Photoprotective Switching Reveals a Thermal Achilles’ Heel in Breviolum minutum at 41 °C
Abstract
1. Introduction
2. Materials and Methods
3. Results
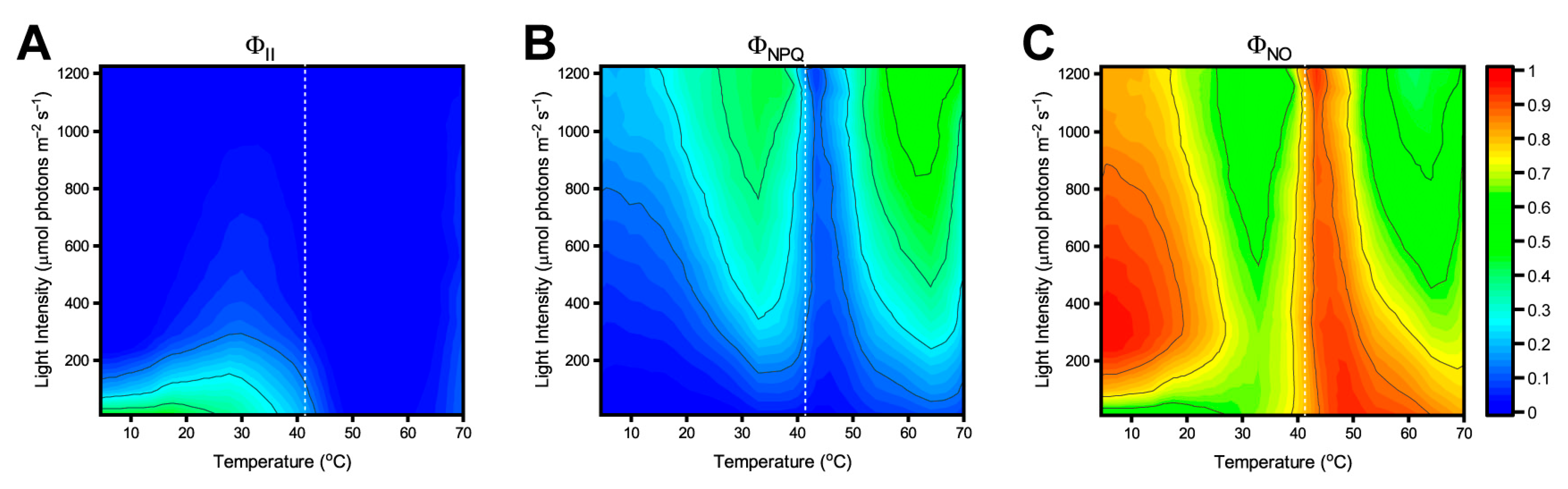
3.1. Phenoplate Measurements
3.2. Energetic Pathways in PSII
3.3. PSII Open Reaction Centres and Post-Stress Survival
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RLC | Rapid Light Curve |
| NPQ | Non-photochemical quenching |
| PSII | Photosystem 2 |
| PSI | Photosystem 1 |
| ΦII | Effective quantum yield of PSII |
| ΦNPQ | Regulated energy quenching in PSII |
| ΦNO | Unregulated energy quenching in PSII |
References
- Baird, A.H.; Bhagooli, R.; Ralph, P.J.; Takahashi, S. Coral bleaching: The role of the host. Trends Ecol. Evol. 2009, 24, 16–20. [Google Scholar] [CrossRef]
- Oakley, C.A.; Schmidt, G.W.; Hopkinson, B.M. Thermal responses of Symbiodinium photosynthetic carbon assimilation. Coral Reefs 2014, 33, 501–512. [Google Scholar] [CrossRef]
- Dang, K.V.; Pierangelini, M.; Roberty, S.; Cardol, P. Alternative Photosynthetic Electron Transfers and Bleaching Phenotypes Upon Acute Heat Stress in Symbiodinium and Breviolum spp. (Symbiodiniaceae) in Culture. Front. Mar. Sci. 2019, 6, 656. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Matta, J.L.; Robins, W.A.; Trench, R.K. Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. USA 1992, 89, 10302–10305. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Wentworth, M. Allosteric Regulation of the Light-Harvesting System of Photosystem II. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1361–1370. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Cao, X.; Xue, S.; Li, C. Liberating photoinhibition through nongenetic drainage of electrons from photosynthesis. Nat. Sci. 2021, 1, e20210038. [Google Scholar] [CrossRef]
- Herdean, A.; Hall, C.; Hughes, D.J.; Kuzhiumparambil, U.; Diocaretz, B.C.; Ralph, P.J. Temperature mapping of non-photochemical quenching in Chlorella vulgaris. Photosynth. Res. 2023, 155, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Evensen, N.R.; Parker, K.E.; Oliver, T.A.; Palumbi, S.R.; Logan, C.A.; Ryan, J.S.; Klepac, C.N.; Perna, G.; Warner, M.E.; Voolstra, C.R.; et al. The Coral Bleaching Automated Stress System (CBASS): A low-cost, portable system for standardized empirical assessments of coral thermal limits. Limnol. Oceanogr. Methods 2023, 21, 421–434. [Google Scholar] [CrossRef]
- Hill, R.; Ulstrup, K.E.; Ralph, P.J. Temperature induced changes in thylakoid membrane thermostability of cultured, freshly isolated, and expelled zooxanthellae from scleractinian corals. Bull. Mar. Sci. 2009, 85, 223–244. [Google Scholar]
- Slavov, C.; Schrameyer, V.; Reus, M.; Ralph, P.J.; Hill, R.; Büchel, C.; Larkum, A.W.D.; Holzwarth, A.R. Super-quenching” state protects Symbiodinium from thermal stress—Implications for coral bleaching. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2016, 1857, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Dove, S.; Ortiz, J.C.; Enríquez, S.; Fine, M.; Fisher, P.; Iglesias-Prieto, R.; Thornhill, D.; Hoegh-Guldberg, O. Response of holosymbiont pigments from the scleractinian coral Montipora monasteriata to short-term heat stress. Limnol. Oceanogr. 2006, 51, 1149–1158. [Google Scholar] [CrossRef]
- Warner, M.; Berry-Lowe, S. Differential xanthophyll cycling and photochemical activity in symbiotic dinoflagellates in multiple locations of three species of Caribbean coral. J. Exp. Mar. Biol. Ecol. 2006, 339, 86–95. [Google Scholar] [CrossRef]
- Suggett, D.J.; Smith, D.J. Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Change Biol. 2020, 26, 68–79. [Google Scholar] [CrossRef]
- Herdean, A.; Sutherland, D.L.; Ralph, P.J. Phenoplate: An innovative method for assessing interacting effects of temperature and light on non-photochemical quenching in microalgae under chemical stress. New Biotechnol. 2022, 66, 89–96. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- England, H.; Herdean, A.; Matthews, J.; Hughes, D.J.; Roper, C.D.; Suggett, D.J.; Voolstra, C.R.; Camp, E.F. A portable multi-taxa phenotyping device to retrieve physiological performance traits. Sci. Rep. 2024, 14, 21826. [Google Scholar] [CrossRef]
- Roper, C.D.; Suggett, D.J.; Songsomboon, K.; Edmondson, J.; England, H.; Haydon, T.D.; Goyen, S.; Duijser, C.M.; Alderdice, R.; Voolstra, C.R.; et al. Coral thermotolerance retained following year-long exposure to a novel environment. Sci. Adv. 2025, 11, eadu3858. [Google Scholar] [CrossRef]
- Deore, P.; Ching, S.J.T.M.; Nitschke, M.R.; Rudd, D.; Brumley, D.R.; Hinde, E.; Blackall, L.L.; van Oppen, M.J.H. Unique photosynthetic strategies employed by closely related Breviolum minutum strains under rapid short-term cumulative heat stress. J. Exp. Bot. 2024, 75, 4005–4023. [Google Scholar] [CrossRef]
- Cook, A.M.; Rezende, E.L.; Petrou, K.; Leigh, A. Beyond a single temperature threshold: Applying a cumulative thermal stress framework to plant heat tolerance. Ecol. Lett. 2024, 27, e14416. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.H.; Price, J.T.; Solomon, S.L.; Grottoli, A.G. Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs 2020, 39, 885–902. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Toonen, R.J.; van Woesik, R.; Thurber, R.V.; Warner, M.E.; McLachlan, R.H.; Price, J.T.; Bahr, K.D.; Baums, I.B.; Castillo, K.D.; et al. Increasing comparability among coral bleaching experiments. Ecol. Appl. 2021, 31, e02262. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.J.V.; Matthews, G.; Frith, K.R.; Harrison, H.B.; Marzonie, M.R.; Slaughter, K.L.; Suggett, D.J.; Bay, L.K. Experimental considerations of acute heat stress assays to quantify coral thermal tolerance. Sci. Rep. 2022, 12, 16831. [Google Scholar] [CrossRef] [PubMed]
- Cunning, R.; Matsuda, S.B.; Bartels, E.; D’aLessandro, M.; Detmer, A.R.; Harnay, P.; Levy, J.; Lirman, D.; Moeller, H.V.; Muller, E.M.; et al. On the use of rapid acute heat tolerance assays to resolve ecologically relevant differences among corals. Coral Reefs 2024, 43, 1793–1801. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
England, H.; Camp, E.F.; Herdean, A. Photoprotective Switching Reveals a Thermal Achilles’ Heel in Breviolum minutum at 41 °C. J. Mar. Sci. Eng. 2025, 13, 1937. https://doi.org/10.3390/jmse13101937
England H, Camp EF, Herdean A. Photoprotective Switching Reveals a Thermal Achilles’ Heel in Breviolum minutum at 41 °C. Journal of Marine Science and Engineering. 2025; 13(10):1937. https://doi.org/10.3390/jmse13101937
Chicago/Turabian StyleEngland, Hadley, Emma F. Camp, and Andrei Herdean. 2025. "Photoprotective Switching Reveals a Thermal Achilles’ Heel in Breviolum minutum at 41 °C" Journal of Marine Science and Engineering 13, no. 10: 1937. https://doi.org/10.3390/jmse13101937
APA StyleEngland, H., Camp, E. F., & Herdean, A. (2025). Photoprotective Switching Reveals a Thermal Achilles’ Heel in Breviolum minutum at 41 °C. Journal of Marine Science and Engineering, 13(10), 1937. https://doi.org/10.3390/jmse13101937






