Preliminary Biological Assessments of Some Algae Basis Biomaterials
Abstract
1. Introduction
- 1.
- Obtaining crude polysaccharide extracts (A1–A4) from four algae species—Porphyra umbilicalis (A1), Undaria pinnatifida (A2), Cystoseira barbata (A3), and Chlorella sp. (A4);
- 2.
- Developing gold-enriched bioproducts by adding Au3+ ions in standardised extracts and characterising gold-enriched bioproducts (A1+Au, A2+Au, A3+Au, A4+Au);
- 3.
- Evaluating the antioxidant or prooxidant activities of the algae extracts;
- 4.
- Assessing bioproducts cytotoxicity by tests performed in vitro on three standardised human cell lines: HUVEC, Caco-2, and HepG2.
2. Materials and Methods
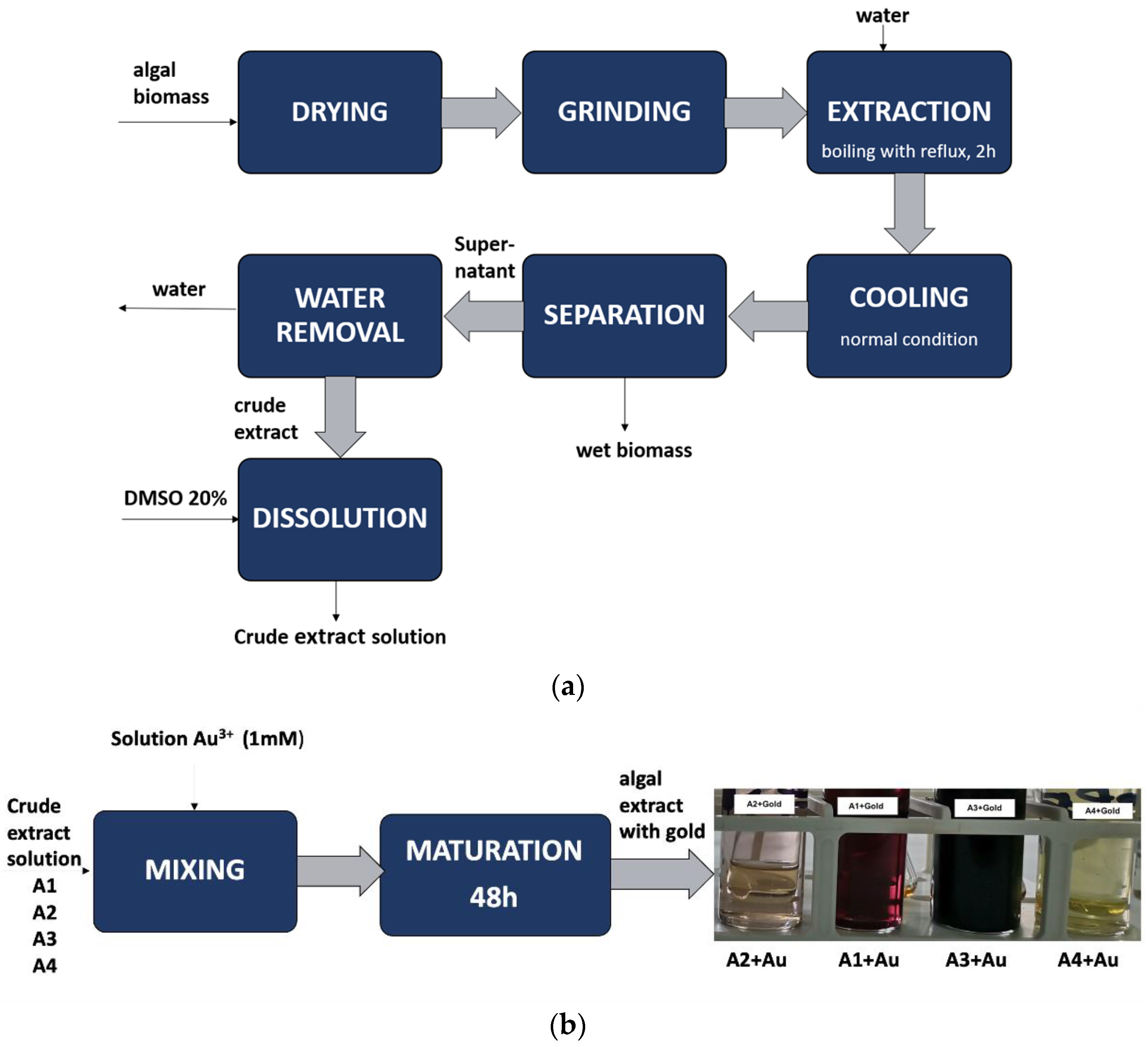
2.1. Obtaining Bioproducts with Polysaccharides
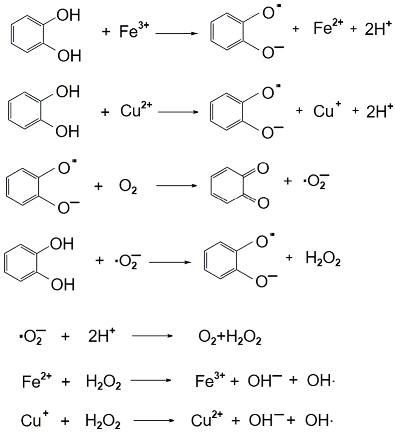
2.2. Antioxidant Methodology
- Luminol (LH2) solution: 5-amino-2,3-dihydro-1,4-phthalazinedione (c = 2.5 × 10−5 M, prepared in DMSO) (Merck, Bucharest, Romania);
- TRIS-HCl buffer solution c = 50 mM, pH = 8.5; (Sigma Aldrich, Bucharest, Romania):
- H2O2, c= 30 mM; (Merck, Bucharest, Romania);
- Witness (control): a mixture containing 200 μL LH2, 750 μL buffer solution, and 50 μL H2O2.
2.3. Polyphenols Content Evaluation
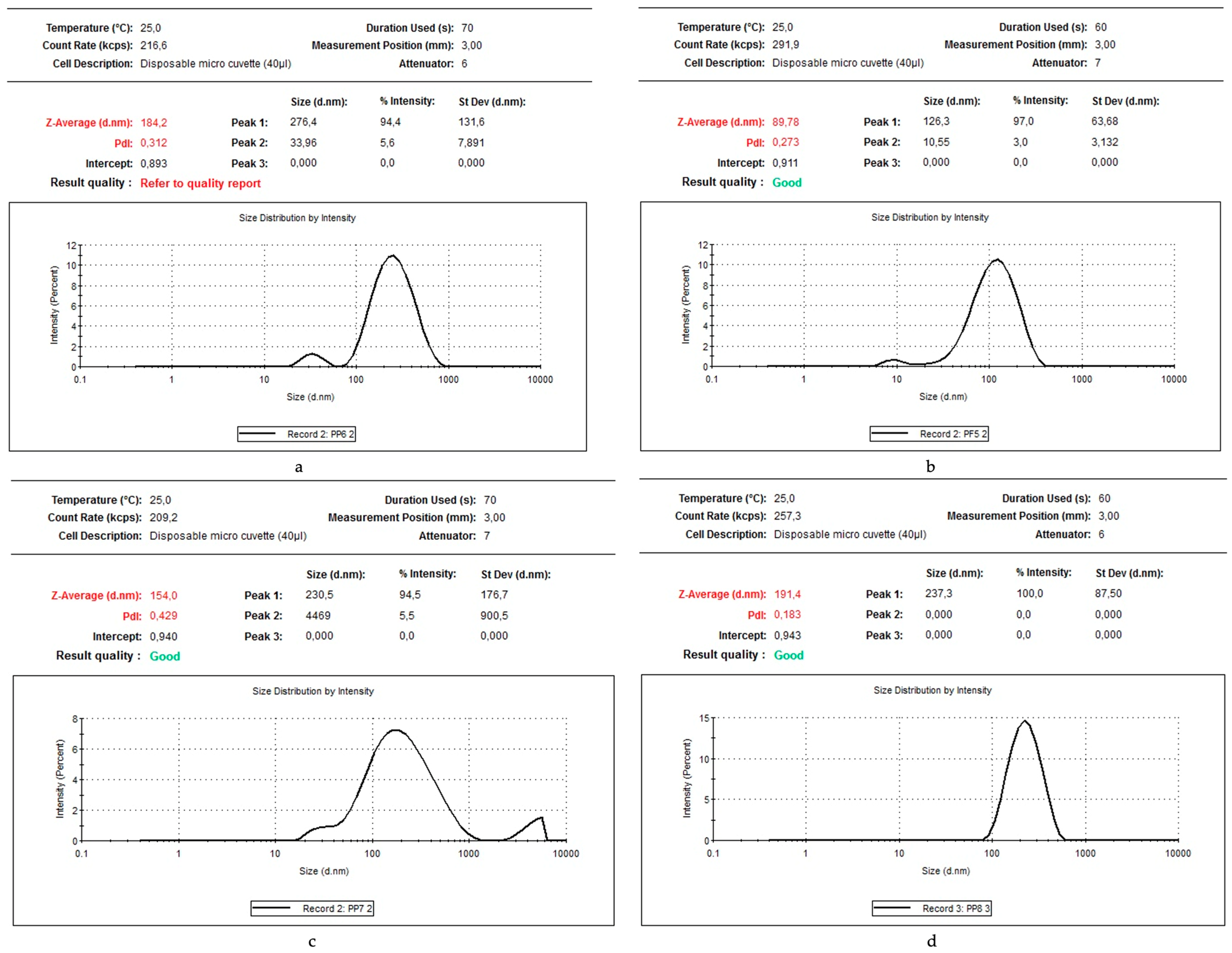
2.4. Characterisation of Bioproducts Obtained from Au3+ and Aqueous Extracts of Algae
2.5. Cell Cultures
2.6. In Vitro Cytotoxicity Assessment
2.7. Gold Content Evaluation
2.8. Statistical Analysis
3. Results
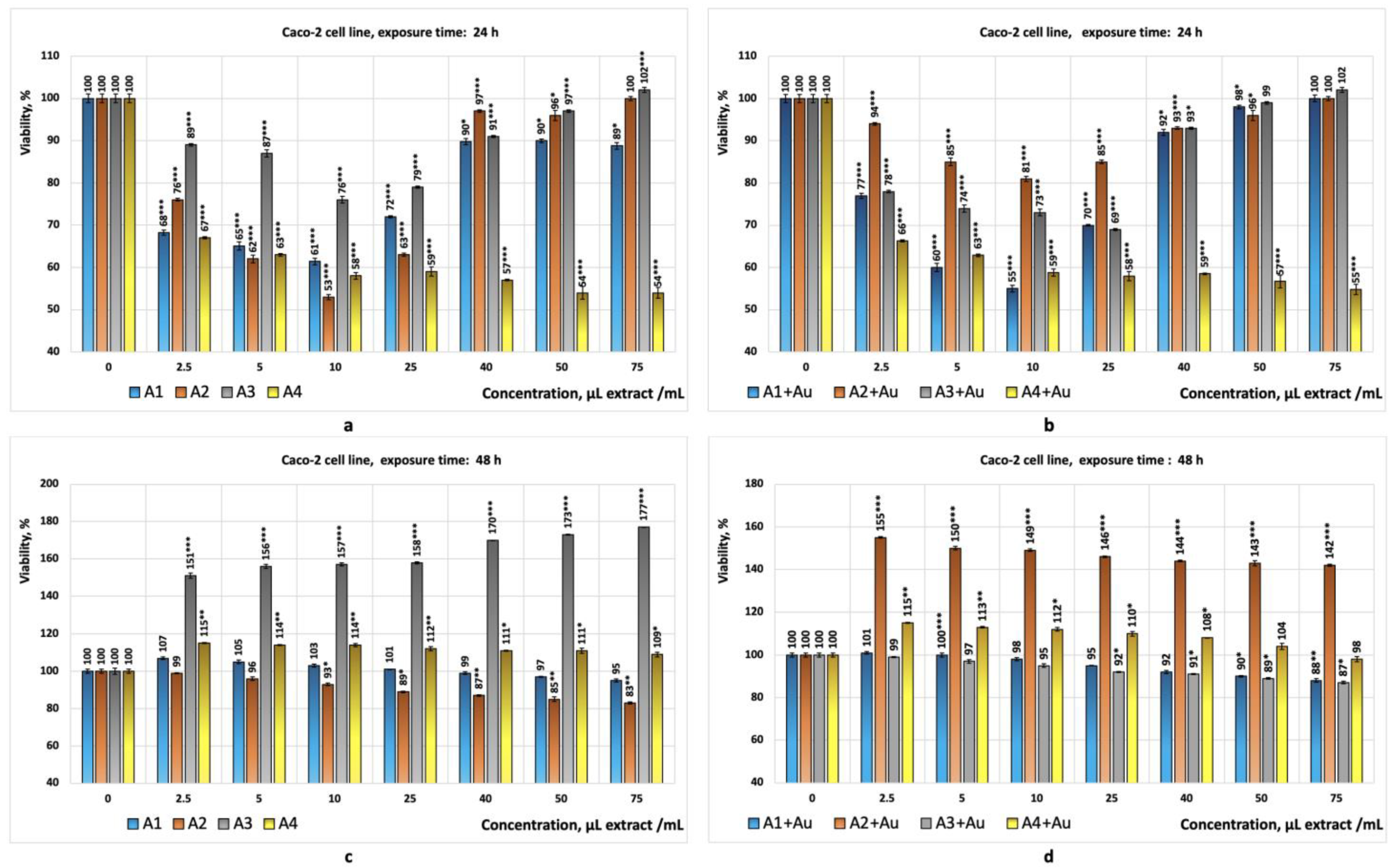
- Bioproduct A1 showed cytotoxic effects at concentrations situated between (2.5–10) µL/mL, with the corresponding cell viabilities ranging from 61% to 68% (statistically significant values);
- Bioproduct A2 exhibited cytotoxicity at concentrations situated between (5–25) µL/mL, with the corresponding cell viabilities ranging from 53% to 65% (statistically significant values);
- Bioproduct A3 did not exhibit cytotoxic effects within the concentration range situated of (2.5–75) µL/mL. In this case, the cell viabilities range from 76% to 102%;
- Bioproduct A4 exhibited cytotoxic effects across the entire concentration range studied (2.5–75 µL/mL), with the corresponding cell viabilities ranging from 54% to 67% (statistically significant results);
- Bioproduct (A1+Au) showed cytotoxic effects at concentrations situated between (5–10) µL/mL, with the corresponding cell viabilities ranging from 55% to 60% (statistically significant values);
- The bioproduct (A4+Au) exhibited cytotoxicity across the entire concentration range studied, with the cell viabilities ranging from 55% to 66% (statistically significant values).
4. Discussion
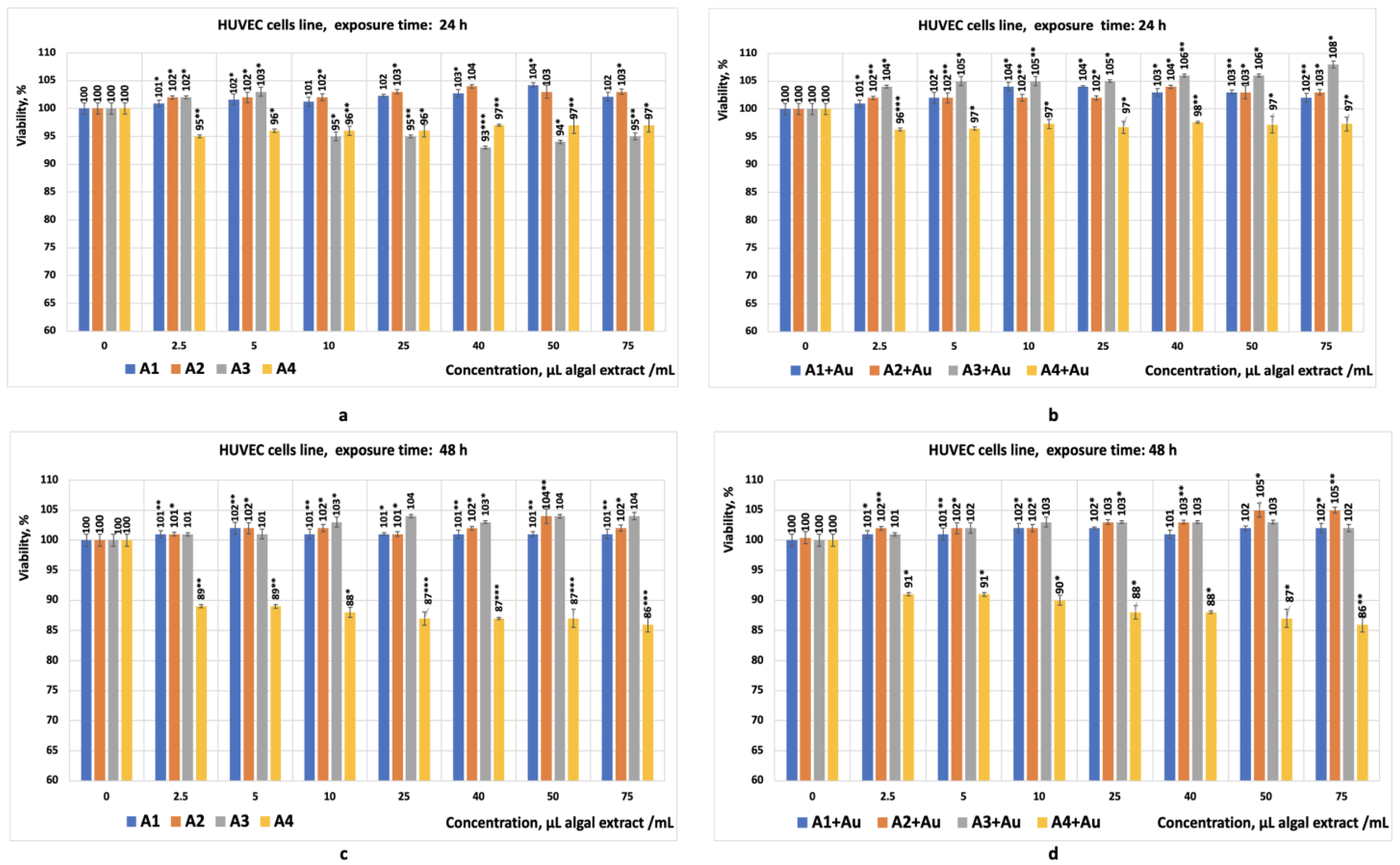
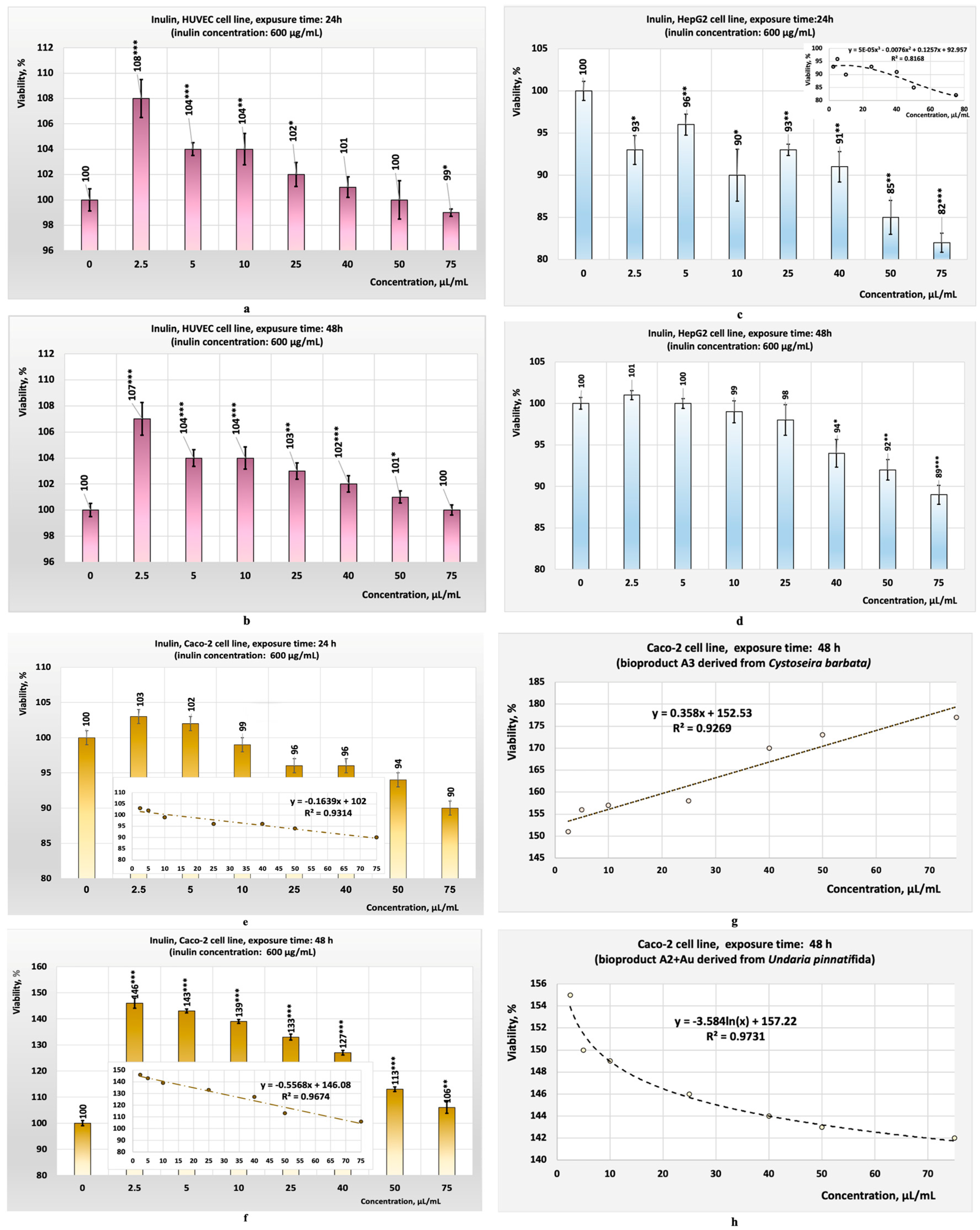
- The HUVEC cell line consists of primary endothelial cells derived from human umbilical veins; they are not transformed or cancer cells. This characteristic recommends them for preliminary in vitro studies aimed at evaluating the cytotoxic effects of specific chemical compounds or bioproducts on normal human cells [59,60,61]. The HUVEC cell line is sensitive to toxic effects such as oxidative stress, apoptosis, and necrosis, making it suitable for detecting subtle cytotoxic effects. Since HUVECs are representative of vascular endothelial cells, they are useful for studying the potential cytotoxicity of a chemical compound or a new bioproduct on normal and healthy endothelial cells [62,63]. Evaluating cytotoxicity on these types of cell lines helps to predict how some specific chemical compounds or some bioproducts might affect normal and healthy tissues in the human body. The response of this cell line to various substances added to the culture medium provides a more accurate model than non-human cell lines for predicting human-specific toxicological effects [60,61]. The results obtained during this study from the test performed on this cell line showed that the eight bioproducts tested did not exhibit cytotoxicity for this cell line (Figure 4a–d). Similarly, the tests performed in parallel with inulin on the same cell line yielded comparable results (Figure 7a–f);
- The Caco-2 and HepG2 cell lines were chosen because they are widely used in cytotoxicity studies. These cell lines can model the physiological processes of the intestinal or liver epithelium, which occur upon their exposure to different compounds. The preliminary results obtained are used to evaluate in vitro the toxicity of specific chemical compounds or bioproducts [64]. The Caco-2 tumour cell line is derived from human colon adenocarcinoma and develops properties similar to differentiated enterocytes. This characteristic underlies the use of this cell line in preliminary in vitro studies to evaluate the cytotoxic effects of ingested substances on the intestinal tract as well as to evaluate the cytotoxic effects on colon tumour cells [64,65,66]. The HepG2 cell line is also used in preliminary in vitro studies aimed at evaluating the toxic effects on liver tumour cells. Both cell lines are complementary and can provide a more comprehensive understanding of the effects of a chemical compound or a bioproducts on intestinal barriers and hepatic metabolism. Studies have shown that their combined use can generate relevant data on the safety of specific chemical compounds or new bioproducts by analysing their effects on cell viability, DNA, or the production of reactive oxygen species in response to the toxicity of a chemical compound or bioproduct [58,64,67]. The results obtained during this study, from the tests performed in vitro on the Caco-2 cell line (all with statistical significance, p < 0.05), showed that the three crude algal bioproducts tested (A1; A2; A4) exhibited cytotoxicity for this cell line (Figure 5a) after 24 h of exposure. Despite this, the tests performed in parallel on the HepG2 cell line suggest that, in the case of the crude bioproduct A3, cytotoxic effects may occur at concentrations higher than 50 µL/mL (Figure 6a) after 24 h of exposure.
- 1.
- Certain monosaccharides or polysaccharides (such as fucose, glucose, fucoidan, alginate, or laminarin) may mimic or enhance the activity of growth factors, thereby promoting tumour cell growth through mechanisms that may include direct binding to growth factor receptors, stabilising receptor–ligand interactions, or extracellular matrix modification to promote signalling, or modulating responses that support tumour growth [84,85].
- 2.
- Crude extracts of algal polysaccharides may contain other water-soluble compounds (proteins, peptides, or other small molecules) that might interact with tumour cell receptors, modulating signalling pathways in tumour cells, which promote their proliferation [86].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, J.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M.; Afonso, C. Seaweed bioactives potential as nutraceuticals and functional ingredients: A review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Taboada, C.; Millan, R.; Miguez, I. Evaluation of marine algae Undaria pinnatifida and Porphyra purpurea as a food supplement: Composition, nutritional value and effect of intake on intestinal, hepatic and renal enzyme activities in rats. J. Sci. Food Agric. 2023, 93, 1863–1868. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Dragan, A.M.L.; Sirbu, R.; Cadar, E. Brown Seaweeds from Black Sea Coast as an Important Source of Bioactive Compounds of Interest for Human Health Brown Seaweeds from Black Sea Coast as an Important Source of Bioactive Compounds of Interest for Human Health. Eur. J. Nat. Sci. Med. 2023, 6, 98–110. [Google Scholar] [CrossRef]
- Mutlu-Durak, H.; Arikan-Algul, Y.; Bayram, E.; Haznedaroglu, B.Z.; Kutman, U.B.; Kutman, B.Y. Various extracts of the brown seaweed Cystoseira barbata with different compositions exert biostimulant effects on seedling growth of wheat. Physiol. Plant 2024, 176, e14503. [Google Scholar] [CrossRef]
- Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods 2023, 12, 3642. [Google Scholar] [CrossRef]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef]
- Gamero-Vega, G.; Palacios-Palacios, M.; Quitral, V. Nutritional Composition and Bioactive Compounds of Red Seaweed: A Mini-Review. J. Food Nutr. Res. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Salomone, V.N.; Riera, M. Proximal Composition of Undaria pinnatifida from San Jorge Gulf (Patagonia, Argentina). Biol. Trace Elem. Res. 2020, 196, 252–261. [Google Scholar] [CrossRef]
- Martinez, M.A.; Becherucci, M.E. Study of the potential use of the invasive marine algae Undaria pinnatifida in the preliminary development of a functional textile. J. Ind.Text. 2022, 51, 8127S–8141S. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Park, S.-W.; Kim, J.-W.; Choi, M.-S.; Lee, S.-M.; Haq, M.; Zhang, W.; Chun, B.-S. Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts. Mar. Drugs 2024, 22, 344. [Google Scholar] [CrossRef] [PubMed]
- Kolb, N.; Vallorani, L.; Milanović, N.; Stocchi, V. Evaluation of Marine Algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as Food Supplements. Food Technol. Biotechnol. 2024, 42, 57–61. [Google Scholar]
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora—Consolidated Version 01/01/2007. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity_en (accessed on 3 December 2024).
- Manev, Z.; Iliev, A.; Vachkova, V. Chemical characterization of brown seaweed—Cystoseira barbata. Bulg. J. Agric. Sci. 2013, 19, 12–15. [Google Scholar]
- Cadar, E.; Sirbu, R.; Ibram, A.; Ionescu, A.M. Evaluation of Total Phenolic Content in Relation to Antioxidant Activity of Brown Algae Cystoseira barbata from Black Sea. Rev. Chim. 2019, 70, 2684–2689. [Google Scholar] [CrossRef]
- Sellimi, S.; Kadri, N.; Barragan-Montero, V.; Laouer, H.; Hajji, M.; Nasri, M. Fucans from a Tunisian brown seaweed Cystoseira barbata: Structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014, 66, 281–288. [Google Scholar] [CrossRef]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the Therapeutic Applications of Algal Polysaccharides. J. Polym. Environ. 2022, 30, 785–809. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef]
- Türkmen, M.; Duran, K. The Effect of Brown Seaweed and Cattle Manure Combinations on The Properties of Eisenia fetida’s Organic Fertilizer. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 1070–1075. [Google Scholar] [CrossRef]
- Ramandani, A.A.; Sun, Y.-M.; Lan, J.C.W.; Lim, J.W.; Chang, J.S.; Srinuanpan, S.; Khoo, K.S. Upcycling food waste as a low-cost cultivation medium for Chlorella sp. microalgae. J. Sci. Food Agric. 2024. early view. [Google Scholar] [CrossRef]
- Zakaria, M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Extraction of antioxidants from Chlorella sp. using subcritical water treatment. In IOP Conference Series: Materials Science and Engineering, Volume 206, 29th Symposium of Malaysian Chemical Engineers (SOMChE) 2016, Miri, Sarawak, Malaysia, 1–3 December 2016; IOP Publishing: Bristol, UK, 2016; Available online: https://iopscience.iop.org/article/10.1088/1757-899X/206/1/012035 (accessed on 30 November 2024).
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella sp. and Spirulina (Arthrospira sp.). Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Toshkova-Yotova, T.; Sulikovska, I.; Djeliova, V.; Petrova, Z.; Ognyanov, M.; Denev, P.; Toshkova, R.; Georgieva, A. Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV-Isolation, Characterization, and Assessment of Anticancer Potential. Curr. Issues Mol. Biol. 2024, 46, 10312–10334. [Google Scholar] [CrossRef]
- Yusof, Y.A.; Saad, S.M.; Makpol, S.; Shamaan, N.A.; Ngah, W.Z. Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics 2010, 65, 1371–1377. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Surendran, L.; Sudhagar, B.; Kumar, D.S.R.S. Facile green synthesis of gold nanoparticles from marine algae Gelidiella acerosa and evaluation of its biological Potential. SN Appl. Sci. 2019, 1, 284. [Google Scholar] [CrossRef]
- He, R.; Zhou, D.; Xiao, L.; Li, Y. Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels 2023, 9, 11. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Maietta, I.; Rey-Méndez, R.; Rodríguez-Argüelles, M.C.; Lastra-Valdor, M.; Cavazza, A.; Grimaldi, M.; Bigi, F.; Simón-Vázquez, R. Gold Nanoparticles Synthesized by an Aqueous Extract of Codium tomentosum as Potential Antitumoral Enhancers of Gemcitabine. Mar. Drugs 2023, 21, 20. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Abd El Maksoud, A.I.; Wageed, M.; Alotaibi, A.S.; Elebeedy, D.; Khalil, H.; Hassan, A.; Abdella, A. Characterization and Anticancer Activity of Biosynthesized Au/Cellulose Nanocomposite from Chlorella vulgaris. Polymers 2021, 13, 3340. [Google Scholar] [CrossRef]
- Gürsoy, N.; Öztürk, B.Y.; Dağ, İ. Synthesis of intracellular and extracellular gold nanoparticles with a green machine and its antifungal activity. Turk. J. Biol. 2021, 45, 196–213. [Google Scholar] [CrossRef]
- Radu, N.; Voicescu, M.; Radu, E.; Ciprian Tanasescu, C. Biomaterial with antioxidant and antifungal activities, obtained from Romanian indigenous plants. Mol. Cryst. Liq. Cryst. 2017, 655, 243–249. [Google Scholar] [CrossRef]
- Radu, N.; Ghita, I.; Rau, I. Therapeutic Effect of Polysaccharides from Plantago Species. Mol. Cryst. Liq. Cryst. 2010, 523, 236–246. [Google Scholar] [CrossRef]
- Zaharie, M.G.O.; Radu, N.; Pirvu, L.; Bostan, M.; Voicescu, M.; Begea, M.; Constantin, M.; Voaides, C.; Babeanu, N.; Roman, V. Studies Regarding the Pharmaceutical Potential of Derivative Products from Plantain. Plants 2022, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Ioan, D.-C.; Rău, I.; Albu Kaya, M.G.; Radu, N.; Bostan, M.; Zgârian, R.G.; Tihan, G.T.; Dinu-Pîrvu, C.E.; Lupuliasa, A.; Ghica, M.V. Ciprofloxacin-Collagen-Based Materials with Potential Oral Surgical Applications. Polymer 2020, 12, 1915. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 system. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef]
- Eghbaliferiz, I. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1397. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Vignaud, J.; Loiseau, C.; Hérault, J.; Mayer, C.; Côme, M.; Martin, I.; Ulmann, L. Microalgae Produce Antioxidant Molecules with Potential Preventive Effects on Mitochondrial Functions and Skeletal Muscular Oxidative Stress. Antioxidants 2023, 12, 1050. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Talbot, G. Food and Beverage Stability and Shelf Life. In The Stability and Shelf Life of Fats and Oils; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 683–715. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Neshovska, H.; Manev, I.; Kirov, V. Heavy metal levels in water, brown algae (Cystoseira barbata), and eelgrass (Zostera marina) from the Southern Black Sea coast of Bulgaria. Int. J. Vet. Sci. Anim. Husb. 2021, 6, 15–18. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu Pîrjol, T.; Tomescu, A.; Paris, S.; Erimia, C.L.; Bogdan, N.S. Heavy Metals Existing in the Seaweed from the Romanian Coast of the Black Sea. Eur. J. Med. Nat. Sci. 2019, 2, 14–21. [Google Scholar] [CrossRef][Green Version]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef]
- Available online: https://www.ceva-algues.com/wp-content/uploads/2021/04/EN-Undaria-pinnatifida-1.pdf (accessed on 5 December 2024).
- van Groenigen, J.; Derksen, G.C.H.; Timmermans, K.R. Review of Presence, Induction and Isolation of Major Cellular Constituents From Porphyra Sensu Lato (Rhodophyceae), Including Mycosporine-Like Amino Acids (MAA’s). J. Mar. Biol. Aquac. Res. 2022, 4, 30–46. [Google Scholar] [CrossRef]
- Maliar, T.; Maliarová, M.; Blažková, M.; Kunštek, M.; Uváčková, Ľ.; Viskupičová, J.; Purdešová, A.; Beňovič, P. Simultaneously Determined Antioxidant and Pro-Oxidant Activity of Randomly Selected Plant Secondary Metabolites and Plant Extracts. Molecules 2023, 28, 6890. [Google Scholar] [CrossRef]
- Le Tutour, B.; Benslimane, F.M.; Gouleau, M.; Gouygou, J.P.; Saadan, B.; Quéméneur, F. Antioxidant and pro-oxidant activities of the brown algae, Laminaria digitata, Himanthalia elongata, Fucus vesiculosus, Fucus serratus and Ascophyllum nodosum. J. Appl. Phycol. 1998, 10, 121–129. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Rzeski, W. Enhancement of chemopreventive properties of young green barley and Chlorella extracts used together against colon cancer cells. Ann. Agric. Environ. Med. AAEM 2020, 27, 591–598. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT, and protein assay in hepatoma cell lines and primary hepatocytes cultured on collagen and on standard plastic plates. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Babeanu, N.; Radu, N.; Enascuta, C.-E.; Alexandrescu, E.; Ganciarov, M.; Mohammed, M.S.O.; Suica-Bunghez, I.R.; Senin, R.; Ursu, M.; Bostan, M. Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine. Polymers 2022, 14, 3544. [Google Scholar] [CrossRef]
- Ciric, A.; Radu, N.; Zaharie, M.G.O.; Neagu, G.; Pirvu, L.C.; Begea, M.; Stefaniu, A. Potential Antitumor Effect of Functional Yogurts Formulated with Prebiotics from Cereals and a Consortium of Probiotic Bacteria. Foods 2023, 12, 1250. [Google Scholar] [CrossRef]
- Fletcher, D.; Mullins, R. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Pfuhler, S.; Fellows, M.; van Benthem, J.; Corvi, R.; Curren, R.; Dearfield, K.; Fowler, P.; Frötschl, R.; Elhajouji, A.; Le Hégarat, L.; et al. In vitro genotoxicity test approaches with better predictivity: Summary of an IWGT workshop. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2011, 723, 101–107. [Google Scholar] [CrossRef]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef]
- Scheers, N.M.; Almgren, A.B.; Sandberg, A.S. Proposing a Caco-2/HepG2 cell model for in vitro iron absorption studies. J. Nutr. Biochem. 2014, 25, 710–715. [Google Scholar] [CrossRef]
- Mora-Navarro, C.; Méndez-Vega, J.; Caraballo-León, J.; Lee, M.; Palecek, S. Hydrophobicity of Antifungal β-Peptides is Associated with Their Cytotoxic Effect on In Vitro Human Colon Caco-2 and Liver HepG2 Cells. PLoS ONE 2016, 11, e0157025. [Google Scholar]
- Roursgaard, M.; Rothmann, M.H.; Schulte, J.; Karadimou, I.; Marinelli, E.; Møller, P. Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2, Cells. Front. Public Health 2022, 10, 906430. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. High-content analysis in toxicology: Screening substances for human toxicity potential, elucidating subcellular mechanisms and in vivo use as translational safety biomarkers. Basic Clin. Pharmacol. Toxicol. 2014, 115, 4–17. [Google Scholar] [CrossRef]
- Sawasdee, N.; Jantakee, K.; Wathikthinnakon, M.; Panwong, S.; Pekkoh, J.; Duangjan, K.; Yenchitsomanus, P.; Panya, A. Microalga Chlorella sp. extract induced apoptotic cell death of cholangiocarcinoma via AKT/mTOR signaling pathway. Biomed. Pharmacother. 2023, 160, 114306. [Google Scholar] [CrossRef]
- Celep, A.G.S.; Demirkaya, A.; Solak, E.K. Antioxidant and Anticancer Activities of Gallic Acid Loaded Sodium Alginate Microspheres on Colon Cancer. Curr. Appl. Phys. 2022, 40, 30–42. [Google Scholar] [CrossRef]
- Koivikko, R. Brown Algal Phlorotannins Improving and Applying Chemical Methods. Doctoral Thesis, University of Turku, Turku, Finland, 2008. Available online: https://urn.fi/URN:ISBN:978-951-29-3503-1 (accessed on 23 December 2024).
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2020, 230, 115644. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C. Interactions between apple (Malus x domestica borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Manuel, A.; Coimbra, A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Zdziebłowska, S.; Czarnecki, M.; Ciosek-Skibińska, P.; Ruzik, L. The microalgae’s ability to accumulate selected trace elements studied by ICP-MS/MS and chemometric methods. J. Trace Elem. Med. Biol. 2024, 81, 127351. [Google Scholar] [CrossRef]
- Hau, L.; Robertson, J.; White, W. Metals in New Zealand Undaria pinnatifida (Wakame). Open J. Mar. Sci. 2014, 4, 163–173. [Google Scholar] [CrossRef][Green Version]
- Jing, Y.; Zhang, S.; Li, M.; Zhang, R.; Zhang, H.; Zheng, Y.; Zhang, D.; Wu, L. Structural characterization and biological activities of polysaccharide iron complex synthesized by plant polysaccharides: A review. Front. Nutr. 2022, 9, 1013067. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-S.; Yao, Y.-F.; Wang, L.-F.; Li, W.-J. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023, 159, 114221. [Google Scholar] [CrossRef]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Ustyuzhanina, N.E.; Vinnitskiy, D.Z.; Tokatly, A.I.; Reshetnikova, V.V.; Chikileva, I.O.; Shubina, I.Z.; Kirgizov, K.I.; Nifantiev, N.E. Perspectives for the Use of Fucoidans in Clinical Oncology. Int. J. Mol. Sci. 2022, 23, 11821. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, N.Y.; Ustyuzhanina, N.E.; Bilan, M.I.; Morozevich, G.E.; Usov, A.I.; Nifantiev, N.E.; Kiselevskiy, M.V. Anti-angiogenic properties of sulfated polysaccharides fucoidans and their analogs. Russ. Chem. Bull. 2022, 71, 2505–2514. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef]
- Dutot, M.; Olivier, E.; Fouyet, S.; Magny, R.; Hammad, K.; Roulland, E.; Rat, P.; Fagon, R. In Vitro Chemopreventive Potential of Phlorotannins-Rich Extract from Brown Algae by Inhibition of Benzo[a]pyrene-Induced P2X7 Activation and Toxic Effects. Mar. Drugs 2021, 19, 34. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Roca-Lema, D.; Martinez-Iglesias, O.; Portela, C.F.d.A.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-proliferative and Anti-invasive Effect of Polysaccharide-rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ramírez, J.; Mondragón-Portocarrero, A.C.; Rodríguez, J.A.; Lorenzo, J.M.; Santos, E.M. Algae as a potential source of protein meat alternatives. Front. Nutr. 2023, 10, 1254300. [Google Scholar] [CrossRef] [PubMed]



| Name | Source | Poly- Saccharides Bioproduct Codification | Extraction Conditions | Concentration, in Crude Extract mg/mL ± STDEV | Bioproduct with Gold Codification | Maturation Time | Volume Between A1:Au3+ | Voucher Specimen Deposited at INCDCF Bucharest, Romania |
|---|---|---|---|---|---|---|---|---|
| Porphyra umbilicalis | Romanian market EAN 13 code: 8858960303134 | A1 | t = 95 °C Time = 2 h | 27.27 ± 0.01 | A1+Au | 48 h | 1:4.5 | Poum19-fractionate |
| Undaria pinnatifida | Romanian market EAN 13 code: 8717703617535 | A2 | t = 95 °C time: 2 h | 60 ± 0.10 | A2+Au | 48 h | 1:4.5 | Unpi19-fractionate |
| Cystoseira barbata | Black Sea | A3 | t = 95 °C time:2 h | 176.4 ± 0.05 | A3+Au | 48 h | 1:4.55 | Cyba19-fractionate |
| Chlorella sp. | Photobioreactor multiplication | A4 | t = 95 °C time: 2 h | 55.55 ± 0.10 | A4+Au | 48 h | 1:4.5 | Chl19-fractionate |
| Bioproduct with Poly-Saccharides | Source | Polyphenols Content, mgGAE/L ± STDEV | Bioproduct with Gold | Polyphenols Content mgGAE/L ± STDEV | The Total Content of Gold in the Bioproduct, mM ± STDEV |
|---|---|---|---|---|---|
| A1 | Porphyra umbilicalis | 19.84 ± 0.10 | A1+Au | 3.67 ± 0.02 | 0.812 ± 0.008 |
| A2 | Undaria pinnatifida | 19.70 ± 0.13 | A2+Au | 3.03 ± 0.01 | 0.849 ± 0.004 |
| A3 | Cystoseira barbata | 714.17 ± 1.26 | A3+Au | 85.70 ± 0.15 | 0.876 ± 0.010 |
| A4 | Chlorella sp. | 54.72 ± 0.11 | A4+Au | 8.42 ± 0.03 | 0.832 ±0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marian, T.; Radu, N.; Voicescu, M.; Nistor, C.L.; Pirvu, L.C.; Mihaila, M.; Bostan, M. Preliminary Biological Assessments of Some Algae Basis Biomaterials. J. Mar. Sci. Eng. 2025, 13, 318. https://doi.org/10.3390/jmse13020318
Marian T, Radu N, Voicescu M, Nistor CL, Pirvu LC, Mihaila M, Bostan M. Preliminary Biological Assessments of Some Algae Basis Biomaterials. Journal of Marine Science and Engineering. 2025; 13(2):318. https://doi.org/10.3390/jmse13020318
Chicago/Turabian StyleMarian, Toader, Nicoleta Radu, Mariana Voicescu, Cristina Lavinia Nistor, Lucia Camelia Pirvu, Mirela Mihaila, and Marinela Bostan. 2025. "Preliminary Biological Assessments of Some Algae Basis Biomaterials" Journal of Marine Science and Engineering 13, no. 2: 318. https://doi.org/10.3390/jmse13020318
APA StyleMarian, T., Radu, N., Voicescu, M., Nistor, C. L., Pirvu, L. C., Mihaila, M., & Bostan, M. (2025). Preliminary Biological Assessments of Some Algae Basis Biomaterials. Journal of Marine Science and Engineering, 13(2), 318. https://doi.org/10.3390/jmse13020318








