Abstract
Well cementing is an important step in oil and gas development. It uses cement to seal the formation and the casing, preventing fluid leakage. However, when conducting offshore oil well cementing operations, deep-water formations are usually weakly consolidated soils, and it is difficult to form a good cementation between the cement and formation. Therefore, enhancing the strength of the formation is one of the effective measures. This study uses the microbial-induced carbonate precipitation technology to cement sandy formations containing clay minerals. The triaxial tests were conducted to evaluate the consolidation effectiveness in the presence of three clay minerals: montmorillonite, illite, and kaolinite. X-ray computed tomography was utilized to characterize microscopic pore parameters, while thermogravimetric analysis, X-ray diffraction, and surface potential measurements were applied to analyze the mechanisms of clay minerals affecting microbial consolidation. The results showed that microbial mineralization mainly affects the cohesion of the samples. The cohesion of the montmorillonite sample increased from 20 kPa to 65.4 kPa, an increase of up to 3.27 times. The other two samples (illite and kaolinite) had increases of only 0.33 times and 1.82 times. Although the strength of the montmorillonite sample increased the most, unexpected large pores appeared with a diameter of over 120 µm, accounting for 7.1%. This is mainly attributed to the mineral expansion property. The expansion of the minerals will trap more microorganisms in the sample, thereby generating more calcium carbonate. And it also reduced the gaps between sand particles, creating favorable conditions for the connection of calcium carbonate. Although the surface charge of the minerals also affects the attachment of microorganisms, all three minerals have negative charges and a difference of no more than 0.84 mV (pH = 9). Therefore, the expansion property of the minerals is the dominant factor affecting the mechanical and microstructure of the sample.
1. Introduction
With the development of oil and gas drilling technology, resource extraction has progressively extended to the ocean. Offshore oil and gas reserves have garnered significant attention due to their substantial exploitation potential. In the South China Sea, the oil and natural gas reserves are approximately 1.7 × 109 t and 5.38 × 1012 m3 [1], with nearly 70% located in deep-water areas. Compared to onshore operations, oil and gas extraction in deep-water areas presents greater challenges, particularly in cementing processes [2,3]. Cementing is a critical progress in exploitation, involving the injection of cement slurry into the annular space between the casing and the formation to create a long-term seal [4,5]. In deep-water operations, the primary challenge is the weak consolidation of formations, which results in poor bonding between the cement ring and the formation. This weak bonding strength makes it difficult to establish an effective seal [6,7], increasing the risk of oil and gas migrate to the ocean, and the environmental problems would occur. It can even lead to the abandonment of the entire generating well [8].
The use of lightweight cement slurry and the incorporation of ultra-fine cement can improve the early bond strength of the cement sheath to the formation, thereby improving the integrity of the well seal [9,10,11]. However, due to the strength difference between the cement sheath and the formation, the interface strength is prone to damage when well disturbances occur. Some researchers have attempted to improve cementing quality by solidifying mud cakes [12,13,14], but the solidified mud cake has a thinner structure and limited improvement in bonding quality. Although the aforementioned methods contribute to improving the cementing quality in weak formations, they do not fundamentally resolve the problem. The primary cause of poor cementing quality in deep water is mainly due to weak consolidation and unconsolidation of the formation. Enhancing formation strength is essential to effectively improve bonding quality [15]. Injecting organic solidifiers into deep-water formations can improve the bearing strength of the formation [16,17]. However, organic materials are typically chemical materials, which can cause environmental pollution. Additionally, their high viscosity requires injection at higher pressures, increasing the risk of fracturing the surrounding formation.
In recent years, microbially induced calcite precipitation (MICP) has attracted much attention as an effective geotechnical reinforcement technique [18]. MICP refers to the process in which microbial metabolites combine with calcium ions to produce calcium carbonate [19]. The primary mechanisms of MICP include denitrification, sulfate reduction, photosynthesis, hydration, and urea hydrolysis. Due to the rapid reaction of urea hydrolysis, most researchers have focused on Sporosarcina pasteurii, which is capable of urea hydrolysis, for their studies [20,21]. The use of microorganisms has been widely applied in research to enhance the stability of weak soils, promote soil and water conservation, and reduce permeability [22,23,24]. Currently, experimental studies have been conducted on the modification of subsea formations by MICP, and this technology can enhance the strength of weakly consolidated formations and reduce sand production during nature gas hydrate extraction [25,26]. However, it does not involve the influence of clay minerals on it. Although there have been a large number of studies on the influence of clay minerals on the consolidation effect of MICP [27,28,29,30], these studies mainly focus on the unconfined compressive strength of the samples and the amount of calcium carbonate generated inside. Some studies focused on microbial cementation of marine clay-containing samples. By testing the performance after microbial cementation, the effectiveness of microbial mineralization cementation was determined [31,32,33]. Regarding the internal pore structure of the calcareous sand particles was also conducted discussions [34]. However, there is a lack of research on the changes in the micro-pore structure and the influence of mineral properties on microbial adsorption and mineralization, as well as a lack of comparative analysis of the consolidation effects of different minerals.
This study aims to investigate the consolidation effect of MICP on the seabed’s weak formation containing different clay minerals and clarify the main ways in which clay minerals enhance the mechanical properties and the influence of clay physical properties on microbial mineralization and the microstructure of the samples. These clay minerals on MICP consolidation effects is determined through triaxial tests and calcium carbonate content measurements. And the effect of clay minerals on the calcium carbonate generation from MICP is used by thermogravimetric analysis. Additionally, X-ray CT provided the microstructural changes in the treated samples. The difference in testing all cases provided indirect information on the internal pore structure of the samples and the bonding effects are influenced by different clay minerals.
2. Materials and Methods
2.1. Materials
In this study, the main materials were simulated geological sand, bacterium solution, and cementation solution. The deep-water fine sand formation was selected as the experimental object, and quartz sand samples suitable for this formation were artificially prepared by 70–110 mesh and 110–160 mesh quartz sand with a mass fraction of 50% each [35]. To represent the primary clay mineral components of different marine strata, 10% wt of montmorillonite, illite, and kaolinite were added to the sand, respectively. The bacterium (Sporosarcina pasteurii, ATCC 11859) was cultivated by YE medium (20 g/L casein peptone, 10 g/L (NH4)2SO4 and 15.73 g/L Tris alkali, from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and the microorganisms were inoculated at a volume of 1% in the sterilized culture medium and placed it in a shaking table at 37 °C and 165 rpm/min for 24 h. The cementation solution was prepared by mixing 0.5 mol/L urea and calcium chloride. And Table 1 shows some detailed parameters of the experiment. For example, M-T represents the sample containing montmorillonite, which undergoes microbial consolidation treatment. The number 10 in the table represents the mineral content.

Table 1.
Specific sample preparation and difference.
2.2. Test and Analysis Methods
2.2.1. Triaxial Compression Test
In this experiment, a triaxial test was employed to assess the shear strength of samples containing various clay minerals. The samples were classified into microbial consolidated and unconsolidated groups. The triaxial test apparatus consisted of a triaxial test kettle, a water bath cabinet, two constant-speed and constant-pressure pumps, and a grouting cylinder (Jiangsu Kedi Petroleum Instrument Co., Ltd., Nantong, China). The confining pressure, axial pressure, the injection pressure, and flow rate of the bacterial solution was controlled by pumps. The grouting cylinder was used to store the bacterial and cementation solutions. During the sample preparation process, 126 g of sand was divided into four layers and compacted to form a Φ38 × 76 mm sample, with its porosity controlled at 40%, and then frozen. Subsequently, the confining pressure water was injected and increased to the predetermined pressure for 2 h after the specimen was placed; the equipment is shown in Figure 1a. Then, the MICP consolidation experiment was carried out by injecting 25 mL bacterial fluid and 25 mL cementing fluid into the sand column at 12 °C. These solutions were injected for 5 injections. In the testing experiment, it was mainly divided into two main steps: consolidation and shear. During the consolidation process of the specimen, the principal stresses were equal: δ1 = δ2 = δ3. The consolidation pressures for the three tests are 0.4, 0.6, and 0.8 MPa, respectively. During the shear of the specimen, loading was performed in a strain-controlled manner (0.023 mm/min), and the intermediate principal stress (δ2) was constant with the minor principal stress (δ3) and equal to the effective confining pressure, while the major principal stress (δ1) was not directly controlled but was measured deviator stress (q = δ1 − δ3). The control group was only treated by cementation solution, and the control groups containing montmorillonite, illite, and kaolinite were designated as M-U, I-U, and K-U, respectively, while the samples treated with microorganisms were labeled M-T, I-T, and K-T.
Figure 1.
Triaxial testing device: (a) test equipment; (b) sample after testing.
2.2.2. Thermogravimetric Analysis and X-Ray Diffraction
In this study, the influence of clay minerals on the calcium carbonate content and crystal morphology induced by MICP was systematically investigated. Three types of clay minerals were individually mixed with bacterial and cementation solutions at 12 °C. And this procedure was repeated five times to ensure consistent with above experiment. Due to the difficulty of effectively separating the calcium carbonate and clay minerals in the samples, a control group was established, in which montmorillonite, illite, and kaolinite were only mixed with the bacterial solution. After sample preparation, the specimens were dried at 60 °C for 24 h and subsequently ground into a fine powder. The calcium carbonate content produced under different mineral conditions was quantified using thermogravimetric analysis (TGA, Mettler TGA2, Greifensee, Switzerland) over a temperature range of 30 °C to 1000 °C. Additionally, the crystal structure of the calcium carbonate was characterized X-ray diffraction (XRD, Rigaku SmartLab SE, Tokyo, Japan) with a step size of 2°/min across a scanning range of 5° to 90°. The resulting data facilitated the analysis of the crystalline forms of calcium carbonate.
2.2.3. Infrared Spectrum and Zeta Potential
To clarify the role of clay minerals at the micro-scale in microbial-induced mineralization, the surface potential of clay minerals was tested to determine the influence of their electrical properties on the MICP process. For the surface potential measurements (Malvern Zetasizer Nano ZS90, Malvern, UK), clay minerals were analyzed under three pH conditions (pH 5, 7, and 9) to represent the transition of solution pH from neutral to alkaline during the MICP reaction. Additionally, Fourier transform infrared (FTIR, Thermo Fisher Scientific Nicolet iS20, Waltham, MA, USA) spectroscopy was employed to analyze the functional group composition on clay mineral surfaces, providing cross-verification with the surface potential test results.
2.2.4. X-Ray CT Test
In this experiment, X-ray CT was employed to analyze the pore structure of specimens (Nano Voxel-3000, Tianjin, China). To ensure the test data can achieve the best resolution, 2 mL syringes were used to prepare samples. The samples consist of three types, each containing montmorillonite, illite, and kaolinite, respectively. During the treatment process, the microbial and cementation solution was injected at 12 °C, following the same consolidation procedure as that used for the triaxial test. After the sample preparation, it was placed on the sample table of the equipment and conducted at 150 kV, 60 mA throughout full rotational scans covering all angles (360°). A total of 1440 projections were obtained during the test, and the obtained data were reconstructed and analyzed. To validate pore structure compatibility with fluid transport pathways, the pore network model (PNM) algorithm was implemented to extract interconnected pore data from the samples. This dataset underwent rigorous algorithmic processing to establish the definitive pore architecture for subsequent hydrodynamic analysis.
3. Results
3.1. Stress Behavior
Building upon previous experimental methodologies, microbial suspensions and cementing solutions were utilized to treat samples containing different clay minerals (montmorillonite, illite, and kaolinite). The shear strengths of these samples were subsequently tested under varying effective confining pressures, with the results presented in Figure 2. The microbial consolidation can effectively enhance the mechanical properties of samples containing different clay minerals, aligning with previous studies [27,28]. However, the strength improvement provided by microbial cementation varied in these samples, and differences were also observed in the control groups. To clarify the reasons of variations, the δ ~ τ coordinate system was constructed and the stress values of the sample at 15% strain under three different confining pressures were adopted. A circle was drawn in the coordinate system with (δ1f′ + δ3f′)/2 as the center and (δ1f′ − δ3f′)/2 as the radius, and the strength envelope line was plotted. The cohesion and friction angle values of the sample were obtained based on the Mohr–Coulomb criterion (τf = c + δtanφ) [36]. Notably, certain stress–strain curves in Figure 2 exhibited anomalous early-stage strength degradation, potentially attributable to the small liquid outlet that hindered complete drainage at the sample apex; it can be clearly seen in Figure 1. Despite these localized deviations, the majority of specimens demonstrated progressive strain hardening behavior. Consequently, the cohesion and internal friction angle were derived from the stabilized stress state at 15% axial strain, with the compiled results systematically presented in Figure 3.
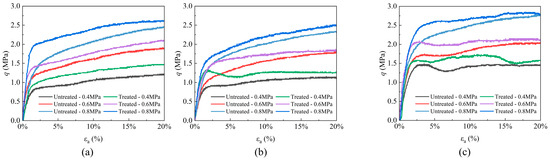
Figure 2.
Shear stress of sand samples containing (a) montmorillonite, (b) illite, and (c) kaolinite.
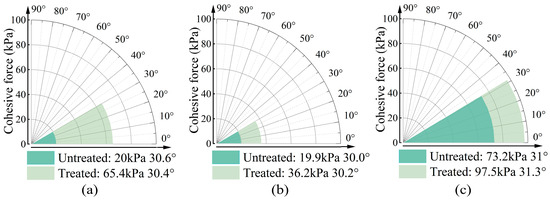
Figure 3.
Cohesive force and fraction angle of sand samples containing (a) montmorillonite, (b) illite, and (c) kaolinite.
The cohesion parameters and internal friction angles of the three specimen types are comparatively presented in Figure 3. While the internal friction angle of clay-amended specimens exhibits no significant variation, a marked enhancement in cohesion is observed with mineral additives. Notably, the montmorillonite-amended specimen demonstrates the most substantial improvement in cohesive strength, achieving a progressive increase from 20.0 kPa to 65.4 kPa. This elevation corresponds to a 3.27-fold enhancement relative to the baseline specimen. The sample containing illite also exhibits increase in cohesion, with a 16.3 kPa rise, corresponding to a 1.82-fold. In contrast, the sample containing kaolinite shows the least improvement, with a 0.33-fold increase in cohesion. Regarding the growth rate of cohesion, montmorillonite demonstrates the most significant effect, while kaolinite shows the least. However, the values of cohesion in these samples are inversely related. The untreated montmorillonite and illite samples exhibit cohesion values of 20 kPa and 19.9 kPa, respectively, which increase to 65.4 kPa and 36.2 kPa after treatment. The untreated kaolinite sample already has a cohesion of 73.2 kPa, which increases to 97.5 kPa following microbial treatment. Previous studies have confirmed that both montmorillonite and kaolinite can enhance sample strength after bacterial treatment by the production of calcium carbonate [28,37,38]. This study corroborates these findings and further reveals that the addition of illite also contributes to increased strength by MICP. Although these clay minerals could improve the sample strength by bacterium, the trends in the growth rate and cohesion values for samples with different clay minerals are not consistent. This discrepancy may be attributed to the expansion characteristics of the clay minerals.
Montmorillonite, illite, and kaolinite are all clay minerals. The basic structural units of clay minerals are silicon–oxygen tetrahedra and aluminum–oxygen octahedra. These basic units are, respectively, connected to form the basic crystal layers of clay minerals. However, for different clay minerals, the combination methods of these two basic crystal layers are different. Montmorillonite and illite have a 2:1 configuration, that is, two layers of silicon–oxygen tetrahedra sandwich one layer of aluminum–oxygen octahedra to form a unit cell. The overlapping unit cells are connected by van der Waals forces with oxygen layers facing each other [39,40]. In addition, the aluminum ions in the aluminum–oxygen octahedra are easily replaced by other ions, which leads to the fact that montmorillonite is prone to expansion when exposed to water, while illite has a lower expansion performance due to the presence of more potassium ions between the unit cells. Kaolinite has a 1:1 configuration, and the unit cells are connected by strong hydrogen bonds. Therefore, kaolinite is not prone to expansion when exposed to water [41].
In this experiment, all samples were subjected to fluid before the shear strength test. The untreated samples were injected with the cementation liquid five times, while the experimental group was injected with the bacterial and the cementation liquid five times each. Therefore, the clay minerals in these groups of samples had undergone expansion before the test. As the clay expanded, it obstructed the flow path, inevitably affecting the pore structure of the samples. The changes in the pores can be observed in CT test. The stronger the expansion performance of the clay, the greater the impact on the cohesion of the samples. Therefore, the kaolinite samples, which are less prone to water expansion, exhibited greater cohesion than the montmorillonite and illite samples. However, the expansion of clay is not entirely negative. The expansion increases its volume and specific surface area [42,43], which can enhance the attachment of microorganisms when the bacterial liquid passes through. A higher retention of microorganisms in the samples can product more calcium carbonate. Additionally, due to the expansion of the clay minerals, the gaps between the sand particles decrease in samples, facilitating the formation of calcium carbonate bridges to connect the surrounding sand particles and enhance the overall cohesion. Therefore, the samples containing montmorillonite and illite, which have better expansion performance, showed a greater increase rate in cohesion compared to the kaolinite samples.
3.2. CaCO3 Content and Crystal Form
The calcium carbonate content and crystal type were measured and characterized by TGA and XRD, respectively. The thermogravimetric analysis curves of the three samples at 30–1000 °C was shown in Figure 4, and the numerical statistics of several major mass loss areas are included in Table 2. In Figure 4, it can be clearly found that there are three obvious mass loss regions in the case of thermal decomposition, and the approximate temperature range of mass loss is 30–150 °C, 150–300 °C, and 450–980 °C. In the first temperature range, the mass loss may be due to the gradual loss of bound water in clay minerals as the temperature rises. In the temperature range of 150–300 °C, it is caused by the decomposition of urea and the loss of water of calcium chloride dihydrate still in the sample, and also includes the thermal decomposition of organic matter in some microbial medium in this temperature range [25]. The decomposition peak at 450–980 °C is caused by the decomposition of calcium carbonate.
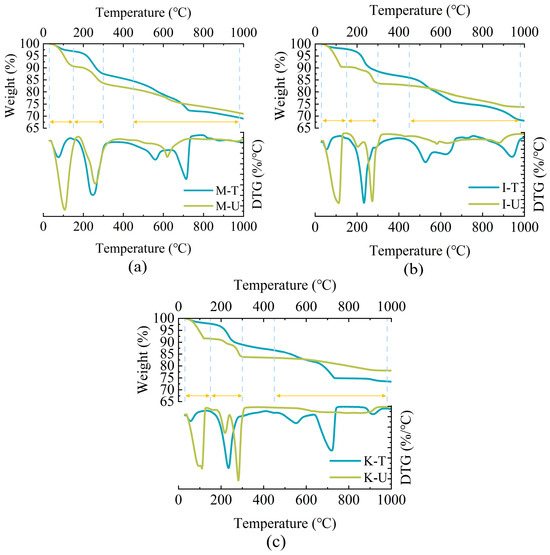
Figure 4.
CaCO3 content in samples: (a) Montmorillonite. (b) Illite. (c) Kaolinite.

Table 2.
Mass loss rate in different temperature range.
Table 2 lists the decomposition mass of calcium carbonate in the samples treated by microorganisms at 450–980 °C, in which the calcium carbonate mass of M-T, I-T, and K-T is 5.23%, 8.75%, and 7.72%, respectively. The microorganisms treated with montmorillonite produced the least calcium carbonate, but showed the largest growth rate of cohesion in Figure 3. It was opposite to the formation rate of calcium carbonate, perhaps due to the evolution of pore structure and ion changes within the sample. In this experiment, these samples were prepared by the same method, the internal pore structure was almost consistent, but the montmorillonite held the strongest expansion performance. Therefore, in the process of grouting, the internal circulation pores of samples containing montmorillonite decrease the most, which can trap more microorganisms in the flow process of bacterial liquid. In addition, due to the expansion of montmorillonite, the gap between sand particles was reduced, and the generated calcium carbonate was easier to bond sand particles to form a stressed skeleton. Meanwhile, this phenomenon may also be the change in ions inside the solution. After the montmorillonite mixes with water, the aluminum ions in the aluminum–oxygen octahedron, which makes up the crystal layer, are replaced by the magnesium, iron, and zinc plasma between the crystal cells [40]. When the aluminum ions are replaced, this causes the aluminum ions to flocculate in the form of Al(OH)3 due to the alkaline conditions of the MICP reaction. Under flocculation, Al(OH)3 has adsorption properties, which can aggregate and adsorb calcium carbonate and bridge the surrounding sand particles, so that less calcium carbonate can provide higher strength [44,45].
The calcium carbonate crystal forms formed by the MICP process under different clay minerals were measured via XRD. The results in Figure 5 show the characteristic peaks of different crystal types of calcium carbonate, indicating that calcium carbonate generated by MICP has two crystal types, calcite and aragonite, which correspond to the results of different thermal decomposition regions in the TGA test in Figure 4.
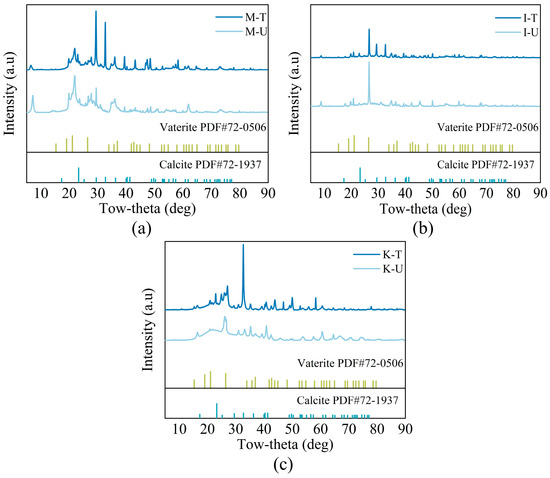
Figure 5.
XRD analysis: (a) Montmorillonite. (b) Illite. (c) Kaolinite.
3.3. Microscopic Pore Structure Variation
X-ray CT was utilized to analysis the changes in the internal pore structure of the three types of samples after microbial treatment, and the results obtained by Avizo Fire 8 software were shown in Figure 6. Figure 6a and Figure 6b, respectively, show the changes in pore and throat size inside the samples after treatment. The maximum pore radius of the M-U sample is 87.91 µm, which can be observed in Figure 6a, indicating that when the expansion of montmorillonite obstructs the passage of water during sample pretreatment, the continuous inflow of water inevitably expands the pore radius of M-U. After microbiological treatment, the pores of all samples were reduced. However, the pore volume of M-T did not decrease completely, but increased. It may be related to the expansion of montmorillonite and the calcium carbonate formation. The expansion of montmorillonite in contact with water will reduce and block part of the flow channel, and the formation of calcium carbonate by microorganisms inside the sample will be exacerbated this situation. Some blocked flow channels and pores in the sample will increase the pressure due to the continuous entry of fluid, thus developing large erosive pores and increasing the average pore volume in the sample. Hydraulic erosion pores can be clearly seen in CT test.
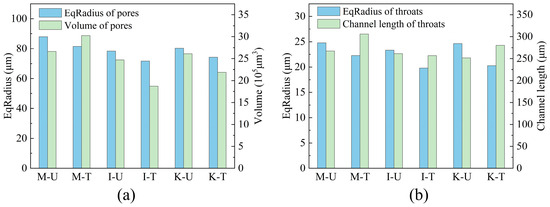
Figure 6.
Pore and throat size characteristics: (a) Pores. (b) Throats.
Figure 6b shows the pore radius and the length of throat channel inside these samples. The throat radius of all samples is below 25 µm. After treatment by microorganisms, the average throat radius decreases by 10.2–17.7%, indicating that calcium carbonate generated by microorganisms is mostly attached to the surface of sand particles, reducing the radius of the flow channel constructed by sand. This phenomenon also shows the contribution of the sample to the increase in cohesion after microbial treatment. Although the throat radius was reduced, the length of M-T and G-T increased by 12.7% and 10.3%, respectively, after microbial treatment. In M-T and G-T, the expansion of the clay and the calcium carbonate may have blocked the smaller pore throats, leaving longer channels. The reason for the difference in pore throat length between the two samples is that there are two factors in M-T: clay expansion and calcium carbonate formation, while G-T is mainly caused by calcium carbonate.
The distribution and change in equivalent radius of pore and throats include all samples are calculated in Figure 7, and the size of bubbles represents the percentage of pores within this radius in the total, and specific data are listed in Table 3. In Figure 7a, the pores of all samples were reduced after microbial treatment, but the samples containing montmorillonite showed a trend different from the other two samples. Some pores with a larger radius appeared in the M-T sample, indicating that the expansion performance of montmorillonite would greatly affect the pore structure of the samples treated by microorganisms, it can be clearly seen in Figure 8. In Figure 7b, the throat radius of the sample containing illite changes most obviously after treatment, and most of the throats of I-U change from above 40 µm to below 40 µm in I-T, which also indicates that the throat length of I-T in Figure 6b does not increase because the calcium carbonate is attached to the inner wall of the throat.
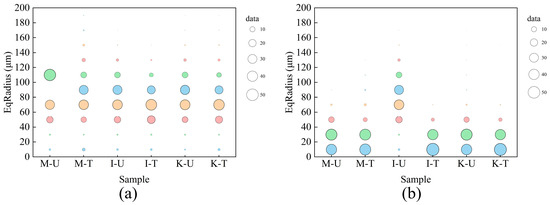
Figure 7.
Equivalent radius distribution and variation: (a) Pores. (b) Throats.

Table 3.
Equivalent radius distribution in samples.
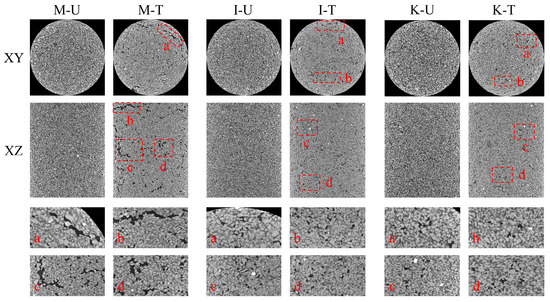
Figure 8.
Partial X-ray CT slice display of the specimen in the XY and XZ directions (mark some erosion pores with red boxes).
Figure 8 shows the X-ray CT slice images of the three samples after the MICP process, four significant pore changes are labeled with letters in the figure and enlarged accordingly according to their numbers. It can be obviously found that the pores of the M-U, I-U, and K-U samples were significantly reduced after being filled with calcium carbonate, but larger pores also appeared, and the M-T sample showed the most prominent performance, which was consistent with the results obtained before. It is also indicated that the cohesive force of M-T and I-T samples is smaller than that of K-T samples after the treatment by bacterial solution, mainly because the internal pore structure of M-T and I-T samples is greatly changed.
3.4. Chemical Composition and Surface Potential
In Figure 9 and Figure 10, the functional group composition and surface potential of clay minerals at different pH values are shown, respectively. In Figure 9, the peaks in the range of 3300–3750 cm−1 wave number correspond to -O-H on the mineral surface, and in the range of 700–1250 cm−1 correspond to the stretching vibration of Si-O and Al-O covalent bonds in the silico-tetrahedron and alumino-octahedron. The peak at 470 cm−1 is caused by the flexural vibration of Si-O and Al-O covalent bonds [46,47,48]. It can be found that only hydroxyl functional groups exist on the surface of the three clay minerals, so all three clay minerals have negative charges, which can also be observed from the surface potential test in Figure 10.
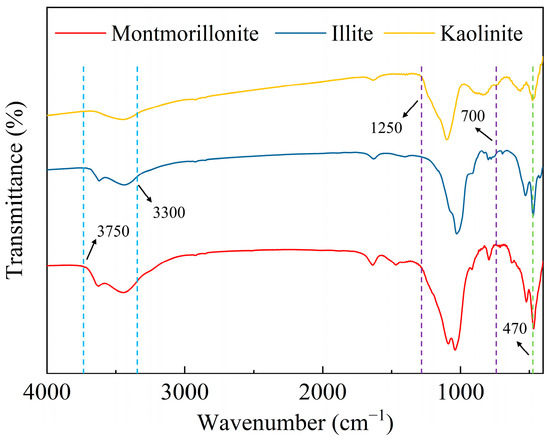
Figure 9.
Infrared spectra of clay.
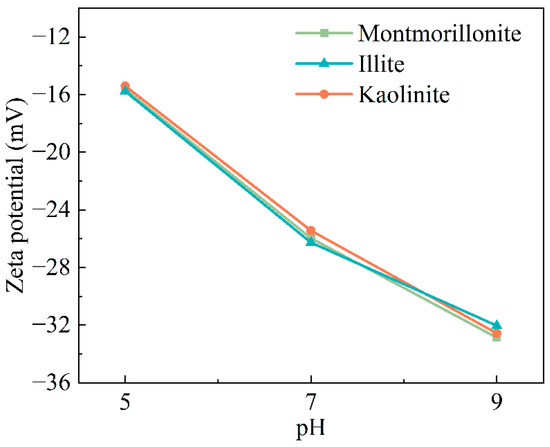
Figure 10.
Surface potential of clay minerals at different pH values.
Figure 10 shows the surface potential test results of the three clay minerals under different pH conditions. It can be obviously found that the surface of the three minerals all have negative charges, and with the increase in pH, their negative charges also increase, which may be related to the increase in the pH value of the solution. The surface of microorganisms is also negatively charged. When the microbial fluid passes through the sand sample containing clay, the clay minerals will attach less microorganisms through the charge, but when the cement fluid passes, more calcium ions may be retained for the subsequent microbial reaction to produce calcium carbonate. However, it can also be found from Figure 10 that the surface potential difference in the three clay minerals is not obvious, indicating that the surface charge of clay minerals plays a small role in the mechanical properties and pore evolution of the entire sample. Thus, it can be inferred that the difference in the swelling properties of different clay minerals is the main factor leading to the change in mechanical properties and microstructure of sand samples.
4. Discussion
The results clearly showed that the samples containing clay minerals all improve strength after microbial consolidation. This result is consistent with previous studies, indicating that the MICP technology can effectively improve the mechanical properties of weak soil. It can be attributed to mineralization products form in the pores and throats between the particles, increasing the contact points between sand particles and thus enhancing the overall performance of the samples [49]. Although the mechanical properties of the samples have been improved, there are still differences among the samples when different clay minerals are present. In this study, when montmorillonite is contained in the samples, the bacterial treatment shows the greatest improvement effect. The reason for this phenomenon can mainly be considered from two aspects. One is that the expansion of montmorillonite leads to a decrease in the overall porosity of the samples, which can increase the attachment of microorganisms in the samples, thereby generating more calcium carbonate precipitation and achieving a better consolidation effect [50]. The montmorillonite exhibited the best water expansion performance. Therefore, during the injection of the bacterial solution, it could adsorb the large of microorganisms [51], and generating more calcium carbonate to enhance the strength. Although the expansion of the minerals would increase the attachment of microorganisms, it would also increase the difficulty of injection. Previous studies have pointed out that the mixture of sand and clay would make it difficult to inject the solution [52]. The same phenomenon also occurred in this study. Due to the difficulty in solution injection, under continuous fluid pass, large pores and fractures could be clearly observed in the CT scan slices of the samples containing montmorillonite. The second reason is the montmorillonite sample has the best consolidation effect; the expansion of the mineral reduces the gaps between the sand particles, making the “bridging” effect of calcium carbonate more obvious. The expanded mineral itself will also adhere to the sand particles. This phenomenon can be confirmed by the fact that the montmorillonite sample shows the maximum cohesion. The mechanical properties of the sample containing kaolinite are higher than illite, which may also be the reason.
Apart from the expansiveness of clay minerals, the surface electrical properties of clay minerals may also have an impact on microbial mineralization. In the tests of surface functional groups of the three minerals, there were a large number of hydroxyl groups on the surface, causing the minerals to carry a negative charge. And this was also confirmed through surface potential tests. However, due to the decomposition of urea by urease, carbonate ions are produced and accumulate on the surface, causing the microorganisms to carry a negative charge, and minerals also carry a negative charge [53,54,55]. When microorganisms come into contact with minerals, their attachment efficiency will be affected. However, all three minerals carry a negative charge, and according to the test results, the surface potential of the three minerals does not differ much. The influence of surface charge on the microbial consolidation effect is relatively small. Therefore, the expansion properties of the minerals to retain more microorganisms for mineral formation is the most important approach to improve the consolidation performance. Although this study investigated the effects of microorganisms on the consolidation performance of samples containing different clay minerals and the changes in microstructure, the existing research still has some shortcomings. The content of clay minerals was a fixed value, and only the influence of different types of clay minerals on the microbial consolidation was explored, but the effect of different gradient contents of clay on the consolidation performance of the samples was not obtained, and the influence of mixed minerals was also not considered. These factors need to be further studied in subsequent research.
5. Conclusions
In this study, the influence of three different clay minerals on the cementation properties of sand samples before and after MICP treatment was studied through experiments, and the evolution of mechanical properties, internal pore structure, and mineral composition (especially calcium carbonate content) of the samples was compared so as to clarify the influence of clay mineral types on the cementation properties of the samples during grouting. The following conclusions can be drawn.
The stronger the expansibility of clay minerals, the more obvious the increased cohesion of the sample after MICP treatment. The cohesion of the montmorillonite-containing sample increased from 20 kPa to 65.4 kPa, a 3.27-fold increase, which was the most significant. This was mainly attributed to the expansibility and the retention of more microorganisms, resulting more calcium carbonate formation.
Compared to illite and kaolinite, the internal pore structure of the montmorillonite-containing sample changed the most significantly. After continuous fluid passage through the sample, pores with a diameter of over 120 µm appeared inside, accounting for 7.1% of the total pores.
The surface potential of clay minerals did not differ significantly under the three pH values (for example, at pH = 9, the surface charge was between −32.03 mV and −32.87 mV). Compared to the surface charge of the minerals, the expansion performance of the minerals was the dominant factor affecting the consolidation effect and pore structure of the sample.
Author Contributions
Methodology, S.Z., C.T., S.Q. and H.L.; Validation, S.Q.; Formal analysis, Z.W.; Investigation, C.T., T.L. and H.L.; Data curation, S.Q. and Z.W.; Writing—original draft, C.T.; Writing—review & editing, S.Z. and T.L.; Supervision, T.L.; Funding acquisition, S.Z. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2024YFC2814300) and the Sub-project (Task No. 2024YFC2814304), the National Natural Science Foundation of China (42072343, 42402318), and the Natural Science Foundation of Hubei Province (2024AFB051).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author (liutianle@cug.edu.cn) on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meierding, E. Joint development in the South China Sea: Exploring the prospects of oil and gas cooperation between rivals. Energy Res. Soc. Sci. 2017, 24, 65–70. [Google Scholar] [CrossRef]
- Yue, J.; Liu, Z.; Wang, J.; Sun, T.; Wu, Z.; Geng, Y. A Low-Cost and Low-Density Cement Slurry System Suitable for a Shallow Unconsolidated Stratum. Adv. Mater. Sci. Eng. 2020, 2020, 1628281. [Google Scholar] [CrossRef]
- Jiapei, D.; Yuhuan, B.; Xuechao, C.; Zhonghou, S.; Baojiang, S. Utilization of alkali-activated slag based composite in deepwater oil well cementing. Constr. Build. Mater. 2018, 186, 114–122. [Google Scholar] [CrossRef]
- Kiran, R.; Teodoriu, C.; Dadmohammadi, Y.; Nygaard, R.; Wood, D.; Mokhtari, M.; Salehi, S. Identification and evaluation of well integrity and causes of failure of well integrity barriers (A review). J. Nat. Gas Sci. Eng. 2017, 45, 511–526. [Google Scholar] [CrossRef]
- Yang, G.; Lei, G.; Liu, T.; Zheng, S.; Qu, B.; Que, C.; Feng, Y.; Jiang, G. Development and application of low-melting-point microencapsulated phase change materials for enhanced thermal stability in cementing natural gas hydrate layers. Geoenergy Sci. Eng. 2024, 238, 212846. [Google Scholar] [CrossRef]
- Okoro, E.E.; Sanni, S.E.; Emetere, M.E.; Omeje, M.; Orodu, K.B. Effect of Stratigraphic features on Deep-Water Cementing Operation—A Review. J. Phys. Conf. Ser. 2019, 1378, 22042. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, D.; Sun, Y.; Li, S. Experimental study on hydraulic fracturing behavior of frozen clayey silt and hydrate-bearing clayey silt. Fuel 2022, 322, 124366. [Google Scholar] [CrossRef]
- Zheng, D.; Ozbayoglu, E.; Miska, S.Z.; Liu, Y.; Li, Y. Cement sheath fatigue failure prediction by ANN-based model. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2022; p. D011S013R009. [Google Scholar]
- Bu, Y.; Hou, X.; Wang, C.; Du, J. Effect of colloidal nanosilica on early-age compressive strength of oil well cement stone at low temperature. Constr. Build. Mater. 2018, 171, 690–696. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Sun, Y.; Zhang, D.; Li, X.; Fan, Z. Experimental investigation of the shear bond strength between HGM cement and shallow formation in deepwater environments. Geoenergy Sci. Eng. 2023, 221, 111174. [Google Scholar] [CrossRef]
- Feng, Q.; Mao, Y.; Peng, Z.; Zheng, Y.; Wu, J. Preparation and properties of low-temperature early strength material for nano-CSH gel seed. Arab. J. Sci. Eng. 2022, 47, 5567–5575. [Google Scholar] [CrossRef]
- Hao, H.; Gu, J.; Huang, J.; Wang, Z.; Wang, Q.; Zou, Y.; Wang, W. Comparative study on cementation of cement-mudcake interface with and without mud-cake-solidification-agents application in oil & gas wells. J. Pet. Sci. Eng. 2016, 147, 143–153. [Google Scholar] [CrossRef]
- Bai, X.; Xu, Y.; Zhang, X.; Yong, X.; Ning, T. Enhancing the solidification between mud cake and wall rock for cementing applications: Experimental investigation and mechanisms. J. Energy Resour. Technol. 2021, 143, 73003. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S. Recent advances in polymers as additives for wellbore cementing applications: A review. Fuel 2024, 357, 129692. [Google Scholar] [CrossRef]
- Skogdalen, J.E.; Vinnem, J.E. Quantitative risk analysis of oil and gas drilling, using Deepwater Horizon as case study. Reliab. Eng. Syst. Saf. 2012, 100, 58–66. [Google Scholar] [CrossRef]
- Du, J.; Bu, Y.; Shen, Z.; Cao, X. A novel fluid for use in oil and gas well construction to prevent the oil and gas leak from the wellbore. Constr. Build. Mater. 2019, 217, 626–637. [Google Scholar] [CrossRef]
- Tian, L.; Bu, Y.; Liu, H.; Zhao, L. An Experimental Study on the Reinforcement of Weakly-Consolidated Shallow Formation in Deep Water Using an Epoxy Resin-Based Fluid. Fluid Dyn. Mater. Process. 2023, 19, 1215–1226. [Google Scholar] [CrossRef]
- Pacheco, V.L.; Bragagnolo, L.; Reginatto, C.; Thomé, A. Microbially induced calcite precipitation (MICP): Review from an engineering perspective. Geotech. Geol. Eng. 2022, 40, 2379–2396. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Ehrlich, H.L.; Newman, D.K.; Kappler, A. Geomicrobiology; Dekker, M., Ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0824707648. [Google Scholar]
- Hamdan, N.; Kavazanjian, E.; Rittmann, B.E.; Karatas, I. Carbonate Mineral Precipitation for Soil Improvement Through Microbial Denitrification. Geomicrobiol. J. 2017, 34, 139–146. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Konstantinou, C.; Soga, K.; Biscontin, G.; Kabla, A.J. Use of microfluidic experiments to optimize MICP treatment protocols for effective strength enhancement of MICP-treated sandy soils. Acta Geotech. 2022, 17, 3817–3838. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.; Jiang, N.; Pan, X.; Liu, B.; Wang, Y.; Shi, B. Microbial-induced carbonate precipitation (MICP) technology: A review on the fundamentals and engineering applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Tang, C.; Liu, T.; Fang, C.; Qin, S.; Yang, G.; Lei, G.; Sun, J. Experimental study of microbially induced carbonate precipitation treatment on seafloor sediment of hydrate formation. Acta Geotech. 2024, 19, 1597–1610. [Google Scholar] [CrossRef]
- Qin, S.; Sun, J.; Liu, T.; Tang, C.; Lei, G.; Dou, X.; Gu, Y. Sand control during gas production from marine hydrate reservoirs by using microbial-induced carbonate precipitation technology: A feasibility study. Energy 2024, 299, 131494. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. Effects of Different Clay’s Percentages on Improvement of Sand-Clay Mixtures with Microbially Induced Calcite Precipitation. Geomicrobiol. J. 2019, 36, 810–818. [Google Scholar] [CrossRef]
- Cardoso, R.; Pires, I.; Duarte, S.O.D.; Monteiro, G.A. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Maston, O.; Ouahbi, T.; Taibi, S.; El Hajjar, A.; Sapin, L.; Esnault-Filet, A.; Duchemin, B.; Fleureau, J. Effect of MICP treatment on the mechanical properties of clay soils. Transp. Geotech. 2025, 50, 101483. [Google Scholar] [CrossRef]
- Teng, F.; Sie, Y.; Ouedraogo, C. Strength improvement in silty clay by microbial-induced calcite precipitation. Bull. Eng. Geol. Environ. 2021, 80, 6359–6371. [Google Scholar] [CrossRef]
- Li, J.; Tian, L.; Xu, Y.; Tian, Z.; Zhang, Z. Study on the Solidification Effect of Dredger Fill by Microbial-Induced Calcium Precipitation (MICP). Materials 2022, 15, 7891. [Google Scholar] [CrossRef]
- Hu, J.; Fan, F.; Huang, L.; Yu, J. Experimental Study on Enhancing the Mechanical Properties of Sandy Soil by Combining Microbial Mineralization Technology with Silty Soil. Materials 2024, 17, 2362. [Google Scholar] [CrossRef]
- Kannan, K.; Bindu, J.; Vinod, P. Engineering behaviour of MICP treated marine clays. Mar. Georesources Geotechnol. 2020, 38, 761–769. [Google Scholar] [CrossRef]
- Lv, Y.; Li, X.; Fan, C.; Su, Y. Effects of internal pores on the mechanical properties of marine calcareous sand particles. Acta Geotech. 2021, 16, 3209–3228. [Google Scholar] [CrossRef]
- Ito, T.; Komatsu, Y.; Fujii, T.; Suzuki, K.; Egawa, K.; Nakatsuka, Y.; Konno, Y.; Yoneda, J.; Jin, Y.; Kida, M.; et al. Lithological features of hydrate-bearing sediments and their relationship with gas hydrate saturation in the eastern Nankai Trough, Japan. Mar. Petrol. Geol. 2015, 66, 368–378. [Google Scholar] [CrossRef]
- Clough, G.W.; Sitar, N.; Bachus, R.C.; Rad, N.S. Cemented sands under static loading. J. Geotech. Eng. Div. 1981, 107, 799–817. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, T.; Liu, Y.; Jiang, Q.; Shang, H.; Zheng, C. Montmorillonite combined with microbially induced carbonate precipitation for wind erosion control of bare surface soil in arid mining area. Process Saf. Environ. 2024, 187, 926–939. [Google Scholar] [CrossRef]
- Hattab, M.; Hammad, T.; Fleureau, J.M. Internal friction angle variation in a kaolin/montmorillonite clay mix and microstructural identification. Géotechnique 2015, 65, 1–11. [Google Scholar] [CrossRef]
- Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; The Mineralogical Society of Great Britain and Ireland: London, UK, 1982; ISBN 0903056089. [Google Scholar]
- Meunier, A. Clays; Springer Science & Business Media: Heidelberg, Germany, 2005; ISBN 3540271414. [Google Scholar]
- Velde, B.; Meunier, A.; Velde, B.; Meunier, A. Fundamentals of clay mineral crystal structure and physicochemical properties. In The Origin of Clay Minerals in Soils and Weathered Rocks; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–73. [Google Scholar]
- Salles, F.; Douillard, J.; Denoyel, R.; Bildstein, O.; Jullien, M.; Beurroies, I.; Van Damme, H. Hydration sequence of swelling clays: Evolutions of specific surface area and hydration energy. J. Colloid Interf. Sci. 2009, 333, 510–522. [Google Scholar] [CrossRef]
- Madsen, F.T.; Mueller-Vonmoos, M.; Mueller-Vonmoos, M. The swelling behaviour of clays. Appl. Clay Sci. 1989, 4, 143–156. [Google Scholar] [CrossRef]
- Wei, R.; Peng, J.; Li, L.; Jiang, Z.; Tang, J. Accelerated Reinforcement of Calcareous sand via Biomineralization with Aluminum Ion Flocculant. Appl. Biochem. Biotech. 2023, 195, 7197–7213. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Peng, J.; He, J.; Li, L.; Jiang, Z.; Tang, J. Effects of adding aluminum ion flocculant on MICP reinforcement of sand. Acta Geotech. 2024, 19, 3505–3517. [Google Scholar] [CrossRef]
- Schroeder, P.A. Infrared spectroscopy in clay science. In Teaching Clay Science; The Clay Mineral Society: Aurora, CO, USA, 2002; pp. 181–206. [Google Scholar]
- Plevova, E.; Vaculikova, L.; Valovicova, V. Thermal analysis and FT-IR spectroscopy of synthetic clay mineral mixtures. J. Therm. Anal. Calorim. 2020, 142, 507–518. [Google Scholar] [CrossRef]
- Djomgoue, P.; Njopwouo, D. FT-IR Spectroscopy Applied for Surface Clays Characterization. J. Surf. Eng. Mater. Adv. Technol. 2013, 03, 275–282. [Google Scholar] [CrossRef]
- Tang, C.; Yin, L.; Jiang, N.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Yuan, M.; Chen, X.; Xiao, Z.; Hao, X.; Zhang, J.; Tang, Q. The effect of MICP on physical and mechanical properties of silt with different fine particle content and pore ratio. Appl. Sci. 2021, 12, 139. [Google Scholar] [CrossRef]
- Ma, G.; Xiao, Y.; Fang, Q.; He, X. Biogrouting and bio-clay grouting: Regulating biocement location with clay. Appl. Clay Sci. 2025, 274, 107860. [Google Scholar] [CrossRef]
- Jiang, N.; Soga, K.; Kuo, M. Microbially induced carbonate precipitation for seepage-induced internal erosion control in sand–clay mixtures. J. Geotech. Geoenviron. 2017, 143, 4016100. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Yang, G.; Zhao, S. Preparation and research on cationic modified vermiculite with strong adsorption capacity for mineralizing bacteria. Mater. Lett. 2024, 363, 136313. [Google Scholar] [CrossRef]
- Ma, L.; Pang, A.; Luo, Y.; Lu, X.; Lin, F. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Fact. 2020, 19, 12. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, S.; Liu, T.; Luo, E.; Tang, C.; Qu, B.; Lei, G.; Jiang, G. Effect of biocarriers on microbially induced carbonate precipitation for sand reinforcement. Acta Geotech. 2025, 20, 3615–3632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).