Bivalves and Gastropods: Models for the Study of Mucomics
Abstract
1. Introduction
2. The Glycosylation of Mucins in Mollusks: Structures, Functions, and Environmental Impacts
3. The Role of Mucins in Mollusks: Immune Protection, Digestion, and Environmental Adaptations
4. Models Study
4.1. Gastropods
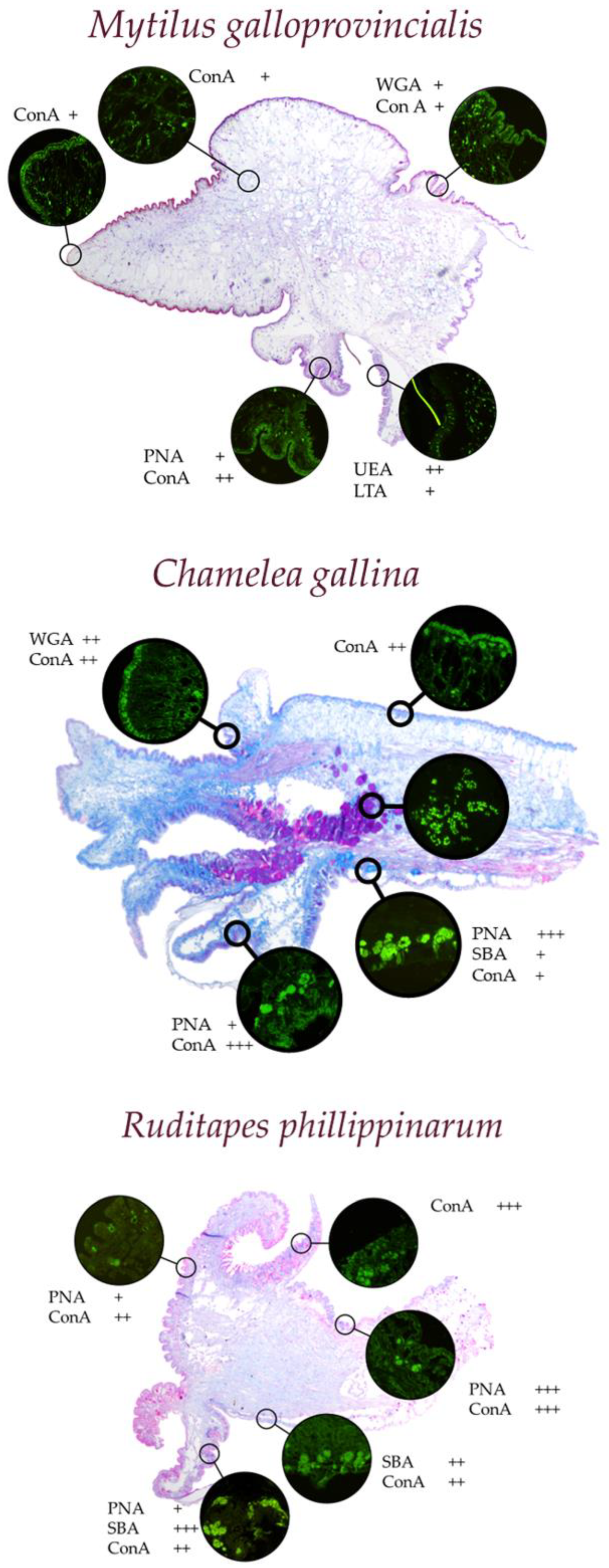
4.2. Bivalves
5. Investigative Techniques and Contaminants
| Lectin | Origin | Buffer | Dilution | Inhibitory Sugar Conc. |
|---|---|---|---|---|
| PNA | Arachis hypogaea | Hepes | 10 mg/mL | 0.2 M Gal |
| SBA | Glycine max | Hepes | 20 mg/mL | 0.2 M GalNAc |
| WGA | Triticum vulgaris | Hepes | 20 mg/mL | 0.5 M GlcNAc |
| LTA | Tetragonolobus purpureus | Hepes | 20 mg/mL | 0.2 M L-Fuc |
| UEA I | Ulex europaeus | Hepes | 10 mg/mL | 0.2 M L-Fuc |
| AAL | Aleuria aurantia | Hepes | 10 mg/mL | 0.2 M L-Fuc |
| ConA | Canavalia ensiformis | Hepes | 20 mg/mL | 0.1 M MαM |
6. Contaminants and Mucus Functionality
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, M.S.; Hawkins, S. Mucus from marine molluscs. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 1–71. [Google Scholar]
- Carlucci, R.; Mentino, D.; Semeraro, D.; Ricci, P.; Sion, L.; Scillitani, G. Comparative histochemical analysis of intestinal glycoconjugates in the blunthead pufferfish Sphoeroides pachygaster and grey triggerfish Balistes capriscus (Teleostei: Tetraodontiformes). J. Fish. Biol. 2019, 94, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Gristina, M.; Bertrandino, S.; Cardone, F.; Mentino, D.; Corriero, G.; Scillitani, G. Skin filament recovery after clipping in Hippocampus guttulatus: Behavioural and histological aspects. Aquat. Biol. 2017, 26, 149–157. [Google Scholar] [CrossRef]
- Liquori, G.E.; Mastrodonato, M.; Zizza, S.; Ferri, D. Glycoconjugate histochemistry of the digestive tract of Triturus carnifex (Amphibia, Caudata). J. Mol. Histol. 2007, 38, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, R.; Cipriano, G.; Fanizza, C.; Gerussi, T.; Maglietta, R.; Petrella, A.; Pietroluongo, G.; Ricci, P.; Semeraro, D.; Guglielmi, M.V.; et al. Glycopatterns of the foregut in the striped dolphin Stenella coeruleoalba, Meyen 1833 from the Mediterranean Sea. Mar. Mammal Sci. 2021, 38, 190–211. [Google Scholar] [CrossRef]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wahltinez, S.J.; Harms, C.A.; Lewbart, G.A. Anesthesia and analgesia in invertebrates. In Anesthesia and Analgesia in Laboratory Animals; Melissa, C., Dyson, P.J., Lofgren, J., Nunamaker, E.A., Pang, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 647–671. [Google Scholar]
- Cerullo, A.R.; McDermott, M.B.; Pepi, L.E.; Liu, Z.-L.; Barry, D.; Zhang, S.; Yang, X.; Chen, X.; Azadi, P.; Holford, M.; et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum Secretions. Nat. Commun. 2023, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Calabro, C.; Albanese, M.P.; Martella, S.; Licata, P.; Lauriano, E.R.; Bertuccio, C.; Licata, A. Glycoconjugate histochemistry and nNOS immunolocalization in the mantle and foot epithelia of Tapes philippinarum (bivalve mollusc). Folia Histochem. Cytobiol. 2005, 43, 151–156. [Google Scholar]
- Eble, A.F. Chapter 4 Anatomy and histology of Mercenaria mercenaria. In Biology of the Hard Clam; Elsevier: Amsterdam, The Netherlands, 2001; pp. 117–220. [Google Scholar]
- Espinosa, E.P.; Perrigault, M.; Ward, J.E.; Shumway, S.E.; Allam, B. Lectins associated with the feeding organs of the oyster Crassostrea virginica can mediate particle selection. Biol. Bull. 2009, 217, 130–141. [Google Scholar] [CrossRef]
- Prezant, R.S. Form, Function and Phylogeny of Bivalve Mucins; Hong Kong University Press: Hong Kong, China, 1990. [Google Scholar]
- Bolognani Fantin, A.; Vigo, E. La mucinogenesi nei Molluschi. IV. Caratteristiche istochimiche dei tipi cellulari presenti nel piede e nel mantello di alcune specie di Gasteropodi. Riv. Istochem. Norm. Pat 1967, 13, 1–28. [Google Scholar]
- Smolowitz, R. Mollusca: Bivalvia. Invertebr. Hist. 2021, 163–183. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, J. Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: A review. IIOAB J. 2011, 2, 49–57. [Google Scholar]
- Cofone, R.; Carraturo, F.; Capriello, T.; Libralato, G.; Siciliano, A.; Del Giudice, C.; Maio, N.; Guida, M.; Ferrandino, I. Eobania vermiculata as a potential indicator of nitrate contamination in soil. Ecotoxicol. Environ. Saf. 2020, 204, 111082. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Batista-Pinto, C. Stomach of Aplysia depilans (Mollusca, Opisthobranchia): A histochemical, ultrastructural, and cytochemical study. J. Morphol. 2003, 256, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Petraccioli, A.; Maio, N.; Guarino, F.M.; Scillitani, G. Seasonal variation in glycoconjugates of the pedal glandular system of the rayed Mediterranean limpet, Patella caerulea (Gastropoda: Patellidae). Zoology 2013, 116, 186–196. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Campbell, M.M.; Thompson, R.P.; McCrohan, C.R.; White, K.N.; Powell, J.J. Mucus secretion by the freshwater snail Lymnaea stagnalis limits aluminum concentrations of the aqueous environment. Environ. Sci. Technol. 1998, 32, 2591–2595. [Google Scholar] [CrossRef]
- Colella, F.; Scillitani, G.; Pierri, C.L. Sweet as honey, bitter as bile: Mitochondriotoxic peptides and other therapeutic proteins isolated from animal tissues, for dealing with mitochondrial apoptosis. Toxicology 2021, 447, 152612. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, M.V.; Semeraro, D.; Ricci, P.; Mastrodonato, M.; Mentino, D.; Carlucci, R.; Mastrototaro, F.; Scillitani, G. First data on the effect of Aluminium intake in Chamelea gallina of exploited stocks in the southern Adriatic Sea (Central Mediterranean Sea). Reg. Stud. Mar. Sci. 2023, 63, 103025. [Google Scholar] [CrossRef]
- Perić, L.; Perusco, V.S.; Nerlović, V. Differential response of biomarkers in the native European flat oyster Ostrea edulis and the non-indigenous Pacific oyster Crassostrea gigas co-exposed to cadmium and copper. J. Exp. Mar. Biol. Ecol. 2020, 523, 151271. [Google Scholar] [CrossRef]
- Spada, L.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; Di Leo, A. Heavy metals monitoring in mussels Mytilus galloprovincialis from the Apulian coasts (Southern Italy). Med. Mar. Sci. 2013, 14, 99–108. [Google Scholar] [CrossRef]
- Moschino, V.; Delaney, E.; Meneghetti, F.; Ros, L.D. Biomonitoring approach with mussel Mytilus galloprovincialis (Lmk) and clam Ruditapes philippinarum (Adams and Reeve, 1850) in the Lagoon of Venice. Environ. Monit. Assess. 2011, 177, 649–663. [Google Scholar] [CrossRef]
- Farrington, J.W.; Tripp, B.W.; Tanabe, S.; Subramanian, A.; Sericano, J.L.; Wade, T.L.; Knap, A.H.; Edward, D. Goldberg’s proposal of “the mussel watch”: Reflections after 40 years. Mar. Poll. Bull. 2016, 110, 501–510. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Bowen, V.T.; Farrington, J.W.; Harvey, G.; Martin, J.H.; Parker, P.L.; Risebrough, R.W.; Robertson, W.; Schneider, E.; Gamble, E. The mussel watch. Environ. Cons. 1978, 5, 101–125. [Google Scholar] [CrossRef]
- Allam, B.; Espinosa, E.P. Mucosal immunity in mollusks. In Mucosal Health in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 325–370. [Google Scholar]
- Beninger, P.; St-Jean, S. The role of mucus in particle processing by suspension-feeding marine bivalves: Unifying principles. Mar. Biol. 1997, 129, 389–397. [Google Scholar] [CrossRef]
- González-Costa, A.; Fernández-Gago, R.; Carid, S.; Molist, P. Mucus characterisation in the Octopus vulgaris skin throughout its life cycle. Anat. Histol. Embryol. 2020, 49, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research—A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49 (Suppl. S2), 1–90. [Google Scholar] [CrossRef] [PubMed]
- Accogli, G.; Scillitani, G.; Mentino, D.; Desantis, S. Characterization of the skin mucus in the common octopus Octopus vulgaris (Cuvier) reared paralarvae. Eur. J. Histochem. EJH 2017, 61, 2518. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, M.W.; Kim, B.H.; Kim, H.; Jeon, M.A.; Lee, J.S. Microanatomy and ultrastructure of outer mantle epidermis of the cuttlefish, Sepia esculenta (Cephalopoda: Sepiidae). Micron 2014, 58, 38–46. [Google Scholar] [CrossRef]
- Strous, G.J.; Dekker, J. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 57–92. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Bivalve filter feeding revisited. Mar. Ecol. Prog. Ser. 1996, 142, 287–302. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bolognani-Fantin, A.; Vigo, E.; Gervaso, M. La mucinogenesi nei Molluschi. VI. Caratteristiche dei tipi cellulari presenti nel piede e nel mantello di alcune specie di Lamellibranchi. Riv. Istochem. Norm. Patol. 1969, 15, 345–370. [Google Scholar]
- Ottaviani, E.; Fantin, A.M.B.; Bolognani, L. Muramic acid as a glycoconjugate component in Mollusca Gastropoda. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 627–632. [Google Scholar] [CrossRef]
- Albanese, M.P.; Calabro, C. Studio strutturale ed istochimico sul mantello e sul piede di natica millepunctata lmk (MOLL. GAST. PROS.). Riv. Idrobiol. 1996, 35, 87–99. [Google Scholar]
- Robledo, Y.; Madrid, J.F.; Leis, O.; Cajaraville, M.P. Analysis of the distribution of glycoconjugates in the digestive gland of the bivalve mollusc Mytilus galloprovincialis by conventional and lectin histochemistry. Cell Tissue Res. 1997, 288, 591–602. [Google Scholar] [CrossRef]
- Bravo Portela, I.; Martínez-Zorzano, V.S.; Molist-Pérez, I.; Molist Garcia, P. Ultrastructure and glycoconjugate pattern of the foot epithelium of the abalone Haliotis tuberculata (Linnaeus, 1758) (Gastropoda, Haliotidae). Sci. World J. 2012, 2012, 960159. [Google Scholar] [CrossRef]
- Ahn, H.-Y.; Sue, L.-F.; Ma, J.K.; Pinkstaff, C.A.; Pore, R.S.; Overman, D.O.; Malanga, C.J. Synthesis and secretion fo mucous glycoprotein by the gill of Mytilus edulis I. Histochemical and chromatographic analysis of [14C] glucosamine bioincorporation. Biochim. Biophys. Acta Gen. Subj. 1988, 966, 122–132. [Google Scholar] [CrossRef]
- Nogarol, L.R.; Brossi-Garcia, A.L.; de Oliveira David, J.A.; Fontanetti, C.S. Morphological and histochemical characterization of gill filaments of the brazilian endemic bivalve Diplodon expansus (Küster, 1856) (Mollusca, Bivalvia, Hyriidae). Microsc. Microanal. 2012, 18, 1450–1458. [Google Scholar] [CrossRef]
- Bilgin, M.; Uluturhan-Suzer, E. Assessment of trace metal concentrations and human health risk in clam (Tapes decussatus) and mussel (Mytilus galloprovincialis) from the Homa Lagoon (Eastern Aegean Sea). Environ. Sci. Pollut. Res. 2017, 24, 4174–4184. [Google Scholar] [CrossRef]
- Davies, M.; Hawkins, S.; Jones, H. Mucus production and physiological energetics in Patella vulgata L. J. Molluscan Stud. 1990, 56, 499–503. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Guarnieri, A.; Pietrangelo, L.; Magnifico, I.; Venditti, N.; Recchia, L.; Mangano, K.; Nicoletti, F.; Di Marco, R.; Petronio, G. Potential mucosal irritation discrimination of surface disinfectants employed against SARS-CoV-2 by Limacus flavus slug mucosal irritation assay. Biomedicines 2021, 9, 424. [Google Scholar] [CrossRef]
- de Oliveira David, J.A.; Fontanetti, C.S. The Role of Mucus in Mytella falcata (Orbigny 1842) gills from polluted environments. Water Air Soil Pollut. 2009, 203, 261–266. [Google Scholar] [CrossRef]
- Goldberg, E.D. The mussel watch—A first step in global marine monitoring. Mar. Pollut. Bull. 1975, 6, 111. [Google Scholar] [CrossRef]
- Kádár, E.; Salánki, J.; Jugdaohsingh, R.; Powell, J.J.; McCrohan, C.R.; White, K.N. Avoidance responses to aluminium in the freshwater bivalve Anodonta cygnea. Aquat. Toxicol. 2001, 55, 137–148. [Google Scholar] [CrossRef]
- March, F.A.; Dwyer, F.J.; Augspurger, T.; Ingersoll, C.G.; Wang, N.; Mebane, C.A. An evaluation of freshwater mussel toxicity data in the derivation of water quality guidance and standards for copper. Environ. Toxicol. Chem. 2007, 26, 2066–2074. [Google Scholar] [CrossRef]
- Helmholz, H.; Ruhnau, C.; Pröfrock, D.; Erbslöh, H.-B.; Prange, A. Seasonal and annual variations in physiological and biochemical responses from transplanted marine bioindicator species Mytilus spp. during a long term field exposure experiment. Sci. Total Environ. 2016, 565, 626–636. [Google Scholar] [CrossRef]
- Robledo, J.A.F.; Yadavalli, R.; Allam, B.; Espinosa, E.P.; Gerdol, M.; Greco, S.; Stevick, R.J.; Gómez-Chiarri, M.; Zhang, Y.; Heil, C.A. From the raw bar to the bench: Bivalves as models for human health. Dev. Comp. Immunol. 2019, 92, 260–282. [Google Scholar] [CrossRef]
- Pinzon Martin, S.; Seeberger, P.H.; Varon Silva, D. Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators During Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019, 7, 710. [Google Scholar]
- Sheng, Y.H.; Hasnain, S.Z. Mucus and Mucins: The Underappreciated Host Defence System. Front. Cell Infect. Microbiol. 2022, 12, 856962. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Montagne, L.; Piel, C.; Lalles, J.P. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr. Rev. 2004, 62, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.V.; Ravindran, C.; Irudayarajan, L. Beneficial properties of mucus in coral adaptations and ecological interactions. Mar. Biol. 2024, 171, 46. [Google Scholar] [CrossRef]
- Hanson, R.; Hollingsworth, M. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling). Biomolecules 2016, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, K.S.; Gad, A.F.; Radwan, M.A. Physiological and behavioral responses of land molluscs as biomarkers for pollution impact assessment: A review. Environ. Res. 2021, 193, 110558. [Google Scholar] [CrossRef]
- Guglielmi, M.V.; Mastrodonato, M.; Semeraro, D.; Mentino, D.; Capriello, T.; La Pietra, A.; Giarra, A.; Scillitani, G.; Ferrandino, I. Aluminum exposure alters the pedal mucous secretions of the chocolate-band snail, Eobania vermiculata (Gastropoda: Helicidae). Microsc. Res. Tech. 2024, 87, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- De Gregori, I.; Pinochet, H.; Gras, N.; Muñoz, L. Variability of cadmium, copper and zinc levels in molluscs and associated sediments from Chile. Environ. Pollut. 1996, 92, 359–368. [Google Scholar] [CrossRef]
- Campanella, L.; Conti, M.E.; Cubadda, F.; Sucapane, C. Trace metals in seagrass, algae and molluscs from an uncontaminated area in the Mediterranean. Environ. Pollut. 2001, 111, 117–126. [Google Scholar] [CrossRef]
- Rainbow, P.; Wolowicz, M.; Fialkowski, W.; Smith, B.; Sokolowski, A. Biomonitoring of trace metals in the Gulf of Gdansk, using mussels (Mytilus trossulus) and barnacles (Balanus improvisus). Water Res. 2000, 34, 1823–1829. [Google Scholar] [CrossRef]
- Lee, C.S.-L.; Li, X.; Shi, W.; Cheung, S.C.-N.; Thornton, I. Metal contamination in urban, suburban, and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Sci. Total Environ. 2006, 356, 45–61. [Google Scholar] [CrossRef]
- Wagner, A.; Boman, J. Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectroch Acta Part B At. Spectrosc. 2003, 58, 2215–2226. [Google Scholar] [CrossRef]
- Camusso, M.; Balestrini, R.; Muriano, F.; Mariani, M. Use of freshwater mussel Dreissena polymorpha to assess trace metal pollution in the lower river Po (Italy). Chemosphere 1994, 29, 729–745. [Google Scholar] [CrossRef]
- Di Leo, A.; Cardellicchio, N.; Giandomenico, S.; Spada, L. Mercury and methylmercury contamination in Mytilus galloprovincialis from Taranto Gulf (Ionian Sea, Southern Italy): Risk evaluation for consumers. Food Chem. Toxicol. 2010, 48, 3131–3136. [Google Scholar] [CrossRef]
- Idris, A.M.; Eltayeb, M.; Potgieter-Vermaak, S.S.; Van Grieken, R.; Potgieter, J. Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem. J. 2007, 87, 104–112. [Google Scholar] [CrossRef]
- Sokolova, I.; Ringwood, A.; Johnson, C. Tissue-specific accumulation of cadmium in subcellular compartments of eastern oysters Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Aquatic Toxicol. 2005, 74, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Chouikh, N.-E.; Alahyane, H.; Mounir, A.; El Hachimi, Y.; Cheggour, M. Trace metal bioaccumulation in Mytilus galloprovincialis from Essaouira protected coastal area (Atlantic coast of Morocco): Implications for marine ecosystem and human health. Mar. Pollut. Bull. 2024, 209, 117126. [Google Scholar] [CrossRef] [PubMed]
- Miglioli, A.; Tredez, M.; Boosten, M.; Sant, C.; Carvalho, J.E.; Dru, P.; Canesi, L.; Schubert, M.; Dumollard, R. The Mediterranean mussel Mytilus galloprovincialis: A novel model for developmental studies in mollusks. Development 2024, 151, dev202256. [Google Scholar] [CrossRef]
- Guglielmi, M.V.; Semeraro, D.; Mentino, D.; Mastrodonato, M.; Mastrototaro, F.; Scillitani, G. Season- and sex-related variation in mucin secretions of the striped Venus clam, Chamelea gallina (Linnaeus, 1758) (Bivalvia: Veneridae). Eur. Zool. J. 2023, 90, 252–269. [Google Scholar] [CrossRef]
- Li, D.; Liu, T.; Pan, L.; Hu, F.; Jin, Q. Bioaccumulation and oxidative damage of polycyclic aromatic hydrocarbon mixtures in Manila clam Ruditapes philippinarum. Ecotoxicol. Environ. Saf. 2020, 197, 110558. [Google Scholar] [CrossRef]
- Liegertova, M.; Maly, J. Gastropod Mucus: Interdisciplinary Perspectives on Biological Activities, Applications, and Strategic Priorities. ACS Biomater. Sci. Eng. 2023, 9, 5567–5579. [Google Scholar] [CrossRef]
- Lotan, R.; Sharon, N. Peanut (Arachis hypogaea) agglutinin. Methods Enzymol. 1978, 50, 361–367. [Google Scholar]
- Bhattacharyya, L.; Haraldsson, M.; Brewer, C.F. Precipitation of galactose-specific lectins by complex-type oligosaccharides and glycopeptides: Studies with lectins from Ricinus communis (agglutinin I), Erythrina indica, Erythrina arborescens, Abrus precatorius (agglutinin), and Glycine max (soybean). Biochemistry 1988, 27, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.T.; Morris, A.; Dexter, T.M. Identification of two binding sites for wheat-germ agglutinin on polylactosamine-type oligosaccharides. Biochem. J. 1985, 231, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Debray, H.; Decout, D.; Strecker, G.; Spik, G.; Montreuil, J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur. J. Biochem. 1981, 117, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Sughii, S.; Kabat, E.A.; Baer, H.H. Further immunochemical studies on the combining sites of Lotus tetragonolobus and Ulex europaeus I and II lectins. Carbohydr. Res. 1982, 99, 99–101. [Google Scholar] [CrossRef]
- Chandrasekaran, E.V.; Xue, J.; Xia, J.; Khaja, S.D.; Piskorz, C.F.; Locke, R.D.; Neelamegham, S.; Matta, K.L. Novel interactions of complex carbohydrates with peanut (PNA), Ricinus communis (RCA-I), Sambucus nigra (SNA-I) and wheat germ (WGA) agglutinins as revealed by the binding specificities of these lectins towards mucin core-2 O-linked and N-linked glycans and related structures. Glycoconj. J. 2016, 33, 819–836. [Google Scholar]
- Debray, H.; Montreuil, J. Aleuria aurantia agglutinin. A new isolation procedure and further study of its specificity towards various glycopeptides and oligosaccharides. Carbohydr. Res. 1989, 185, 15–26. [Google Scholar] [CrossRef]
- Finne, J.; Krusius, T. Preparation and fractionation of glycopeptides. Methods Enzymol. 1982, 83, 269–277. [Google Scholar]
- Menon, M.; Mohanraj, R.; Vb, J.; Prasath Rv, A. Bioaccumulation of heavy metals in a gastropod species at the Kole wetland agroecosystem, a Ramsar site. J. Environ. Manag. 2023, 329, 117027. [Google Scholar] [CrossRef]
- Conti, M.E.; Tudino, M.; Muse, J.O.; Cecchetti, G. Biomonitoring of heavy metals and their species in the marine environment: The contribution of atomic absorption spectroscopy and inductively coupled plasma spectroscopy. Res. Trends Appl. Spectrosc. 2002, 4, 295–324. [Google Scholar]
- Dallinger, R.; Zerbe, O.; Baumann, C.; Egger, B.; Capdevila, M.; Palacios, O.; Albalat, R.; Calatayud, S.; Ladurner, P.; Schlick-Steiner, B.C.; et al. Metallomics reveals a persisting impact of cadmium on the evolution of metal-selective snail metallothioneins. Metallomics 2020, 12, 702–720. [Google Scholar] [CrossRef]
- Jebali, J.; Chouba, L.; Banni, M.; Boussetta, H. Comparative study of the bioaccumulation and elimination of trace metals (Cd, Pb, Zn, Mn and Fe) in the digestive gland, gills and muscle of bivalve Pinna nobilis during a field transplant experiment. J. Trace Elem. Med. Biol. 2014, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Tanhan, P.; Imsilp, K.; Lansubsakul, N.; Tantiwisawaruji, S.; Thong-Asa, W. Oxidative response to accumulation of trace metals in tissue of two bivalves, the Asian green mussel Perna viridis and the blood cockle Tegillarca granosa, living in Pattani Bay, Thailand. J. Aquat. Anim. Health 2024, 36, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Podgurskaya, O.V.; Kavun, V.Y.; Lukyanova, O.N. Heavy Metal Accumulation and Distribution in Organs of the Mussel Crenomytilus grayanus from Upwelling Areas of the Sea of Okhotsk and the Sea of Japan. Russ. J. Mar. Biol. 2004, 30, 188–195. [Google Scholar] [CrossRef]
- Chahouri, A.; Yacoubi, B.; Moukrim, A.; Banaoui, A. Bivalve molluscs as bioindicators of multiple stressors in the marine environment: Recent advances. Contin. Shelf Res. 2023, 264, 105056. [Google Scholar] [CrossRef]
- De Roma, A.; Neola, B.; Serpe, F.P.; Sansone, D.; Picazio, G.; Cerino, P.; Esposito, M. Land snails (Helix aspersa) as bioindicators of trace element contamination in Campania (Italy). Open Access Libr. J. 2017, 4, 1–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentino, D.; De Blasi, C.; Semeraro, D.; Mastrodonato, M.; Guglielmi, M.V. Bivalves and Gastropods: Models for the Study of Mucomics. J. Mar. Sci. Eng. 2025, 13, 566. https://doi.org/10.3390/jmse13030566
Mentino D, De Blasi C, Semeraro D, Mastrodonato M, Guglielmi MV. Bivalves and Gastropods: Models for the Study of Mucomics. Journal of Marine Science and Engineering. 2025; 13(3):566. https://doi.org/10.3390/jmse13030566
Chicago/Turabian StyleMentino, Donatella, Carlotta De Blasi, Daniela Semeraro, Maria Mastrodonato, and Marco Vito Guglielmi. 2025. "Bivalves and Gastropods: Models for the Study of Mucomics" Journal of Marine Science and Engineering 13, no. 3: 566. https://doi.org/10.3390/jmse13030566
APA StyleMentino, D., De Blasi, C., Semeraro, D., Mastrodonato, M., & Guglielmi, M. V. (2025). Bivalves and Gastropods: Models for the Study of Mucomics. Journal of Marine Science and Engineering, 13(3), 566. https://doi.org/10.3390/jmse13030566






