Verification of Interaction Between Cl− Erosion and Carbonation in Marine Concrete
Abstract
1. Introduction
2. Experimental Overview
2.1. Experimental Materials
2.1.1. Cement
2.1.2. Aggregates
2.1.3. Water-Reducing Admixture
2.1.4. NaCl Solution
2.1.5. Concrete Mix Proportion
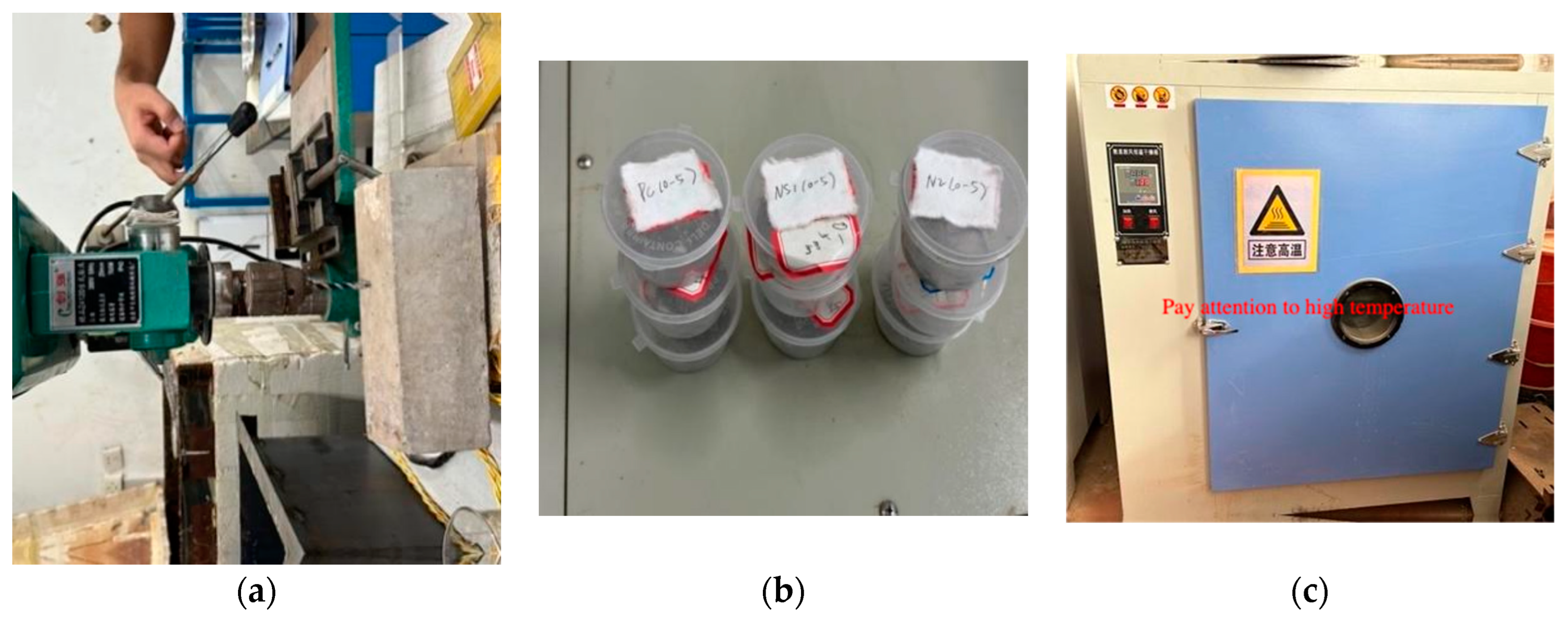
2.2. Experimental Program
2.2.1. Validation Test for the Interaction of Chloride Erosion and Carbonation Effect of Chloride Erosion on Carbonation
Effect of Chloride Erosion on Carbonation
Effect of Carbonation on Cl− Erosion
2.2.2. Experimental Testing IndicatorsFree Chloride Ion Content
Free Chloride Ion Content
- (1)
- Place 10 g of precisely measured dry material into a conical flask, add 100 milliliters of distilled water, and shake vigorously for 1 to 2 min. Allow the mixture to soak for 24 h to ensure complete dissolution of the material.
- (2)
- Filter the soaked powder using filter paper, then transfer 10 mL (V2) of the filtrate into a clean conical flask and add one drop of phenolphthalein, resulting in a pale pink color.
- (3)
- Add dilute sulfuric acid solution until the solution is colorless.
- (4)
- Add an appropriate amount of potassium chromate solution to the conical flask, resulting in a yellow color.
- (5)
- Quickly titrate with AgNO3 solution (V3) using a burette until the solution turns brick red. After completing the experiment, calculate the concentration of free Cl− using Formula (1).
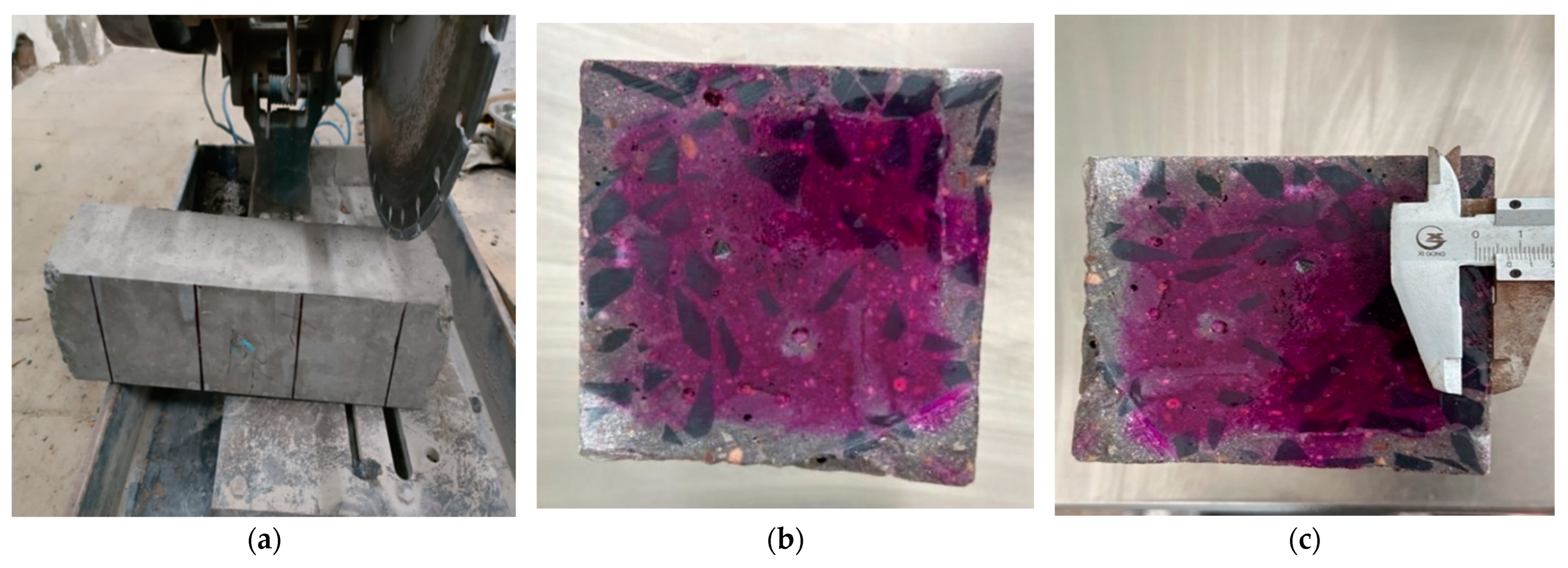
Carbonation Depth Measurement
3. Experimental Results and Analysis
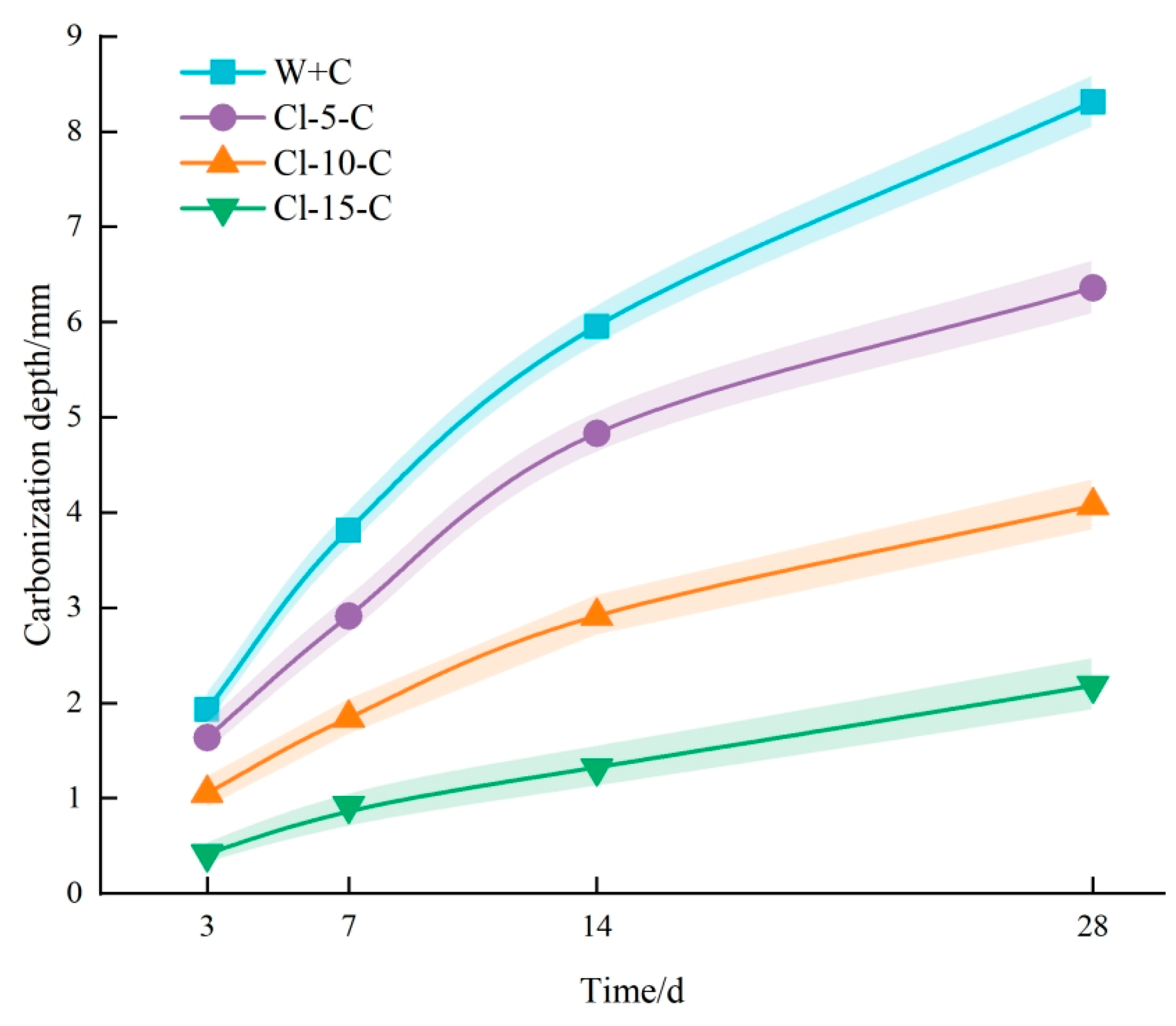
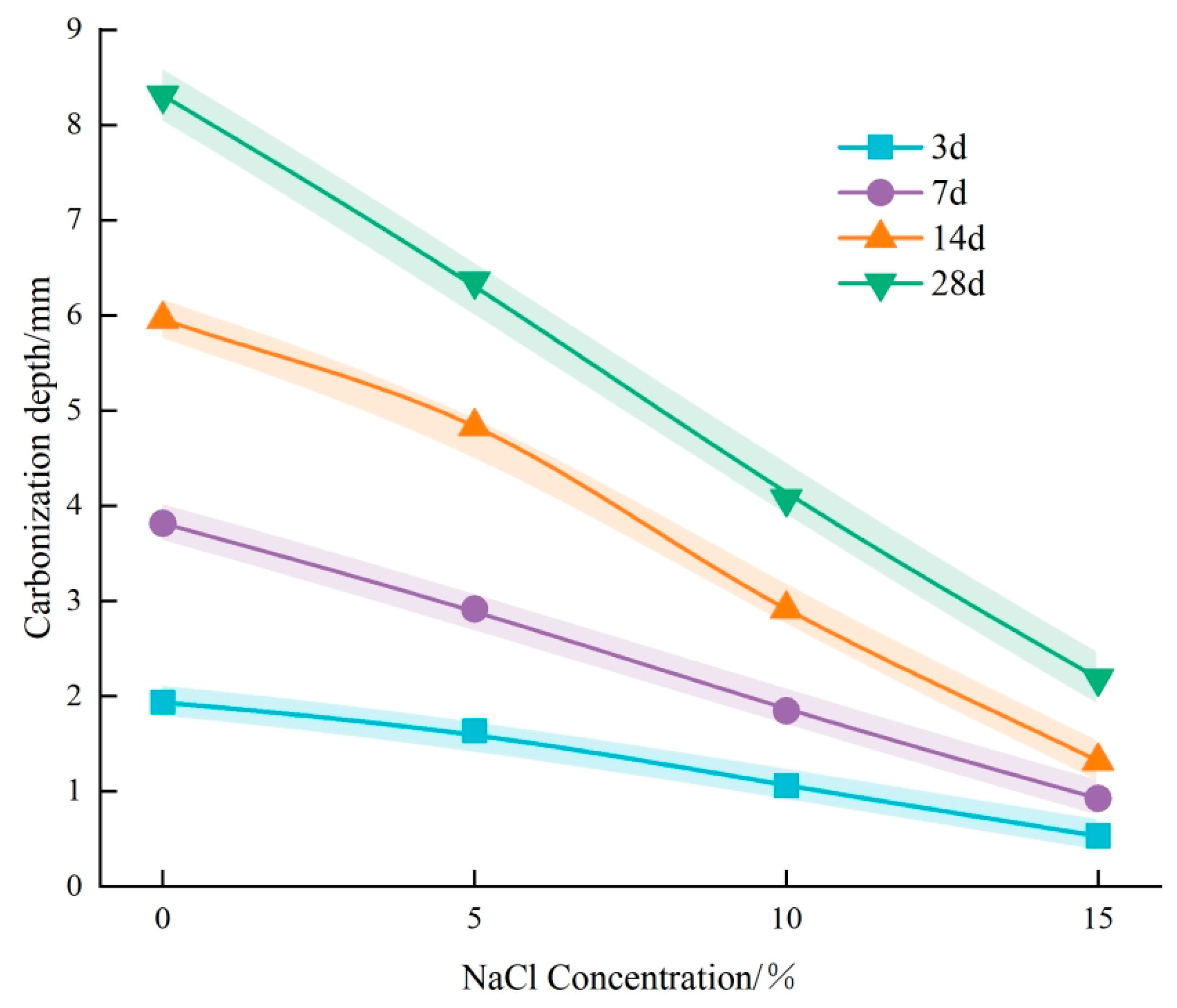
3.1. Effect of Cl− Erosion on Carbonation
- (1)
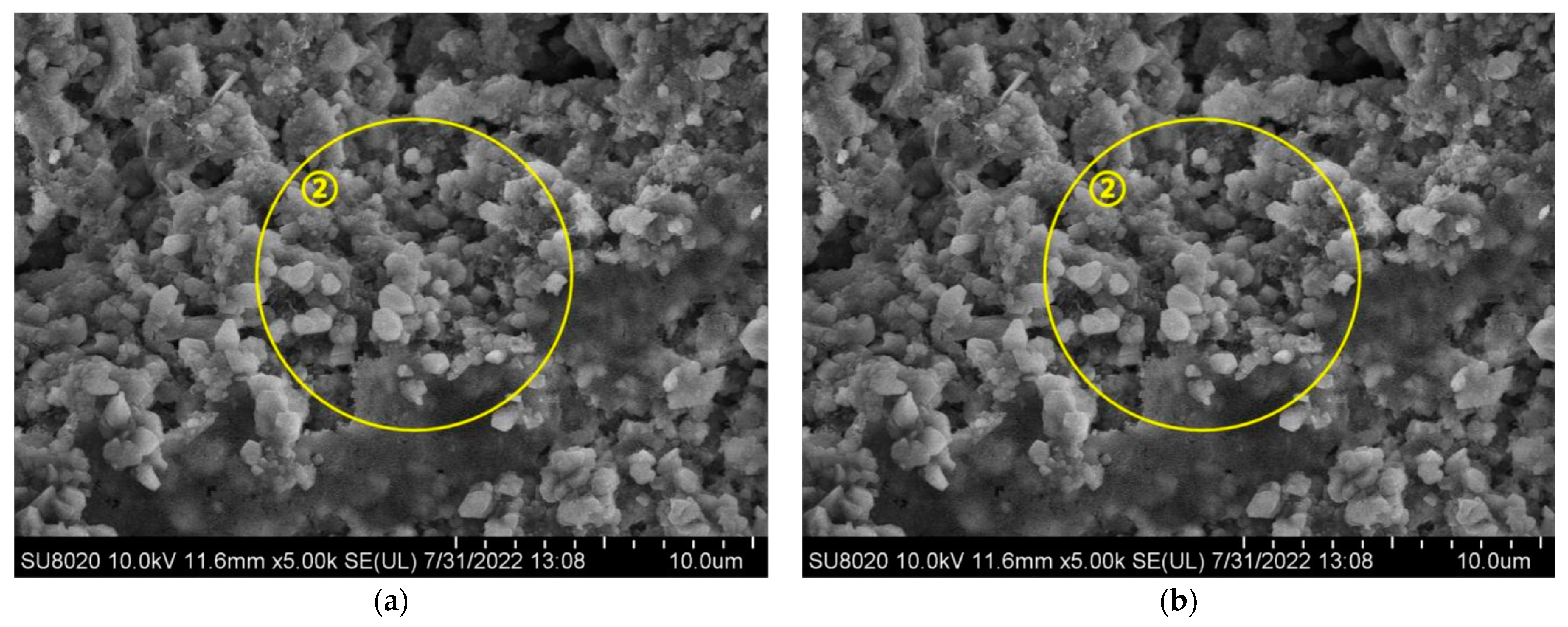
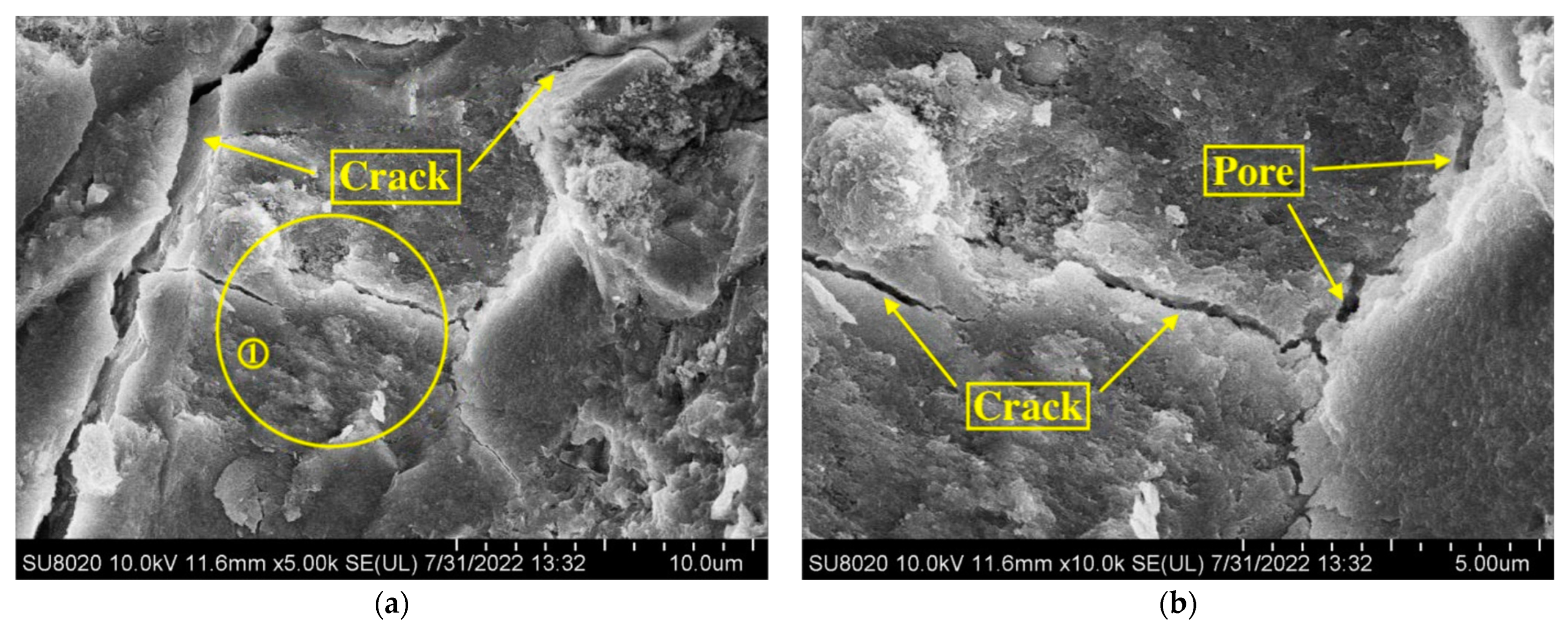
- Physical Mechanism: During the experiment, concrete specimens were initially soaked in a NaCl solution, subsequently dried in an oven, and finally subjected to carbonation treatment. Following immersion in NaCl solution, the concrete stayed dry for a prolonged period, causing surface moisture to evaporate and chloride salts to crystallize and remain within the concrete’s pores. As drying continued, the accumulation of chloride salt crystals gradually filled the internal pores of the concrete [34]. In practical engineering environments, this physical mechanism predominantly occurs in coastal tidal zones and splash zones. Concrete structures in these areas frequently undergo cycles of wetting and drying, with chloride salts infiltrating the concrete from seawater. As moisture evaporates during the drying phase, chloride salt crystals remain in the pores, resulting in gradual accumulation over multiple cycles in the surface pores. This accumulation effectively fills the concrete’s pores and reduces pore connectivity, thereby hindering further CO2 intrusion [35]. This phenomenon is one of the primary reasons for the less pronounced carbonation effects observed in concrete structures situated in coastal tidal and splash zones [36,37].
- (2)
- Chemical Mechanism: Upon entering the concrete, Cl− ions can follow three main pathways [38]. Initially, Cl− chemically interacts with cement hydration products to produce Friedel’s salt, a process known as the chemical binding of Cl−. Second, Cl− can be physically adsorbed onto the surface of calcium silicate hydrate (C-S-H) gel. Third, Cl− can exist freely within the concrete’s pore solution, referred to as free Cl− [39]. The presence of Friedel’s salt in the pores refines the pore structure by decreasing the number and size of interconnected pores, thereby improving the concrete’s compactness [40]. The densification of the structure obstructs CO2 ingress and diffusion, thereby slowing down the carbonation reaction [41].
3.2. The Effect of Carbonation on Chloride Ingress
4. Conclusions
- (1)
- After Cl− erosion, the carbonation depth of concrete notably reduced across all ages. Additionally, the reduction in carbonation depth became more pronounced with increasing concentrations of the NaCl solution. Microscopic examinations revealed the formation of crystalline substances within the concrete, which filled the pores and impeded further CO2 ingress. This indicates that Cl− erosion acts to inhibit carbonation.
- (2)
- The inhibition of carbonation by Cl− erosion can be attributed to the accumulation of NaCl crystals within the concrete pores as surface water evaporates after immersion in the NaCl solution. Furthermore, the expansive Friedel’s salt generated during Cl− erosion contributes to pore filling, resulting in a denser internal structure that restricts CO2 penetration.
- (3)
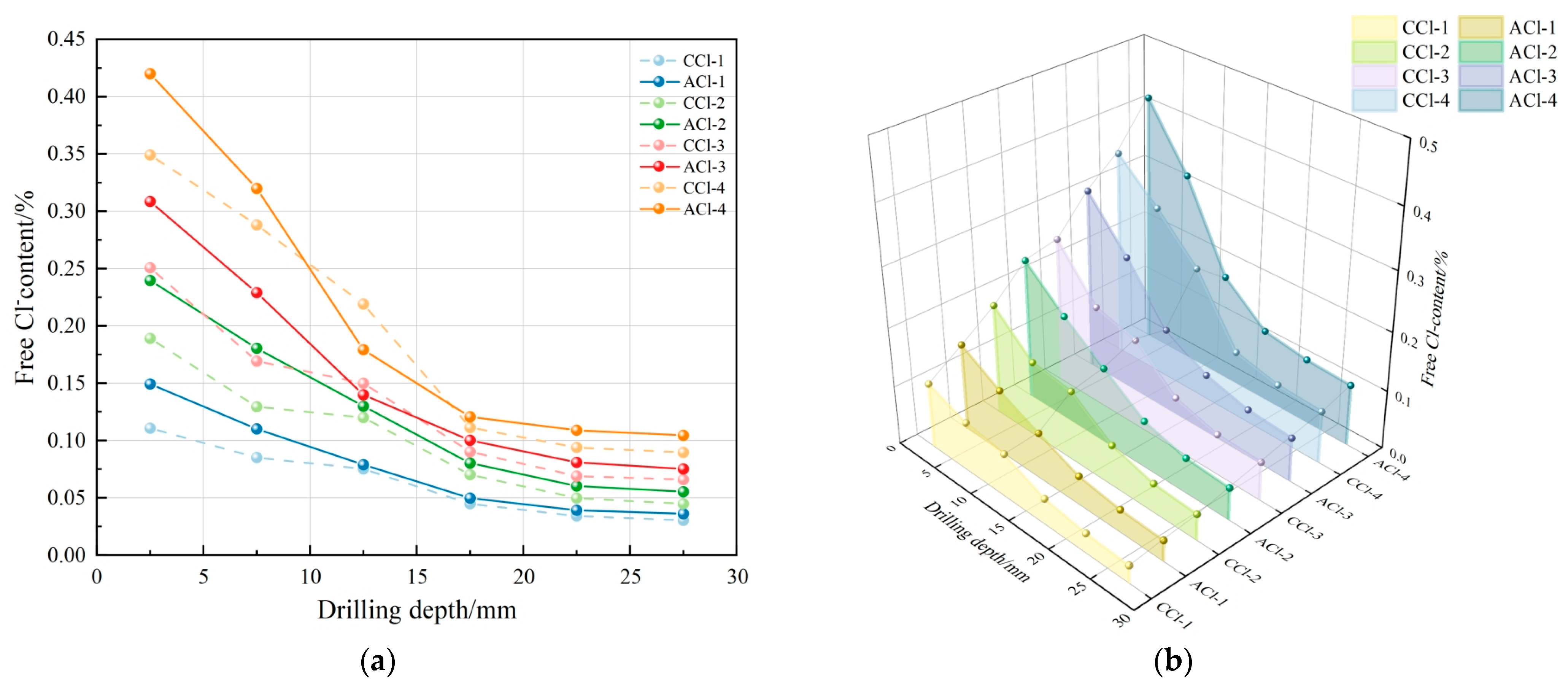
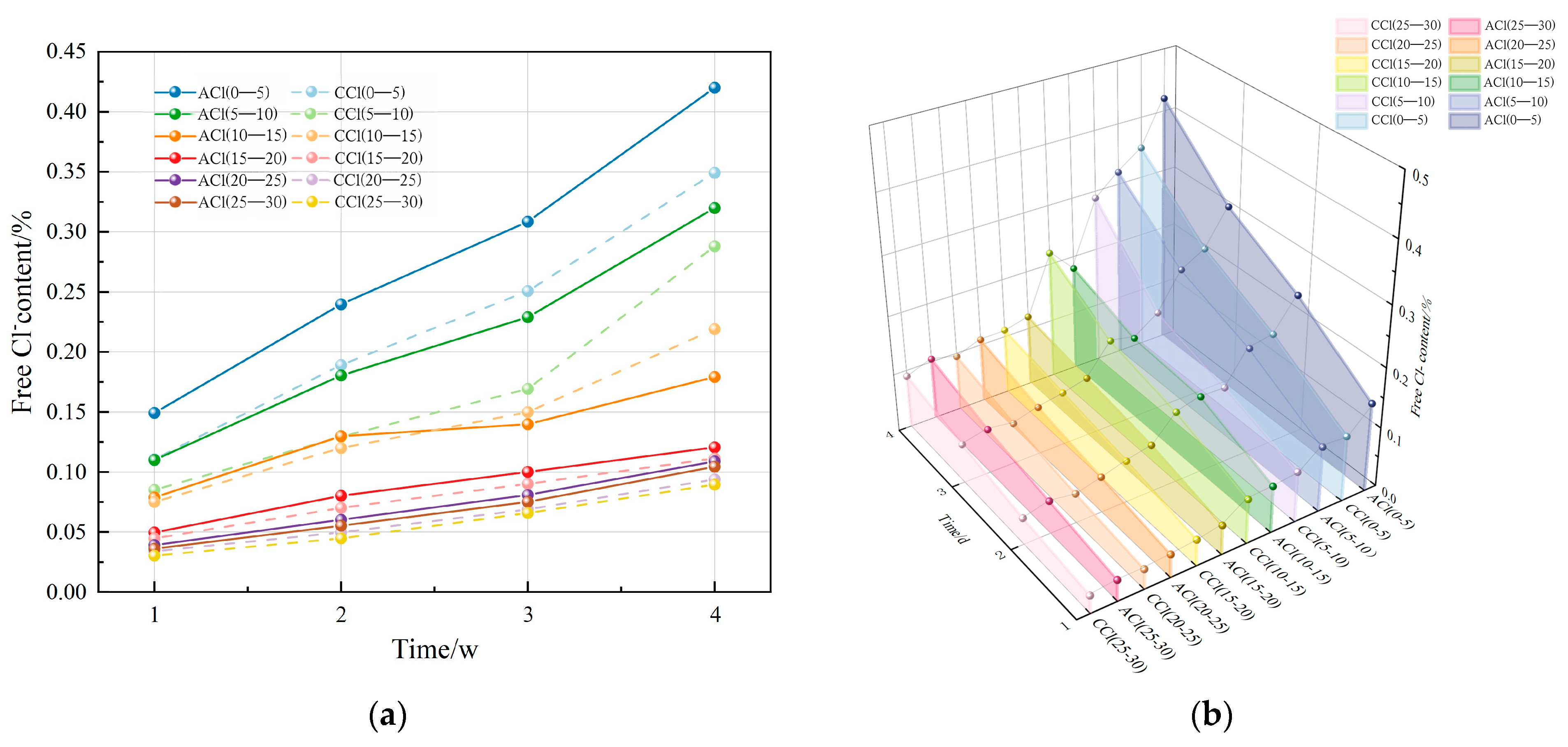
- After carbonation, the free chloride content in the concrete exhibited a decreasing trend across all ages, with levels generally diminishing as drilling depth increased. In the fully carbonated zone (0–10 mm drilling depth), the CCl group exhibited a lower free chloride content compared to the ACl group. In the transition zone between carbonated and uncarbonated areas (10–15 mm drilling depth), the CCl group exhibited higher free chloride content compared to the ACl group. In the uncarbonated zone, Cl− diffusion was unaffected by carbonation, and the free chloride content between the two groups showed minimal variation.
- (4)
- Carbonation exerts both positive and negative effects on Cl− erosion. The inhibitory effect primarily occurs in the fully carbonated zone, where carbonation products fill the concrete’s internal pores, obstructing Cl− diffusion. In contrast, the promoting effect is observed at the interface between the carbonated and uncarbonated zones, where carbonation consumes Ca(OH)2, reducing the formation of Friedel’s salt and thus weakening Cl− binding capacity. This causes a rise in free chloride content at the interface, resulting in localized Cl− concentration build-up and further promoting Cl− diffusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, Y.; Yang, L.; Gao, D.; Kang, K.; Cao, Z.; Zhao, P. Properties Evolution and Deterioration Mechanism of Steel Fiber Reinforced Concrete (SFRC) under the Coupling Effect of Carbonation and Chloride Attack. J. Build. Eng. 2024, 95, 110275. [Google Scholar] [CrossRef]
- Qu, H.; Feng, M.; Li, M.; Tian, D.; Zhang, Y.; Chen, X.; Li, G. Enhancing the Carbonation and Chloride Resistance of Concrete by Nano-Modified Eco-Friendly Water-Based Organic Coatings. Mater. Today Commun. 2023, 37, 107284. [Google Scholar] [CrossRef]
- Ortolan, T.L.P.; Borges, P.M.; Silvestro, L.; da Silva, S.R.; Possan, E.; de Oliveira Andrade, J.J. Durability of Concrete Incorporating Recycled Coarse Aggregates: Carbonation and Service Life Prediction under Chloride-Induced Corrosion. Constr. Build. Mater. 2023, 404, 133267. [Google Scholar] [CrossRef]
- Li, T.; Yang, W.; Zhan, M.; Chen, X.; Zhang, L.; Wang, C.; Yang, Y.; Lyv, H. Study on the Microscopic Transmission Characteristics and Coupled Erosion Depth of Tailings Recycled Concrete under the Coupling Effect of Carbonization and Salt-Fog. J. Build. Eng. 2024, 95, 110051. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Mi, T.; Ding, X.; Tang, L.; Xing, F. Durability Study of Seawater and Sea-Sand Concrete under the Combined Effects of Carbonation and Chloride Redistribution. J. Build. Eng. 2024, 89, 109294. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, M.; Hang, M.; Yang, Y.; Zhou, X.; Liu, X.; Xu, G. Inhibition Resistance and Mechanism of Migrating Corrosion Inhibitor on Reinforced Concrete under Coupled Carbonation and Chloride Attack. J. Build. Eng. 2023, 76, 107398. [Google Scholar] [CrossRef]
- Geng, Y.J.; Sun, C.T.; Sun, M.; Zhang, Y.G.; Duan, J.Z. Review of binding mechanisms and influencing factors of chloride ions in cement-based materials. Bull. Chin. Ceram. Soc. 2022, 41, 2604–2617. [Google Scholar]
- Ihekwaba, N.M.; Hope, B.B.; Hansson, C.M. Carbonation and electrochemical chloride extraction from concrete. Cem. Concr. Res. 1996, 26, 1095–1107. [Google Scholar] [CrossRef]
- Tumidajski, P.J.; Chan, G.W. Effect of sulfate and carbon dioxide on chloride diffusivity. Cem. Concr. Res. 1996, 26, 551–556. [Google Scholar] [CrossRef]
- Puatatsananon, W.; Saouma, V. Nonlinear coupling of carbonation and chloride diffusion in concrete. J. Mater. Civ. Eng. 2005, 17, 264–275. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zheng, J.Q.; Zhang, M. Influence of carbonation degree on chloride ion diffusion coefficient in concrete. J. Tongji Univ. (Nat. Sci. Ed.) 2010, 38, 412–416. [Google Scholar]
- Chindaprasirt, P.; Rukzon, S.; Sirivivatnanon, V. Effect of carbon dioxide on chloride penetration and chloride ion diffusion coefficient of blended Portland cement mortar. Constr. Build. Mater. 2008, 22, 1701–1707. [Google Scholar] [CrossRef]
- Goñi, S.; Guerrero, A. Accelerated carbonation of Friedel’s salt in calcium aluminate cement paste. Cem. Concr. Res. 2003, 33, 21–26. [Google Scholar] [CrossRef]
- Hassnan, Z. Binding of External Chloride by Cement Pastes. Master’s Thesis, Department of Building Materials, University of Toronto, Toronto, ON, Canada, 2001. [Google Scholar]
- Yuan, C.F.; Niu, D.T.; Qi, G.Z. Experimental study on chloride ion erosion of carbonated concrete under wet-dry cycle mechanism. J. Xi’an Univ. Archit. Technol. Nat. Sci. Ed. 2012, 44, 339–344. [Google Scholar]
- Dang, V.Q.; Ogawa, Y.; Bui, P.T.; Kawai, K. Effects of chloride ions on the durability and mechanical properties of sea sand concrete incorporating supplementary cementitious materials under accelerated carbonation conditions. Constr. Build. Mater. 2021, 274, 122016. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar]
- Suryavanshi, A.K.; Narayan Swamy, R. Stability of Friedel’s salt in carbonated concrete structural elements. Cem. Concr. Res. 1996, 26, 729–741. [Google Scholar] [CrossRef]
- Oh, B.-H.; Lee, S.-K.; Lee, M.-K.; Jung, S.-H. Influence of carbonation on chloride diffusion in concrete. J. Korea Concr. Inst. 2003, 15, 829–839. [Google Scholar] [CrossRef]
- Niu, D.T.; Sun, C.T. Study on the combined effects of concrete carbonation and chloride ion erosion. Bull. Chin. Ceram. Soc. 2013, 41, 1094–1099. [Google Scholar]
- Jin, Z.Q.; Sun, W.; Li, Q.Y. Influence of carbonation on chloride ion diffusion in concrete. J. Beijing Univ. Sci. Technol. 2008, 8, 921–925. [Google Scholar]
- Sun, C.T. Research on Concrete Durability and Life Prediction Based on Chloride Ion Erosion. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2011. [Google Scholar]
- Wan, X.M. Research on the Deterioration Mechanism of Concrete Structures Under Combined Mechanical Loads and Environmental Factors. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2011. [Google Scholar]
- Zhang, P.; Zhao, T.J.; Guo, P.G.; Wittmann, F.H. Effects of freeze-thaw and carbonation on chloride ion erosion of concrete. J. Southeast Univ. Nat. Sci. Ed. 2006, S2, 238–242. [Google Scholar]
- Yuan, C.F.; Niu, D.T. Durability research of fly ash concrete in marine atmospheric environment. Bull. Chin. Ceram. Soc. 2012, 31, 1–6. [Google Scholar]
- Liu, J.-Z.; Ba, M.-F.; Du, Y.-G.; He, Z.-M.; Chen, J.-B. Effects of chloride ions on the carbonation rate of hardened cement paste by X-ray CT techniques. Constr. Build. Mater. 2016, 122, 619–627. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Q.; Chen, X.; Xing, F.; Han, N.; He, Y.; Ma, Y. Understanding the interacted mechanism between carbonation and chloride aerosol attack in ordinary Portland cement concrete. Cem. Concr. Res. 2017, 95, 217–225. [Google Scholar] [CrossRef]
- GB 175-2007; General Portland Cement. Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- GB/T 14685-2011; Standard for Pebble and Crushed Stone. Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- GB/T 14684-2022; Standard for Construction Sand. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 50476-2008; Code for Durability Design of Concrete Structures. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- SL 352-2018; Specifications for Hydraulic Concrete Testing. Ministry of Water Resources of the People’s Republic of China: Beijing, China, 2018.
- GB/T 50476-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Li, P.; Li, C.; Li, D.; Chen, R.; Chen, J. Modelling Chloride Diffusion in Concrete with Carbonated Surface Layer. Mag. Concr. Res. 2024, 76, 1048–1058. [Google Scholar] [CrossRef]
- Jiang, Z.-L.; Gu, X.-L.; Huang, Q.-H.; Zhang, W.-P. Statistical analysis of concrete carbonation depths considering different coarse aggregate shapes. Constr. Build. Mater. 2019, 229, 116856. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Fang, Y.; Song, L.; Xu, N.; Kang, Z. Relationship between the pore structure of concrete and chloride ion diffusion properties. Bull. Chin. Ceram. Soc. 2022, 41, 2716–2727. [Google Scholar]
- Hu, D.G.; Wang, X.H.; Fang, C.Q. Equivalent diffusion coefficient model of chloride ions in cracked concrete structures. Concrete 2022, 393, 24–28. [Google Scholar]
- Xu, F.Y.; Liang, Y.Z.; Dai, M.; Lin, L.; Liu, Q.; Dai, L. Research on the effects of chloride ions in seawater on the durability of concrete. Mod. Salt Chem. Ind. 2022, 49, 56–58. [Google Scholar]
- Wang, D.Y.; Dong, H.J.; Pang, B.; Chen, J.; Dai, Y. Analysis of the diffusion process of chloride ions in unsaturated flow of concrete beams. MECH-Q 2022, 43, 863–875. [Google Scholar]
- Gao, G.Y.; Zhang, C.H.; Ma, F.H. Experimental study on the influencing factors of concrete anti-carbonation performance. Concrete 2020, 368, 30–32. [Google Scholar]
- Wang, Y.F. Research on the influence of curing age on the anti-carbonation performance of fly ash concrete. Railw. Constr. Technol. 2022, 8, 10–14. [Google Scholar]
- Liu, B.; Chen, D.; Lü, Z.; Rong, H.; Liu, D. Durability Performance of Low-Calcium High-Strength Clinker Cement Concrete under the Combined Action of Chloride Ions and Carbonation. J. Anhui Univ. Technol. (Nat. Sci. Ed.) 2024, 1–7. Available online: http://kns.cnki.net/kcms/detail/34.1254.N.20240726.1505.004.html (accessed on 17 November 2024).
- Li, Y. Study on the Deterioration Mechanism of Chloride Salt-Containing Reinforced Concrete Under the Coupled Action of Carbonation and Chloride Ions. Ph.D. Thesis, Institute of Engineering Mechanics, China Earthquake Administration, Harbin, China, 2023. [Google Scholar] [CrossRef]
- Mei, K.; He, Z.; Yi, B.; Lin, X.; Wang, J.; Wang, H.; Liu, J. Study on Electrochemical Characteristics of Reinforced Concrete Corrosion under the Action of Carbonation and Chloride. Case Stud. Constr. Mater. 2022, 17, e01351. [Google Scholar] [CrossRef]
- Al-Ameeri, A.S.; Rafiq, M.I.; Tsioulou, O. Combined Impact of Carbonation and Crack Width on the Chloride Penetration and Corrosion Resistance of Concrete Structures. Cem. Concr. Compos. 2021, 115, 103819. [Google Scholar] [CrossRef]
- Sun, M.; Bao, Q.; Ding, G.J.; Zhang, P.; Sun, C. Influence of fly ash on the carbonation and chloride ion erosion characteristics of concrete. Highway 2021, 66, 295–299. [Google Scholar]



| Performance Indicator | Standard Consistency | Initial Setting Time | Final Setting Time | Stabilizing Properties | Compressive Strength (MPa) | |
|---|---|---|---|---|---|---|
| Water Demand (%) | (min) | (min) | 3 d | 28 d | ||
| Standard Requirement | ≤28 | ≥45 | ≤600 | Must pass | ≥3.5 | Standard Requirement |
| Test Results | 25.8 | 139 | 346 | Passed | 11.1 | Test Results |
| Chemical Component | SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | MgO | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| Standard Requirement | - | - | - | - | ≤3.5 | ≤5.0 | ≤3.0 |
| Actual Content | 21.08 | 61.96 | 5.12 | 3.68 | 2.46 | 1.85 | 1.57 |
| Technical Indicator | Apparent Density (kg/m3) | Bulk Density (kg/m3) | Mud Content (%) | Moisture Content (%) | Crushing Value (%) | Needle-Flake Content (%) |
|---|---|---|---|---|---|---|
| Measured Value | 2705 | 1602 | 0.38 | 0 | 8.12 | 3.1 |
| Performance Indicators | Water Reduction Rate at 20 °C | Plastic Consistency | Drying Shrinkage | Water-Binder Ratio | Applicable Temperature Range | Air Content | Effect on Setting Time |
|---|---|---|---|---|---|---|---|
| Units | % | mm | mm/m | —— | °C | % | min |
| Reference Values | ≥15% | 180–220 | ≤0.05 | ≤0.4 | −5 to 40 | ≤5 | ≤30 |
| Material | Designation | Water | Cement | Sand | Aggregate | Superplasticizer |
|---|---|---|---|---|---|---|
| Ordinary Concrete | PC | 205 | 465.9 | 576 | 1175.8 | 1.86 |
| Designation | Immersion Duration | Carbonation Periods | Number of Specimens |
|---|---|---|---|
| W + C | Water (28 days) | 3, 7, 14, 28 days | 3 |
| Cl-5 + C | 5% NaCl (28 days) | 3, 7, 14, 28 days | 3 |
| Cl-10 + C | 10% NaCl (28 days) | 3, 7, 14, 28 days | 3 |
| Cl-15 + C | 15% NaCl (28 days) | 3, 7, 14, 28 days | 3 |
| Sample ID | Exposure Conditions | Immersion Age | Number of Specimens |
|---|---|---|---|
| CCl | Carbonation (28 days) | 1, 2, 3, 4 weeks | 8 |
| ACl | Air under same conditions (28 days) | 3, 7, 14, 28 days | 3 |
| Number | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks |
|---|---|---|---|---|
| ACl | 27.63 | 10 | 5.025 | 4.847 |
| CCl | 13.85 | 7.556 | 3.462 | 2.356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Du, L.; Xu, R. Verification of Interaction Between Cl− Erosion and Carbonation in Marine Concrete. J. Mar. Sci. Eng. 2025, 13, 97. https://doi.org/10.3390/jmse13010097
Zhang M, Du L, Xu R. Verification of Interaction Between Cl− Erosion and Carbonation in Marine Concrete. Journal of Marine Science and Engineering. 2025; 13(1):97. https://doi.org/10.3390/jmse13010097
Chicago/Turabian StyleZhang, Maohua, Lin Du, and Ronghua Xu. 2025. "Verification of Interaction Between Cl− Erosion and Carbonation in Marine Concrete" Journal of Marine Science and Engineering 13, no. 1: 97. https://doi.org/10.3390/jmse13010097
APA StyleZhang, M., Du, L., & Xu, R. (2025). Verification of Interaction Between Cl− Erosion and Carbonation in Marine Concrete. Journal of Marine Science and Engineering, 13(1), 97. https://doi.org/10.3390/jmse13010097





