Tidal Zonation Shapes Microbial Communities and Sediment Properties in a UNESCO World Heritage Site (Gomso Bay, Korea)
Abstract
1. Introduction
2. Materials and Methods
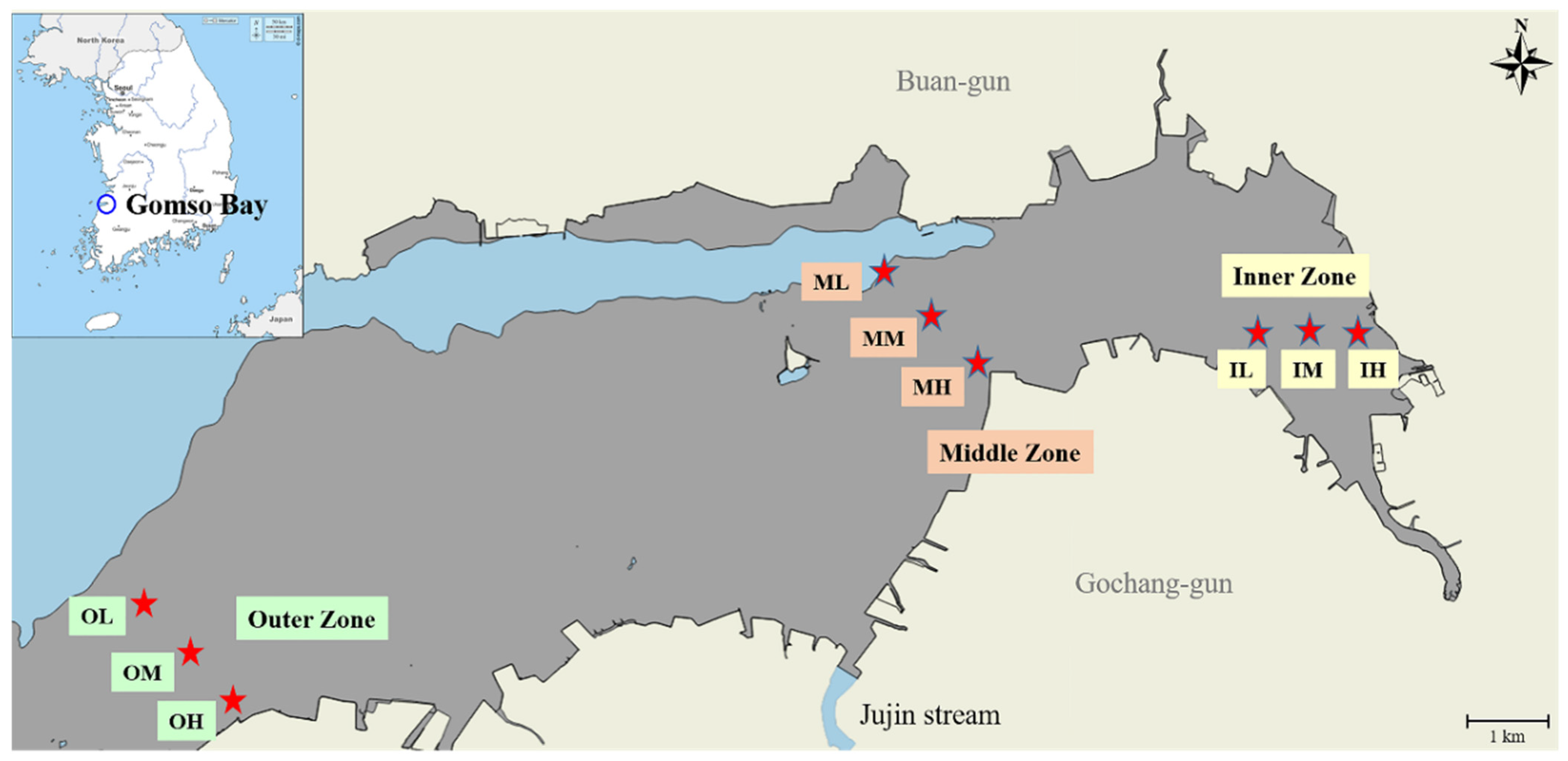
2.1. Study Area and Sediment Sampling
2.2. Physicochemical Analysis
2.3. Metabarcoding Analysis
2.4. Statistical Analysis
3. Results
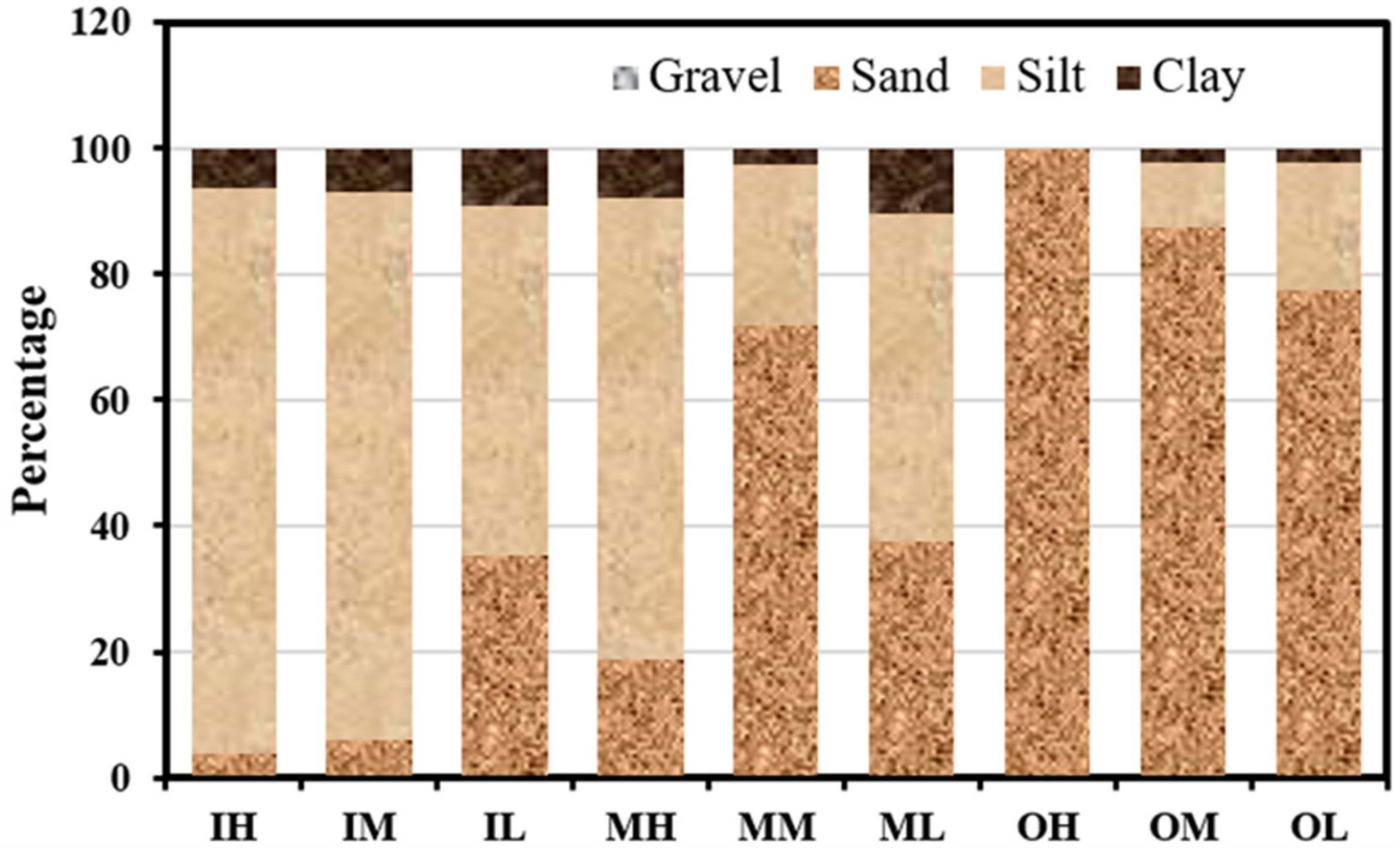
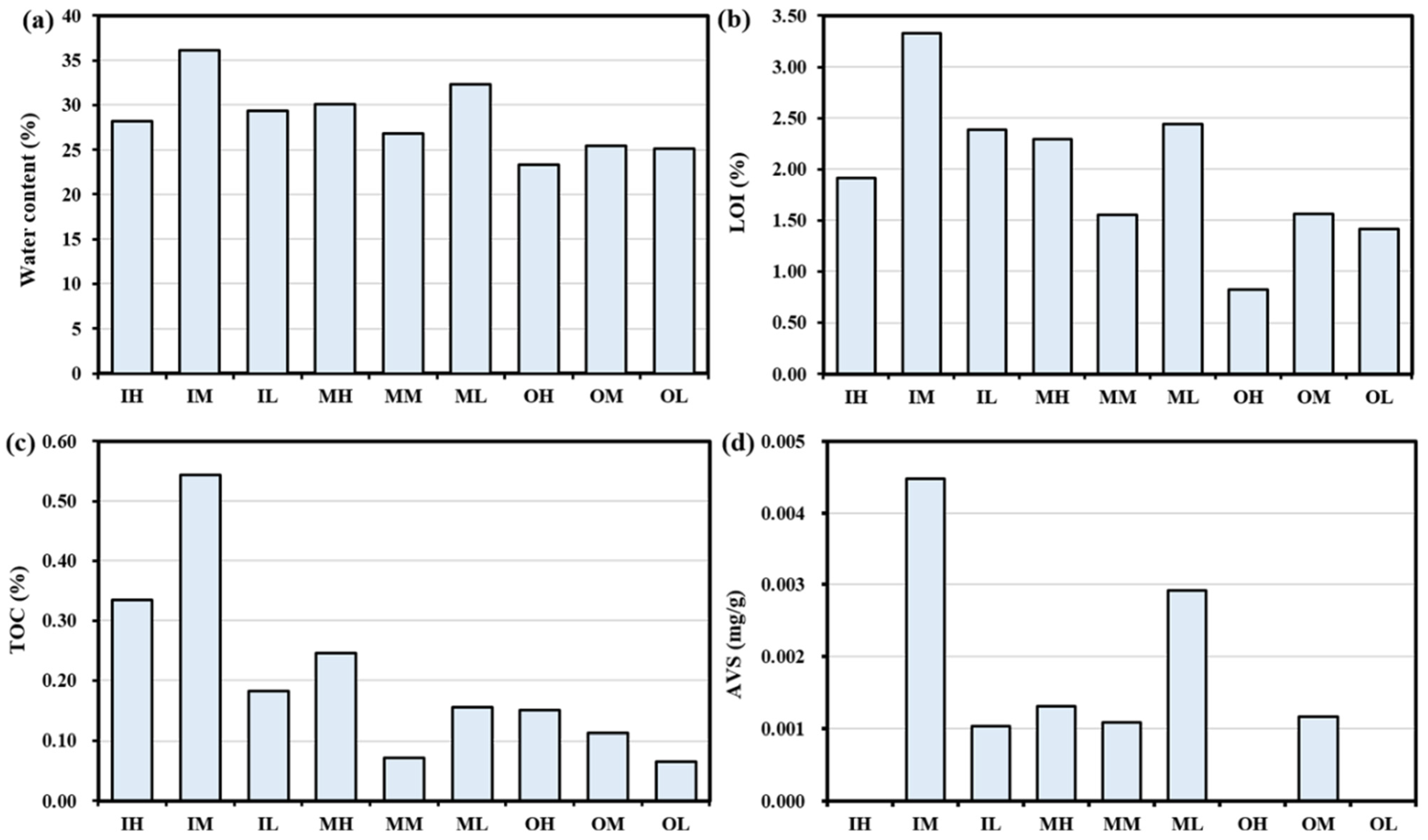
3.1. Sediment Properties
3.2. Spatial Distribution of Metals in Sediment
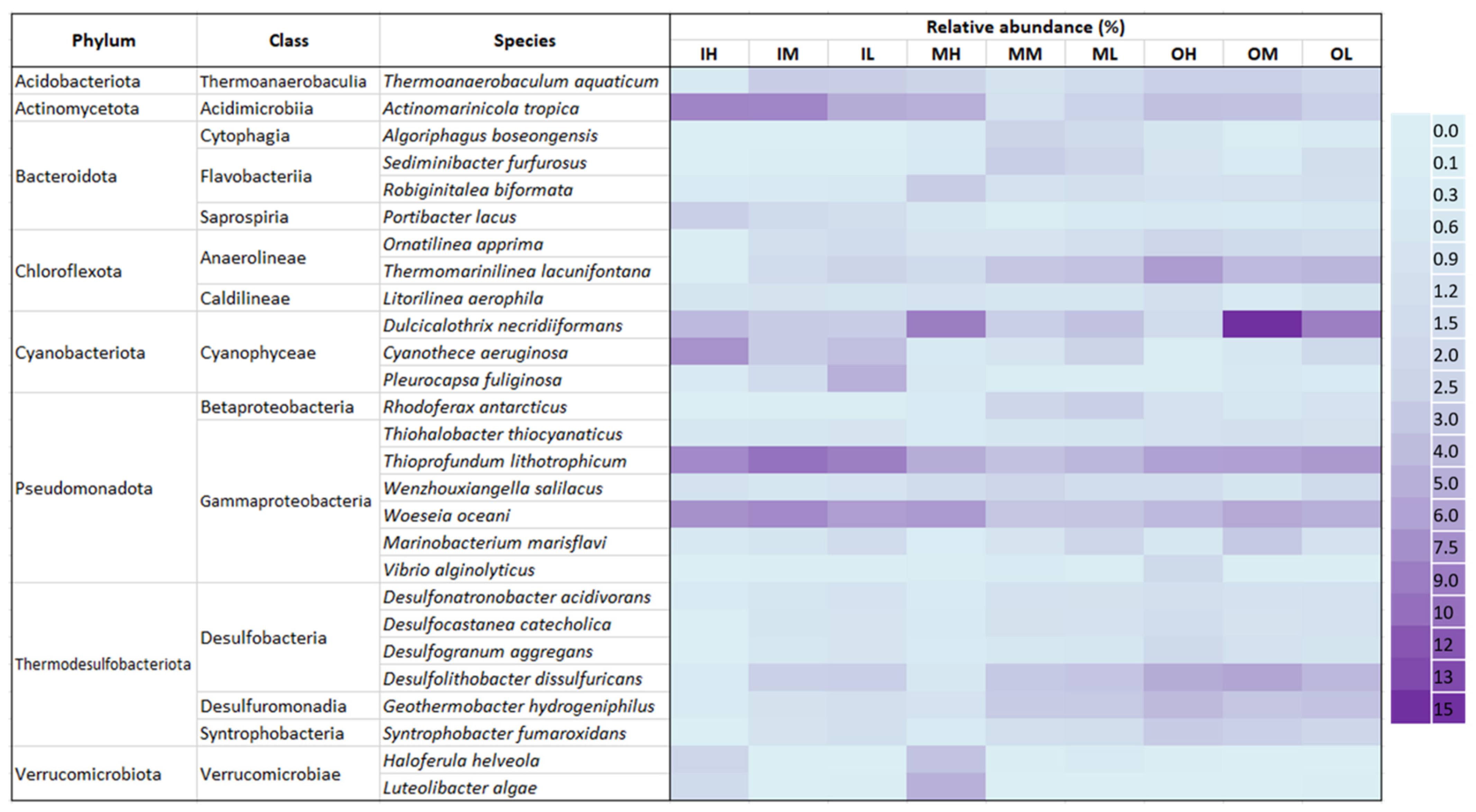
3.3. Overall Intertidal Sediment Microbial Community Composition
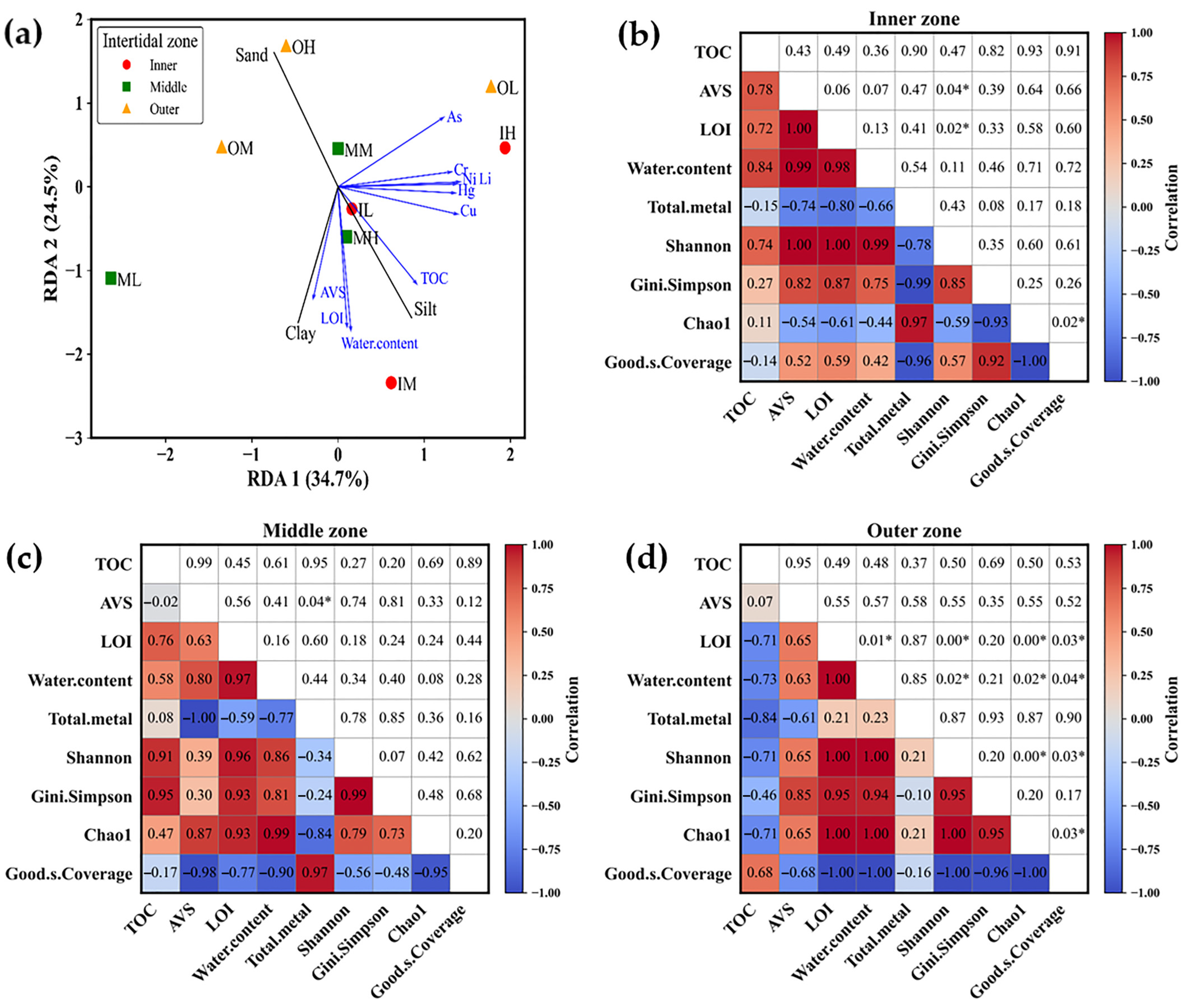
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ortega-Morales, B.O.; Chan-Bacab, M.J.; De la Rosa, S.D.C.; Camacho-Chab, J.C. Valuable processes and products from marine intertidal microbial communities. Curr. Opin. Biotechnol. 2010, 21, 346–352. [Google Scholar] [CrossRef]
- Rios-Yunes, D.; Tiano, J.C.; van Rijswijk, P.; De Borger, E.; van Oevelen, D.; Soetaert, K. Long-term changes in ecosystem functioning of a coastal bay expected from a shifting balance between intertidal and subtidal habitats. Cont. Shelf Res. 2023, 254, 104904. [Google Scholar] [CrossRef]
- Rios-Yunes, D.; Grandjean, T.; di Primio, A.; Tiano, J.; Bouma, T.J.; van Oevelen, D.; Soetaert, K. Sediment resuspension enhances nutrient exchange in intertidal mudflats. Front. Mar. Sci. 2023, 10, 1155386. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, G.; Yang, H.S. Active exchange of water and nutrients between seawater and shallow pore water in intertidal sandflats. Ocean Sci. J. 2008, 43, 223–232. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, J.; Li, L.; Huang, L.; Manzoor, M.; Yang, J.; Wu, G.; Sun, X.; Wang, B.; Egamberdieva, D.; et al. Onshore soil microbes and endophytes respond differently to geochemical and mineralogical changes in the Aral Sea. Sci. Total Environ. 2021, 765, 142675. [Google Scholar] [CrossRef]
- Yue, Y.; Rong, H.; Yang, Z.; Pan, X.; Chen, Y.; Yang, M. Microbial diversity and functional profiling in coastal tidal flat sediment with pollution of nutrients and potentially toxic elements. J. Soils Sediment. 2023, 23, 2935–2950. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, T.; Jiang, H.; Ye, K.; Dai, Z. Bacterial community driven nitrogen cycling in coastal sediments of intertidal transition zone. Sci. Total Environ. 2024, 908, 168299. [Google Scholar] [CrossRef]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef]
- Köpke, B.; Wilms, R.; Engelen, B.; Cypionka, H.; Sass, H. Microbial diversity in coastal subsurface sediments: A cultivation approach using various electron acceptors and substrate gradients. Appl. Environ. Microbiol. 2005, 71, 7819–7830. [Google Scholar] [CrossRef]
- Acosta-González, A.; Marqués, S. Bcterial diversity in oil-polluted marine coastal sediments. Curr. Opin. Biotechnol. 2016, 38, 24–32. [Google Scholar] [CrossRef]
- Webb, S.J.; Rabsatt, T.; Erazo, N.; Bowman, J.S. Impacts of Zostera eelgrasses on microbial community structure in San Diego coastal waters. Elem. Sci. Anth. 2019, 7, 11. [Google Scholar] [CrossRef]
- Degenhardt, J.; Merder, J.; Heyerhoff, B.; Simon, H.; Engelen, B.; Waska, H. Cross-shore and depth zonations in bacterial diversity are linked to age and source of dissolved organic matter across the intertidal area of a sandy beach. Microorganisms 2021, 9, 1720. [Google Scholar] [CrossRef]
- Lee, H.J. Preliminary results on suspended sediment transport by tidal currents in Gomso Bay, Korea. Ocean Sci. J. 2010, 45, 187–195. [Google Scholar] [CrossRef]
- Yang, B.; Dalrymple, R.W.; Gingras, M.K.; Chun, S.; Lee, H. Up-estuary variation of sedimentary facies and ichnocoenoses in an open-mouthed, macrotidal, mixed-energy estuary, Gomso Bay, Korea. J. Sediment. Res. 2007, 77, 757–771. [Google Scholar] [CrossRef]
- Chang, J.H.; Ryu, S.O.; Jo, Y.J. Long-term variation of tidal-flat sediments in Gomso bay, west coast of Korea. J. Korean Earth Sci. Soc. 2007, 28, 357–366. [Google Scholar] [CrossRef]
- Ingram, R.L. Sieve analysis. In Procedures in Sedimentary Petrology; Carver, R.E., Ed.; Wiley-Interscience: New York, NY, USA, 1971; pp. 49–67. [Google Scholar]
- Patil, M.P.; Jeong, I.; Woo, H.E.; Kim, J.O.; Lee, D.I.; Kim, K. Natural variations in the benthic environment and bacterial communities of coastal sediments around aquaculture farms in South Korea. Indian J. Microbiol. 2023, 63, 100–105. [Google Scholar] [CrossRef]
- Song, Y.H.; Choi, M.S. REE Geochemistry of fine-grained sediments from major rivers around the Yellow Sea. Chem. Geol. 2009, 266, 328–342. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Wilson, A.M.; Huettel, M.; Klein, S. Grain size and depositional environment as predictors of permeability in coastal marine sands. Estuar. Coast. Shelf Sci. 2008, 80, 193–199. [Google Scholar] [CrossRef]
- Bryant, W.R. Permeability of clays, silty-clays and clayey-silts. Gulf Coast Assoc. Geol. Soc. Trans. 2002, 52, 1069–1077. [Google Scholar]
- Cinco-Castro, S.; Herrera-Silveira, J.; Comín, F. Sedimentation as a support ecosystem service in different ecological types of mangroves. Front. For. Glob. Change 2022, 5, 733820. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, S.H.; Lee, W.C.; Lee, D.H. Spatial variability of water quality and sedimentary organic matter during winter season in coastal aquaculture zone of Korea. Mar. Pollut. Bull. 2022, 182, 113991. [Google Scholar] [CrossRef]
- Wu, S.; Tao, S.; Ye, X.; Wang, A.; Liu, Z.; Ran, C.; Liang, H.; Li, H.; Yang, Y.; Zhang, W.; et al. Characteristics of sedimentary organic matter in tidal estuaries: A case study from the Minjiang river estuary. Water 2023, 15, 1682. [Google Scholar] [CrossRef]
- LaRowe, D.E.; Arndt, S.; Bradley, J.A.; Estes, E.R.; Hoarfrost, A.; Lang, S.Q.; Lloyd, K.G.; Mahmoudi, N.; Orsi, W.D.; Walter, S.S.; et al. The fate of organic carbon in marine sediments—New insights from recent data and analysis. Earth-Sci. Rev. 2020, 204, 103146. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, P.J.; Kim, S.G.; Sun, C.I.; Koh, B.S.; Ryu, S.O.; Kim, T.H. Spatial distribution and pollution assessment of metals in intertidal sediments, Korea. Environ. Sci. Pollut. Res. 2019, 26, 19379–19388. [Google Scholar] [CrossRef] [PubMed]
- National Fisheries Research and Development Institute (NFRDI). Technical Report of National Fisheries Research and Development Institute in 2014; Report No. TR-2015-PM-002; NFRDI: Busan, Republic of Korea, 2015; p. 1366. (In Korean)
- Patil, M.P.; Woo, H.E.; Kim, J.O.; Kim, K. Field study on short-term changes in benthic environment and benthic microbial communities using pyrolyzed oyster shells. Sci. Total Environ. 2022, 824, 153891. [Google Scholar] [CrossRef]
- Lee, J.H.; Patil, M.P.; Kim, J.O.; Woo, H.E.; Kim, K. Diversity of microbial communities in sediment from Yeosu bay, republic of Korea, as determined by 16s rRNA gene amplicon sequencing. Microbiol. Resour. Announc. 2022, 11, e00363-22. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Kirchman, D.L. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 2000, 66, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Beaupré, S.R.; Steen, A.D.; Pearson, A. Sequential bioavailability of sedimentary organic matter to heterotrophic bacteria. Environ. Microbiol. 2017, 19, 2629–2644. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.; Van Kuijk, B.L.; Plugge, C.M.; Akkermans, A.D.; De Vos, W.M.; Stams, A.J. Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 1998, 48, 1383–1387. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Tourova, T.P.; Panteleeva, A.N.; Muyzer, G. Desulfonatronobacter acidivorans gen. nov., sp. nov. and Desulfobulbus alkaliphilus sp. nov., haloalkaliphilic heterotrophic sulfate-reducing bacteria from Soda Lakes. Int. J. Syst. Evol. Microbiol. 2012, 62, 2107–2113. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, I.; Choi, J.K.; Abuyen, K.; Tyler, M.; Ronkowski, C.; Romero, E.; Trujillo, A.; Tremblay, J.; Viney, I.; Savalia, P.; et al. Geothermobacter hydrogeniphilus sp. nov., a mesophilic, Iron (III)-reducing bacterium from seafloor/subseafloor environments in the Pacific Ocean, and emended description of the genus Geothermobacter. Int. J. Syst. Evol. Microbiol. 2021, 71, 004739. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Shimamura, S.; Tame, A.; Sawayama, S.; Miyazaki, J.; Takai, K.; Nakagawa, S. Physiological and comparative proteomic characterization of Desulfolithobacter dissulfuricans gen. nov., sp. nov., a novel mesophilic, sulfur-disproportionating chemolithoautotroph from a deep-sea hydrothermal vent. Front. Microbiol. 2022, 13, 1042116. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, B.; Richter, M.; Schüler, M.; Pinhassi, J.; Acinas, S.G.; González, J.M.; Pedrós-Alió, C. Ecology of marine Bacteroidetes: A comparative genomics approach. ISME J. 2013, 7, 1026–1037. [Google Scholar] [CrossRef]
- Yoon, J.; Matsuo, Y.; Kasai, H.; Yokota, A. Portibacter lacus gen. nov., sp. nov., a new member of the family Saprospiraceae isolated from a Saline Lake. J. Gen. Appl. Microbiol. 2012, 58, 191–197. [Google Scholar] [CrossRef]
- Golubic, S.; Seong-Joo, L.; Browne, K.M. Cyanobacteria: Architects of sedimentary structures. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 57–67. [Google Scholar] [CrossRef]
- He, Y.Q.; Chen, R.W.; Li, C.; Shi, S.B.; Cui, L.Q.; Long, L.J.; Tian, X.P. Actinomarinicola tropica gen. nov. sp. nov., a new marine actinobacterium of the family Iamiaceae, isolated from South China Sea sediment environments. Int. J. Syst. Evol. Microbiol. 2020, 70, 3852–3858. [Google Scholar] [CrossRef]
- Yoon, J.; Matsuo, Y.; Katsuta, A.; Jang, J.H.; Matsuda, S.; Adachi, K.; Kasai, H.; Yokota, A. Haloferula rosea gen. nov., sp. nov., Haloferula harenae sp. nov., Haloferula phyci sp. nov., Haloferula helveola sp. nov. and Haloferula sargassicola sp. nov., five marine representatives of the family Verrucomicrobiaceae within the phylum ‘Verrucomicrobia’. Int. J. Syst. Evol. Microbiol. 2008, 58, 2491–2500. [Google Scholar] [CrossRef]
- Yoon, J.; Matsuo, Y.; Adachi, K.; Nozawa, M.; Matsuda, S.; Kasai, H.; Yokota, A. Description of Persicirhabdus sediminis gen. nov., sp. nov., Roseibacillus ishigakijimensis gen. nov., sp. nov., Roseibacillus ponti sp. nov., Roseibacillus persicicus sp. nov., Luteolibacter pohnpeiensis gen. nov., sp. nov. and Luteolibacter algae sp. nov., six marine members of the phylum ‘Verrucomicrobia’, and emended descriptions of the class Verrucomicrobiae, the order Verrucomicrobiales and the family Verrucomicrobiaceae. Int. J. Syst. Evol. Microbiol. 2008, 58, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Podosokorskaya, O.A.; Bonch-Osmolovskaya, E.A.; Novikov, A.A.; Kolganova, T.V.; Kublanov, I.V. Ornatilinea apprima gen. nov., sp. nov., a cellulolytic representative of the Class Anaerolineae. Int. J. Syst. Evol. Microbiol. 2013, 63, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nunoura, T.; Hirai, M.; Miyazaki, M.; Kazama, H.; Makita, H.; Hirayama, H.; Furushima, Y.; Yamamoto, H.; Imachi, H.; Takai, K. Isolation and characterization of a thermophilic, obligate anaerobic and heterotrophic marine Chloroflexi bacterium from a Chloroflexi-dominated microbial community associated with a Japanese shallow hydrothermal system, and proposal for Thermomarinilinea lacunofontalis gen. nov., sp. nov. Microbes Environ. 2013, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]

| Gomso Bay, Republic of Korea | Heavy Metal Concentration (mg/kg) in Sediment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Sample | Cu | Li | Zn | Ni | Cr | Cd | Pb | As | Hg | Total | |
| Location | ID | |||||||||||
| Inner zone | High tidal flat | IH | 11.656 | 44.132 | 59.814 | 18.573 | 51.314 | 0.054 | 24.266 | 7.230 | 0.013 | 217.05 |
| Mid tidal flat | IM | 10.949 | 29.419 | 39.869 | 14.411 | 40.016 | 0.055 | 21.737 | 5.453 | 0.007 | 161.91 | |
| Low tidal flat | IL | 7.263 | 30.804 | 45.431 | 13.969 | 41.322 | 0.048 | 21.414 | 5.484 | 0.007 | 165.74 | |
| Middle zone | High tidal flat | MH | 6.886 | 31.644 | 52.115 | 15.511 | 40.377 | 0.035 | 19.724 | 5.514 | 0.007 | 171.81 |
| Mid tidal flat | MM | 6.607 | 31.042 | 45.511 | 15.514 | 39.988 | 0.046 | 20.519 | 5.871 | 0.006 | 165.10 | |
| Low tidal flat | ML | 1.436 | 9.943 | 18.644 | 3.674 | 9.543 | 0.015 | 21.723 | 3.442 | 0.001 | 68.42 | |
| Outer zone | High tidal flat | OH | 4.246 | 21.072 | 25.927 | 11.275 | 33.228 | 0.044 | 23.920 | 6.580 | 0.003 | 126.29 |
| Mid tidal flat | OM | 2.978 | 20.251 | 20.939 | 10.308 | 30.105 | 0.032 | 20.569 | 5.072 | 0.003 | 110.26 | |
| Low tidal flat | OL | 12.720 | 43.530 | 60.500 | 18.704 | 56.145 | 0.061 | 22.871 | 7.154 | 0.012 | 221.70 | |
| Average | 7.19 | 29.09 | 40.97 | 13.55 | 38.00 | 0.04 | 21.86 | 5.75 | 0.006 | 156.48 | ||
| Standard deviation (± SD) | 3.95 | 10.94 | 15.92 | 4.66 | 13.35 | 0.01 | 1.55 | 1.17 | 0.004 | 48.92 | ||
| Sample ID | Bacterial Community Analysis by 16S rRNA | SRA Accession No. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Reads | Bacterial Richness and Diversity | ||||||||
| Raw | After Primer Trimming | After Quality Filter | No. of ASVs | Chao1 | Shannon | Gini-Simpson | Good’s Coverage | ||
| IH | 83,312 | 82,288 | 69,508 | 1412 | 1500.0 | 9.1680 | 0.9956 | 0.9893 | SRX22497941 |
| IM | 77,055 | 75,830 | 58,700 | 1209 | 1226.9 | 9.2178 | 0.9966 | 0.9949 | SRX22497943 |
| IL | 72,241 | 71,120 | 55,469 | 1147 | 1154.7 | 9.1834 | 0.9964 | 0.9966 | SRX22497942 |
| MH | 77,171 | 76,060 | 62,717 | 1261 | 1305.1 | 9.3795 | 0.9969 | 0.9934 | SRX22497944 |
| MM | 68,952 | 68,006 | 55,107 | 1138 | 1168.6 | 8.8752 | 0.9900 | 0.9943 | SRX22497937 |
| ML | 80,816 | 79,673 | 66,722 | 1376 | 1447.0 | 9.3134 | 0.9953 | 0.9899 | SRX22497936 |
| OH | 57,857 | 56,944 | 42,728 | 747 | 747.12 | 8.6320 | 0.9955 | 0.9997 | SRX22497938 |
| OM | 83,720 | 82,533 | 68,986 | 1424 | 1515.5 | 9.4821 | 0.9974 | 0.9891 | SRX22497940 |
| OL | 75,451 | 74,452 | 62,362 | 1317 | 1368.7 | 9.3198 | 0.9965 | 0.9916 | SRX22497939 |
| Phylum * | Class | Relative Abundance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IH | IM | IL | MH | MM | ML | OH | OM | OL | ||
| Pseudomonadota | - | 33.29 | 30.29 | 31.98 | 27.93 | 26.24 | 29.45 | 35.07 | 32.01 | 29.93 |
| Thermodesulfobacteriota | - | 4.91 | 11.40 | 13.35 | 22.51 | 22.53 | 16.55 | 3.01 | 11.77 | 12.57 |
| Bacteroidota | - | 13.34 | 16.05 | 13.43 | 9.33 | 7.95 | 12.30 | 14.31 | 9.24 | 8.98 |
| Cyanobacteriota | - | 9.65 | 7.43 | 7.59 | 2.37 | 14.82 | 10.50 | 14.56 | 7.42 | 11.48 |
| Actinomycetota | - | 10.52 | 7.21 | 9.30 | 8.09 | 5.94 | 5.99 | 11.23 | 12.39 | 9.09 |
| Chloroflexota | - | 3.31 | 4.65 | 5.76 | 9.30 | 6.00 | 6.74 | 1.07 | 3.23 | 3.93 |
| Pseudomonadota | Gammaproteobacteria | 23.00 | 16.13 | 16.65 | 20.18 | 21.47 | 21.44 | 20.06 | 26.93 | 25.12 |
| Alphaproteobacteria | 9.44 | 9.39 | 9.95 | 6.72 | 3.80 | 6.21 | 11.59 | 4.74 | 4.42 | |
| Betaproteobacteria | 0.85 | 4.76 | 5.38 | 1.03 | 0.97 | 1.79 | 3.42 | 0.34 | 0.39 | |
| Sample | Bacterial Diversity Based on ASV Assigned | |||||
|---|---|---|---|---|---|---|
| Phylum | Classes | Orders | Families | Genera | Species | |
| IH | 26 | 71 | 124 | 220 | 407 | 529 |
| IM | 27 | 72 | 127 | 236 | 451 | 605 |
| IL | 26 | 68 | 120 | 220 | 418 | 527 |
| MH | 28 | 68 | 117 | 201 | 370 | 469 |
| MM | 25 | 61 | 104 | 187 | 334 | 405 |
| ML | 25 | 64 | 112 | 222 | 410 | 515 |
| OH | 22 | 47 | 78 | 130 | 256 | 321 |
| OM | 26 | 67 | 107 | 193 | 345 | 425 |
| OL | 27 | 65 | 108 | 186 | 332 | 412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, M.P.; Ryu, S.O.; Woo, H.-E.; Lee, C.-G.; Oh, H.N.; Jang, S.Y.; Kim, J.-O.; Kim, K. Tidal Zonation Shapes Microbial Communities and Sediment Properties in a UNESCO World Heritage Site (Gomso Bay, Korea). J. Mar. Sci. Eng. 2025, 13, 2222. https://doi.org/10.3390/jmse13122222
Patil MP, Ryu SO, Woo H-E, Lee C-G, Oh HN, Jang SY, Kim J-O, Kim K. Tidal Zonation Shapes Microbial Communities and Sediment Properties in a UNESCO World Heritage Site (Gomso Bay, Korea). Journal of Marine Science and Engineering. 2025; 13(12):2222. https://doi.org/10.3390/jmse13122222
Chicago/Turabian StylePatil, Maheshkumar Prakash, Sang Ock Ryu, Hee-Eun Woo, Chang-Gun Lee, Ha Neul Oh, So Yun Jang, Jong-Oh Kim, and Kyunghoi Kim. 2025. "Tidal Zonation Shapes Microbial Communities and Sediment Properties in a UNESCO World Heritage Site (Gomso Bay, Korea)" Journal of Marine Science and Engineering 13, no. 12: 2222. https://doi.org/10.3390/jmse13122222
APA StylePatil, M. P., Ryu, S. O., Woo, H.-E., Lee, C.-G., Oh, H. N., Jang, S. Y., Kim, J.-O., & Kim, K. (2025). Tidal Zonation Shapes Microbial Communities and Sediment Properties in a UNESCO World Heritage Site (Gomso Bay, Korea). Journal of Marine Science and Engineering, 13(12), 2222. https://doi.org/10.3390/jmse13122222







