Seasonal Dynamics of Microalgal Biomass and Its Biomethanation Potential: A Case Study from the Bay of Gdansk, Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Concept of Research Works
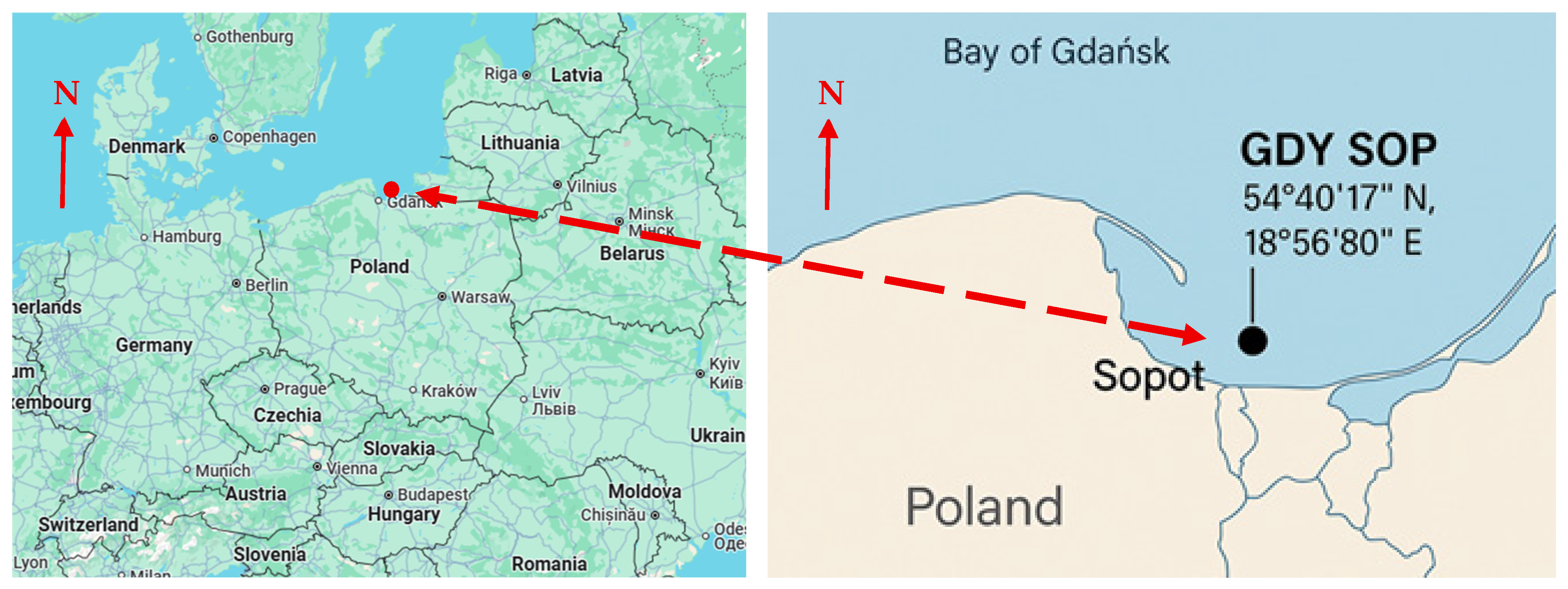
2.2. Study Area
2.3. Microalgal Biomass
2.4. Inoculum of Anaerobic Respirometric Digesters
2.5. Respirometric Measurements of Biogas Production
- -
- V(t) is the cumulative volume of biogas or methane produced at time t [mL/g VS],
- -
- Vmax is the maximum (asymptotic) biogas/methane yield [mL/g VS],
- -
- Rmax is the maximum production rate [mL/g VS·d],
- -
- λ is the lag phase duration [d],
- -
- t is the fermentation time [d],
- -
- e is Euler’s constant (≈2.71828).
2.6. Analytical Methods
2.7. Statistical Methods
3. Results and Discussion
3.1. Water Quality Parameters
3.2. Quantitative and Taxonomic Characteristics of the Biomass
3.3. Chemical Composition of Microalgal Biomass
3.4. Anaerobic Digestion
3.5. Dependencies and Correlations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amorim, C.A.; Moura, A.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H. Urgent Necessity for Algal Bloom Mitigation and Derived Resource Recycling. Water 2025, 17, 853. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Hou, X.; Qin, B.; Kuster, T.; Qu, F.; Chen, N.; Paerl, H.W.; Zheng, C. Harmful algal blooms in inland waters. Nat. Rev. Earth Environ. 2024, 5, 631–644. [Google Scholar] [CrossRef]
- Węsławski, J.M.; Urbański, J.; Piwowarczyk, J.; Kotwicki, L.; Piskozub, J.; Kuliński, K.; Pazdro, K.; Wiktor, J.; Sagan, S.; Psuty, I.; et al. Environmental change between 1980 and 2020 followed by societal change in the Gulf of Gdansk, Southern Baltic, a review. Front. Earth Sci. 2025, 13, 1557993. [Google Scholar] [CrossRef]
- Dybowski, D.; Dzierzbicka-Glowacka, L.A.; Pietrzak, S.; Juszkowska, D.; Puszkarczuk, T. Estimation of nitrogen leaching load from agricultural fields in the Gdansk Commune with an interactive calculator. PeerJ Life Environ. 2020, 8, e8899. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Walery, M. Aquatic Macrophyte Biomass Periodically Harvested Form Shipping Routes and Drainage Systems in a Selected Region of Poland as a Substrate for Biogas Production. Appl. Sci. 2023, 13, 4184. [Google Scholar] [CrossRef]
- Kalinichenko, A.; Pisarenko, P.; Kulyk, M. Algae in urban water bodies—Control of growth and use as a biomass. E3S Web Conf. 2018, 45, 00028. [Google Scholar] [CrossRef]
- Amalapridman, V.; Ofori, P.A.; Abbey, L. Valorization of Algal Biomass to Biofuel: A Review. Biomass 2025, 5, 26. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar] [CrossRef]
- Huo, S.; Dong, R.; Wang, Z.; Pang, C.; Yuan, Z.; Zhu, S.; Chen, L. Available Resources for Algal Biofuel Development in China. Energies 2011, 4, 1321–1335. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Dudek, M.; Grala, A. Acquisition feasibility and methane fermentation effectiveness of biomass of microalgae occurring in eutrophicated aquifers on the example of the Vistula Lagoon. Int. J. Green Energy 2016, 13, 395–407. [Google Scholar] [CrossRef]
- Mosharov, S.A.; Mosharova, I.V.; Dmitrieva, O.A.; Semenova, A.S.; Ulyanova, M.O. Seasonal Variability of Plankton Production Parameters as the Basis for the Formation of Organic Matter Flow in the Southeastern Part of the Baltic Sea. Water 2022, 14, 4099. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Sonne, C.; Vasseghian, Y. Algae biogas production focusing on operating conditions and conversion mechanisms—A review. Heliyon 2023, 9, e17757. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Kalita, N.; Wall, D.; Xia, A.; Murphy, J.D. Optimised biogas production from microalgae through co-digestion with carbon-rich co-substrates. Bioresour. Technol. 2016, 214, 328–337. [Google Scholar] [CrossRef]
- Helsinki Commission, Baltic Marine Environment Protection Commission. Manual for Marine Monitoring in the Combine Programme of HELCOM. 2023. Available online: https://archive.iwlearn.net/helcom.fi/groups/monas/CombineManual/en_GB/main/index.html (accessed on 12 May 2024).
- Mantoura, R.F.C. SCOR/UNESCO WG 78 on Determination of Photosynthetic Pigments in Seawater. 1984. Available online: https://www.scor-int.org/Working_Groups/WG%2078.htm (accessed on 14 April 2024).
- Cheregi, O.; Engelbrektsson, J.; Andersson, M.X.; Strömberg, N.; Ekendahl, S.; Godhe, A.; Spetea, C. Marine microalgae for outdoor biomass production—A laboratory study simulating seasonal light and temperature for the west coast of Sweden. Physiol. Plant. 2021, 173, 543–554. [Google Scholar] [CrossRef]
- HELCOM/Baltic Earth. Climate Change in the Baltic Sea 2021 Fact Sheet. Available online: https://helcom.fi/wp-content/uploads/2021/09/Baltic-Sea-Climate-Change-Fact-Sheet-2021.pdf (accessed on 14 April 2024).
- Beltran-Perez, O.D.; Waniek, J.J. Inter-annual variability of Spring and summer blooms in the eastern Baltic Sea. Front. Mar. Sci. 2022, 9, 928633. [Google Scholar] [CrossRef]
- Sokołowski, A.; Jankowska, E.; Balazy, P.; Jędruch, A. Distribution and extent of benthic habitats in Gdansk Bay (Gulf of Gdansk, southern Baltic Sea). Oceanologia 2021, 63, 301–320. [Google Scholar] [CrossRef]
- Rak, D.; Walczowski, W.; Dzierzbicka-Głowacka, L.; Shchuka, S. Dissolved oxygen variability in the southern Baltic Sea in 2013–2018. Oceanologia 2020, 62, 525–537. [Google Scholar] [CrossRef]
- Pryputniewicz-Flis, D.; Burska, D.; Bolałek, J. Warunki tlenowe w Wodach Zatoki Gdanskiej. In Zatoka Gdanska Part 2; Bolałek, J., Burska, D., Eds.; Wydawnictwo Uniwersytetu Gdanskiego: Gdansk, Poland, 2022; ISBN 978-83-8206-345-5. [Google Scholar]
- Conley, D.J.; Carstensen, J.; Aigars, J.; Axe, P.; Bonsdorff, E.; Eremina, T.; Haahti, B.M.; Humborg, C.; Jonsson, P.; Kotta, J.; et al. Hypoxia is increasing in the coastal zone of the Baltic Sea. Environ. Sci. Technol. 2011, 45, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Węsławski, J.M.; Kryla-Straszewska, L.; Piwowarczyk, J.; Urbański, J.; Warzocha, J.; Kotwicki, L.; Włodarska-Kowalczuk, M.; Wiktor, J. Habitat modelling limitations—Gdansk Bay, Baltic Sea—A case study. Oceanologia 2013, 55, 167–183. [Google Scholar] [CrossRef]
- Kahru, M.; Cahill, B.; Elmgren, R.; Rehder, G. What initiates cyanobacterial blooms in the Baltic Sea? Harmful Algae 2025, 148, 102924. [Google Scholar] [CrossRef]
- Somogyi, B.; Li, H.; Tapolczai, K.; Kovács, A.W.; Tóth, L.G.; Horváth, H.; Krassován, K.; Fodor-Kardos, A.; Vörös, L. Regime shift in microalgal dynamics: Impact of water level changes on planktonic and benthic algal biomass. Sci. Total Environ. 2024, 929, 172351. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R.; Kaiser, J.; Wasmund, N.; Savchuk, O. Cyanobacterial blooms in the Baltic Sea: Correlations with environmental factors. Harmful Algae 2020, 92, 101739. [Google Scholar] [CrossRef] [PubMed]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Przednowek, I.; Ogrodowczyk, I.; Kozic, S. Composition of phytoplankton in the Puck Bay and the open Baltic Sea. Ann. Univ. Paedagog. Cracoviensis Stud. Naturae 2017, 2, 27–35. [Google Scholar] [CrossRef]
- Su, M.; Andersen, T.; Burch, M.; Jia, Z.; An, W.; Yu, J.; Yang, M. Succession and interaction of surface and subsurface cyanobacterial blooms in oligotrophic/mesotrophic reservoirs: A case study in Miyun Reservoir. Sci. Total Environ. 2019, 649, 1553–1562. [Google Scholar] [CrossRef]
- Svedén, J.B.; Walve, J.; Larsson, U.; Elmgren, R. The bloom of nitrogen-fixing cyanobacteria in the northern Baltic Proper stimulates summer production. J. Mar. Syst. 2016, 163, 102–112. [Google Scholar] [CrossRef]
- Canuti, E.; Penna, A. Dynamics of phytoplankton communities in the Baltic Sea: Insights from a multi-dimensional analysis of pigment and spectral data—Part I, pigment dataset. Front. Mar. Sci. 2024, 11, 1425347. [Google Scholar] [CrossRef]
- Camarena-Gómez, M.T.; Ruiz-González, C.; Piiparinen, J.; Lipsewers, T.; Sobrino, C.; Logares, R.; Spilling, K. Bacterioplankton dynamics driven by interannual and spatial variation in diatom and dinoflagellate spring bloom communities in the Baltic Sea. Limnol. Oceanogr. 2021, 66, 255–271. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Skrobek, I.; Ziułkiewicz, M.; Adamski, M.; Kaminski, A.; Żmudzki, P. Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. Int. J. Environ. Res. Public Health 2022, 19, 6990. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Perez, O.D.; Waniek, J.J. Environmental window of cyanobacteria bloom occurrence. J. Mar. Syst. 2021, 224, 103618. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: http://data.europa.eu/eli/dir/2000/60/oj (accessed on 9 May 2024).
- Enberg, S.; Majaneva, M.; Autio, R.; Blomster, J.; Rintala, J.M. Phases of microalgal succession in sea ice and the water column in the Baltic Sea from autumn to spring. Mar. Ecol. Prog. Ser. 2018, 599, 19–34. [Google Scholar] [CrossRef]
- Hjerne, O.; Hajdu, S.; Larsson, U.; Downing, A.; Winder, M. Climate Driven Changes in Timing, Composition and Size of the Baltic Sea Phytoplankton Spring Bloom. Front. Mar. Sci. 2019, 6, 482. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Baracho, D.H.; Lombardi, A.T. Study of the growth and biochemical composition of 20 species of cyanobacteria cultured in cylindrical photobioreactors. Microb. Cell Fact. 2023, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga-Astudillo, D.; Ruge, J.C.; Caicedo-Hormaza, B. Micromechanical Characterization of Diatom Frustules of Multiple Origin. Appl. Sci. 2025, 15, 749. [Google Scholar] [CrossRef]
- Chai, X.; Li, X.; Hii, K.S.; Zhang, Q.; Deng, Q.; Wan, L.; Zheng, L.; Lim, P.T.; Tan, S.N.; Mohd-Din, M.; et al. Blooms of diatom and dinoflagellate associated with nutrient imbalance driven by cycling of nitrogen and phosphorus in anaerobic sediments in Johor Strait (Malaysia). Mar. Environ. Res. 2021, 169, 105398. [Google Scholar] [CrossRef]
- Fernández-Juárez, V.; Bennasar-Figueras, A.; Sureda-Gomila, A.; Ramis-Munar, G.; Agawin, N.S.R. Differential effects of varying concentrations of phosphorus, iron, and nitrogen in N2-fixing cyanobacteria. Front. Microbiol. 2020, 11, 541558. [Google Scholar] [CrossRef]
- Mills, M.M.; Brown, Z.W.; Laney, S.R.; Ortega-Retuerta, E.; Lowry, K.E.; van Dijken, G.L.; Arrigo, K.R. Nitrogen Limitation of the Summer Phytoplankton and Heterotrophic Prokaryote Communities in the Chukchi Sea. Front. Mar. Sci. 2018, 5, 362. [Google Scholar] [CrossRef]
- Sandman, A.N.; Näslund, J.; Gren, I.M.; Norling, K. Effects of an invasive polychaete on benthic phosphorus cycling at sea basin scale: An ecosystem disservice. Ambio 2018, 47, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Laamanen, M.; Huttunen, M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 71, 580–592. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Physiological changes of Parachlorella kessleri TY02 in lipid accumulation under nitrogen stress. Int. J. Environ. Public Health 2019, 16, 1188. [Google Scholar] [CrossRef]
- Kumar, R.; Biswas, K.; Singh, P.K.; Singh, P.K.; Elumalai, S.; Shukla, P.; Pabbi, S. Lipid production and molecular dynamics simulation for regulation of accD gene in cyanobacteria under different N and P regimes. Biotechnol. Biofuels 2017, 10, 94. [Google Scholar] [CrossRef]
- Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, & Komárek by using modified nutrient media. Aquacult. Int. 2020, 28, 1371–1388. [Google Scholar] [CrossRef]
- Han, F.; Pei, H.Y.; Hu, W.R.; Han, L.; Zhang, S.; Ma, G.X. Effect of high temperature stress on microalgae at the end of the logarithmic phase for the efficient production of lipid. Environ. Technol. 2016, 37, 2649–2657. [Google Scholar] [CrossRef]
- Wu, J.T.; Chiang, Y.R.; Huang, W.Y.; Jane, W.N. Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat. Toxicol. 2006, 80, 338–345. [Google Scholar] [CrossRef]
- Jensen, E.L.; Yangüez, K.; Carrière, F.; Gontero, B. Storage compound accumulation in diatoms as response to elevated CO2 concentration. Biology 2019, 9, 5. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Chen, T.; Hao, T.; Balamurugan, S.; Yang, W.; Liu, J.; Li, H. Overproduction of bioactive algal chrysolaminarin by the critical carbon flux regulator phosphoglucomutase. Biotechnol. J. 2019, 14, 1800220. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, T.B.; Medvedeva, N.G. Impact of biogenic elements on the growth of bloom-forming filamentous cyanobacteria and formation of metabolites. Inland Water Biol. 2022, 15, 305–314. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Dubey, N.K. Polysaccharides from cyanobacteria: Response to biotic and abiotic stress and their antiviral activity. Indian J. Mar. Sci. 2018, 47, 21–33. Available online: http://scholar.cu.edu.eg/?q=emad-shalaby/files/ijms_471_21-33.pdf (accessed on 10 May 2024).
- Ankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, J.; Wan, L.; Zhou, Z.; Wang, Z.; Song, C.; Zhou, Y.; Cao, X. Mutual dependence of nitrogen and phosphorus as key nutrient elements: One facilitates Dolichospermum flos-aquae to overcome limitation by the other. Environ. Sci. Technol. 2018, 52, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Manandhar-Shrestha, K.; Abbriano, R. Effects of chrysolaminarin synthase knockdown in the diatom Thalassiosira pseudonana: Implications of reduced carbohydrate storage relative to green algae. Algal Res. 2017, 23, 66–77. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Bartkowska, I. Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Appl. Sci. 2022, 12, 7291. [Google Scholar] [CrossRef]
- Park, J.M.; You, Y.H.; Kang, N.S.; Cho, E.; Back, C.G.; Hong, J.W. Potential of freshwater cyanobacterial harmful algal bloom biomass for biomethane production via anaerobic digestion. Microbiol. Biotechnol. Lett. 2024, 52, 343–357. [Google Scholar] [CrossRef]
- Kato, Y.; Hidese, R.; Matsuda, M.; Ohbayashi, R.; Ashida, H.; Kondo, A.; Hasunuma, T. Glycogen deficiency enhances carbon partitioning into glutamate for an alternative extracellular metabolic sink in cyanobacteria. Commun. Biol. 2024, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 156415. [Google Scholar] [CrossRef]
- Yuan, X.; Shi, X.; Zhang, D.; Qiu, Y.; Guo, R.; Wang, L. Biogas Production and Microcystin Biodegradation in Anaerobic Digestion of Blue Algae. Energy Environ. Sci. 2011, 4, 1511–1515. [Google Scholar] [CrossRef]
- Kowthaman, C.N.; Selvan, V.A.M.; Kumar, P.S. Optimization strategies of alkaline thermo-chemical pretreatment for the enhancement of biogas production from de-oiled algae. Fuel 2021, 303, 121242. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Vergara-Fernández, A.; Vargas, G.; Alarcón, N.; Velasco, A. Evaluation of Marine Algae as a Source of Biogas in a Two-Stage Anaerobic Reactor System. Biomass Bioenergy 2008, 32, 338–344. [Google Scholar] [CrossRef]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.P. Experimental Study on a Coupled Process of Production and Anaerobic Digestion of Chlorella Vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef]
- Samson, R.; Leduy, A. Multistage Continuous Cultivation of Blue-Green Alga Spirulina Maxima in the Flat Tank Photobioreactors with Recycle. Can. J. Chem. Eng. 1985, 63, 105–112. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as Substrates for Fermentative Biogas Production in a Combined Biorefinery Concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Zamalloa, C.; Boon, N.; Verstraete, W. Anaerobic Digestibility of Scenedesmus Obliquus and Phaeodactylum Tricornutum under Mesophilic and Thermophilic Conditions. Appl. Energy 2012, 92, 733–738. [Google Scholar] [CrossRef]
- Yen, H.W.; Brune, D.E. Anaerobic Co-Digestion of Algal Sludge and Waste Paper to Produce Methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F. Total organic carbon as a predictor of biological wastewater treatment efficiency and kinetic reaction rates. Water Sci. Technol. 1997, 35, 119–126. [Google Scholar] [CrossRef]
- Loganath, R.; Mazumder, D. Performance study on organic carbon, total nitrogen, suspended solids removal and biogas production in hybrid UASB reactor treating real slaughterhouse wastewater. J. Environ. Chem. Eng. 2018, 6, 3474–3484. [Google Scholar] [CrossRef]
- Shao, Z.; Fan, Q.; Gao, F.; Xia, T.; Wang, Y.; Liang, Y.; Guo, X.; Yang, X.; Yao, Y.; Qiu, L.; et al. Sustained methane production enhancement by magnetic biochar and its recovery in semi-continuous anaerobic digestion with varying substrate C/N ratios. Chem. Eng. J. 2025, 514, 163050. [Google Scholar] [CrossRef]
- Budiyono, B.; Matin, H.H.A.; Yasmin, I.Y.; Priogo, I.S. Effect of pretreatment and C/N ratio in anaerobic digestion on biogas production from coffee grounds and rice husk mixtures. Int. J. Renew. Energy Dev. 2023, 12, 209. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Kopachevsky, A.M.; Chow, S.; Bouwer, E.J.; Betenbaugh, M.J. Synergistic co-digestion of wastewater grown algae-bacteria polyculture biomass and cellulose to optimize carbon-to-nitrogen ratio and application of kinetic models to predict anaerobic digestion energy balance. Bioresour. Technol. 2018, 269, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Ammar, M.; Korai, R.M.; Ahmad, N.; Ali, A.; Khalid, M.S.; Zou, D.; Li, X. Impact of C/N ratios and organic loading rates of paper, cardboard and tissue wastes in batch and CSTR anaerobic digestion with food waste on their biogas production and digester stability. SN Appl. Sci. 2020, 2, 1436. [Google Scholar] [CrossRef]
- de Castro, I.M.P.; de Alencar Neves, T.; Rosa, A.P.; da Cunha, F.F.; Passos, F. Long-term assessment of anaerobic co-digestion of food waste and microalgae: Process stabilization, methane yield and agronomic properties of digestate. Algal Res. 2025, 86, 103947. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Zhao, L. Comprehensive study of the combined effects of biochar and iron-based conductive materials on alleviating long chain fatty acids inhibition in anaerobic digestion. Environ. Res. 2023, 239, 117446. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Zieliński, M. Methane Production from Confectionery Wastewater Treated in the Anaerobic Labyrinth-Flow Bioreactor. Energies 2023, 16, 571. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Betenbaugh, M.J.; Bouwer, E.J. The effects of alternative pretreatment strategies on anaerobic digestion and methane production from different algal strains. Bioresour. Technol. 2014, 155, 366–372. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Month | |||||
|---|---|---|---|---|---|---|---|
| May | June | July | August | September | October | ||
| Salinity | [PSU] | 6.5 ± 0.7 | 7.0 ± 0.2 | 7.5 ± 0.3 | 7.8 ± 0.6 | 7.6 ± 0.4 | 6.9 ± 0.5 |
| Temperature | [°C] | 10 ± 4.2 | 12 ± 2.2 | 15 ± 0.8 | 17 ± 2.8 | 15 ± 0.7 | 12 ± 2.3 |
| Oxygen | [mg/L] | 8.2 ± 0.5 | 7.8 ± 0.1 | 7.5 ± 0.2 | 7.3 ± 0.4 | 7.6 ± 0.1 | 8.0 ± 0.3 |
| Transparency | [m] | 4.5 ± 0.3 | 4.2 ± 0.1 | 4.0 ± 0.2 | 3.8 ± 0.4 | 4.1 ± 0.1 | 4.3 ± 0.2 |
| Chlorophyll-a | [µg/L] | 7.1 ± 0.6 | 10.5 ± 1.2 | 25.0 ± 1.3 | 31.4 ± 2.8 | 12.9 ± 1.5 | 6.2 ± 0.5 |
| Feopigments | [µg/L] | 1.54 ± 0.23 | 2.62 ± 0.11 | 3.71 ± 0.09 | 4.83 ± 0.15 | 3.77 ± 0.12 | 1.69 ± 0.07 |
| Month | Prevailing Groups and Species and Their Contribution in TS | Chlorophyll-a Content and Biomass Concentration |
| May |
| Chlorophyll-a: 7.1 ± 0.6 µg/L Biomass: 270 ± 31 mg TS/m3 |
| June |
| Chl-a: 10.5 ± 1.2 µg/L Biomass: 430 ± 39 mg TS/m3 |
| July |
| Chl-a: 25.0 ± 1.3 µg/L Biomass: 1130 ± 84 mg TS/m3 |
| August |
| Chl-a: 31.4 ± 2.8 µg/L Biomass: 1250 ± 107 mg TS/m3 |
| September |
| Chl-a: 12.9 ± 1.5 µg/L Biomass: 580 ± 64 mg TS/m3 |
| October |
| Chl-a: 6.2 ± 0.5 µg/L Biomass: 340 ± 21 mg TS/m3 |
| Parameter | Unit | Month | |||||
|---|---|---|---|---|---|---|---|
| May | June | July | August | September | October | ||
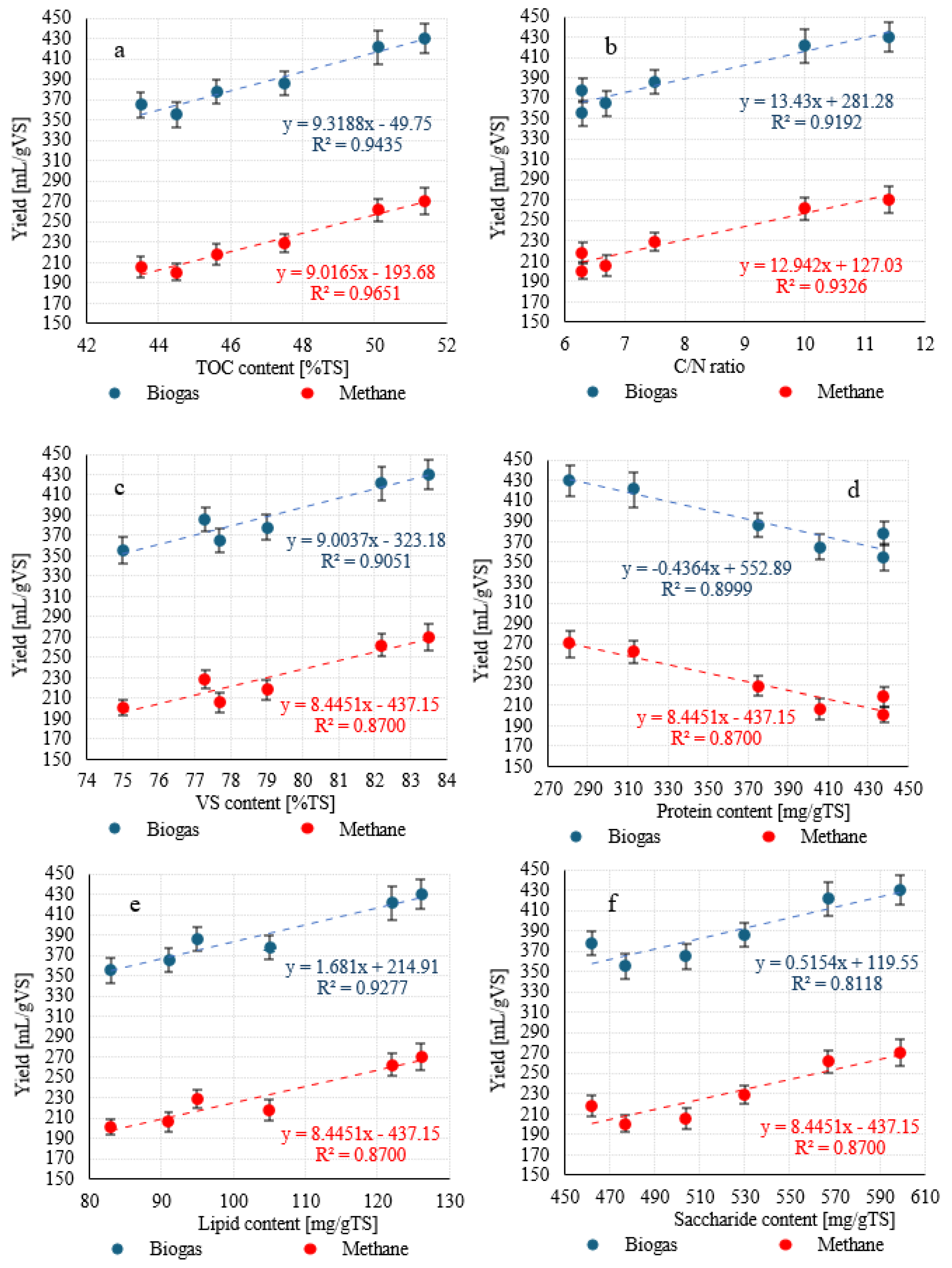
| TOC | [% TS] | 43.5 ± 1.5 a | 45.6 ± 1.7 a | 50.1 ± 2.0 b | 51.4 ± 2.1 b | 47.5 ± 1.8 ab | 44.5 ± 1.6 a |
| TN | [% TS] | 6.5 ± 1.1 ab | 7.2 ± 0.9 b | 6.0 ± 0.3 a | 5.5 ± 0.9 a | 6.3 ± 0.4 ab | 7.1 ± 0.6 b |
| TP | [% TS] | 1.21 ± 0.11 ab | 1.35 ± 0.15 b | 1.00 ± 0.12 a | 0.83 ± 0.10 a | 1.15 ± 0.14 ab | 1.32 ± 0.15 b |
| C/N | - | 6.7 ± 0.3 a | 6.3 ± 0.3 a | 10.0 ± 0.5 b | 11.4 ± 0.6 c | 7.5 ± 0.4 ab | 6.3 ± 0.3 a |
| VS | [%] | 77.7 ± 1.5 ab | 79.0 ± 1.7 b | 82.2 ± 2.0 c | 83.5 ± 2.1 c | 77.3 ± 1.8 ab | 75.0 ± 1.6 a |
| Protein | [mg/g TS] | 406 ± 31 b | 438 ± 38 b | 313 ± 37 a | 281 ± 40 a | 375 ± 32 ab | 438 ± 44 b |
| Lipids | [mg/g TS] | 91 ± 9 a | 105 ± 11 ab | 122 ± 12 b | 126 ± 13 b | 95 ± 10 a | 83 ± 9 a |
| Sugars | [mg/g TS] | 504 ± 33 ab | 462 ± 36 a | 567 ± 39 c | 599 ± 42 c | 530 ± 36 bc | 477 ± 32 ab |
| Parameter | Unit | Month | |||||
|---|---|---|---|---|---|---|---|
| May | June | July | August | September | October | ||
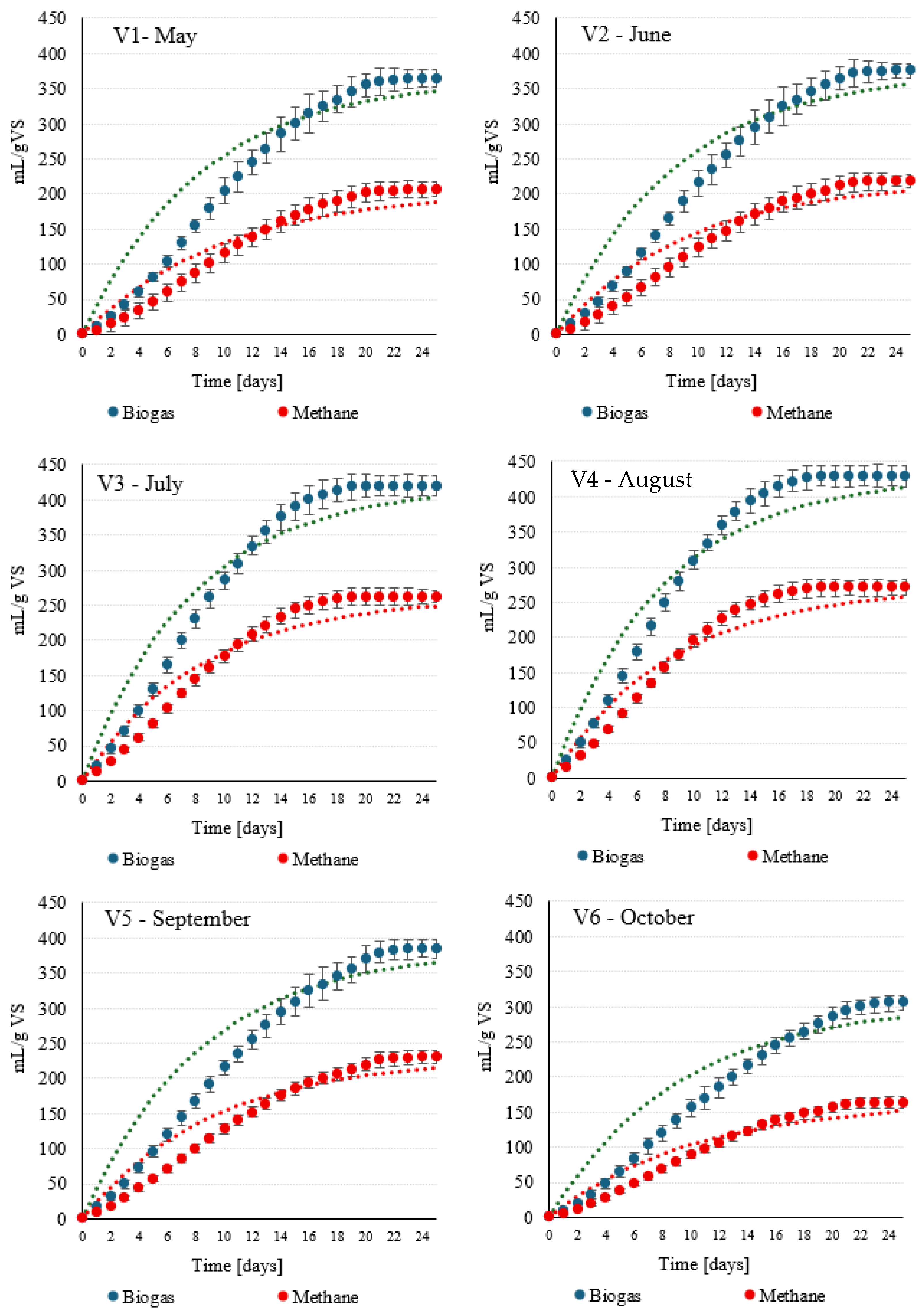
| Biogas yield | mL/g VS | 365 ± 12 a | 378 ± 12 a | 421 ± 17 b | 430 ± 15 b | 386 ± 12 a | 355 ± 13 a |
| Biogas production rate | mL/g VS·day | 47.5 ± 2.0 ab | 45.0 ± 2.3 a | 54.6 ± 2.5 c | 55.9 ± 2.7 c | 46.2 ± 1.6 ab | 33.6 ± 1.8 d |
| Biogas production rate constant | 1/day | 0.13 ± 0.01 a | 0.12 ± 0.01 b | 0.13 ± 0.02 a | 0.13 ± 0.02 a | 0.12 ± 0.01 b | 0.11 ± 0.01 c |
| CH4 content | % | 56.5 ± 1.7 a | 58.1 ± 1.2 ab | 62.4 ± 1.9 c | 63.0 ± 2.1 c | 59.3 ± 1.2 b | 56.8 ± 1.7 a |
| CH4 yield | mL/g VS | 206 ± 10 a | 218 ± 10 a | 262 ± 11 b | 270 ± 13 b | 229 ± 9 a | 201 ± 8 a |
| Biogas production rate | mL/g VS·day | 20.6 ± 1.1 a | 23.9 ± 1.3 ab | 31.4 ± 1.9 c | 32.5 ± 1.6 c | 25.3 ± 1.3 b | 18.0 ± 1.0 d |
| Biogas production rate constant | 1/day | 0.10 ± 0.01 a | 0.11 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.01 a |
| Month | Biogas/CH4 | Gompertz A (L/kg VS) | Rm (L/kg VS·d) | λ (Days) | R2 | RMSE (L/kg VS) |
|---|---|---|---|---|---|---|
| May | Biogas | 365 | 32 | 1 | 0.996 | 6.48 |
| CH4 | 206 | 18 | 1 | 0.999 | 1.57 | |
| June | Biogas | 365 | 35 | 1 | 0.995 | 7.71 |
| CH4 | 206 | 19 | 1 | 0.998 | 2.44 | |
| July | Biogas | 420 | 40 | 1 | 0.991 | 10.16 |
| CH4 | 262 | 24 | 1 | 0.993 | 5.18 | |
| August | Biogas | 430 | 42 | 1 | 0.998 | 4.37 |
| CH4 | 270 | 25 | 1 | 0.995 | 4.21 | |
| September | Biogas | 383 | 38 | 1 | 0.999 | 1.65 |
| CH4 | 228 | 20 | 1 | 0.992 | 5.47 | |
| October | Biogas | 305 | 28 | 1 | 0.997 | 5.97 |
| CH4 | 164 | 15 | 1 | 0.998 | 2.95 |
| Taxon | Biogas/CH4 Content | Methane Content (%) | T (°C) | OLR (g VS/L·d) | HRT (Days) | Reference |
|---|---|---|---|---|---|---|
| Durvillea antarctica | 179.3 ± 80.2 mL CH4/g TS·d | – | 37 | 3 | 1 | [67] |
| Macrocystis pyrifera + Durvillea antarctica | 164.2 ± 54.9 mL CH4/g TS·d | – | 37 | 3 | 1 | |
| Macrocystis pyrifera | 181.4 ± 52.3 mL CH4/g TS·d | – | 37 | 3 | 1 | |
| Chlorella vulgaris | 240 mL CH4/g VS | – | 35 | 1.0 | 28 | [68] |
| 150 mL CH4/g VS | – | 35 | 1.0 | 16 | ||
| Spirulina maxima | 240 mL CH4/g VS | – | 35 | 1.0 | 33 | [69] |
| Arthrospira platensis | 481 ± 13.8 mL CH4/g VS | 61 | 38 | – | – | [70] |
| Euglena gracilis | 485 ± 3 mL biogas/g VS | 67 | 38 | – | – | |
| Chlorella kessleri | 335 ± 7.8 mL biogas/g VS | 65 | 38 | – | – | |
| Dunaliella salina | 505 ± 24.8 mL biogas/g VS | 64 | 38 | – | – | |
| Chlamydomonas reinhardtii | 587 ± 8.8 mL biogas/g VS | 66 | 38 | – | – | |
| Phaeodactylum tricornutum | 800 ± 30 mL/L·d | 78.6 ± 5.0 | 33 ± 2 | 1.9 | 1.9 | [71] |
| 800 ± 30 mL/L·d | 75.1 ± 8.9 | 33 ± 2 | 1.9 | 1.9 | ||
| Scenedesmus obliquus | 287 ± 10.1 mL biogas/g VS | 62 | 38 | – | – | [70] |
| 600 ± 20 mL biogas/L·d | 77.1 ± 3.9 | 33 ± 2 | 2.8 | 2.2 | [71] | |
| 400 ± 0 mL biogas/L·d | 74.3 ± 2.5 | 33 ± 2 | 2.8 | 2.2 | ||
| Scenedesmus sp. + Chlorella sp. | 818 ± 96 mL biogas/L·d | – | 35 | 6.0 | 10 | [72] |
| 573 ± 28 cm3 mL biogas/L·d | – | 35 | 4.0 | 10 | ||
| 180 ± 8 mL biogas/L·d | – | 35 | 2.0 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Zieliński, M. Seasonal Dynamics of Microalgal Biomass and Its Biomethanation Potential: A Case Study from the Bay of Gdansk, Poland. J. Mar. Sci. Eng. 2025, 13, 1880. https://doi.org/10.3390/jmse13101880
Dębowski M, Kisielewska M, Kazimierowicz J, Zieliński M. Seasonal Dynamics of Microalgal Biomass and Its Biomethanation Potential: A Case Study from the Bay of Gdansk, Poland. Journal of Marine Science and Engineering. 2025; 13(10):1880. https://doi.org/10.3390/jmse13101880
Chicago/Turabian StyleDębowski, Marcin, Marta Kisielewska, Joanna Kazimierowicz, and Marcin Zieliński. 2025. "Seasonal Dynamics of Microalgal Biomass and Its Biomethanation Potential: A Case Study from the Bay of Gdansk, Poland" Journal of Marine Science and Engineering 13, no. 10: 1880. https://doi.org/10.3390/jmse13101880
APA StyleDębowski, M., Kisielewska, M., Kazimierowicz, J., & Zieliński, M. (2025). Seasonal Dynamics of Microalgal Biomass and Its Biomethanation Potential: A Case Study from the Bay of Gdansk, Poland. Journal of Marine Science and Engineering, 13(10), 1880. https://doi.org/10.3390/jmse13101880









