Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru
Abstract
1. Introduction
2. Materials and Methods
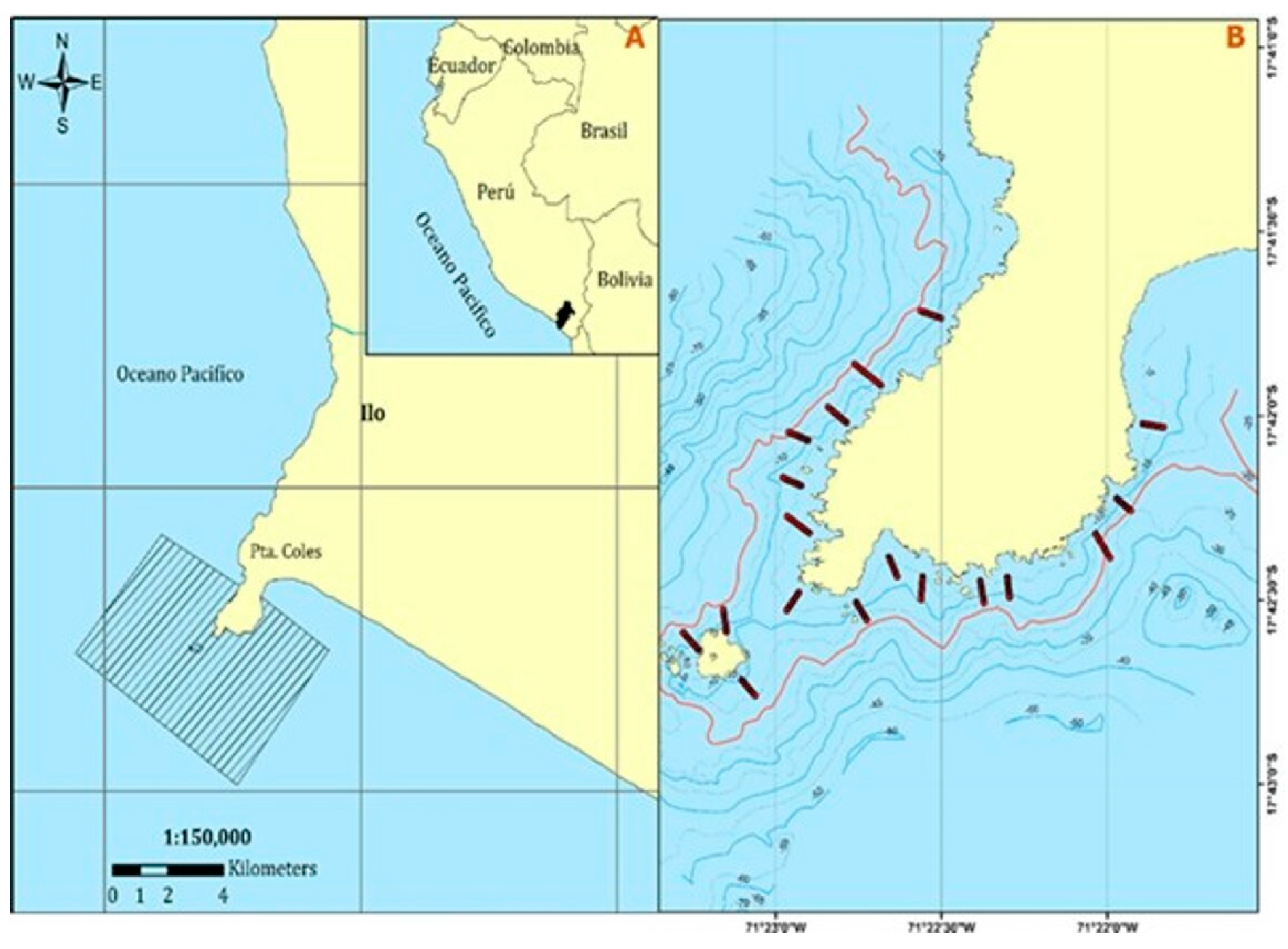
2.1. Study Area
2.2. Collection of Bathilithological Information
2.3. Data Processing
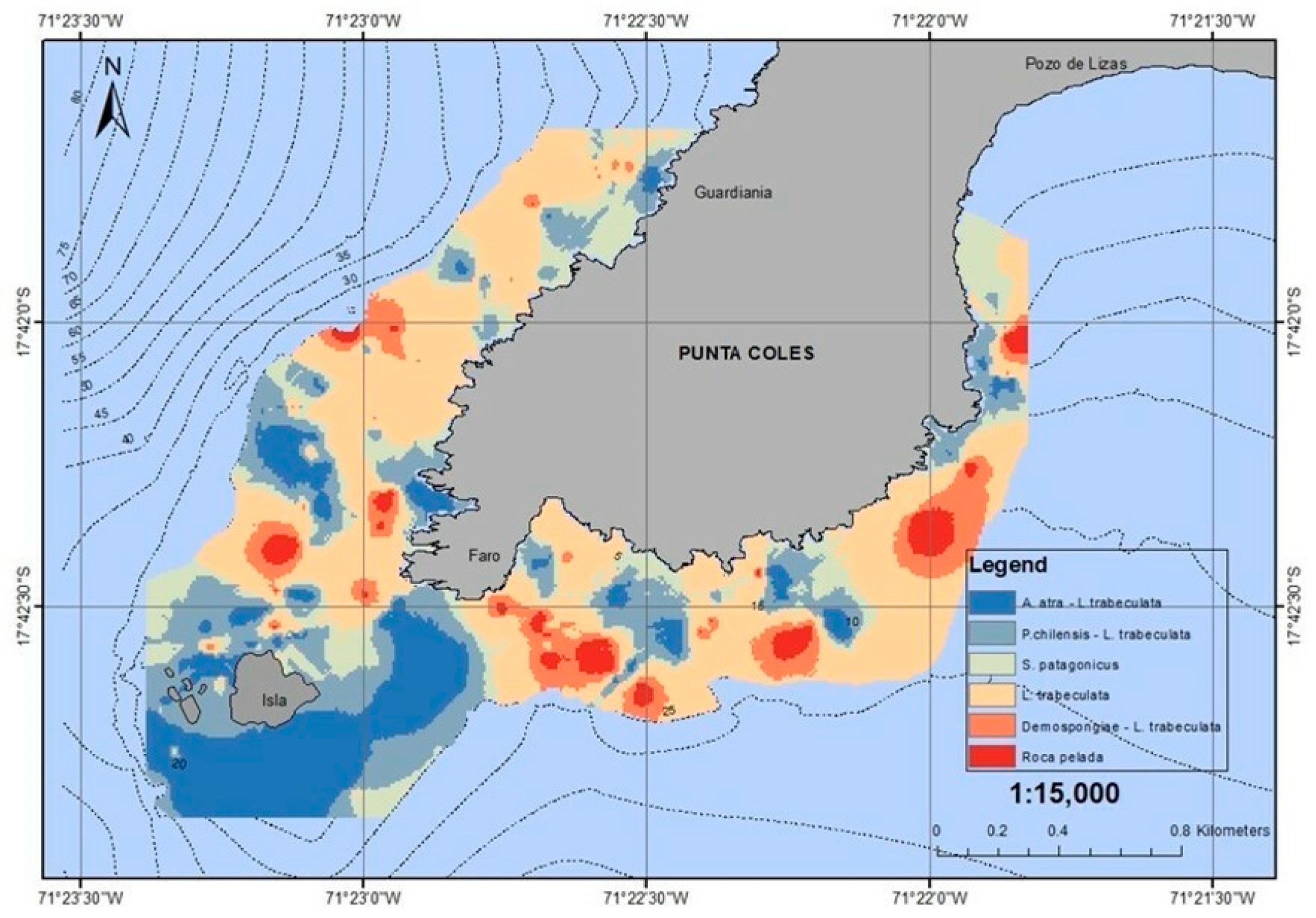
3. Results
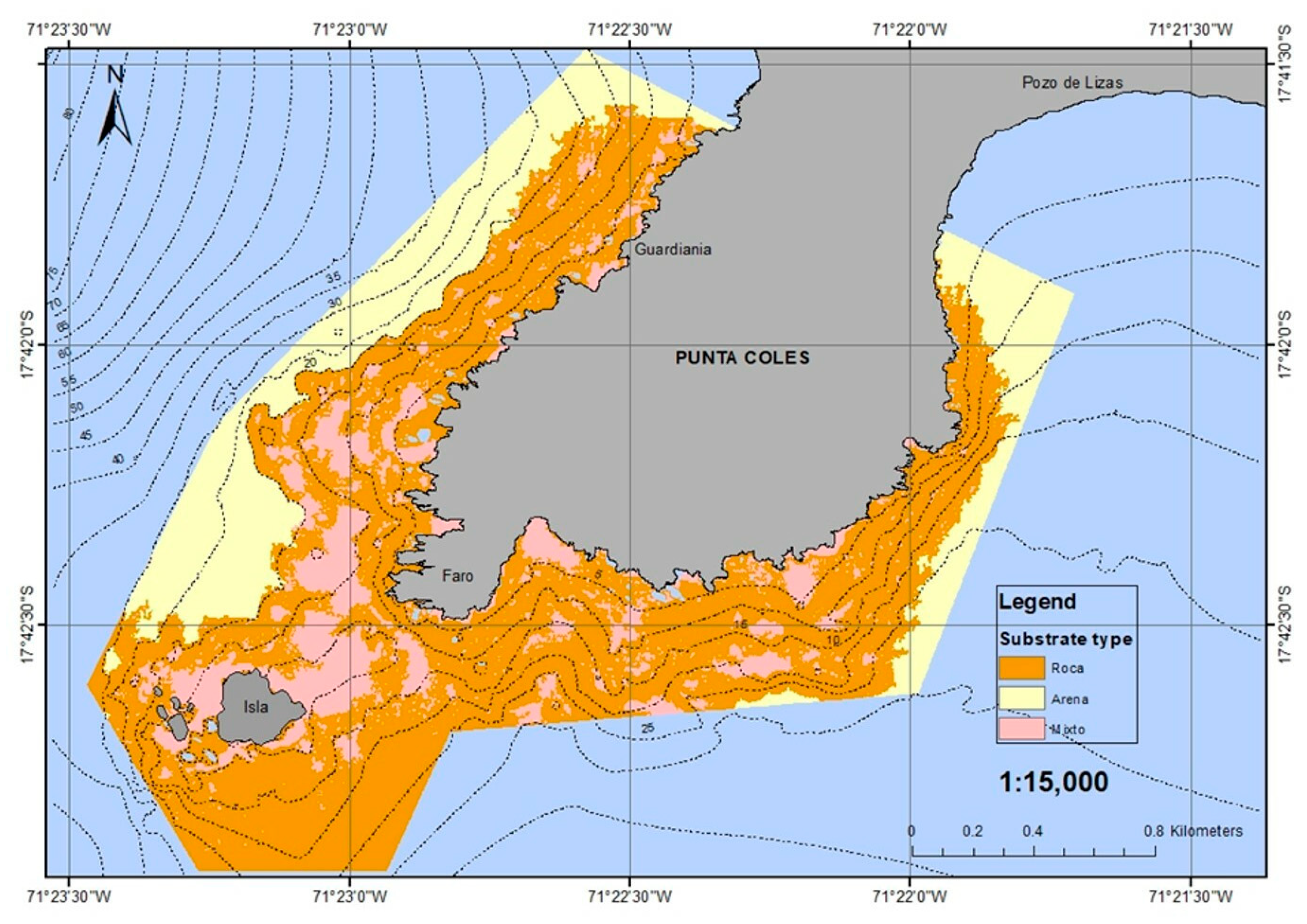
3.1. Bathilithological Survey
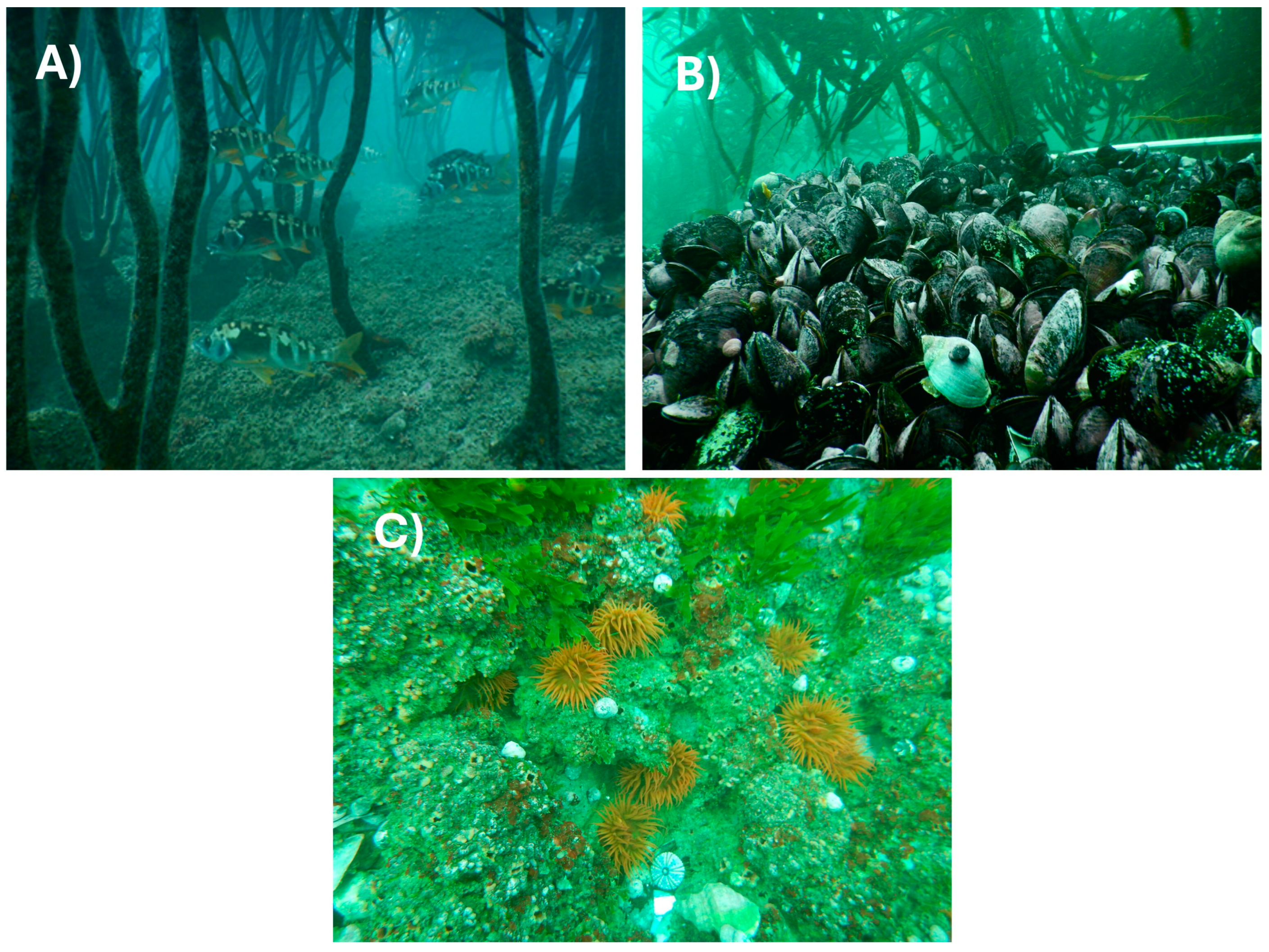
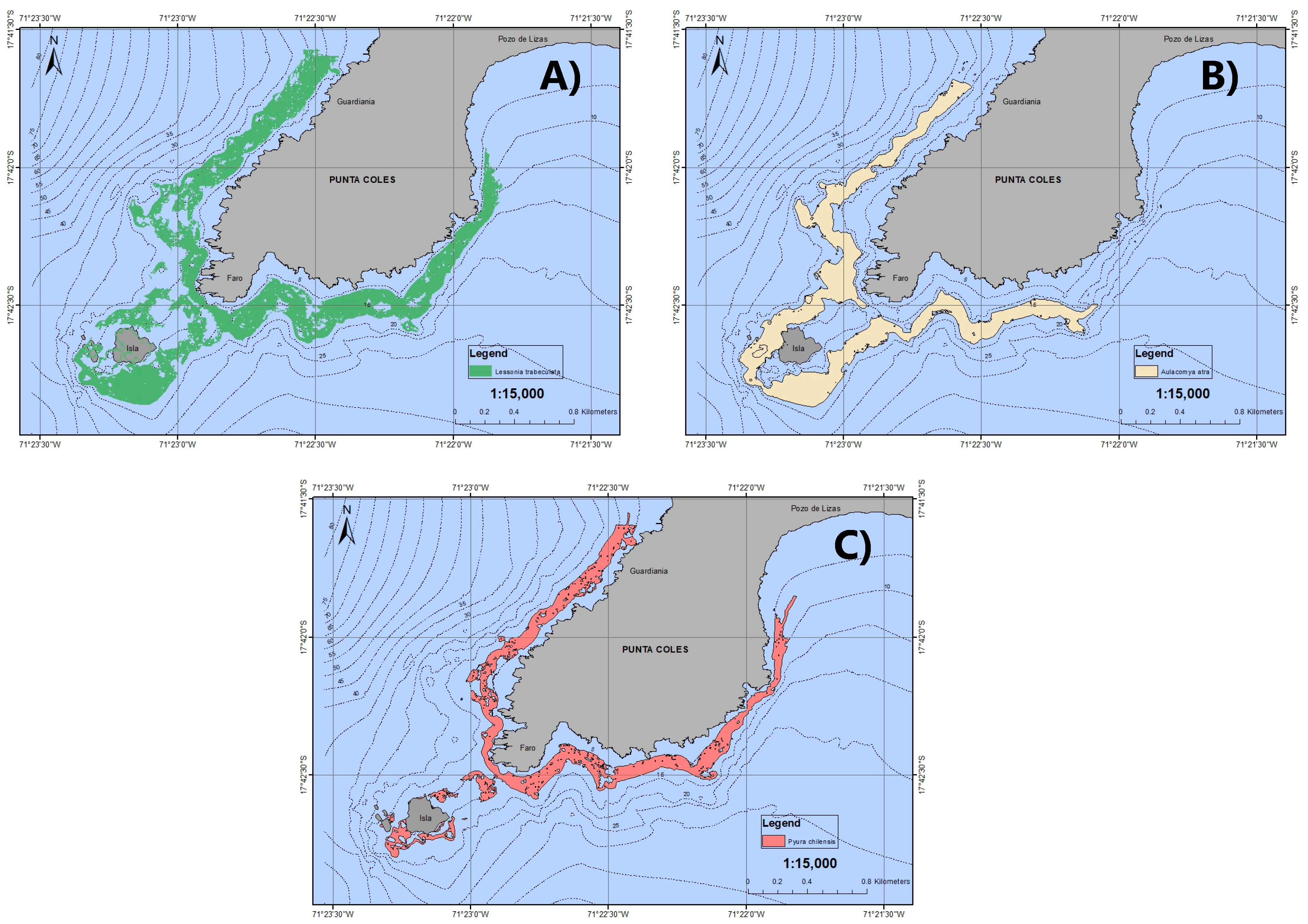
3.2. Main Habitat-Structuring Organisms
3.3. Lithology and Patches of Bioengineering Organisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.; Fox, H.; Allen, G.; Davison, N.; Ferdana, Z.; Finlayson, M.; Halpern, B.; Jorge, M.; Lombona, A.; Lourie, S.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Tarazona, J.; Gutiérrez, D.; Paredes, C.; Indacochea, A. Overview and challenges of marine biodiversity research in Peru una revision y desafios Para la investigacion en biodiversidad marina en Peru. Gayana 2003, 67, 206–231. [Google Scholar]
- Figueroa, E. Biodiversidad Marina: Valoración, Usos y Perspectivas: Hacia Dónde va Chile? Editorial Universitaria: Santiago de Chile, Chile, 2005. [Google Scholar]
- Gómez, R. Cadenas de valor, comercio exterior y diversidad biológica/Value Chains, Trade and Biodiversity. Apuntes 2013, 40, 143–174. [Google Scholar] [CrossRef][Green Version]
- UNDP United Nations Development Programme. Latin America and the Caribbean: A Biodiversity Superpower. 2010. Available online: https://www.undp.org/publications/latin-america-and-caribbean-biodiversity-super-power (accessed on 20 April 2024).
- SERNANP—Servicio Nacional de Áreas Naturales Protegidas por el Estado. Plan de Sitio de Punta Coles, Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras; SERNANP: Lima, Perú, 2014; 104p. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 2002, 279, 860–863. [Google Scholar] [CrossRef]
- Hilborn, R.; Stokes, K.; Maguire, J.J.; Smith, T.; Botsford, L.W.; Mangel, M.; Orensanz, J.; Parma, A.; Rice, J.; Bell, J.; et al. When can marine reserves improve fisheries management? Ocean. Coast. Manag. 2004, 47, 197–205. [Google Scholar] [CrossRef]
- Hilborn, R. Are MPAs effective? ICES J. Mar. Sci. 2018, 75, 1160–1162. [Google Scholar] [CrossRef]
- Sala, E.; Giakoumi, S. No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 2018, 75, 1166–1168. [Google Scholar] [CrossRef]
- Sarker, S.; Rahman, M.J.; Wahab, M.A. Modeling the role of marine protected area in biodiversity conservation. J. Sea Res. 2023, 196, 102457. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Thomson, K.T. Plant diversity and ecosystem productivity: Theoretical considerations. Proc. Natl. Acad. Sci. USA 1997, 94, 1857–1861. [Google Scholar] [CrossRef]
- Naeem, S.; Wright, J.P. Disentangling biodiversity effects on ecosystem functioning: Deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 2003, 6, 567–579. [Google Scholar] [CrossRef]
- Micheli, F.; Saenz-Arroyo, A.; Greenley, A.; Vazquez, L.; Espinoza Montes, J.A.; Rossetto, M.; De Leo, G.A. Evidence that marine reserves enhance resilience to climatic impacts. PLoS ONE 2012, 7, e40832. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J.; Pauly, D.; Sáenz-Arroyo, A.; Sumaila, U.R.; Wilson, R.W.; et al. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef] [PubMed]
- Blanluet, A.; Game, E.T.; Dunn, D.C.; Everett, J.D.; Lombard, A.T.; Richardson, A.J. Evaluating ecological benefits of oceanic protected areas. Trends Ecol. Evol. 2024, 39, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organism as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- IMARPE—Instituto del Mar del Perú. Identificación y Delimitación de Bancos Naturales de Recursos Bentónicos en el Litoral de la Región Moquegua; Informe Técnico; Instituto del Mar del Perú: Callao, Peru, 2003. [Google Scholar]
- Cerda, M.; Castilla, J.C. Diversity and biomass of the macroinfauna and meiofauna associated with Pyura praeputialis (Heller 1878) (Tunicata, Ascidiacea) in the Bay of Antofagasta, Chile. Rev. Chil. De Hist. Nat. 2001, 74, 841–853. [Google Scholar]
- Carbajal, P.; Gamarra Salazar, A.; Moore, P.J.; Pérez-Matus, A. Different kelp species support unique macroinvertebrate assemblages, suggesting the potential community-wide impacts of kelp harvesting along the Humboldt current system. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 14–27. [Google Scholar] [CrossRef]
- Sepúlveda, R.D.; Camus, P.A.; Moreno, C.A. Diversity of faunal assemblages associated with ribbed mussel beds along the South American coast: Relative roles of biogeography and bioengineering. Mar. Ecol. 2016, 37, 943–956. [Google Scholar] [CrossRef]
- Villegas, M.J.; Laudien, J.; Sielfeld, W.; Arntz, W.E. Macrocystis integrifolia and Lessonia trabeculata (Laminariales; Phaeophyceae) kelp habitat structures and associated macrobenthic community off northern Chile. Helgol. Mar. Res. 2008, 62, 33–43. [Google Scholar] [CrossRef]
- Zaixso, H.E. Cholga banks Aulacomya atra atra (Molina) (Bivalvia: Mytilidae) from the San José Gulf (Chubut, Argentina): Diversity and relationships with related facies. J. Mar. Biol. Oceanogr. 2004, 39, 61–78. [Google Scholar]
- Vásquez, J.; Vega, J. Macroinvertebrados asociados a discos de adhesión de algas pardas: Biodiversidad de comunidades discretas como indicadora de perturbaciones locales y de gran escala. In Biodiversidad Marina: Valoración, Uso y Perspectivas. ¿Hacia Dónde va Chile? Editorial Universitaria: Santiago de Chile, Chile, 2005; pp. 429–450. [Google Scholar]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J Exp Mar Biol Ecol 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Villouta, E.; Santelices, B. Estructura de la comunidad submareal de Lessonia (Phaeophyta, Laminariales) en Chile norte y central. Rev. Chil. De Hist. Nat. 1984, 57, 111–122. [Google Scholar]
- Smith, S.; Simpson, R.; Cairns, S. The macrofaunal community of Ecklonia radiata holdfast: Description of the faunal assemblage and variation associated with differences in holdfast volume. Aust. J. Ecol. 1996, 21, 81–95. [Google Scholar] [CrossRef]
- Cacino, J.M.; Santelices, B. Importancia ecológica de los discos adhesivos de Lessonia nigrescens Bory (Phaeophyta) en chile central. Rev. De Hist. Nat. 1984, 56, 23–33. [Google Scholar]
- Cacino, J.M.; Santelices, B. The importance of kelp-like holdfasts as a habitat of invertebrates in central Chile. II, Factors affecting community organization. In Proceedings of the Xth International Seaweed Symposium, Göteborg, Sweden, 11–15 August 1980; Levring, T., Ed.; Walter de Gruyter y Co.: New York, NY, USA, 1981; pp. 241–246. [Google Scholar]
- Andrews, H.L. The kelp beds of the Monterey region. Ecology 1945, 26, 24–37. [Google Scholar] [CrossRef]
- Bakun, A.; Weeks, S.J. The marine ecosystem off Peru: What are the secrets of its fishery productivity and what might its future hold? Prog. Oceanogr. 2008, 79, 290–299. [Google Scholar] [CrossRef]
- Graco, M.; Ledesma, J.; Flores, G.; Girón, M. Nutrientes, oxígeno y procesos biogeoquímicos en el sistema de surgencias de la corriente de Humboldt frente a Perú. Rev. Peru. Biol. 2007, 14, 117–128. [Google Scholar] [CrossRef]
- Greenstreet, S.; Hall, S. Fishing and the ground-fish assemblage structure in the northwestern North Sea: An analysis of long-term and spatial trends. J. Anim. Ecol. 1996, 65, 577–598. [Google Scholar] [CrossRef]
- Jennings, S.; Greenstreet, S.; Reynolds, J. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Ibarra, A.A.; Reid, C.; Thorpe, A. Neo-liberalism and its impact on overfishing and overcapitalization in the marine fisheries of Chile, Mexico and Peru. Food Policy 2000, 25, 599–622. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Echevin, V.; Tam, J.; Takahashi, K.; Bertrand, A. Impacto del Cambio Climático Sobre el mar Peruano: Tendencias Actuales y Futuras; Grégoire, A., Ed.; El Perú frente al Cambio Climático resultados de investigaciones franco-peruanas (143-151); Instituto de Investigación para el Desarrollo-IRD: Montpellier, France, 2014; Available online: https://repositoriodigital.minam.gob.pe/handle/123456789/1029 (accessed on 24 April 2024).
- Jigena-Antelo, B.; Estrada-Ludeña, C.; Howden, S.; Rey, W.; Paz-Acosta, J.; Lopez-García, P.; Salazar-Rodriguez, E.; Endrina, N.; Muñoz-Pérez, J.J. Evidence of sea level rise at the Peruvian coast (1942–2019). Sci. Total Environ. 2023, 859, 160082. [Google Scholar] [CrossRef]
- Loaiza, I.; De Boeck, G.; De Troch, M. Peruvian marine ecosystems under metal contamination: First insights for marine species consumption and sustainable management. Sci. Total Environ. 2022, 826, 154132. [Google Scholar] [CrossRef] [PubMed]
- Tejada, A.; Baldarrago, D.; Pastor, R.; Ortiz, M. Assessing the benefits of the marine protected area of Punta Coles, southern Peru (south-east Pacific) on productivity of conspicuous benthic species using pre-image population analysis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 29–43. [Google Scholar] [CrossRef]
- Valqui, J.; Ibañez-Erquiaga, B.; Pacheco, A.S.; Wilbur, L.; Ochoa, D.; Cardich, J.; Pérez-Huaranga, M.; Salas-Gismondi, R.; Pérez, A.; Indacochea, A.; et al. Changes in rocky intertidal communities after the 2015 and 2017 El Niño events along the Peruvian coast. Estuar. Coast. Shelf Sci. 2021, 250, 107142. [Google Scholar] [CrossRef]
- Caza, F.; Cledon, M.; St-Pierre, Y. Biomonitoring climate change and pollution in marine ecosystems: A review on Aulacomya ater. J. Mar. Sci. 2016, 2016, 7183813. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Vega, J.A.; Buschmann, A.H. Long term variability in the structure of kelp communities in northern Chile and the 1997–1998 ENSO. In Eighteenth International Seaweed Symposium, Proceedings of the Eighteenth International Seaweed Symposium, Held in Bergen, Norway, 20–25 June 2004; Springer: Dordrecht, The Netherlands, 2007; pp. 279–293. [Google Scholar]
- Calderón-Aguilera, L.E.; Pérez-España, H.; Cabral-Tena, R.A.; Norzagaray-Lopez, C.O.; Lopez-Pérez, A.; Alvarez-Filip, L.; Reyes-Bonilla, H. Ecological modeling and conservation on the coasts of México. In Marine Coastal Ecosystems Modeling and Conservation, Latin American Experiences; Ortiz, M., Jordan, F., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 3–25. [Google Scholar]
- Hermosillo-Núñez, B.; Ortiz, M.; Jordán, F.; Endrédi, A. Macroscopic properties and keystone species complexes in kelp forest ecosystems along the north-central Chilean Coast. In Marine Coastal Ecosystems Modeling and Conservation, Latin American Experiences; Ortiz, M., Jordan, F., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 95–119. [Google Scholar]
- Castilla, J.C.; Guiñez, R.; Caro, A.U.; Ortiz, V. Invasion of a rocky intertidal shore by the tunicate Pyura praeputialis in the Bay of Antofagasta, Chile. Proc. Natl. Acad. Sci. USA 2004, 101, 8517–8524. [Google Scholar] [CrossRef]
- Schmiing, M.; Diogo, H.; Santos, R.S.; Afonso, P. Assessing hotspots within hotspots to conserve biodiversity and support fisheries management. Mar. Ecol. Prog. Ser. 2014, 513, 187–199. [Google Scholar] [CrossRef]
- Tovar, H.; Fuentes, H. Observaciones de aves marinas en la zona sur del Perú en diciembre de 1978. Inst. Mar Perú 1980, 68, 1–9. Available online: https://hdl.handle.net/20.500.12958/293 (accessed on 24 April 2024).
- Tovar, H. Áreas de producción y distribución de las aves marinas en el litoral peruano. Bol. Inst. Mar Perú 1968, 1, 523–546. [Google Scholar]
- Baldarrago, D.; Pastor, R.; Aragón, B.; Liza, C.; Tejada, A. Diversidad y abundancia de las comunidades bentónicas en matrices de organismos bioingenieros de las regiones de Moquegua y Tacna—2015. Inst. Mar Perú 2017, 44, 429–441. [Google Scholar]
- Baldarrago, D.; Aragón, B.; Vizcarra, Y.; Tejada, A. Estructura bentónica en el submareal somero de Punta Coles (Ilo, Moquegua) en el 2017. Inst. Mar Perú 2019, 46, 578–600. [Google Scholar]
- Journel, A.G.; Huijbregts, C.J. Mining Geostatistics; Academic Press: Cambridge, UK, 1978. [Google Scholar]
- cFolk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Company: Austin, TX, USA, 1980. [Google Scholar]
- Shepard, D. A Two-Dimensional Interpolation Function for Irregularly Spaced Data. In Proceedings of the 1968 ACM National Conference, New York, NY, USA, 27–29 August 1968; pp. 517–524. [Google Scholar]
- Open Geospatial Consortium OGC. 2012. Available online: https://www.ogc.org/es/press-release/ogc-adopts-netcdf-enhanced-data-model-extension-standard/ (accessed on 24 April 2024).
- ESRI ArcGIS, el Software SIG de Representación Cartográfica líder en el Mundo. 2010. Available online: https://www.esri.com/es-es/home (accessed on 24 August 2023).
- Bae, S.; Ubagan, M.D.; Shin, S.; Kim, D.G. Comparación de los patrones de reclutamiento de invertebrados marinos sésiles According to las características del sustrato. Int. J. Environ. Res. Public Health 2022, 19, 1083. [Google Scholar] [CrossRef]
- Freeman, J.B.; Semmens, B.X.; Thompson, A.R. Impactos de las áreas marinas protegidas y el medio ambiente en la riqueza de especies de peces rocosos larvarios y la estructura de los ensambles en la bahía del sur de California. Mar. Ecol. Prog. Ser. 2022, 698, 125–137. [Google Scholar] [CrossRef]
- Tarazona, J.; Salzwedel, H.; Arntz, W.E. Oscillations of macrobenthos in shallow waters of the Peruvian central coast induced by El Niño 1982–1983. J. Mar. Res. 1988, 46, 593–611. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Bouchon, M. El Niño Costero 2017 y sus impactos en el ecosistema marino. Bol. Inst. Mar Perú 2021, 36, 313–328. Available online: https://hdl.handle.net/20.500.12958/4244 (accessed on 24 August 2023). [CrossRef]
- Lathlean, J.A. Not all space is created equal: Distribution of free space and its influence on heat-stress and the limpet Patelloida latistrigata. J. Therm. Biol. 2014, 46, 16–23. [Google Scholar] [CrossRef]
- Manríquez, P.; Guiñez, R.; Olivares, A.; Clarke, M.; Castilla, J.C. Effects of interannual temperature variability, including ENSO and post-ENSO events, on reproductive traits in the tunicate Pyura praeputialis. Mar. Biol. Res. 2018, 14, 462–477. [Google Scholar] [CrossRef]
- Meira, A.; Byers, J.E.; Sousa, R. A global synthesis of predation on bivalves. Biol. Rev. 2024, 99, 1015–1057. [Google Scholar] [CrossRef]
- Navarrete, S.A.; Manzur, T. Individual- and population-level responses of a keystone predator to geographic variation in prey. Ecology 2008, 89, 2005–2018. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D. Ecological effects of marine protected areas on rocky reef communities—A continental-scale analysis. Mar. Ecol. Prog. Ser. 2009, 388, 51–62. [Google Scholar] [CrossRef]
- Branch, G.M.; Griffiths, C.L. The Benguela ecosystem Part V. The coastal zone. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 395–486. [Google Scholar]
- Kostylev, V.E.; Todd, B.J.; Fader, G.B.J.; Courtney, R.C.; Cameron, G.D.M.; Pickrill, R.A. Benthic habitat mapping on the Scotian Shelf based on multibeam bathymetry, surficial geology and sea floor photographs. Mar. Ecol. Prog. Ser. 2001, 219, 121–137. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Logan, J.B.; Field, M.E. Quantitative morphological analysis of the fringing reef of Molokai, Hawaii. Coral Reefs 2016, 25, 51–66. [Google Scholar]
- Peleg, O.; Blain, C.O.; Shears, N.T. Long-term marine protection enhances kelp forest ecosystem stability. Ecol. Appl. 2023, 33, e2895. [Google Scholar] [CrossRef] [PubMed]
- 6Filbee-Dexter, K.; Starko, S.; Pessarrodona, A.; Wood, G.; Norderhaug, K.M.; Piñeiro-Corbeira, C.; Wernberg, T. Marine protected areas can be useful but are not a silver bullet for kelp conservation. J. Phycol. 2024, 60, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.R.; Bailey, D.M.; Potts, T. Perceptions of practitioners: Managing marine protected areas for climate change resilience. Ocean Coast. Manag. 2016, 128, 18–28. [Google Scholar] [CrossRef]
- Bularz, B.; Fernández, M.; Subida, M.D.; Wieters, E.A.; Pérez-Matus, A. Effects of Harvesting on Subtidal Kelp Forests (Lessonia Trabeculata) in Central Chile. Ecosphere 2022, 13, e3958. [Google Scholar] [CrossRef]
- Topor, Z.M.; Rasher, D.B.; Duffy, J.E.; Brandl, S.J. Marine protected areas enhance coral reef functioning by promoting fish biodiversity. Conserv. Lett. 2019, 12, e12638. [Google Scholar] [CrossRef]
- Jorquera-Jaramillo, C.; Vega, J.A.; Aburto, J.; Martínez-Tillería, K.; León, M.; Perez, M.; Gaymer, C.; Squeo, F.A. Conservación de la biodiversidad en Chile: Nuevos desafíos y oportunidades en ecosistemas terrestres y marinos costeros. Rev. Chil. Hist. Nat. 2012, 85, 267–280. [Google Scholar] [CrossRef]
- Gómez-Aguayo, A.M.; Estruch-Guitart, V. Valoración económica de los servicios ecosistémicos marinos: Un caso de estudio de La Safor, Golfo de Valencia, España. Ecosistemas 2019, 28, 100–108. [Google Scholar] [CrossRef]
- Nakandakari, A.; Caillaux, M.; Zavala, J.; Gelcich, S.; Ghersi, F. The importance of understanding self-governance efforts in coastal fisheries in Peru: Insights from La Islilla and Ilo. Bull. Mar. Sci. 2017, 93, 199–216. [Google Scholar] [CrossRef]
- Castrejón, M.; Defeo, O. Perceptions and attitudes of residents toward small-scale longline tuna fishing in the Galapagos Marine Reserve: Conservation and management implications. Front. Mar. Sci. 2023, 10, 1235926. [Google Scholar] [CrossRef]
- Defeo, O.; Castrejón, M.; Pérez-Castañeda, R.; Castilla, J.C.; Gutiérrez, N.L.; Essington, T.E.; Folke, C. Comanagement in Latin American small-scale shellfisheries: Assessment from long-term case studies. Fish Fish. 2016, 17, 176–192. [Google Scholar] [CrossRef]
| Function | Species | Common Name | Habitat |
|---|---|---|---|
| Benthos of commercial importance | Concholepas concholepas Bruguière, 1789 | False abalone, Barnacke rock shell | It was observed mainly in shallow areas, at depths less than 10 m, with rocky substratum, inhabiting cracks and vertical rocky walls, in P. chilensis matrices and among the algae L. trabeculata. |
| Loxechinus albus Molina, 1782 | Red sea urchin, Green sea urchin | It was recorded mainly in shallow areas with rocky substrate, at depths less than 10 m, forming aggregations or solitary, feeding on the remains (fronds) of L. trabeculata algae and calcareous algae. It was also found in areas without coverage (white backgrounds). | |
| Thaisella chocolata Duclos, 1832 | Chocolate rock shell | It was recorded in deep areas with rocky substratum, more than 12 m deep, forming aggregations, each called an “egg cup”, or solitary on the matrices of A. atra. | |
| Fissurella latimarginata G. B. Sowerby I, 1835 | Black limpet | It presented a bathymetric distribution similar to that observed for L. albus, concentrating on shallow areas with rocky substrate and associated with the disks of L. trabeculata. | |
| Fissurella pulchra G. B. Sowerby I, 1834 Fissurella cumingi Reeve, 1849 Fissurella maxima G. B. Sowerby I, 1834 | Limpet | They presented a bathymetric distribution similar to that observed for F. latimarginata, recorded in the rocky substrate and associated with red calcareous algae and B. laevis barnacles. | |
| Romaleon setosum Molina, 1782 | Rock-crabs, cancer crab | It was recorded both in rocky substrates and in soft bottoms (sandy), at depths that ranged from 5 m to 15 m, in groups or alone. | |
| Predators Competitor | Heliaster helianthus Lamarck, 1816 | Sea sun | It was recorded mainly in deep rocky areas, between 12 and 18 m deep, feeding in groups or alone on the matrices of A. atra, as well as other small mollusks and barnacles. |
| Stichaster striatus Müller and Troschel, 1840 | Starfish | It was recorded in shallow rocky areas, at depths less than 10 m, solitary or forming aggregations, feeding mainly on small bivalves and barnacles. | |
| Arbacia nigra Molina, 1782 | Black sea urchin | It presented a wide bathymetric distribution, with the greatest abundances in shallow rocky areas devoid of cover, where it formed aggregations and shared habitat with L. albus. It was also recorded feeding on the stipe and fronds of L. trabeculata. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Ancca, S.; Pepe-Victoriano, R.; Gonzales, H.H.S.; Aguilar, J.L.C.; Meza, Y.A.; Pacho, M.A.Q.; Cáceres, A.T.; Baldarrago Centeno, D.E.; Aguilera, J.G. Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru. J. Mar. Sci. Eng. 2024, 12, 2265. https://doi.org/10.3390/jmse12122265
Méndez-Ancca S, Pepe-Victoriano R, Gonzales HHS, Aguilar JLC, Meza YA, Pacho MAQ, Cáceres AT, Baldarrago Centeno DE, Aguilera JG. Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru. Journal of Marine Science and Engineering. 2024; 12(12):2265. https://doi.org/10.3390/jmse12122265
Chicago/Turabian StyleMéndez-Ancca, Sheda, Renzo Pepe-Victoriano, Hebert Hernán Soto Gonzales, Juan Luis Ccamapaza Aguilar, Yesica Alvarez Meza, Marco Antonio Quispe Pacho, Alex Tejada Cáceres, Danny Efraín Baldarrago Centeno, and Jorge González Aguilera. 2024. "Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru" Journal of Marine Science and Engineering 12, no. 12: 2265. https://doi.org/10.3390/jmse12122265
APA StyleMéndez-Ancca, S., Pepe-Victoriano, R., Gonzales, H. H. S., Aguilar, J. L. C., Meza, Y. A., Pacho, M. A. Q., Cáceres, A. T., Baldarrago Centeno, D. E., & Aguilera, J. G. (2024). Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru. Journal of Marine Science and Engineering, 12(12), 2265. https://doi.org/10.3390/jmse12122265










