Abstract
Elasmobranchs (Chondrichthyes, Elasmobranchii) are exposed to a variety of gastrointestinal parasites acquired through the ingestion of infected prey. An increasing amount of evidence suggests the usefulness of parasitological information to elucidate aspects of the biology and ecology of sharks and rays, to inform the correct management and conservation of their stocks and the appropriate husbandry of captive specimens. This study aims to identify at the morphological and molecular level the helminth parasites found in the stomachs and intestines of various elasmobranchs accidentally caught by Mediterranean fisheries, with the aim of updating and providing new information on the parasitic fauna of these species. Specimens of smooth-hound Mustelus mustelus, blackspotted smooth-hound Mustelus punctulatus, blue shark Prionace glauca, spiny dogfish Squalus acanthias, lesser-spotted dogfish Scyliorhinus canicula, pelagic stingray Pteroplatytrygon violacea and Mediterranean starry ray Raja asterias were examined. The parasitological examination allowed us to identify the nematode Acanthocheilus rotundatus in the two species of smooth-hounds analyzed, the tapeworm species Scyphophyllidium exiguum, S. prionacis, Anthobothrium caseyi and Nybelinia indica in P. glauca, the nematodes Hysterothylacium aduncum and Proleptus obtusus in S. acanthias and S. canicula, respectively, and finally the nematode Pseudanisakis rajae and the tapeworm Nybelinia sp. in Raja asterias. Some observations represent new reports at a geographical level, in particular, those on A. caseyi in P. glauca and H. aduncum in S. acanthias from the Adriatic Sea, or first host records, such as S. exiguum and N. indica in P. glauca or P. rajae. in R. asterias. The results of this survey represent a contribution to broadening the knowledge of the parasitic fauna of these elasmobranchs in the Mediterranean Sea. From more in-depth future studies, it will be possible to reach more solid evidence and general conclusions on aspects relating to the biology, ecology, and health of the investigated species, offering useful information for their conservation and management.
1. Introduction
Elasmobranchs are exposed to a variety of gastrointestinal parasites acquired through the ingestion of infected prey and are classified as definitive hosts for several groups of parasitic helminths, including nematodes, acanthocephalans, and flatworms [1]. Often displaying complex life cycles that rely on trophic interactions for transmission, these helminths can be used to elucidate the role of their hosts in the food web, providing important ecological information about the host and its interactions in the ecosystem [2,3,4,5].
The biology of elasmobranchs, their late sexual maturity, long life cycle, low fecundity, long lifespan, and their role at the top of the food chain make them particularly vulnerable to fishing activities compared to most teleost species [6]. According to the International Union for Conservation of Nature (IUCN) Red List, approximately 24% of cartilaginous fish belong to a threatened category (IUCN Red List of Threatened Species).
In this framework, information obtained from the parasitological analysis of by-caught sharks and rays is particularly valuable, providing insight into the biology and ecology of analyzed species while minimizing impacts on their wild populations. Among Mediterranean sharks, critical species with respect to their conservation status are the blue shark Prionace glauca (Linnaeus 1758), the spiny dogfish Squalus acanthias Linnaeus, 1758, the smooth-hound Mustelus mustelus (Linnaeus, 1758) and the blackspotted smooth-hound M. punctulatus Risso, 1827.
In particular, blue sharks (Carcharhinidae) are found in all major oceans, as well as in the Mediterranean Sea [7,8]; nevertheless, during the last century, their abundance and biomass has decreased by approximately 98% across the Mediterranean Sea, which represents a decline due to fishing activities [9]. The blue shark was considered “vulnerable” in the Mediterranean in 2007 and “almost threatened” in 2009 by the IUCN [10]. In 2016, its status was updated to “critically endangered” [11].
The spiny dogfish S. acanthias is a critically endangered species, with an estimated population decline higher than 80% [12], and is now considered a “vulnerable” species; it is distributed in the Mediterranean and extra-Mediterranean areas, where it is frequently by-caught by bottom trawl fisheries.
Among smooth-hounds (Triakidae), M. mustelus has been classified as “endangered” since 2013 (IUCN, 2024); this species is found in the Mediterranean Sea and the eastern Atlantic from the British Isles to South Africa [13,14,15]. Generally, it constitutes the by-catch of bottom trawling or pelagic fishing; however, seasonally, it represents a target species of artisanal fishing with gillnets in some coastal areas [16]. A large number of immature individuals are legally fished and landed. Little is known about the distribution of the congeneric M. punctulatus, as this species is often confused, especially in juvenile sizes, with M. mustelus. It is distributed in the eastern North Atlantic and, according to some authors, also in the Mediterranean Sea [13]. Like other Mustelus species, blackspotted smooth-hounds are caught as part of the by-catch in trawls and other demersal fisheries. For many years, despite frequent catches, this species was never evaluated on the IUCN Red List for Mediterranean chondrichthyes, as available information was considered lacking and limited [10]. Similarly to the congeneric species, the conservation status of M. punctulatus is currently “endangered” (EN) according to the IUCN.
In addition to these species, which have been highly impacted by fishing activities and other anthropogenic stressors, there is also a paucity of epidemiological data for common elasmobranch species considered of “less concern” by the IUCN. These include a number of dogfish and ray species, which, despite their abundance and, in some instances, commercial interest, are relatively less known with respect to their parasite distribution.
The lesser-spotted dogfish Scyliorhinus canicula (Linnaeus 1758) is an extremely popular food species in some Mediterranean regions [17] and is treated as a by-catch in most of the remaining fisheries [18,19].
The pelagic stingray Pteroplatytrygon violacea (Bonaparte 1832) is known for having a cosmopolitan distribution [20,21]; in the Mediterranean, P. violacea has been reported from several areas, including the Maghreb coast and the Tyrrhenian Sea [22], the Ligurian Sea [23], and Ionian and Adriatic Seas [24,25]. Although not endangered, this species is listed among the main incidental captures in certain fishing systems, and by-caught individuals are discarded at sea in such poor conditions that their survival probabilities are low [26].
Similarly, the Mediterranean starry ray Raja asterias (Delaroche 1809) is widely distributed in the Mediterranean Sea, where its abundance seems to increase in certain areas [27]. This species is subjected to frequent catches, particularly by bottom trawl fisheries at the adult stage [28,29], and by trammel nets at the juvenile stage [30], therefore experiencing higher fishing pressure in certain areas.
In this study, we gathered parasitological information on the above-mentioned elasmobranch species and carried out morphological and molecular analyses to identify the gastrointestinal parasites of by-caught individuals, with the aim of providing updated data informative of their biology and health status without damaging their stocks.
2. Materials and Methods
Several subjects of elasmobranchs fished as by-catch in the north-western Adriatic Sea and in the Gulf of Lion (FAO 37.2.1 and FAO 37.1.2) were subjected to parasitological examination. The samples were also acquired through collaboration with the Experimental Center for the Protection of Habitats (CESTHA). The analysis was performed on fresh and frozen material. A total of 57 specimens of elasmobranchs were examined, of which 16 were demersal sharks (6 M. mustelus, 8 M. punctulatus, 1 P. glauca, 2 S. acanthias, 2 S. canicula) and 38 were batoid sharks (9 P. violacea and 29 R. asterias, the latter species fished in the Gulf of Lion). Each specimen was identified using specific taxonomic keys [31,32], weighed, and measured; in particular, specimens of M. mustelus, M. punctulatus, P. glauca, and S. canicula were measured from the snout to the caudal fin, while P. violacea and R. asterias were measured from one end to the other of the pectoral fins (wings), from the rostrum to the beginning of the tail, and from the rostrum to the end of the tail [33].
All the elasmobranchs were subjected to parasitological examination focused on metazoan parasites of the stomach and intestine. All the collected parasites were washed in saline and stored in 70% ethanol or 10% buffered formalin for subsequent morphological and molecular analyses aimed at identifying the parasites at the lowest possible taxonomic level. Two parasite taxa, namely nematodes and cestodes, were macroscopically distinguished.
Collected parasites were subjected to microscopic observation after clarification in Amman lactophenol to study their morphology [34,35]. Before clarification, a small portion devoid of taxonomic characters was removed with a sterile scalpel and stored at -20 °C for molecular analysis. Only parasites in the best-preserved conditions were analyzed. Measurements were obtained with the help of image analysis software (Nis-Elements D 4.60.00 64-bit, Nikon) and are reported as a range, followed by the mean and standard deviation. Measurements are given in micrometers (µm) unless otherwise indicated.
For SEM analysis, formalin-fixed samples were dehydrated through a graded ethanol series, critical point dried, sputter-coated with gold palladium, and observed using a Phenom XL G2 Desktop SEM (Thermo Fisher Scientific, Eindhoven, The Netherlands) operating at 5 kV.
Based on the observation of different morphological characters, the parasites were identified through the use of taxonomic keys [34,36,37] and were specific to the taxa identified.
For molecular analysis, genomic DNA was extracted using a PureLink® genomic DNA kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions.
For tapeworms, amplification of the 28S rDNA region was performed with primers U178_f (5′-GCACCCGCTGAAYTTAAG-3′) and L1642_r (5′-CCAGCGCCATCCATTTTCA-3′) [38]. Conditions for the PCR reaction were as follows: 40 cycles of 30 sec at 94 °C, 30 sec at 52 °C and 2 min at 72 °C, preceded by a denaturation step at 94 °C for 2 min and followed by an extended elongation step at 72 °C for 10 min.
For nematodes, the amplification of the complete rDNA ITS region was performed with primers NC5_f (50-GTAGGTGAACCTGCGGAAGGATCATT-30) and NC2_r (50-TTAGTTTCTTCCTCCGCT-30) [39]. Conditions for the PCR reaction were as follows: 35 cycles of 30 sec at 94 °C, 30 sec at 50 °C and 2 min at 72 °C, preceded by a denaturation step at 94 °C for 2 min and followed by an extended elongation step at 72 °C for 10 min.
Amplicons were purified with Nucleo-Spin Gel and PCR Cleanup (Mackerey-Nagel, Düren, Germany). Among the nematode samples collected within the study, only larval stages morphologically referable to Raphidascarididae (e.g., Hysterothylacium spp.) were subjected to polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) following existing protocols [40,41]: briefly, 10 µL of the PCR product was digested with 1.5 µL of restriction enzymes HinfI, and AluI, in a volume of 20 µL at 37 °C for 90 min. The restriction fragments were separated in 3% agarose gel stained with SYBR Safe DNA Gel Stain in 0.5X TBE (Thermo Fisher Scientific, Waltham, Massachusetts, USA). To better support the PCR-RFLP analysis, amplified products of sequenced Raphidascarididae were always included in the electrophoresis as positive controls.
Samples were sequenced with an ABI 3730 DNA analyzer (Applied Biosystems, Waltham, MA, USA) at StarSEQ (Mainz, Germany). The sequences were assembled with ContigExpress (VectorNTI Advance 11 software, Invitrogen, Carlsbad, CA, USA), and the sequences were compared with the sequences available on GenBank using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 19 December 2024).
The sequences generated in this study were deposited in GenBank, and the accession numbers are reported below.
3. Results
Helminth parasites were identified in the gastrointestinal tract of 14 (24.6%) out of 57 elasmobranchs examined, including 11 (57.9%) out of 19 demersal sharks and 3 (7.89%) out of 38 batoid sharks.
Overall, five cestode species (adult stages of Scyphophyllidium exiguum, S. prionacis, Anthobothrium caseyi, Nybelinia indica, and Nybelinia sp.) and four nematode species (adult stages of Acanthocheilus rotundatus, Proleptus obtusus, Pseudanisakis rajae and larval stages of Hysterothylacium aduncum) were identified. Their morphological and morphometric description is provided below.
3.1. Scyphophyllidium exiguum (Yamaguti, 1935) Caira, Jensen and Ruhnke, 2020
(Phyllobothriidea: Phyllobothriidae)
Host: Prionace glauca
Locality: Northern Adriatic Sea (FAO 37.2.1)
GenBank accession number: PV156506, PV156512, PV156515 (28S rDNA)
Adult morphology (n = 3): Scolex with four bothridia (Figure 1A), each with single loculus and one round apical sucker; apical suckers 55.19–63.31 (59.33 ± 4.06) wide. Distal surfaces of bothridia with filiform microtriches and microtriches resembling an “ear of corn”. Cephalic peduncle absent. Neck with dorsal and ventral surfaces scutellate (Figure 1B). Mature proglottids 553.20–818.24 (645.54 ± 149.68) long and 295.72–298 (296.90 ± 1.14) wide. Testes 33–59 in number, arranged in two irregular columns and in a medullary position. Cirrus sac oval, containing an armed and coiled cirrus. Vas deferens convoluted and median, bordering the proximal portion of the cirrus sac, anterior to it. Genital pores, generally marginal, irregularly alternating between one proglottid and the next. Vagina median, extending anteriorly from ovary to middle level of the proglottids, then laterally along the anterior margin of cirrus sac to genital pore. Ovary near the posterior end of the proglottids, H-shaped in frontal view and lobed in cross section. Uterus ventral to vagina, extending from anterior edge of ovary to posterior edge of cirrus sac in mature proglottids. Uterine duct present in mature proglottids. Follicles in two lateral bands.
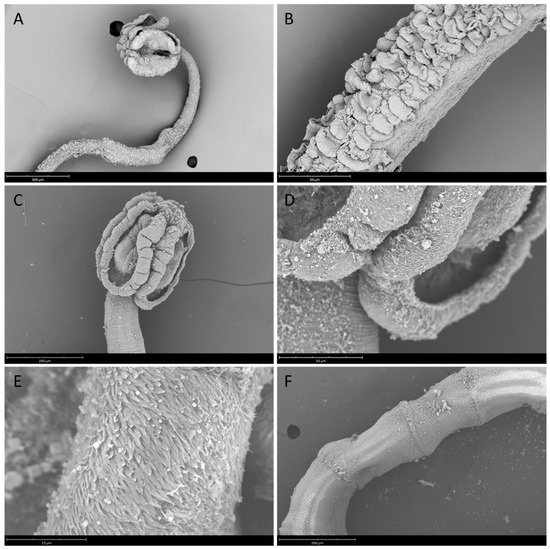
Figure 1.
Scyphophyllidium spp. adult stages from Prionace glauca: (A) S. exiguum, anterior end showing the appearance of scolex and neck region; (B) S. exiguum, detail of neck with scutellate surface; (C) S. prionacis, scolex; (D) S. prionacis, detail of bothridial margin; (E) S. prionacis, detail of serrated microtriches and filiform microtriches over bothridial margin; and (F) S. prionacis, proglottids.
The 28S rDNA sequence obtained from four specimens S. exiguum returned a similarity of between 99.9% and 100% (100% coverage) with Paraorygmatobothrium exiguum (syn S. exiguum) (KF685769, [42]) from the common thresher Alopias vulpinus sampled in the USA, confirming the morphological observations.
3.2. Scyphophyllidium prionacis (Yamaguti, 1934) Caira, Jensen and Ruhnke, 2020
(Phyllobothriidea: Phyllobothriidae)
Host: Prionace glauca
Locality: Northern Adriatic Sea (FAO 37.2.1)
GenBank accession number: PV156509-11, PV156513-14, PV156516 (28S rDNA)
Adult morphology (n = 1): Scolex with four bothridia (Figure 1C), 516 long and 312 wide, each with single loculus and round apical sucker; apical suckers 94 wide. Distal surface of bothridia covered with serrated microtriches and filiform microtriches (Figure 1D,E). Cephalic peduncle absent. Neck with dorsal and ventral surfaces scutellate. Mature proglottids 970–1119.47 long and 308.4–314.73 wide (Figure 1F). Testes 44–61 in number, arranged in two irregular columns and in a medullary position. Cirrus sac pyriform, containing an armed and coiled cirrus. Vas deferens convoluted and median, bordering the proximal portion of the cirrus sac, anterior to it. Genital pores lateral, irregularly alternating. Vagina median, extending anteriorly from ovary to middle level of the proglottids, then laterally along the anterior margin of cirrus sac to genital pore. Ovary near the posterior end of the proglottids, H-shaped in frontal view and lobed in cross section. Uterus ventral to vagina, extending from anterior edge of ovary to posterior edge of cirrus sac in mature proglottids. Uterine duct present in mature proglottids. Follicles in two lateral bands.
The 28S rDNA sequence obtained from four specimens of S. prionacis was compared by BLAST, returning similarity values between 99.4% and 100% (74% coverage) with Paraorygmatobothrium prionacis (syn S. prionacis) (KF685892, [42]).
3.3. Anthobothrium caseyi (Ruhnke and Caira, 2009)
(Tetraphyllidea: Tetraphyllidea incertae sedis)
Host: Prionace glauca
Locality: Northern Adriatic Sea (FAO 37.2.1)
GenBank accession number: PV156508 (28S rDNA)
Adult morphology (n = 5): Worms laciniate, euapolytic. Scolex with four stalked bothridia. Immature proglottid laciniations approximately as long as wide (Figure 2A); immature proglottids 74.72–107.93 long (91.71 ± 13.45) and 213.92–232.53 wide (223.13 ± 7.69). Cirrus sac J-shaped, containing a convoluted cirrus (Figure 2B). Genital pores lateral, irregularly alternated. Vagina sinuous, extending along midline of proglottids. Ovary near posterior end of segment, H-shaped in front view. Uterus extending from anterior edge of ovary to posterior edge of cirrus. Vitellogen in two lateral bands, extending from posterior to anterior end of proglottids.
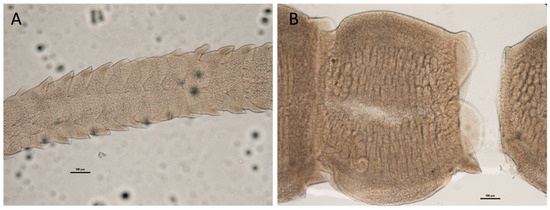
Figure 2.
Antobothrium caseyi adult stage from Prionace glauca: (A) immature proglottids (scale bar 100 µm); (B) mature proglottids (scale bar 100 µm).
The 28S rDNA sequence obtained for one specimen of A. caseyi gave a BLAST identity of 100% (100% coverage) with A. caseyi from P. glauca sampled in the USA (KF685879, [42]).
3.4. Nybelinia indica (Chandra, 1986)
(Trypanorhyncha: Tentaculariidae)
Host: Prionace glauca
Locality: Northern Adriatic Sea (FAO 37.2.1)
GenBank accession number: PV156507 (28S rDNA)
Adult morphology (n = 1): Scolex 1898.47 long, 1619.80 wide, with four long, narrow, and sessile bean-shaped bothridia (Figure 3A,B), 1087.40 long, 333.55 µm wide; bothridial surface covered with spinitriches and filitriches (Figure 3C,D). Neck region short with small velum. Tentacles short, without basal swelling, not totally everted; armature homeomorphic (Figure 3E); basal and metabasal hooks variable in size, basal hooks (4–5 rows) smaller in size, metabasal hooks larger. Hooks 325.95–366.59 (341.91 ± 17.35) long, 140.41–168.95 (155.65 ± 11.92) wide. Immature proglottids 247.93 long, 986.73 wide. Mature proglottids 566.37 long, 1202.98 wide (Figure 3F). Testes occupying the entire proglottids on both sides of ovary and below ovarian lobes. Cirrus pouch conspicuous, elongated in anterior half, and inclined posteriorly. Genital pores irregularly alternating, opening into middle margin of proglottids. Ovary large, bilobed, with lobes connected by narrow isthmus. Vitellaria large, follicles located on both edges of proglottid. Uterus a long blind tube extending anteriorly.
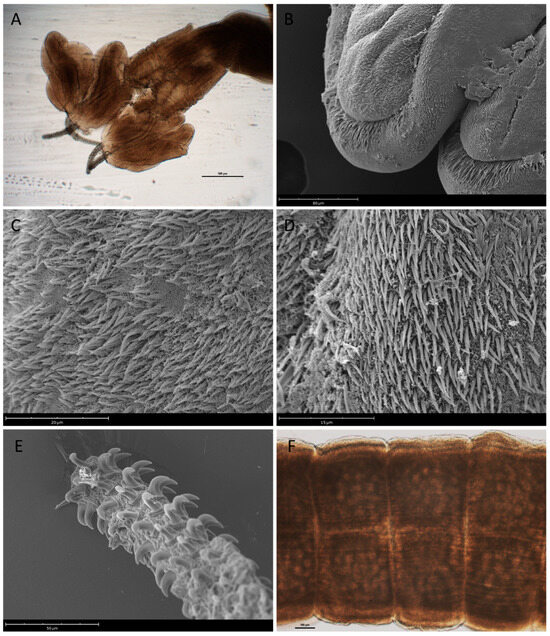
Figure 3.
Nybelinia indica adult stage from Prionace glauca: (A) scolex (scale bar 500 µm); (B) detail of bothridial margin; (C) detail of microtriches over bothridial surface; (D) detail of microtriches over bothridial margin; (E) detail of hooked tentacle; and (F) mature proglottids (scale bar 100 µm).
The 28S rDNA sequence obtained from our specimen of N. indica gave 99% similarity (62% coverage) with N. indica (FJ572930, [43]) from Heteropriacanthus cruentatus sampled in the USA.
3.5. Nybelinia sp. Poche, 1926
(Trypanorhyncha: Tentaculariidae)
Host: Raja asterias
Locality: Gulf of Lion (FAO 37.1.2)
GenBank accession number: PV156517 (28S rDNA)
Adult morphology (n = 1): Body large, acraspedotes. Scolex 1236.97 long, 1023.44 wide, with four bothridia (Figure 4A,B). Neck region 556.55 long, 997.24 wide. Bothridial pits absent. Four retractile tentacles, long and thin. Pre-bulbar organs and muscle rings absent in the basal part of tentacle sheath (Figure 4C). Retractile muscles 920.09 long, originating in basal part of bulbs. Muscle bulbs 255.97 long, 162.02 wide. Tentacular armature homeoacanth, homeomorphic (Figure 4D). Small hooks in shape of “rose thorns”, 21.82–29.85 (26.35 ± 2.60) long, 11.5–15.75 (13.99 ± 1.73) wide at base (Figure 4C,D). Intercalary hooks absent. Mature proglottids 1167–1410.6 (1291.19 ± 90.66) long, 489.83–611.15 (537.65 ± 47.70) wide. Genital pore equatorial or pre-equatorial. Cirrus pouch located near the female genital complex. Ovary located in central position within proglottids.
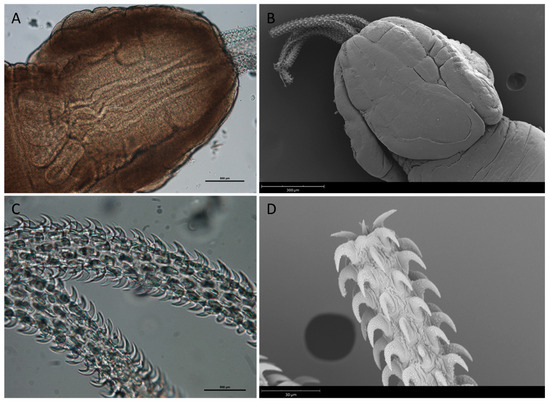
Figure 4.
Nybelinia sp. adult stage from Raja asterias: (A) and (B) scolex; (C) and (D) details of hooked tentacles.
The 28S rDNA sequence of Nybelinia from R. asterias returned a similarity of 100% (69% coverage) with Tentacularidae sp. (KY909272, [44]) from New Zealand sole Peltorhamphus novaezeelandiae; therefore, it was not possible to confirm its specific identity.
3.6. Acanthocheilus rotundatus (Rudolphi, 1819)
(Rhabditida: Acanthocheilidae)
Host: Mustelus mustelus, M. punctulatus
Locality: Northern Adriatic Sea (FAO 37.2.1)
Adult general morphology: Body thick, tapering towards anterior end (Figure 5A). Three small semicircular lips, devoid of labial wings, each lip provided with two indented bifid teeth on the inside (Figure 5B,C); labial pulp-penetrating teeth tips. Interlabia absent. Dorsal lip with two double papillae, latero-ventral lips with a double papilla, a single latero-ventral papilla, and an amphid. Deirids small, rounded, slightly posterior to nerve ring. Excretory pore posterior to nerve ring. Esophagus enlarged at anterior end, measuring 6.5–9.3% of body length; intestinal cecum and esophageal appendix absent (Figure 5D).
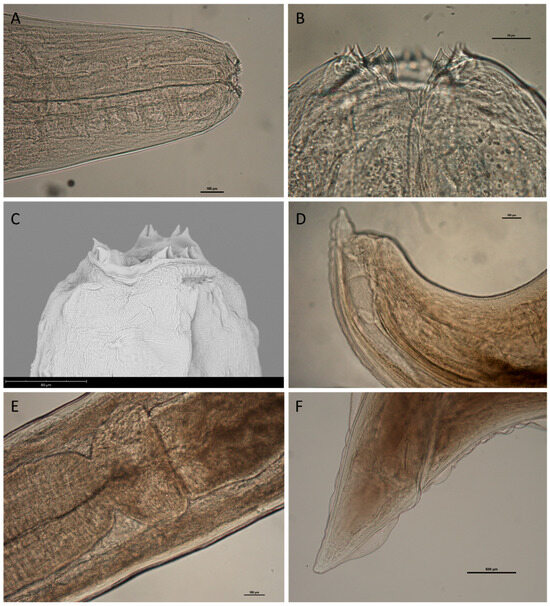
Figure 5.
Acanthocheilus rotundatus adult stages from Mustelus spp. (A) Anterior end (scale bar 100 µm); (B) details of anterior end showing bifid teeth (scale bar 50 µm); (C) scanning electron micrograph showing surface details of anterior end; (D) caudal end of male specimen (scale bar 100 µm); (E) Ventriculus (scale bar 100 µm); and (F) caudal end of female specimen (scale bar 500µm).
Adult males (n = 5): Maximum body width 484.12–1209.03 (990.54 ± 296.58). Ventriculus 339.86–419.19 (385.89 ± 29.57) long, 404.46–760.74 (550.17 ± 141.05) wide. Ventriculus 3075.70–5460.20 (4130.74 ± 922.35) from anterior extremity. Spicules short, robust, equal, with pointed tip (Figure 5D). Gubernaculum absent. Spicules subequal 323.62–594.23 (451.96 ± 104.47) long, caudal end with 30–36 pre-cloacal papillae.
Adult females (n = 10): Maximum body width 534.69–1870.04 µm (1385.43 ± 483.37). Vulva 557.78–3887.62 µm (1602.28 ± 1440.23) from posterior extremity vagina muscular, directed posteriorly from vulva; two long uteri. Ventriculus 353.21–796.07 µm (460.23 ± 131.26) long and 461.57–683.74 µm (723.46 ± 464.80) wide (Figure 5E). Ventriculus 1340.63–5191.85 µm (3501.19 ± 1029.44) from anterior extremity. Rectal gland not prominent; Tail conical, with small terminal mucron (Figure 5F). Eggs oval with thin shell.
3.7. Proleptus obtusus Dujardin, 1845
(Rhabditida: Physalopteridae)
Host: Scyliorhinus canicula
Locality: Northern Adriatic Sea (FAO 37.2.1)
Adult gravid females (n = 1): Body 46.6 mm long, 0.97 mm wide, tapering anteriorly (Figure 6A). Pseudolabia 57 long. Cephalic collar 107 long, 380 wide (Figure 6B); muscular esophagus 639 long, 113 wide; glandular esophagus 4.84 mm long. Nerve-ring, deirids, and excretory pore 506, 521, and 934, respectively, from anterior extremity. Vulva 1.9 mm from posterior end of body. Two parallel uteri contain numerous ovals, thick-shelled eggs, eggs 41 long, 32 wide (Figure 6C). Tail conical, 853 long, with rounded tip (Figure 6D).
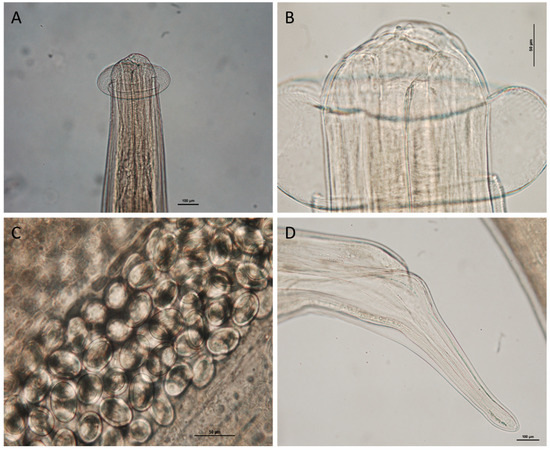
Figure 6.
Proleptus obtusus female adult stage from Scyliorhinus canicula: (A) anterior end; (B) details of cephalic collar; (C) eggs; and (D) caudal end.
3.8. Pseudanisakis rajae Layman and Borovkova, 1926
(Rhabditida: Acanthocheilidae)
Host: Raja asterias
Locality: Gulf of Lion (FAO 37.1.2)
GenBank accession numbers: PV153499-501 (ITS rDNA)
Adult general morphology: Body large, tapering anteriorly (Figure 7A); one dorsal and two sub-ventral lips, sub-ventral lips with one small and one double papilla, dorsal lip with two double papillae. Each lip with large dome-shaped (dome) protrusion. Interlabia absent. One or two rings of denticles surrounding the triradiate mouth (Figure 7B). One amphid on each sub-ventral lip. Long narrow esophagus. Ventriculus oval or oblong. Ventricular appendix and intestinal cecum absent. Excretory pore just posterior to nerve-ring (Figure 7C).
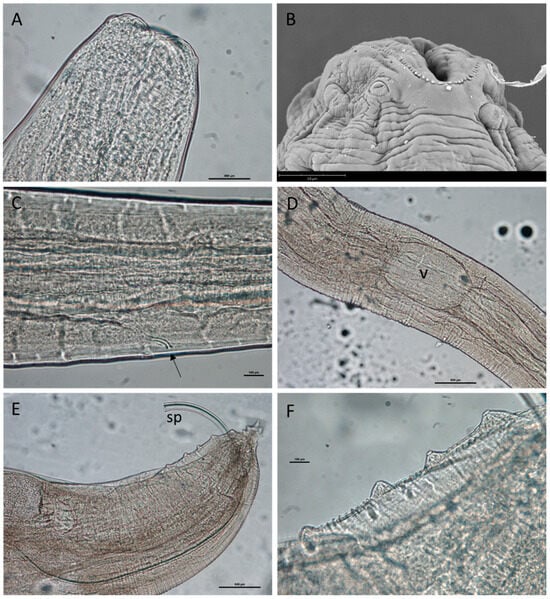
Figure 7.
Pseudanisakis from Raja asterias: (A) anterior end; (B) scanning electron micrograph showing single ring of denticles surrounding the triradiate mouth; (C) excretory pore (arrow); (D) ventriculus (v); (E) caudal end of male specimen with everted spicules (sp); and (F) details of caudal papillae of male specimen.
Adult males (n = 1): Tail conical (Figure 7D), 6–9 pre-anal papillae on each side of body, five pairs of ad-anal and post-anal papillae (Figure 7E); spicules similar in length; distinct and glandular ejaculatory duct; gubernaculum absent. Esophagus 2678.44 long; ventriculus 425.98 long, 273.20 µm wide. Excretory pore 550.44 from anterior end. Non-everted male apparatus 1861.35 long, everted male apparatus 1450.03 long.
Adult females (n = 1): Tail tapering to a sharp point (Figure 7F); vulva in anterior half of body posterior to ventriculus. Esophagus 2841.47 long; ventriculus 432.54 long, 330.87 wide. Excretory pore 126.19 from anterior end.
The ITS rDNA of 3 P. rajae were successfully sequenced and gave a 99.3% BLAST identity with P. rajae (JN392470, [45]).
3.9. Hysterothylacium aduncum (Rudolphi, 1802) Deardorff and Overstreet, 1981
(Rhabditida: Raphidascarididae)
Host: Squalus acanthias
Locality: Northern Adriatic Sea (FAO 37.2.1)
Third-stage larvae (n =1): Body 7240 long, 323.58 wide, with finely striated cuticle. Cephalic end rounded with conical boring tooth. Esophagus 1064.23 long, slightly broader posteriorly. Ventriculus almost spherical, 195.99 wide, approximately as wide as esophagus. Ventricular appendix narrow, 954.17 long. Intestinal cecum 654.37 long. Excretory pore 428.29 from anterior end, nerve ring 329.25 from anterior end.
Fourth-stage larvae (n =3): Body 8316.29–9346.9 (8749.29 ± 534.66) long, 236.6–262.09 (248.55 ± 12.82) wide, tapering anteriorly (Figure 8A), with finely striated cuticle. Lateral alae narrow, extending from cephalic end to about tail tip. Cephalic end with three rounded lips 33.77–50.71 (39.71 ± 9.54) long, two ventral and one dorsal, each ventral lip bearing one double and one single papillae; dorsal lip larger, bearing two double papillae. Interlabium 13.7–16.13 (14.94 ± 1.22) long. Esophagus 889.45–989,49 (934.94 ± 50.63) long, broader posteriorly. Ventriculus almost spherical 85.06–136.66 (106.53 ± 26.87) wide, narrower than the esophagus. Ventricular appendix narrow, 747.38–877.29 (825.95 ± 69.10) long. Intestinal cecum 581.39–664.03 (633.51 ± 45.35) long. Excretory pore 322.01–399.34 (352.80 ± 41) from anterior end (Figure 8B), nerve ring 289.38–319.88 (301.53 ± 16.17) from anterior end. Tail conical (Figure 8C), 116.59–169.08 (134.94 ± 29.59) from cloaca to tip, with characteristic “cactus” appearance (Figure 8D).
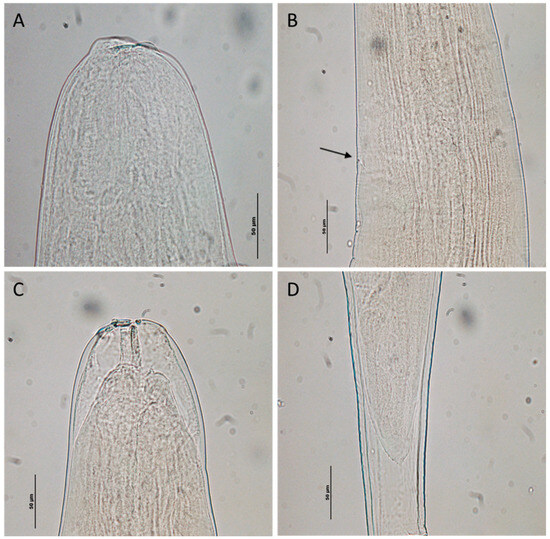
Figure 8.
Hysterothylacium aduncum larval stage from Squalus acanthias: (A) anterior end of third-stage larva; (B) excretory pore of fourth-stage larva (arrow); (C) anterior end of molting fourth-stage larva; and (D) posterior end of molting fourth-stage larva.
The RFLP pattern obtained with HinfI showed two fragments (700–360 bp) for both H. fabri and H. aduncum positive controls, while AluI produced different fragments, such as 303-156-144-139 bp for H. fabri and 307-183-155-121 for H. aduncum, showing that Hysterothylacium specimens collected from S. acanthias belong to the species H. aduncum.
4. Discussion
The present research provides preliminary parasitological data on different elasmobranch species from the Mediterranean Sea. From an ecological point of view, the gastrointestinal helminths of elasmobranchs represent an ideal study system as they are directly connected, through their heteroxenous life cycle, to the trophic network of the marine environment and the potential intermediate hosts involved.
The parasitological examination of M. mustelus and M. punctulatus fished in the north-western Adriatic enabled the identification of the nematode A. rotundatus, a species originally described by Rudolphi (1819) in the Tope shark Galeorhinus galeus (Linnaeus, 1758) from the Adriatic Sea and subsequently redescribed in M. mustelus from the same area [36,40]; it was also reported in spotless smooth-hound Mustelus griseus (Pietschmann, 1908) and in the star-spotted smooth-hound Mustelus manazo (Bleeker, 1855) from Japanese waters [46]. The morphometric data of A. rotundatus generated in this study are consistent with the values already reported [36,40,47]; this parasite species has a two-host life cycle, with hermit crabs as the intermediate host and sharks as the definitive host [47]; therefore, it is not normally reported in teleost fish, although sporadic records of Acanthocheilus sp. in teleosts and cephalopods may be found in the literature.
Studies on blue shark parasitic helminths are scarce and, in some instances, limited to specific anatomical sites, such as the spiral valve [48,49]. From the single specimen of P. glauca examined, the results of the morphological and molecular analyses identified four different species of cestodes: S. prionacis, S. exiguum, A. caseyi, and N. indica. Nevertheless, some of the parasitic elements recovered from this host were in poor conservation status, which prevented their morphological characterization; therefore, the occurrence of a higher diversity of parasites in the examined P. galuca cannot be excluded. The genus Scyphophyllidium can be distinguished from all other genera on the basis of the presence of serrated microtriches on the proximal surface of the bothridia, as well as the very small triangular structures (<500 nm) present on the dorsal and ventral scutes of the worm’s neck; within this genus, the species S. prionacis and S. exiguum, formerly assigned to the genus Paraorygmatobothrium, are distinguished from each other by the appearance of the distal bothridial surface, with particular respect to the morphology of microtriches [50] and by the morphometry of different structures (accessory suckers, bothridia, genital pores, ovaries, eggs). Both species share many morphological features [50]; therefore, molecular data are of great help for their correct identification, as also confirmed by our data.
Scyphophyllidium prionacis has been identified in P. glauca from different parts of the world, possibly following the worldwide distribution of these host species. In the Mediterranean Sea, this association has been reported by Euzet [51] and confirmed in the present study; S. exiguum has been so far identified in the common thresher A. vulpinus [50,52,53]; therefore, this is the first record of this cestode species in P. glauca. The tetraphyllidean A. caseyi was described for the first time in P. glauca and differs conspicuously from other species of Anthobothrium van Beneden, 1850, in the shape of its proglottid laciniations [54].
The morphological analysis of our specimens allowed us to confirm only the presence of laciniate proglottids since the scoleces showed autolytic degenerations incompatible with correct morphological identification. Nevertheless, molecular analysis of the 28S rDNA established the specific identity of our Anthobothrium specimens. A. caseyi was previously reported mainly in P. glauca from extra-Mediterranean areas [54,55] and more recently in the western Mediterranean Sea [56]; therefore, its presence has now also been confirmed in the Adriatic Sea.
The morphology of the trypanorhynch Nybelinia indica found in P. glauca in our study is in accordance with previous descriptions [57,58]; the results of the molecular analyses confirmed the morphological observations. Nybelinia indica was previously identified by Palm [37] in other sharks, including Alopias superciliosus, Carcharhinus leucas, C. limbatus, and Rhizoprionodon acutus. It has also been found in the gray bamboo shark Chiloscyllium griseum [59] and in the Ganges shark Glyphis gangeticus [58]. Up until this date, N. indica had never been reported in P. glauca; therefore, this represents a new host record.
Overall, a high diversity of cestode parasites has been reported in P. glauca from around the world; in our study, three different species of cestodes were identified in a single specimen of blue shark. Such high rates of parasitic infection in P. glauca are likely the result of the consumption of a large amount of prey parasitized by the larval stages of these cestodes [48]. The blue shark is known to regularly feed on a wide variety of teleost and cephalopod species and occasionally even mammals [60]. Generalist hosts are expected to have a great diversity of parasites [61]: Curran and Caira [48] recorded four species of tapeworms in the blue sharks examined, while Henderson and colleagues [62] reported eight species of parasites, four of which were tapeworms.
The consumption of teleosts plays a crucial role in the transmission and species richness of helminth parasites [63]; concerning tapeworms, teleosts are important links in the life cycles of Paraorygmatobothrium (=Scyphophyllidium) and Anthobothrium spp. [64]. Members of the Tetraphyllidea and Trypanorhyncha are also frequently reported in Cephalopod molluscs [65,66]; however, the larval stages of cestodes found in these hosts are difficult to identify at the species level due to their relatively simple morphology compared to their adult counterparts. As a result, reports of Scyphophyllidium and Anthobothrium in teleosts and invertebrates often lack specific identification, complicating life cycle reconstructions of species belonging to these genera. On the other hand, the morphology of plerocercoid stages of Nybelinia found in teleosts and invertebrates has been used for its specific diagnosis in some cases. In particular, N. indica is widely distributed in several teleosts as intermediate hosts, including Diodon hystrix, Istiompax indica, and Istiophorus platypterus [37]. The 28S rDNA sequence obtained from our specimen of N. indica gave 99% similarity (62% coverage) with N. indica from a teleost host, namely H. cruentatus (Sciaenidae) [43].
As the life cycles of S. exiguum, A. caseyi, and N. indica are still not completely known, further studies should investigate the food webs involving blue sharks as top predators, providing new information on the biology of these parasite species.
During our parasitological examination of specimens of R. asterias fished in the Gulf of Lion (France), we identified the nematode Pseudanisakis sp. and a Trypanorhynch cestode belonging to the genus Nybelinia. The latter has already been described by Palm [37], although at a larval stage, in the same ray species; on the other hand, no species of the genus Pseudanisakis has ever been reported in R. asterias; therefore, this represents the first host record.
The parasitic fauna of S. acanthias in the northern Adriatic Sea has been recently investigated [67]. The results of this study highlight the presence of several species of helminths, including larvae of Anisakis spp. as the most represented taxon, pseudophyllidean tapeworms, and adult specimens of C. micropapillatus and Ptychogonimus megastomum. A higher prevalence of anisakid nematodes was observed during the spring season, coinciding with the higher production of zooplankton and with the consumption of pelagic fish such as anchovies and sardines [68]. In our work, we identified a parasite fauna different from the one reported by Gračan and colleagues [67]; in particular, the only parasite species identified was H. aduncum. The presence of this species in S. acanthias has already been reported off the west coast of Ireland [62]. Our data, therefore, also report the presence of H. aduncum in S. acanthias in the Adriatic Sea. H. aduncum is among the most abundant and widely distributed Hysterothylacium species, with larval stages reported in a wide variety of commercially important teleosts.
Finally, a single gravid female of P. obtusus was found inside the stomach of the only lesser-spotted dogfish examined. The morphometric values obtained for the specimen of P. obtusus examined are consistent with those reported by Moravec and colleagues [69], based on five gravid specimens infecting S. canicula from extra-Mediterranean areas. Our data also confirm the presence of this species in dogfish from the Adriatic Sea, as already indicated by other authors [70,71]. Due to its two-host life cycle, with a crustacean intermediate host and an elasmobranch definitive host, this parasite is not reported in teleosts.
5. Conclusions
From the elasmobranchs examined in this study (P. glauca, M. mustelus, M. punctulatus, S. acanthias, S. canicula, P. violacea, and R. asterias), several species of nematode parasites (P. rajae and A. rotundatus) and tapeworms (S. exiguum, S. prionacis, A. caseyi, N. indica, Nybelinia sp.) have been identified. Among these helminths, some represent new geographical records, in particular, A. caseyi in P. glauca and H. aduncum in S. acanthias from the Adriatic Sea, or new host records, such as S. exiguum and N. indica in P. glauca or P. rajae in R. asterias. The results of this survey represent a contribution to broadening the knowledge of the parasitic fauna of elasmobranchs in the Mediterranean Sea and provide useful information to understand their feeding ecology and health conditions, with the ultimate goal of informing better conservation and management policies of vulnerable and endangered species.
Author Contributions
Conceptualization, P.T. and A.G.; methodology, P.T., M.C. and A.G.; investigation, P.T., E.L.Q. and A.G.; resources P.T., L.A., F.M., M.C., M.L.F. and A.G.; data curation, P.T. and E.L.Q.; writing—original draft preparation, P.T. and E.L.Q.; writing—review and editing, P.T., E.L.Q., L.A., F.M., M.C., M.L.F., F.T. and A.G.; visualization, P.T.; supervision, A.G.; project administration, F.T and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the research was carried out under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—Next Generation EU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP J33C22001190001 (Department of Veterinary Medical Sciences, Alma Mater Studiorum Università di Bologna).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
The authors are grateful to Sara Segati, Simone D’Acunto, Elia Bueloni (CESTHA, Ravenna) and Carlotta Mazzoldi (Università di Padova) for their help with the collection of by-caught specimens. The research leading to these results has been conceived under the International PhD Program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources” (www.FishMed-PhD.org).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Caira, J.N.; Healy, C.J. Elasmobranchs as hosts of metazoan parasites. In Biology of Sharks and Their Relatives; CRC Press: Boca Raton, FL, USA, 2004; pp. 523–551. [Google Scholar]
- Marcogliese, D.J.; Cone, D.K. Food webs: A plea for parasites. Trends Ecol. Evol. 1997, 12, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J. Food webs and the transmission of parasites to marine fish. J. Parasitol. 2002, 124, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J. Food webs and biodiversity: Are parasites the missing link. J. Parasitol. 2003, 89, 106–113. [Google Scholar]
- Marcogliese, D.J. Parasites: Small players with crucial roles in the ecological theater. EcoHealth 2004, 1, 151–164. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Stevens, J.D. The occurrence and significance of tooth cuts on the blue shark (Prionace glauca L.) from British waters. J. Mar. Biol. Assoc. UK 1974, 54, 373–378. [Google Scholar] [CrossRef]
- Megalofonou, P.; Damalas, D.; De Metrio, G. Biological characteristics of blue shark, Prionace glauca, in the Mediterranean Sea. J. Mar. Biol. Assoc. UK 2009, 89, 1233–1242. [Google Scholar] [CrossRef]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef]
- Cavanagh, R.D.; Gibson, C. Overview of the Conservation Status of Cartilaginous Fishes (Chrondrichthyans) in the Mediterranean Sea (No. 3); IUCN: Gland, Switzerland, 2007. [Google Scholar]
- Dulvy, N.K.; Davidson, L.N.; Kyne, P.M.; Simpfendorfer, C.A.; Harrison, L.R.; Carlson, J.K.; Fordham, S.V. Ghosts of the coast: Global extinction risk and conservation of sawfishes. Aquat. Conserv. 2016, 26, 134–153. [Google Scholar] [CrossRef]
- Finucci, B.; Cheok, J.; Chiaramonte, G.E.; Cotton, C.F.; Dulvy, N.K.; Kulka, D.W.; Neat, F.C.; Pacoureau, N.; Rigby, C.L.; Tanaka, S.; et al. Squalus acanthias. The IUCN Red List of Threatened Species 2020: E.T91209505A124551959. 2020. Available online: https://www.iucnredlist.org/species/91209505/124551959#assessment-information (accessed on 30 October 2024).
- Compagno, L.J. FAO Species Catalogue. Vol. 4. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Part 2 Carcharhiniformes; FAO: Rome, Italy, 1984; FAO Fisheries Synopsis No.125; Volume 4, Part 2; pp. 251–655. [Google Scholar]
- Saïdi, B.; Bradaï, M.N.; Bouaïn, A. Reproductive biology of the smooth-hound shark Mustelus mustelus (L.) in the Gulf of Gabès (south-central Mediterranean Sea). J. Fish Biol. 2008, 72, 1343–1354. [Google Scholar] [CrossRef]
- da Silva, C.; McCord, M.E. Blackspotted smoothhound. In South African Marine Linefish Species Profiles; Special Publication No. 9; South African Association for Marine Biological Research: Durban, South Africa, 2013; pp. 289–290. [Google Scholar]
- Bradaï, M.N.; Saïdi, B.; Ghorbel, M.; Bouaïn, A.; Guélorget, O.; Capapé, C. Observations sur les requins du golfe de Gabès (Tunisie méridionale, Méditerranée centrale). Mésogée 2002, 60, 61–77. [Google Scholar]
- Capapé, C.; Vergne, Y.; Reynaud, C.; Guélorget, O.; Quignard, J.P. Maturity, fecundity and occurrence of the smallspotted catshark Scyliorhinus canicula (Chondrichthyes: Scyliorhinidae) off the Languedocian coast (southern France, north-western Mediterranean). Vie Et Milieu 2008, 58, 47–55. [Google Scholar]
- Fowler, S.L.; Cavanagh, R.D. (Eds.) Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes: Status Survey (Vol. 63); IUCN: Gland, Switzerland, 2005; 476p. [Google Scholar]
- Rodrıguez-Cabello, C.; Sánchez, F.; Fernández, A.; Olaso, I. Is the lesser spotted dogfish (Scyliorhinus canicula) population from the Cantabrian Sea a unique stock? Fish. Res. 2004, 69, 57–71. [Google Scholar] [CrossRef]
- Mollet, H.F. Distribution of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832). Mar. Fresh Res. 2002, 53, 531–541. [Google Scholar] [CrossRef]
- Neer, J.A. The biology and ecology of the pelagic stingray, Pteroplatytrygon violacea (Bonaparte, 1832). In Sharks of the Open Ocean: Biology, Fisheries and Conservation; Camhi, M.D., Pikitch, E.K., Babcock, E.A., Eds.; Blackwell Science: Oxford, UK, 2008; pp. 152–159. [Google Scholar]
- McEachran, J.D.; Capapé, C. Myliobatidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; Unesco: Paris, France, 1984; Volume 1, pp. 205–209. [Google Scholar]
- Orsi Relini, L.; Garibaldi, B.; Digitali, B.; Lanteri, L.; Vacchi, M.; La Mesa, G.; Serena, F.; Séret, B. Abundance of the pelagic stingray, Pteroplatytrygon (Dasyatis) violacea, in the Ligurian Sea, with preliminary notes about its feeding and growth. In Proceedings of the European Elasmobranch Association, Livorno, Italy, 27–30 September 2000; p. 209. [Google Scholar]
- Mavrič, B.; Jenko, R.; Makovec, T.; Lipej, L. On the occurrence of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832), in the Gulf of Trieste (Northern Adriatic). Ann. Ser. Hist. Nat. 2004, 14, 181–186. [Google Scholar]
- Jardas, I.; Pallaoro, A.; Vrgoč, N.; Jukić-Peladić, S.; Dadić, V. Crvena Knjiga Morskih Riba; Državni Zavod Za Zaštitu Prirode: Zagreb, Croatia, 2008. [Google Scholar]
- Piovano, S.; Clò, S.; Giacoma, C. Reducing longline bycatch: The larger the hook, the fewer the stingrays. Biol. Conserv. 2010, 143, 261–264. [Google Scholar] [CrossRef]
- Ferrá, C.; Fabi, G.; Polidori, P.; Tassetti, A.N.; Leoni, S.; Pellini, G.; Scarcella, G. Raja asterias population assessment in FAO GFCM GSA17 area. Mediterr. Mar. Sci. 2016, 17, 651–660. [Google Scholar] [CrossRef][Green Version]
- Barone, M.; De Ranieri, S.; Fabiani, O.; Pirone, A.; Serena, F. Gametogenesis and maturity stages scale of Raja asterias Delaroche, 1809 (Chondrichthyes, Rajidae) from the South Ligurian Sea. Hydrobiologia 2007, 580, 245–254. [Google Scholar] [CrossRef]
- Fortuna, C.M.; Vallini, C.; Filidei, E., Jr.; Ruffino, M.; Consalvo, I.; Di Muccio, S.; Gion, C.; Scacco, U.; Tarulli, E.; Giovanardi, O.; et al. By-catch of cetaceans and other species of conservation concern during pair trawl fishing operations in the Adriatic Sea (Italy). Chem. Ecol. 2010, 26, 65–76. [Google Scholar] [CrossRef]
- Serena, F.; Abella, A.; Walls, R.; Dulvy, N. Raja asterias. The IUCN Red List of Threatened Species 2015. 2015. Available online: https://www.iucnredlist.org/species/63120/48913317 (accessed on 6 June 2018).
- Manzoni, P.; Tepedino, V. Grande enciclopedia illustrata dei pesci. Vet. Ital. 2008, 44, 691. [Google Scholar]
- Serena, F.; Mancusi, C.; Barone, M. Field identification guide to the skates (Rajidae) of the Mediterranean Sea. Guidelines for data collection and analysis. Biol. Mar. Mediterr. 2010, 17, 204. [Google Scholar]
- Camhi, M.D.; Pikitch, E.K.; Babcock, E.A. Sharks of the Open Ocean: Biology, Fisheries and Conservation; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 15, 536p. [Google Scholar]
- Khalil, L.F. Keys to the Cestode Parasites of Vertebrates; CABI: Wallingford, UK, 1994; 768p. [Google Scholar]
- Gibbons, L.M. (Ed.) Keys to the Nematode Parasites of Vertebrates: Supplementary Volume; Cabi: Wallingford, UK, 2010; Volume 10, 424p. [Google Scholar]
- Petter, A.J.; Paradiznik, V.; Radujkovic, B.M.; Cassone, J. Redescription d’Acanthocheilus rotundatus (Ascaridoidea, Nematoda), parasite de requins (Pleurotremata, Selachii). Considérations sur les affinités du genre Acanthocheilus et conclusions taxonomiques. Ann. Parasitol. Hum. Comp. 1991, 66, 187–194. [Google Scholar] [CrossRef][Green Version]
- Palm, H.W. The Trypanorhyncha Diesing, 1863; PKSPL-IPB Press: Bogor, Indonesia, 2004; 710p. [Google Scholar]
- Lockyer, A.E.; Olson, P.D.; Littlewood, D.T.J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. J. Linn. Soc. Lond. 2003, 78, 155–171. [Google Scholar] [CrossRef]
- Zhu, X.; Gasser, R.B.; Podolska, M.; Chilton, N.B. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. J. Parasitol. 1998, 28, 1911–1921. [Google Scholar]
- Tedesco, P.; Gustinelli, A.; Caffara, M.; Marsella, A.; Toffan, A.; Fioravanti, M.L. Morphological and molecular characterization of two gastrointestinal parasites in Mustelus mustelus (Linnaeus, 1758) from Adriatic Sea. Eur. Zool. J. 2020, 87, 616–623. [Google Scholar] [CrossRef]
- Macchioni, F.; Tedesco, P.; Cocca, V.; Massaro, A.; Sartor, P.; Ligas, A.; Pretti, C.; Monni, G.; Cecchi, F.; Caffara, M. Anisakid and Raphidascaridid parasites in Trachurus trachurus: Infection drivers and possible effects on the host’s condition. Parasitol. Res. 2021, 120, 3113–3122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caira, J.N.; Jensen, K.; Waeschenbach, A.; Olson, P.D.; Littlewood, D.T. Orders out of chaos-molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. Int. J. Parasitol. 2014, 44, 55–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palm, H.W.; Waeschenbach, A.; Olson, P.D.; Littlewood, D.T. Molecular phylogeny and evolution of the Trypanorhyncha Diesing, 1863 (Platyhelminthes: Cestoda). Mol. Phylogenet. Evol. 2009, 52, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Anglade, T.; Randhawa, H.S. Gaining insights into the ecological role of the New Zealand sole (Peltorhamphus novaezeelandiae) through parasites. J. Helminthol. 2018, 92, 187–196. [Google Scholar] [CrossRef]
- Li, L.; Gibson, D.I.; Liu, Y.Y.; Zhang, L.P. Morphological and molecular study of the poorly known species Pseudanisakis rajae (Yamaguti, 1941)(Nematoda: Acanthocheilidae) from elasmobranchs in the Yellow Sea and Taiwan Strait off the coast of China. Syst. Parasitol. 2012, 81, 115–123. [Google Scholar] [CrossRef]
- Moravec, F.; Nagasawa, K. Two remarkable nematodes from sharks in Japan. J. Nat. Hist. 2000, 34, 1–13. [Google Scholar] [CrossRef]
- Diaz, J.P. Cycle évolutif d’Acanthoceilus quadridentatus Molin, 1988 (Nematoda). Vie Milieu 1971, 22, 289–304. [Google Scholar]
- Curran, S.; Caira, J.N. Attachment site specificity and the tapeworm assemblage in the spiral intestine of the blue shark (Prionace glauca). J. Parasitol. 1995, 81, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Borucinska, J.; Dunham, A. Lesions associated with attachment of the cestode Tentacularia sp. to the duodeno-spiral junction in the blue shark, Prionace glauca (L.), with a description of the intestinal morphology of the shark. J. Fish Dis. 2000, 23, 353–359. [Google Scholar] [CrossRef]
- Ruhnke, T.R. Resurrection of Anthocephalum Linton, 1890 (Cestoda: Tetraphyllidea) and taxonomic information on five proposed members. Syst. Parasitol. 1994, 29, 159–176. [Google Scholar] [CrossRef]
- Euzet, L. Recherches sur les Cestodes Tétraphyllides des Sélaciens des Côtes de France. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 1959. [Google Scholar]
- Yamaguti, S. Studies on the helminth fauna of Japan. Part 4. Cestodes of fishes. Jpn. J. Zool. 1934, 6, 1–112. [Google Scholar]
- Yamaguti, S. Studies on the helminth fauna of Japan. Part 6. Cestodes of birds, I. Jpn. J. Zool. 1935, 6, 183–232. [Google Scholar]
- Ruhnke, T.R.; Caira, J.N. Two new species of Anthobothrium van Beneden, 1850 (Tetraphyllidea: Phyllobothriidae) from carcharhinid sharks, with a redescription of Anthobothrium laciniatum Linton, 1890. Syst. Parasitol. 2009, 72, 217–227. [Google Scholar] [CrossRef]
- Méndez, O.; Galván-Magaña, F. Cestodes of the blue shark, Prionace glauca (Linnaeus 1758), (Carcharhiniformes: Carcharhinidae), off the west coast of Baja California Sur, Mexico. Zootaxa 2016, 4085, 438–444. [Google Scholar] [CrossRef]
- Penadés-Suay, J.; Jarque-Rico, A.E.; Tomás, J.; Aznar, F.J. Determinants of diversity and composition of the tapeworm fauna of blue sharks, Prionace glauca: A geographical and host-specificity analysis. J. Helminthol. 2022, 96, e87. [Google Scholar] [CrossRef]
- Vijayalakshmi, C.; Vijayalakshmi, J.; Gangadharam, T. Some trypanorhynch cestodes from the shark Scoliodon palasorrah (Cuvier) with the description of a new species, Tentacularia scoliodoni. Riv. Parassitol 1996, 13, 83–89. [Google Scholar]
- Palm, H.W. Nybelinia Poche, 1926, Heteronybelinia gen. nov. and Mixonybelinia gen. nov. (Cestoda, Trypanorhyncha) in the collections of the Natural History Museum, London. Bull. Nat. Hist. Mus. Lond. Zool. 1999, 65, 133–153. [Google Scholar]
- Jensen, K.; Caira, J.N. A revision of Uncibilocularis Southwell, 1925 (Tetraphyllidea: Onchobothriidae) with the description of four new species. Comp. Parasitol. 2008, 75, 157–173. [Google Scholar] [CrossRef]
- Kohler, N.E. Aspects of the feeding ecology of the blue shark, Prionace glauca in the Western North Atlantic. Mar. Biol. 1987, 106, 329–342. [Google Scholar]
- Chen, H.W.; Liu, W.C.; Davis, A.J.; Jordán, F.; Hwang, M.J.; Shao, K.T. Network position of hosts in food webs and their parasite diversity. Oikos 2008, 117, 1847–1855. [Google Scholar] [CrossRef]
- Henderson, A.C.; Flannery, K.; Dunne, J.J. An investigation into the metazoan parasites of the spiny dogfish (Squalus acanthias L.), off the west coast of Ireland. J. Nat. Hist. 2002, 36, 1747–1760. [Google Scholar] [CrossRef]
- Jensen, K.; Bullard, S.A. Characterization of a diversity of tetraphyllidean and rhinebothriidean cestode larval types, with comments on host associations and life-cycles. J. Parasitol. 2010, 40, 889–910. [Google Scholar] [CrossRef]
- Chambers, C.B.; Cribb, T.H.; Jones, M.K. Tetraphyllidean metacestodes of teleosts of the Great Barrier Reef, and the use of in vitro cultivation to identify them. Folia Parasitol. 2000, 47, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, P.; Bevilacqua, S.; Fiorito, G.; Terlizzi, A. Global patterns of parasite diversity in cephalopods. Sci. Rep. 2020, 10, 11303. [Google Scholar] [CrossRef]
- Tedesco, P.; Caffara, M.; Gustinelli, A.; Fiorito, G.; Fioravanti, M.L. Metacestodes of elasmobranch tapeworms in Octopus vulgaris (mollusca, Cephalopoda) from central Mediterranean-SEM and molecular data. Animals 2020, 10, 2038. [Google Scholar] [CrossRef]
- Gračan, R.; Culinovic, M.; Mladineo, I.; Lackovic, C.; Lazar, B. Trophic ecology shapes gastrointestinal helminth communities of two sympatric mesopredatory sharks in the Adriatic Sea. J. Zool. 2016, 299, 172–182. [Google Scholar] [CrossRef]
- Gračan, R.; Zavodnik, D.; Krstinic, P.; Dragicevic, B.; Lackovic, G.; Lazar, B. Feeding ecology and trophic segregation of two sympatric shark species, Mustelus punctulatus and Squalus acanthias, in the north–central Adriatic Sea. In Programme and Abstract Book, Proceedings of the International Workshop on Conservation Biology, Koper, Slovenija, 14 May 2013; Department of Biodiversity Faculty of Mathematics, Natural Sciences and Information Technologies University of Primorska: Koper, Slovenia, 2013; 6p. [Google Scholar]
- Moravec, F.; Van As Jo, G.; Dykovà, I. Proleptus obtusus Dujardin, 1845 (Nematoda: Physalopteridae) from the puffadder shyshark Haploblepharus edwardsii (Scyliorhinidae) from off South Africa. Syst. Parasitol. 2002, 53, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, B.M. Nematode parasites of fishes from the Adriatic sea. Bull. Du Mus. Natl. Hist. Nat. Zool. Biol. Ecoogie Anaimales 1986, 457–500. [Google Scholar]
- Radujkovic, B.M.; Sundic, D. Parasitic flatforms (Platyhelminthes: Monogenea, Digenea, Cestoda) of fishes from the Adriatic Sea. Nat. Montenegrina 2014, 13, 7–280. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).