and the top part of the Bulimina jacksonensis Zone [66] from north Eastern Desert (Figure 10). It is matched with the top part of the Halkyardia minima Zone from the Helwan area [32] and with the Nonion scaphum/Cibicides pygmous Zone in the Nile Valley [39]. It also correlates with the middle and upper parts of the Uvigerina continusa/Bulimina jacksonensis Zone at Western Sinai [69]. Moreover, it fits with the lower part of the Quinqueloculina seminula/Q. triangularis Zone [42] and with the middle and top parts of the Nonion scaphum/Cancris subconicus Zone [36] in the Fayum area. Regarding the large benthic foraminiferal zonation, this zone is well correlated to the Operculina pyramidium Zone, which was recognized from the Nile Valley [72], as shown in Figure 10.
- Age: Based on the stratigraphic position of this zone, besides the occurrence of the index macrofauna (Carolia spp.), and the previous studies that have been carried out on the studied section, a late Eocene (Priabonian) age is attributed to this zone.
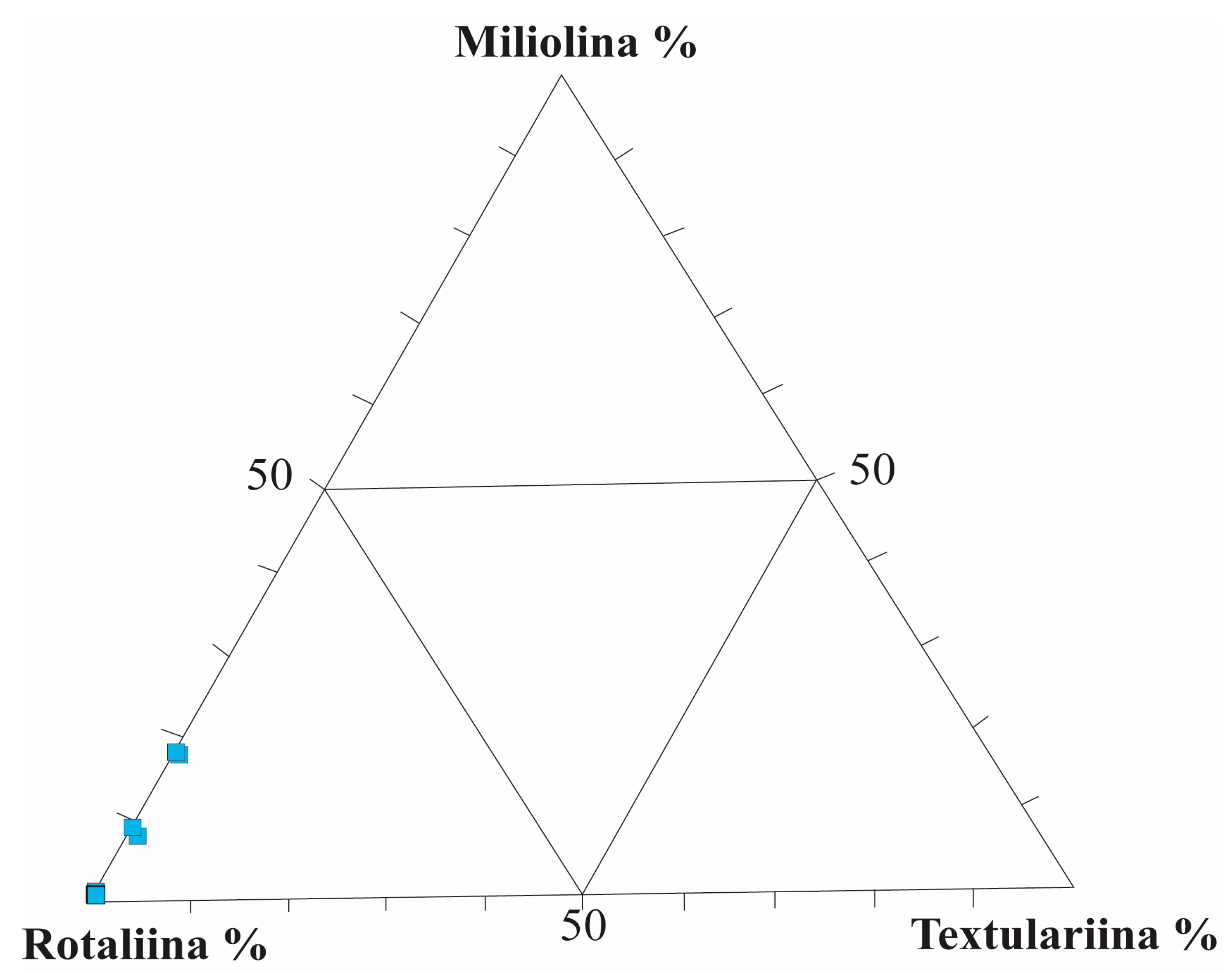
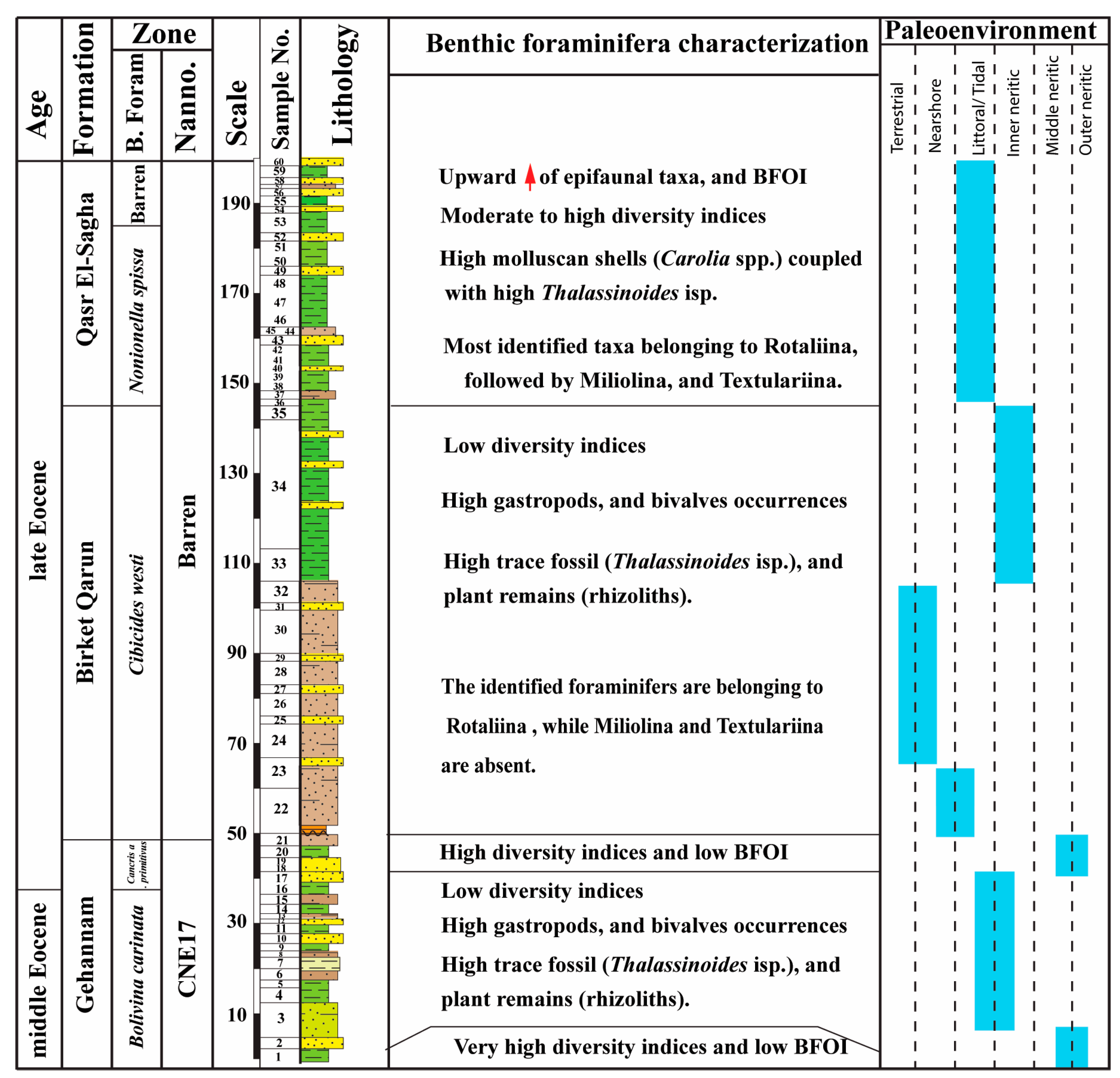
4.2. Ecological Parameters and Diversity Indices
5. Discussion
5.1. Depositional Environments
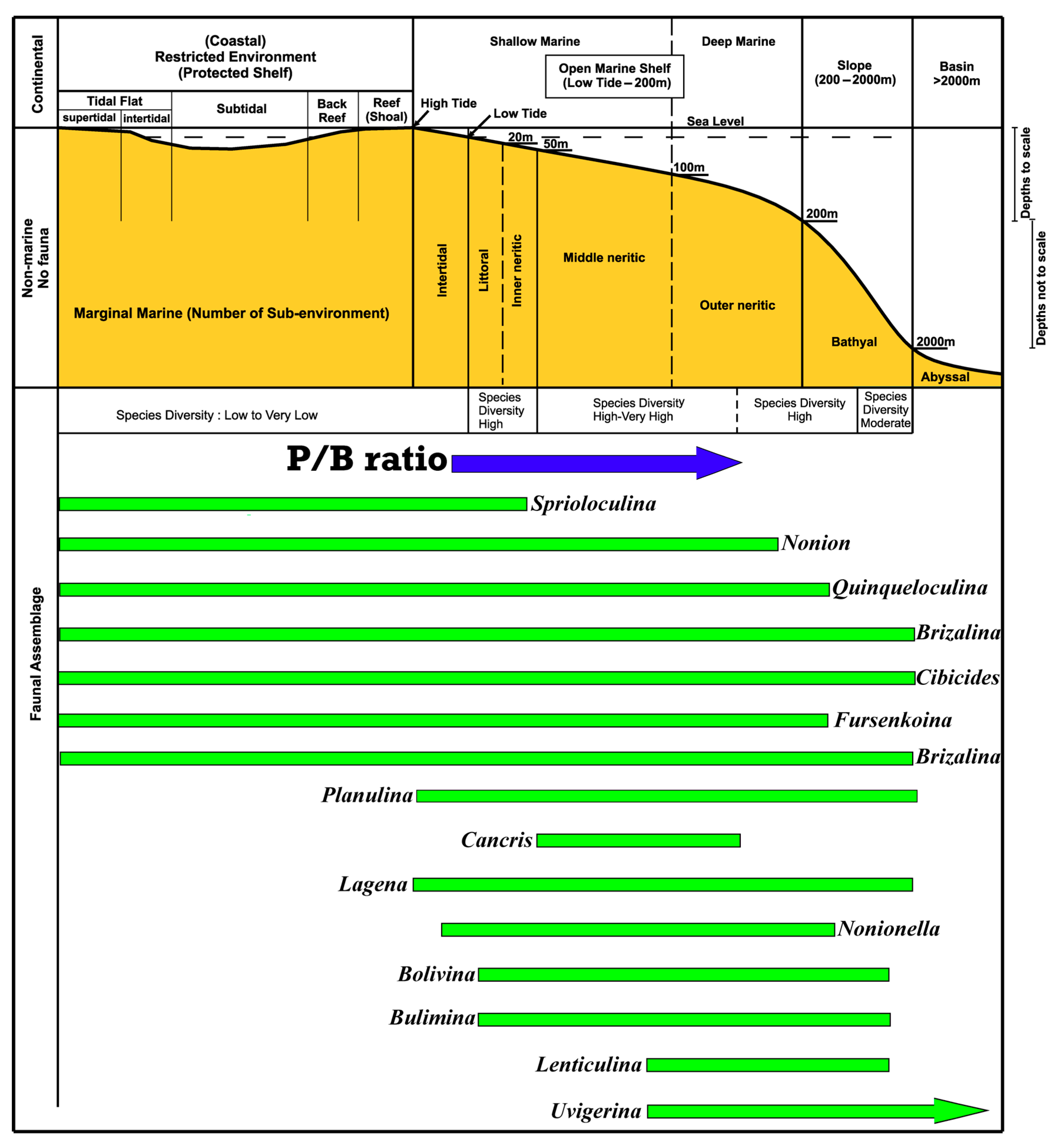
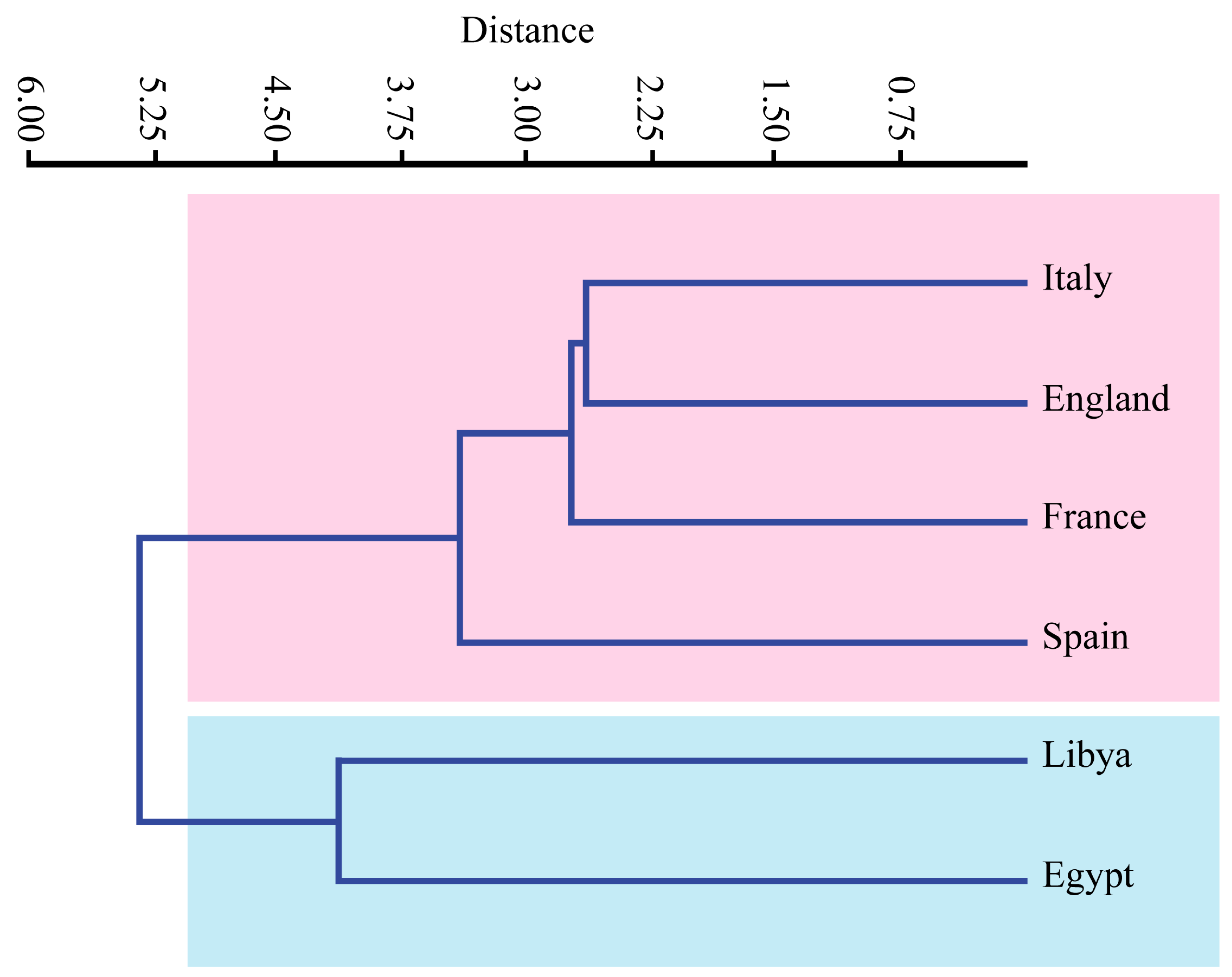
5.2. Paleobiogeography
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gradstein, F.M.; Ogg, J.; Schmitz, M.D.; Ogg, G.E. The Geologic Time Scale; Elsevier: Boston, MA, USA, 2012. [Google Scholar]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [PubMed]
- Wang, P.; Du, Y.; Yu, W.; Algeo, T.J.; Zhou, Q.; Xu, Y.; Qi, L.; Yuan, L.; Pan, W. The chemical index of alteration (CIA) as a proxy for climate change during glacial-interglacial transitions in Earth history. Earth Sci. Rev. 2020, 201, 103032. [Google Scholar]
- Sayed, M.M.; Heinz, P.; Abd El-Gaied, I.M.; Wagreich, M. Paleoclimate and paleoenvironment reconstructions from middle eocene successions at beni-suef, Egypt: Foraminiferal assemblages and geochemical approaches. Diversity 2023, 15, 695. [Google Scholar] [CrossRef]
- Scheibner, C.; Speijer, R. Late Paleocene—Early Eocene Tethyan carbonate platform evolution—A response to long-and short-term paleoclimatic change. Earth Sci. Rev. 2008, 90, 71–102. [Google Scholar]
- Alhejoj, I.; Farouk, S.; Bazeen, Y.S.; Ahmad, F. Depositional sequences and sea-level changes of the upper Maastrichtian-middle Eocene succession in central Jordan: Evidence from foraminiferal biostratigraphy and paleoenvironments. J. Afr. Earth Sci. 2020, 161, 103663. [Google Scholar]
- Messadi, A.M.; Touir, J.; Mardassi, B.; Ouali, J.A. Factors controlling sedimentation and sequence stratigraphy evolution in shallow marine (carbonates) platform: Example of Middle Eocene deposits from Gafsa Basin. Carbonates Evaporites 2020, 35, 58. [Google Scholar]
- Messaoud, J.H.; Thibault, N.; Aljahdali, M.H.; Yaich, C. Middle Eocene to early Oligocene biostratigraphy in the SW Neo-Tethys (Tunisia): Large-scale correlations using calcareous nannofossil events and paleoceanographic implications. J. Afr. Earth Sci. 2023, 198, 104805. [Google Scholar] [CrossRef]
- Messaoud, J.H.; Thibault, N.; Aljahdali, M.H.; Yaich, C. Evolution of the Eocene Shallow Carbonate Platform in Tunisia: Carbon Isotopes Stratigraphy and Petroleum Implications; Research Square Platform LLC.: Durham, NC, USA, 2022. [Google Scholar]
- Messaoud, J.H.; Thibault, N.; De Vleeschouwer, D.; Monkenbusch, J. Benthic biota (nummulites) response to a hyperthermal event: Eccentricity-modulated precession control on climate during the middle Eocene warming in the Southern Mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 626, 111712. [Google Scholar] [CrossRef]
- Hüneke, H.; Hernández-Molina, F.J.; Rodríguez-Tovar, F.J.; Llave, E.; Chiarella, D.; Mena, A.; Stow, D.A. Diagnostic criteria using microfacies for calcareous contourites, turbidites and pelagites in the Eocene-Miocene slope succession, southern Cyprus. Sedimentology 2021, 68, 557–592. [Google Scholar]
- Hadi, M.; Less, G.; Vahidinia, M. Eocene larger benthic foraminifera (alveolinids, nummulitids, and orthophragmines) from the eastern Alborz region (NE Iran): Taxonomy and biostratigraphy implications. Rev. Micropaléontol. 2019, 63, 65–84. [Google Scholar]
- Abdel-Fattah, Z.A. Bioerosion in the middle Eocene larger foraminifer Nummulites in the Fayum depression, Egypt. Proc. Geol. Assoc. 2018, 129, 774–781. [Google Scholar]
- Messaoud, J.H.; Thibault, N.; Yaich, C.; Monkenbusch, J.; Omar, H.; Jemai, H.F.B.; Watkins, D.K. The Eocene-Oligocene Transition in the South-Western Neo-Tethys (Tunisia): Astronomical Calibration and Paleoenvironmental Changes. Paleoceanogr. Paleoclimatol. 2020, 35, e2020PA003887. [Google Scholar] [CrossRef]
- Messaoud, J.H.; Thibault, N.; Bomou, B.; Adatte, T.; Monkenbusch, J.; Spangenberg, J.E.; Aljahdali, M.H.; Yaich, C. Integrated stratigraphy of the middle-upper Eocene Souar Formation (Tunisian dorsal): Implications for the middle eocene climatic optimum (MECO) in the SW Neo-Tethys. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 581, 110639. [Google Scholar]
- Less, G.; Özcan, E.; Okay, A. Stratigraphy and larger foraminifera of the Middle Eocene to Lower Oligocene shallow-marine units in the northern and eastern parts of the Thrace Basin, NW Turkey. Turk. J. Earth Sci. 2011, 20, 793–845. [Google Scholar]
- Ben İsmail-Lattrache, K.; Özcan, E.; Boukhalfa, K.; Saraswati, P.K.; Soussi, M.; Jovane, L. Early Bartonian orthophragminids (Foraminiferida) from Reineche Limestone, north African platform, Tunisia: Taxonomy and paleobiogeographic implications. Geodin. Acta 2013, 26, 94–121. [Google Scholar]
- Özcan, E.; Abbasi, İ.A.; Drobne, K.; Govindan, A.; Jovane, L.; Boukhalfa, K. Early Eocene orthophragminids and alveolinids from the Jafnayn Formation, N Oman: Significance of Nemkovella stockari Less & Özcan, 2007 in Tethys. Geodin. Acta 2016, 28, 160–184. [Google Scholar]
- Höntzsch, S.; Scheibner, C.; Kuss, J.; Marzouk, A.M.; Rasser, M.W. Tectonically driven carbonate ramp evolution at the southern Tethyan shelf: The Lower Eocene succession of the Galala Mountains, Egypt. Facies 2011, 57, 51–72. [Google Scholar]
- King, C.; Dupuis, C.; Aubry, M.-P.; Berggren, W.A.; Robert, O.B.K.; Galal, W.F.; Baele, J.-M. Anatomy of a mountain: The Thebes limestone formation (lower Eocene) at Gebel Gurnah, Luxor, Nile valley, upper Egypt. J. Afr. Earth Sci. 2017, 136, 61–108. [Google Scholar]
- Saber, S.G.; Salama, Y.F. Facies analysis and sequence stratigraphy of the Eocene successions, east Beni Suef area, eastern Desert, Egypt. J. Afr. Earth Sci. 2017, 135, 173–185. [Google Scholar]
- Tawfik, M.; El-Sorogy, A.S.; Moussa, M. Relationships between sequence stratigraphy and diagenesis of corals and foraminifers in the Middle Eocene, northern Egypt. Turk. J. Earth Sci. 2017, 26, 147–169. [Google Scholar]
- Sayed, M.M.; Abd El-Gaied, I.M.; Abdelhady, A.A.; Abd El-Aziz, S.M.; Wagreich, M. Ostracods sensitivity to reconstructing water depths and oxygen levels: A case study from the Middle-Late Eocene of the Beni Suef area (Egypt). Mar. Micropaleontol. 2022, 175, 102155. [Google Scholar] [CrossRef]
- Gingerich, P.D. Marine Mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: Stratigraphy, Age and Paleoenvironments; The University of Michigan: Ann Arbor, MI, USA, 1992. [Google Scholar]
- El-Shazly, S.H.; Abdel-Gawad, G.I.; Salama, Y.F.; Sayed, D.M. Paleontology, paleobiogeography and paleoecology of Carolia-bearing beds from the Late Eocene rocks at Nile-Fayum Divide, Egypt. J. Afr. Earth Sci. 2016, 124, 447–477. [Google Scholar] [CrossRef]
- Sayed, D.M.; El-Shazly, S.H.; Salama, Y.F.; Abd El-Gaied, I.M.; Badawy, H.S. Symbiosis of genus Hydractinia and in situ hermit crab in Kerunia cornuta Mayer-Eymar, 1899 from the Upper Eocene rocks in northwest Qarun Lake, Fayum area, Egypt. J. Afr. Earth Sci. 2023, 198, 104789. [Google Scholar] [CrossRef]
- El Baz, S.M.; Wanas, H.A.; Abou Awad, H.A.; Assal, E.M. The Middle-Upper Eocene benthic foraminifera from north-west Fayoum area, Egypt: Paleoecology and their similarity to the Tethyan provinces. J. Afr. Earth Sci. 2023, 204, 104962. [Google Scholar] [CrossRef]
- Strougo, A.; Faris, M.; Abul-Nasr, R.A.; Gingerich, P.D.; Haggag, M.A. Planktonic Foraminifera and Calcareous Nannofossil Biostratigraphy Through the Middle to Late Eocene Transition at Wadi Hitan, Fayum Province, Egypt; The University of Michigan: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Marzouk, A.M.; El Shishtawy, A.M.; Kasem, A.M. Calcareous nannofossil and planktonic foraminifera biostratigraphy through the Middle to Late Eocene transition of Fayum area, Western Desert, Egypt. J. Afr. Earth Sci. 2014, 100, 303–323. [Google Scholar] [CrossRef]
- Abd El-Gaied, I.M.; Salama, Y.F.; Saber, S.G.; Sayed, M.M. Benthic foraminiferal communities of the Eocene platform, north Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 151, 121–135. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Abd El-Gaied, I.M.; Attia, G.M.; Mahmoud, A.E.-A.A.; Bakr, S.A. Foraminiferal biostratigraphy and paleoenvironment of the middle and Upper Eocene succession at Cairo-Helwan area, north Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 158, 103516. [Google Scholar] [CrossRef]
- Papazzoni, C.A.; Fornaciari, E.; Giusberti, L.; Vescogni, A.; Fornaciari, B. Integrating shallow benthic and calcareous nannofossil zones: The lower Eocene of the Monte Postale section (northern Italy). Palaios 2017, 32, 6–17. [Google Scholar] [CrossRef]
- Pipperr, M.; Reichenbacher, B. Biostratigraphy and paleoecology of benthic foraminifera from the Eggenburgian ”Ortenburger Meeressande” of southeastern Germany (Early Miocene, Paratethys).(With 8 figures and 2 tables). Neues Jahrb. Geol. Palaontol. Abh. 2009, 254, 41. [Google Scholar] [CrossRef]
- Serra-Kiel, J.; Hottinger, L.; Caus, E.; Drobne, K.; Ferrandez, C.; Jauhri, A.K.; Less, G.; Pavlovec, R.; Pignatti, J.; Samso, J.M. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bull. De La Société Géologique De Fr. 1998, 169, 281–299. [Google Scholar]
- Helal, S. Contribution to the Eocene benthic foraminifera and ostracoda of the Fayoum Depression, Egypt. Egypt. J. Paleontol. 2002, 2, 105–155. [Google Scholar]
- Helal, S.; Holcová, K. Response of foraminiferal assemblages on the middle Eocene climatic optimum and following climatic transition in the shallow tropical sea (the south Fayoum area, Egypt). Arab. J. Geosci. 2017, 10, 43. [Google Scholar]
- Sayed, D.M. Stratigraphical and Macropaleontological Study of the Eocene Succession at the Northern Nile Valley, Egypt. PhD Thesis, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt, 2023. [Google Scholar]
- Abd El-Aziz, S. Stratigraphy of the Eocene Sequences of Fayoum-Minia District, Egypt. PhD Thesis, Faculty of Science, Cairo University, Cairo, Egypt, 2002. [Google Scholar]
- Abd El-Aziz, S.M.; Abd El-Gaied, I.M. Middle and Late Eocene planktonic foraminiferal stydy of Wadi El Nazla and Wadi El Batts sections, Fayoum Depression, Egypt. Gremena 2007, 2, 887–910. [Google Scholar]
- Abdallah, A.; Helal, S.; Abdel Aziz, S. Planktic foraminiferal biostratigraphy of the Eastern Fayoum Depressioin, Egypt. In Proceedings of the 3rd International of Conference on the Geology of Africa, Assiut, Egypt, 7–10 December 2003; pp. 571–598. [Google Scholar]
- Abd El-Azeam, S. Stratigraphy and paleoenvironments of the Eocene rocks at wadi El hitan area, Fayoum depression, Egypt. Egypt. J. Paleontol. 2008, 8, 49–62. [Google Scholar]
- Abdel-Fattah, Z.A.; Gingras, M.K.; Caldwell, M.W.; George Pemberton, S. Sedimentary environments and depositional characteristics of the Middle to Upper Eocene whale-bearing succession in the Fayum Depression, Egypt. Sedimentology 2010, 57, 446–476. [Google Scholar]
- Al Menoufy, S.; Shreif, A. Benthic foraminifera and biostratigraphy of the Middle–Upper Eocene transition at Wadi Hitan area, Fayum, Egypt. Arab. J. Geosci. 2021, 14, 2004. [Google Scholar]
- Anan, T.; El Shahat, A. Provenance and sequence architecture of the Middle–Late Eocene Gehannam and Birket Qarun formations at Wadi Al Hitan, Fayum province, Egypt. J. Afr. Earth Sci. 2014, 100, 614–625. [Google Scholar]
- Peters, S.E.; Antar, M.S.M.; Zalmout, I.S.; Gingerich, P.D. Sequence stratigraphic control on preservation of late Eocene whales and other vertebrates at Wadi Al-Hitan, Egypt. Palaios 2009, 24, 290–302. [Google Scholar]
- Morsi, A.-M.M.; Boukhary, M.; Strougo, A. Middle–upper Eocene ostracods and nummulites from Gebel Na’alun, southeastern Fayum, Egypt. Rev. De Micropaléontol. 2003, 46, 143–160. [Google Scholar]
- Smith, A.G. Alpine deformation and the oceanic areas of the Tethys, Mediterranean, and Atlantic. Geol. Soc. Am. Bull. 1971, 82, 2039–2070. [Google Scholar]
- Beadnell, H.J.L. The Topography and Geology of the Fayum Province of Egypt; National Print. Department: Cairo, Egypt, 1905. [Google Scholar]
- Said, R. The Geology of Egypt; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1962; p. 377. [Google Scholar]
- King, C.; Underwood, C.; Steurbaut, E. Eocene stratigraphy of the Wadi Al-Hitan world heritage site and adjacent areas (Fayum, Egypt). Stratigraphy 2014, 11, 185–234. [Google Scholar] [CrossRef]
- Haggag, M.A.; Bolli, H.M. The origin of Globigerinatheka semiinvoluta (Keijzer), Upper Eocene, Fayoum area, Egypt. Neues Jahrb. Für Geol. Und Paläontologie-Monatshefte 1996, 6, 365–374. [Google Scholar] [CrossRef]
- Ghandour, I.M.; Bălc, R.; Faris, M.; Helal, S.; Mosa, G.A.; Aljahdali, M.H. New insight into the middle eocene calcareous nannoplankton biostratigraphy and paleoenvironment from fayoum and beni suef areas, Egypt. Riv. Ital. Di Paleontol. E Stratigr. 2023, 129. [Google Scholar] [CrossRef]
- Haggag, M.A. A comprehensive Egyptian Middle/Upper Eocene planktonic foraminiferal zonation. Egypt. J. Geol. 1992, 36, 97–118. [Google Scholar]
- Abdel-Fattah, Z.A. Facies transition and depositional architecture of the Late Eocene tide-dominated delta in northern coast of Birket Qarun, Fayum, Egypt. J. Afr. Earth Sci. 2016, 119, 185–203. [Google Scholar]
- Loeblich, A.R.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nosrand Reinhold Co.: New York, NY, USA, 1988; Volume 2, p. 970. [Google Scholar]
- Murray, J.W. Distribution and Ecology of Living Benthic Foraminiferids; Crane and Russak: New York, NY, USA, 1973; p. 274. [Google Scholar]
- Kaiho, K. Benthic foraminiferal dissolved-oxygen index and dissolved-oxygen levels in the modern ocean. Geology 1994, 22, 719–722. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Agnini, C.; Fornaciari, E.; Raffi, I.; Catanzariti, R.; Pälike, H.; Backman, J.; Rio, D. Biozonation and biochronology of Paleogene calcareous nannofossils from low and middle latitudes. Newsl. Stratigr. 2014, 47, 131–181. [Google Scholar] [CrossRef]
- Perch-Nielsen, K. Cenozoic calcareous nannofossils. In Plankton Stratigraphy; Cambridge University Press: Cambridge, UK, 1985; pp. 427–455. [Google Scholar]
- Wade, B.S.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth Sci. Rev. 2011, 104, 111–142. [Google Scholar]
- Wynd, J. Biofacies of the Iranian oil consortium agreement area. IOOC Rep. 1965, 1082, 89. [Google Scholar]
- Babazadeh, S.A.; Cluzel, D. New biostratigraphy and microfacies analysis of Eocene Jahrum Formation (Shahrekord region, High Zagros, West Iran). A carbonate platform within the Neo-Tethys oceanic realm. BSGF-Earth Sci. Bull. 2023, 194, 1. [Google Scholar]
- Changaei, K.; Babazadeh, S.A.; Arian, M.; Pirbaloti, B.A. Systematic paleontology of Bartonian larger benthic Foraminifera from Shahrekord region in High Zagros, Iran. Paleontol. Res. 2022, 27, 73–84. [Google Scholar] [CrossRef]
- Aly, H.H.; Abd El-Aziz, S.M.; Abd EL-Gaied, I.M. Middle and Upper Eocene benthic foraminifera from Wadi Bayad El Arab-Gebel Homret Shaibon area, Northeastern Beni Suef, Nile Valley, Egypt. Egypt. J. Paleontol 2011, 11, 79–131. [Google Scholar]
- El-Dawy, M.; Dakrory, A. Biostratigraphy and paleoecology of the middle Eocene benthic foraminifers of east Beni Suef area, Nile Valley, Egypt. In Proceedings of the 4th International Conference on the Geology of Africa, Assiut, Egypt, 15–17 November 2005; pp. 623–656. [Google Scholar]
- Shahin, A.; Bassal, A.; El-Halaby, O.; El-Baz, S. Middle Eocene benthonic foraminiferal biostratigraphy and paleoenvironment at the Qattamia area, northern Eastern Desert, Egypt. Egypt. J. Paleontol 2007, 7, 29. [Google Scholar]
- Shahin, A. Biostratigraphic significance, paleobiogeography and paleobathymetry of Tertiary Buliminacea and Bolivinacea in the western Sinai, Egypt. Neues Jahrb. Geol. Paläontologie Abh. 2000, 216, 195–231. [Google Scholar]
- El Dawy, M. Middle Eocene benthic foraminiferal biostratigraphy and paleoecology of east Beni Mazar area, Nile Valley, Egypt. Egypt. J. Geol. 1997, 41, 413–464. [Google Scholar]
- Elewa, A.; Omar, A.; Dakrory, A. Biostratigraphical and paleoenvironmental studies on some Eocene ostracodes and forminifers from the Fayum depression, western desert, Egypt. Egypt. J. Geol. 1998, 42, 439–469. [Google Scholar]
- Mansour, H.; Philobbos, E.; Abdu, F. Contribution to the geology of the east and northeast of Beni Suef, Nile Valley, Egypt. Qatar Univ. Sci. Bull. 1982, 11, 52–65. [Google Scholar]
- Berggren, W.A.; Pearson, P.N. A revised tropical to subtropical Paleogene planktonic foraminiferal zonation. J. Foraminifer. Res. 2005, 35, 279–298. [Google Scholar]
- Strougo, A. The middle Eocene/upper Eocene transition in Egypt reconsidered. Neues Jahrb. Geol. Paläontologie. Abh. 1992, 186, 71–89. [Google Scholar]
- Mukhopadhyay, S.K. Turborotalia cerroazulensis group in the Paleogene sequence of Cambay Basin, India with a note on the evolution of Turborotalia cunialensis (Toumarkine & Bolli). Rev. Paléobiologie 2005, 24, 29. [Google Scholar]
- Toumarkine, M.; Luterbacher, H.P. Paleocene and Eocene planktonic foraminifera. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perc-Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 87–154. [Google Scholar]
- Berggren, W.A.; Kent, D.V.; Swisher, C.C.; Aubry, M.-P. A Revised Cenozoic Geochronology and Chronostratigraphy; GeoScience World: McLean, VA, USA, 1995. [Google Scholar]
- Karoui-Yaakoub, N.; Grira, C.; Mtimet, M.S.; Negra, M.H.; Molina, E. Planktic foraminiferal biostratigraphy, paleoecology and chronostratigraphy across the Eocene/Oligocene boundary in northern Tunisia. J. Afr. Earth Sci. 2017, 125, 126–136. [Google Scholar]
- Özcan, E.; Less, G.; Jovane, L.; Catanzariti, R.; Frontalini, F.; Coccioni, R.; Giorgioni, M.; Rodelli, D.; Rego, E.S.; Kayğılı, S. Integrated biostratigraphy of the middle to upper Eocene Kırkgeçit Formation (Baskil section, Elazığ, eastern Turkey): Larger benthic foraminiferal perspective. Mediterr. Geosci. Rev. 2019, 1, 55–90. [Google Scholar]
- Abu Bakr, S.; Abd El-Gaied, I.M.; Abd El-Aziz, S.M.; Sayed, M.M.; Mahmoud, A. Planktonic Foraminifera of the Middle and Upper Eocene Successions at the Northwestern and Northeastern Sides of the Nile Valley, Egypt: Stratigraphic and Paleoenvironmental Implications. Diversity 2025, 17, 116. [Google Scholar] [CrossRef]
- Salama, Y.; Sayed, M.; Saber, S.; Abd El-Gaied, I. Eocene planktonic foraminifera from the north Eastern Desert, Egypt: Biostratigraphic, paleoenvironmental and sequence stratigraphy implications. Palaeontol. Electron. 2021, 24, a11. [Google Scholar]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In Proceedings of the Proceedings Second Planktonic Conference, Rome, Italy, 23–28 September 1970; Edizioni Tecnoscienza: Rome, Italy, 1971; pp. 739–785. [Google Scholar]
- Agnini, C.; Backman, J.; Boscolo-Galazzo, F.; Condon, D.; Fornaciari, E.; Galeotti, S.; Giusberti, L.; Grandesso, P.; Lanci, L.; Luciani, V. Proposal for the Global Boundary Stratotype Section and Point (GSSP) for the Priabonian Stage (Eocene) at the Alano section (Italy). Epis. J. Int. Geosci. 2020, 44, 151–173. [Google Scholar]
- Sayed, M.M.; Heinz, P.; El-Gaied, A.; Ibrahim, M.; Gier, S.; El-Kahawy, R.M.; Sayed, D.M.; Salama, Y.F.; Abuamarah, B.A.; Wagreich, M. Paleoenvironments and Paleoclimate Reconstructions of the Middle–Upper Eocene Rocks in the North–West Fayum Area (Western Desert, Egypt): Insights from Geochemical Data. Minerals 2025, 15, 227. [Google Scholar] [CrossRef]
- Bassiouni, M.e.A.A.; Boukhary, M.; Shamah, K.; Blondeau, A. Middle Eocene ostracodes from Fayoum, Egypt. Géologie Méditerranéenne 1984, 11, 181–192. [Google Scholar]
- Hadi, M.; Sarkar, S.; Vahidinia, M.; Bayet-Goll, A. Microfacies analysis of Eocene Ziarat Formation (eastern Alborz zone, NE Iran) and paleoenvironmental implications. All Earth 2021, 33, 66–87. [Google Scholar]
- Wilson, J.L. Carbonate Facies in Geologic History; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nisha, N.; Singh, A. Benthic foraminiferal biofacies on the shelf and upper continental slope off North Kerala (Southwest India). J. Geol. Soc. India 2012, 80, 783–801. [Google Scholar]
- Holbourn, A.; Henderson, A.S.; MacLeod, N. Atlas of Benthic Foraminifera; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Murray, J. Ecology and Palaeoecology of Benthic Foraminifera; Longman Scientific and Technical: Harlow, UK, 1991; p. 397. [Google Scholar]
- Miller, K.G.; Lohmann, G. Environmental distribution of Recent benthic foraminifera on the northeast United States continental slope. Geol. Soc. Am. Bull. 1982, 93, 200–206. [Google Scholar] [CrossRef]
- Saint-Marc, P. Qualitative and quantitative analysis of benthic foraminifers in Paleocene deep-sea sediments of the Sierra Leone Rise, central Atlantic. J. Foraminifer. Res. 1986, 16, 244–253. [Google Scholar] [CrossRef]
- El Ashwah, A.; El Deep, W. Late Cretaceous foraminiferal paleobathymetric study of Wadi El Natrun Well no.1 succession, northeastern part of Western Desert, Egypt. J. Geol. 2000, 44, 1–12. [Google Scholar]
- Smith, C.R.; Levin, L.A.; Hoover, D.J.; McMurtry, G.; Gage, J.D. Variations in bioturbation across the oxygen minimum zone in the northwest Arabian Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 227–257. [Google Scholar] [CrossRef]
- Vallim, A.L.; Schenone, S.; Thrush, S.F. Megafauna: The ignored bioturbators. Mar. Ecol. Prog. Ser. 2024, 733, 137–144. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, C.; Liu, J.P.; Jia, Y. Sediment Dynamics in Coastal and Marine Environments: Scientific Advances. Water 2023, 15, 1404. [Google Scholar] [CrossRef]
- Hardenbol, J.; Thierry, J.; Farley, M.B.; Jacquin, T.; De Graciansky, P.-C.; Vail, P.R. Mesozoic and Cenozoic Sequence Chronostratigraphic Framework of European Basins; GeoScience World: McLean, VA, USA, 1998. [Google Scholar]
- Buatois, L.A.; Mángano, M.G. Ichnology: Organism-Substrate Interactions in Space and Time; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Sharafi, M.; Rodriguez-Tovar, F.J.; Janočko, J.; Bayet-Goll, A.; Mohammadi, M.; Khanehbad, M. Environmental significance of trace fossil assemblages in a tide—Wave-dominated shallow-marine carbonate system (Lower Cretaceous), northern Neo-Tethys margin, Kopet-Dagh Basin, Iran. Int. J. Earth Sci. 2022, 111, 103–126. [Google Scholar] [CrossRef]
- Allmon, W.D. Whence Southern Gastropods? Paleobiogeography of Turritella in the Neogene of Florida. Paleobiology 1992, 18, 252–269. [Google Scholar]
- Anderson, L.C.; Hartman, J.H.; Wesselingh, F.P. Paleoecology and taphonomy of high-density Turritella accumulations. Palaios 2017, 32, 770–790. [Google Scholar]
- Fürsich, F.T.; Palmer, T.J.; Goodyear, K.L. Growth and life habits of the Jurassic bivalve Carolia from the Indian Himalayas. Palaeontology 2001, 44, 125–147. [Google Scholar]
- Kirby, M.X. Differences in growth rate and environmental responses among fossil and modern Ostrea from Chesapeake Bay. Mar. Biol. 2001, 139, 1057–1065. [Google Scholar]
- Kidwell, S.M. The stratigraphy of shell concentrations. Taphon. Releas. Data Locked Foss. Rec. 1991, 9, 211–290. [Google Scholar]
- Taylor, P.D.; Wilson, M.A. Palaeoecology and evolution of marine hard substrate communities. Earth Sci. Rev. 2003, 62, 1–103. [Google Scholar]
- Fürsich, F.; Oschmann, W. Shell beds as tools in basin analysis: The Jurassic of Kachchh, western India. J. Geol. Soc. 1993, 150, 169–185. [Google Scholar] [CrossRef]
- Morkhoven, V. Cenozoic cosmopolitan deep-water benthic foraminifera. Bull. Cent. Rech. Explor. -Prodroduction Elf-Aquitaine Mémoire V. 1986, 11, 90–91. [Google Scholar]
- Culver, S.J. New foraminiferal depth zonation of the northwestern Gulf of Mexico. Palaios 1988, 3, 69–85. [Google Scholar] [CrossRef]
- Van der Zwaan, G.; Jorissen, F.; De Stigter, H. The depth dependency of planktonic/benthic foraminiferal ratios: Constraints and applications. Mar. Geol. 1990, 95, 1–16. [Google Scholar] [CrossRef]
- Dumas, S.; Arnott, R. Origin of hummocky and swaley cross-stratification—The controlling influence of unidirectional current strength and aggradation rate. Geology 2006, 34, 1073–1076. [Google Scholar] [CrossRef]
- Tribovillard, N.; Bout-Roumazeilles, V.; Abraham, R.; Ventalon, S.; Delattre, M.; Baudin, F. The contrasting origins of glauconite in the shallow marine environment highlight this mineral as a marker of paleoenvironmental conditions. Comptes Rendus. Géoscience 2023, 355, 213–228. [Google Scholar] [CrossRef]
- Andreetto, F.; Flecker, R.; Aloisi, G.; Mancini, A.; Guibourdenche, L.; de Villiers, S.; Krijgsman, W. High-amplitude water-level fluctuations at the end of the Mediterranean Messinian Salinity Crisis: Implications for gypsum formation, connectivity and global climate. Earth Planet. Sci. Lett. 2022, 595, 117767. [Google Scholar]
- MacEachern, J.A.; Gingras, M.K. Recognition of Brackish-Water Trace-Fossil Suites in the Cretaceous Western Interior Seaway of Alberta, Canada; GeoScience World: McLean, VA, USA, 2007. [Google Scholar]
- Catuneanu, O.; Galloway, W.E.; Kendall, C.G.S.C.; Miall, A.D.; Posamentier, H.W.; Strasser, A.; Tucker, M.E. Sequence stratigraphy: Methodology and nomenclature. Newsl. Stratigr. 2011, 44, 173–245. [Google Scholar]
- Bhattacharya, J.P.; MacEachern, J.A. Hyperpycnal rivers and prodeltaic shelves in the Cretaceous seaway of North America. J. Sediment. Res. 2009, 79, 184–209. [Google Scholar]
- Ekdale, A. Muckraking and mudslinging: The joys of deposit-feeding. Short Courses Paleontol. 1992, 5, 145–171. [Google Scholar]
- Seilacher, A. Sedimentary structures tentatively attributed to synsedimentary tectonics. J. Sediment. Petrol. 1982, 52, 125–136. [Google Scholar]
- MacEachern, J.A.; Bann, K.L.; Pemberton, S.G.; Gingras, M.K. The Ichnofacies Paradigm: High-Resolution Paleoenvironmental Interpretation of the Rock Record; GeoScience World: McLean, VA, USA, 2007. [Google Scholar]
- Gingras, M.K.; MacEachern, J.A.; Dashtgard, S.E.; Pemberton, S.G. Process Ichnology and the Application of Ichnofacies in Paleoenvironmental Analysis; Taylor and Francis: Abingdon, UK, 2011; Volume 103, pp. 19–38. [Google Scholar]
- Rodrigues, C.F.; Buatois, L.A. Deep-sea trace fossils and their implications for the evolution of deep-marine benthic communities. Earth Sci. Rev. 2020, 209, 103307. [Google Scholar]
- Pemberton, S.G.; Frey, R.W. The Glossifungites Ichnofacies: Modern Examples from the Georgia Coast, USA; GeoScience World: McLean, VA, USA, 1985. [Google Scholar]
- Nara, M.; Seike, K. Taphonomy of crustacean burrows: Fossilization potential of modern Thalassinoides in a tidal flat. Lethaia 2019, 52, 574–586. [Google Scholar]
- Knaust, D. Atlas of Trace Fossils in Well Core: Appearance, Taxonomy and Interpretation; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Gentzis, T.; Carvajal-Ortiz, H.; Selim, S.S.; Tahoun, S.S.; El-Shafeiy, M.; Ocubalidet, S.; Ali, A.A.-M. Depositional environment and characteristics of Late Eocene carbonaceous swampy tidal flat facies in the Fayoum Basin, Egypt. Int. J. Coal Geol. 2018, 200, 45–58. [Google Scholar]
- Haq, B.U.; Hardenbol, J.; Vail, P.R. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar]
- Snedden, J.W.; Liu, C. Recommendations for a uniform chronostratigraphic designation system for Phanerozoic depositional sequences. AAPG Bull. 2011, 95, 1095–1122. [Google Scholar]
- Guiraud, R.; Bosworth, W. Phanerozoic geodynamic evolution of northeastern Africa and the northwestern Arabian platform. Tectonophysics 1999, 315, 73–104. [Google Scholar]
- Bosworth, W.; Huchon, P.; McClay, K. The red sea and gulf of aden basins. J. Afr. Earth Sci. 2005, 43, 334–378. [Google Scholar] [CrossRef]
- Moustafa, A.R.; Khalil, S.M.; El-Araby, H.M. Tectonic evolution of the northern Red Sea rift: Insights from the NW Red Sea and Gulf of Suez regions. J. Afr. Earth Sci. 2018, 142, 34–52. [Google Scholar]
- Miller, K.G.; Kominz, M.A.; Browning, J.V.; Wright, J.D.; Mountain, G.S.; Katz, M.E.; Sugarman, P.J.; Cramer, B.S.; Christie-Blick, N.; Pekar, S.F. The Phanerozoic record of global sea-level change. Science 2005, 310, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, R.; Bosworth, W.; Thierry, J.; Delplanque, A. Phanerozoic geological evolution of Northern and Central Africa: An overview. J. Afr. Earth Sci. 2005, 43, 83–143. [Google Scholar] [CrossRef]
- El Baz, S.M.; Wanas, H.A.; Abou Awad, H.A.; Assal, E.M. Biostratigraphy, palaeoecology, and palaeobiogeography of the Middle-Late Eocene ostracods, north-west Fayoum area, Egypt. Geol. J. 2022, 57, 3686–3705. [Google Scholar] [CrossRef]
- Morsi, A.-M.M.; Speijer, R.P. High-resolution ostracode records of the paleocene/eocene transition in the South Eastern Desert of Egypt—Taxonomy, biostratigraphy, paleoecology and paleobiogeography. Senckenberg. Lethaea 2003, 83, 61–93. [Google Scholar] [CrossRef]
- Youssef, M.; Ismail, A.; El-Sorogy, A. Paleoecology and paleobiogeography of Paleocene ostracods in Dineigil area, south Western Desert, Egypt. J. Afr. Earth Sci. 2017, 131, 62–70. [Google Scholar] [CrossRef]
- Bassiouni, M.E.-A.A.; Luger, P. Maastrichtian to early Eocene Ostracoda from southern Egypt. Palaeontol. Palaeoecol. Paleobiogeography Biostratigraphy Berl. Geowiss. Abh A 1990, 120, 755–928. [Google Scholar]
- Hamdi, A.A.; Lattrache, K.B.I. Les ostracodes et foraminifères associés des dépôts de l’Éocène moyen et supérieur de la coupe de Jebel Serj (Tunisie centrale). Intérêt biostratigraphique, paléoécologique et paléobiogéographique. Rev. De Micropaléontologie 2013, 56, 159–174. [Google Scholar] [CrossRef]
- Amami-Hamdi, A.; Dhahri, F.; Jomaa-Salmouna, D.; Ismail-Lattrache, K.B.; Chaabane, N.B. Quantative analysis and paleoecology of middle to upper Eocene ostracods from Jebel Jebil, central Tunisia. Rev. De Micropaléontologie 2016, 59, 409–424. [Google Scholar] [CrossRef]


| Sample No. | FM | Taxa_S | Individuals | Dominance_D | Shannon_H |
|---|---|---|---|---|---|
| 1 | Gehannam Fm | 49 | 406 | 0.06309 | 3.227 |
| 2 | 5 | 12 | 0.2361 | 1.517 | |
| 4 | 12 | 76 | 0.1908 | 2.025 | |
| 5 | 2 | 3 | 0.5556 | 0.6365 | |
| 6 | 11 | 45 | 0.1625 | 2.04 | |
| 7 | 3 | 3 | 0.3333 | 1.099 | |
| 9 | 1 | 1 | 1 | 0 | |
| 10 | 10 | 40 | 0.16 | 2.008 | |
| 11 | 5 | 6 | 0.2222 | 1.561 | |
| 13 | 4 | 6 | 0.2778 | 1.33 | |
| 14 | 1 | 2 | 1 | 0 | |
| 15 | 2 | 3 | 0.5556 | 0.6365 | |
| 16 | 2 | 3 | 0.5556 | 0.6365 | |
| 18 | 32 | 188 | 0.05794 | 3.137 | |
| 19 | 33 | 190 | 0.05518 | 3.159 | |
| 20 | 24 | 339 | 0.1542 | 2.364 | |
| 21 | 31 | 577 | 0.1188 | 2.672 | |
| 22 | Birket Qarun Fm | 4 | 9 | 0.2593 | 1.369 |
| 25 | 8 | 20 | 0.14 | 2.016 | |
| 27 | 8 | 21 | 0.1429 | 2.004 | |
| 29 | 8 | 14 | 0.1429 | 2.008 | |
| 33 | 9 | 19 | 0.1302 | 2.114 | |
| 34 | 9 | 21 | 0.1202 | 2.153 | |
| 35 | 4 | 8 | 0.2813 | 1.321 | |
| 37 | Qasr El Sagha Fm | 14 | 62 | 0.1733 | 2.177 |
| 40 | 12 | 39 | 0.1006 | 2.373 | |
| 46 | 27 | 66 | 0.06336 | 3.035 | |
| 47 | 23 | 88 | 0.1059 | 2.631 | |
| 48 | 16 | 158 | 0.378 | 1.656 | |
| 50 | 1 | 2 | 1 | 0 | |
| 51 | 2 | 3 | 0.5556 | 0.6365 | |
| 52–60 | Barren | ||||
| Species | Egypt | Libya | Italy | France | Spain | England |
|---|---|---|---|---|---|---|
| Cancris a. primitivus | 1 | 0 | 1 | 1 | 0 | 0 |
| Cancris danvillensis | 1 | 0 | 0 | 0 | 0 | 0 |
| Cancris amplus | 1 | 1 | 0 | 0 | 0 | 0 |
| Cancris subconicus | 1 | 0 | 1 | 1 | 0 | 0 |
| Spiroloculina dorsata | 1 | 0 | 0 | 1 | 0 | 1 |
| Spiroloculina esnaensis | 1 | 0 | 0 | 0 | 0 | 0 |
| Quinqueloculina seminula | 1 | 0 | 1 | 1 | 0 | 1 |
| Cibicides mabahethi | 1 | 1 | 0 | 0 | 0 | 0 |
| Cibicidoides laurisae | 1 | 1 | 0 | 0 | 0 | 0 |
| Cibicides libycus | 1 | 1 | 0 | 0 | 0 | 0 |
| Planulina cocoaensis | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina alabamensis | 1 | 0 | 0 | 0 | 0 | 0 |
| Lenticulina alatolimbata | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina clerici | 1 | 1 | 0 | 0 | 1 | 0 |
| Lenticulina cultrata | 1 | 1 | 0 | 0 | 1 | 0 |
| Lenticulina cuvillieri | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina cf. ellisori | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina isidis | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina limbata | 1 | 1 | 1 | 0 | 0 | 0 |
| Lenticulina turbinata | 1 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina yaguatensis | 1 | 1 | 0 | 0 | 1 | 0 |
| Stillostomella jacksonensis | 1 | 1 | 0 | 0 | 0 | 0 |
| Bolivina anglica | 1 | 1 | 0 | 1 | 0 | 0 |
| Bolivina brabantica | 1 | 0 | 0 | 0 | 0 | 0 |
| Bolivina carinata | 1 | 1 | 0 | 1 | 1 | 0 |
| Bolivina jacksonensis | 1 | 1 | 0 | 0 | 0 | 0 |
| Bolivina j. striatella | 1 | 0 | 0 | 0 | 0 | 0 |
| Bolivina kuriani | 1 | 0 | 0 | 0 | 0 | 0 |
| Brizalina cookei | 1 | 0 | 0 | 0 | 0 | 0 |
| Nonionella spissa | 1 | 0 | 0 | 0 | 0 | 0 |
| Lagena striata | 1 | 0 | 1 | 1 | 0 | 0 |
| Fursenkoina dibolensis | 1 | 0 | 0 | 1 | 1 | 0 |
| Fursenkoina squamosa | 1 | 0 | 0 | 0 | 0 | 0 |
| Uvigerina cocoaensis | 1 | 1 | 0 | 1 | 0 | 0 |
| Uvigerina hispidocostata | 1 | 1 | 0 | 0 | 1 | 0 |
| Uvigerina mexicana | 1 | 1 | 1 | 0 | 0 | 0 |
| Uvigerina rippensis | 1 | 1 | 0 | 0 | 1 | 0 |
| Uvigerina seriata | 1 | 0 | 0 | 0 | 0 | 0 |
| Bulimina jacksonensis | 1 | 1 | 0 | 0 | 0 | 0 |
| Textularia adalta | 1 | 0 | 1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, M.M.; Heinz, P.; Abd El-Gaied, I.M.; El-Kahawy, R.M.; Sayed, D.M.; Salama, Y.F.; Al-Hashim, M.H.; Wagreich, M. Paleobiodiversity, Paleobiogeography, and Paleoenvironments of the Middle–Upper Eocene Benthic Foraminifera in the Fayum Area, Western Desert, Egypt. J. Mar. Sci. Eng. 2025, 13, 663. https://doi.org/10.3390/jmse13040663
Sayed MM, Heinz P, Abd El-Gaied IM, El-Kahawy RM, Sayed DM, Salama YF, Al-Hashim MH, Wagreich M. Paleobiodiversity, Paleobiogeography, and Paleoenvironments of the Middle–Upper Eocene Benthic Foraminifera in the Fayum Area, Western Desert, Egypt. Journal of Marine Science and Engineering. 2025; 13(4):663. https://doi.org/10.3390/jmse13040663
Chicago/Turabian StyleSayed, Mostafa M., Petra Heinz, Ibrahim M. Abd El-Gaied, Ramadan M. El-Kahawy, Dina M. Sayed, Yasser F. Salama, Mansour H. Al-Hashim, and Michael Wagreich. 2025. "Paleobiodiversity, Paleobiogeography, and Paleoenvironments of the Middle–Upper Eocene Benthic Foraminifera in the Fayum Area, Western Desert, Egypt" Journal of Marine Science and Engineering 13, no. 4: 663. https://doi.org/10.3390/jmse13040663
APA StyleSayed, M. M., Heinz, P., Abd El-Gaied, I. M., El-Kahawy, R. M., Sayed, D. M., Salama, Y. F., Al-Hashim, M. H., & Wagreich, M. (2025). Paleobiodiversity, Paleobiogeography, and Paleoenvironments of the Middle–Upper Eocene Benthic Foraminifera in the Fayum Area, Western Desert, Egypt. Journal of Marine Science and Engineering, 13(4), 663. https://doi.org/10.3390/jmse13040663