Environmental Factors Influencing Annual Changes in Bycatch per Unit Effort of Delphinus delphis around Their Main Hotspot in Korean Waters
Abstract
1. Introduction
2. Materials and Methods
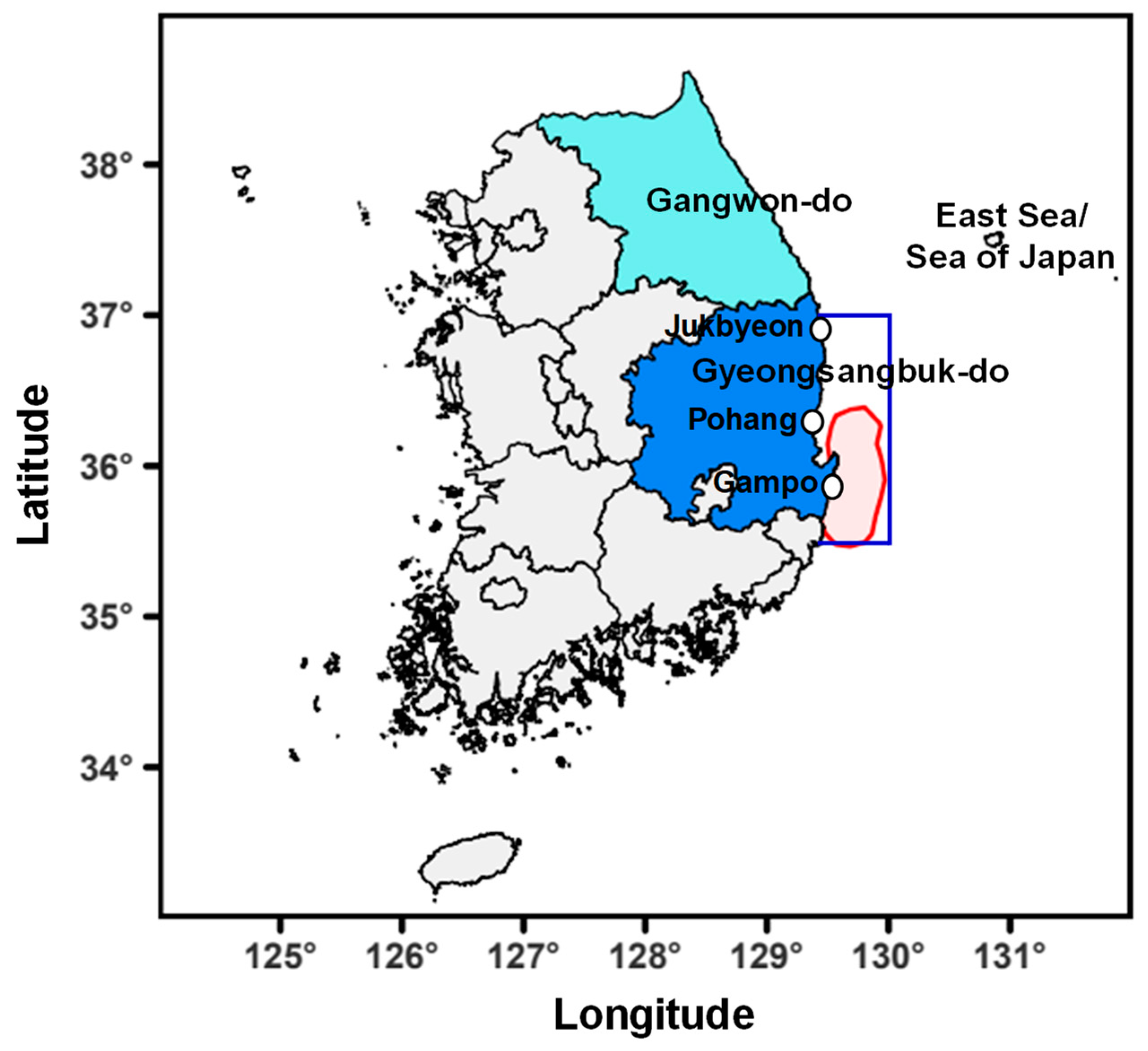
2.1. Data Collection
2.2. Data Analysis
3. Results
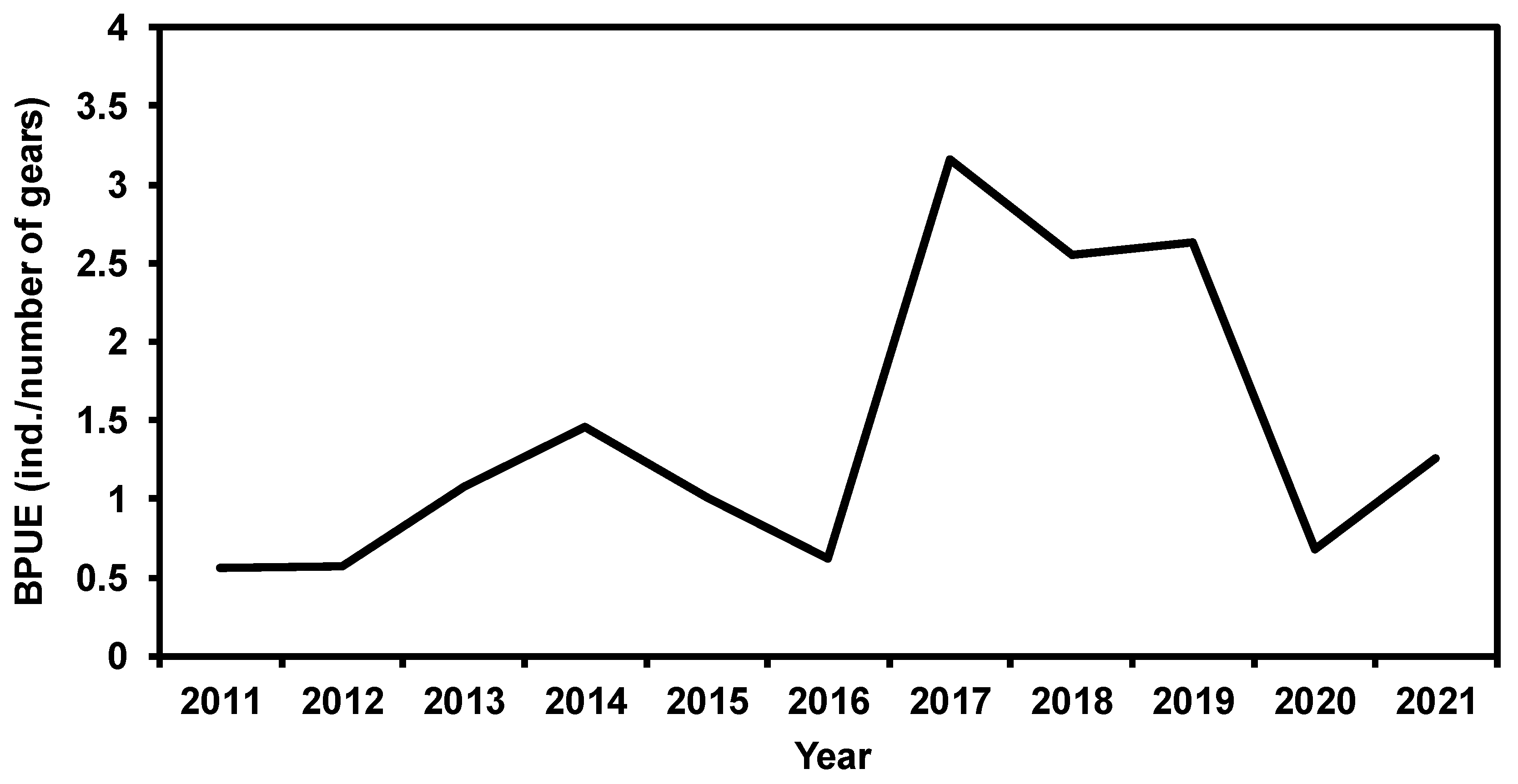
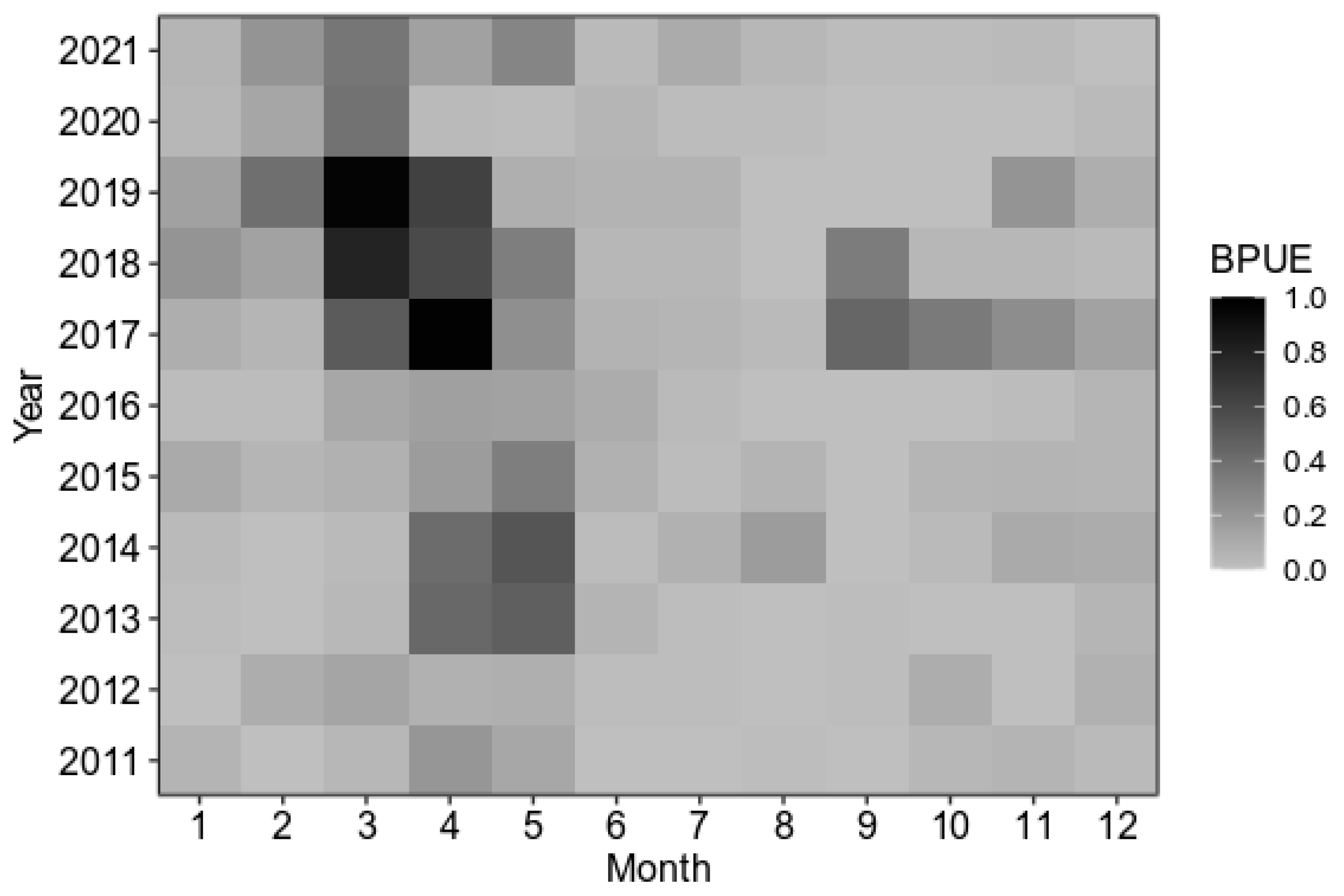
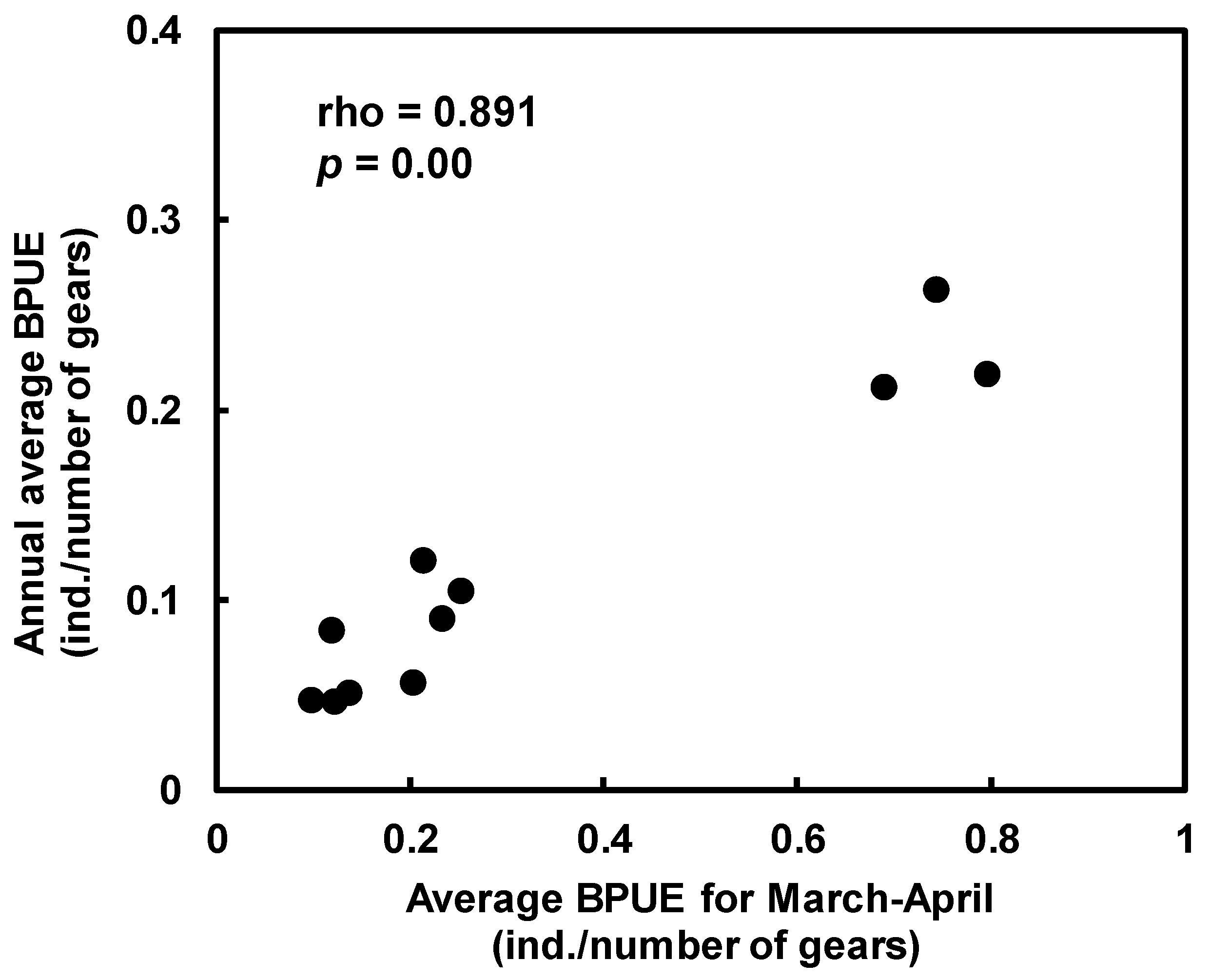
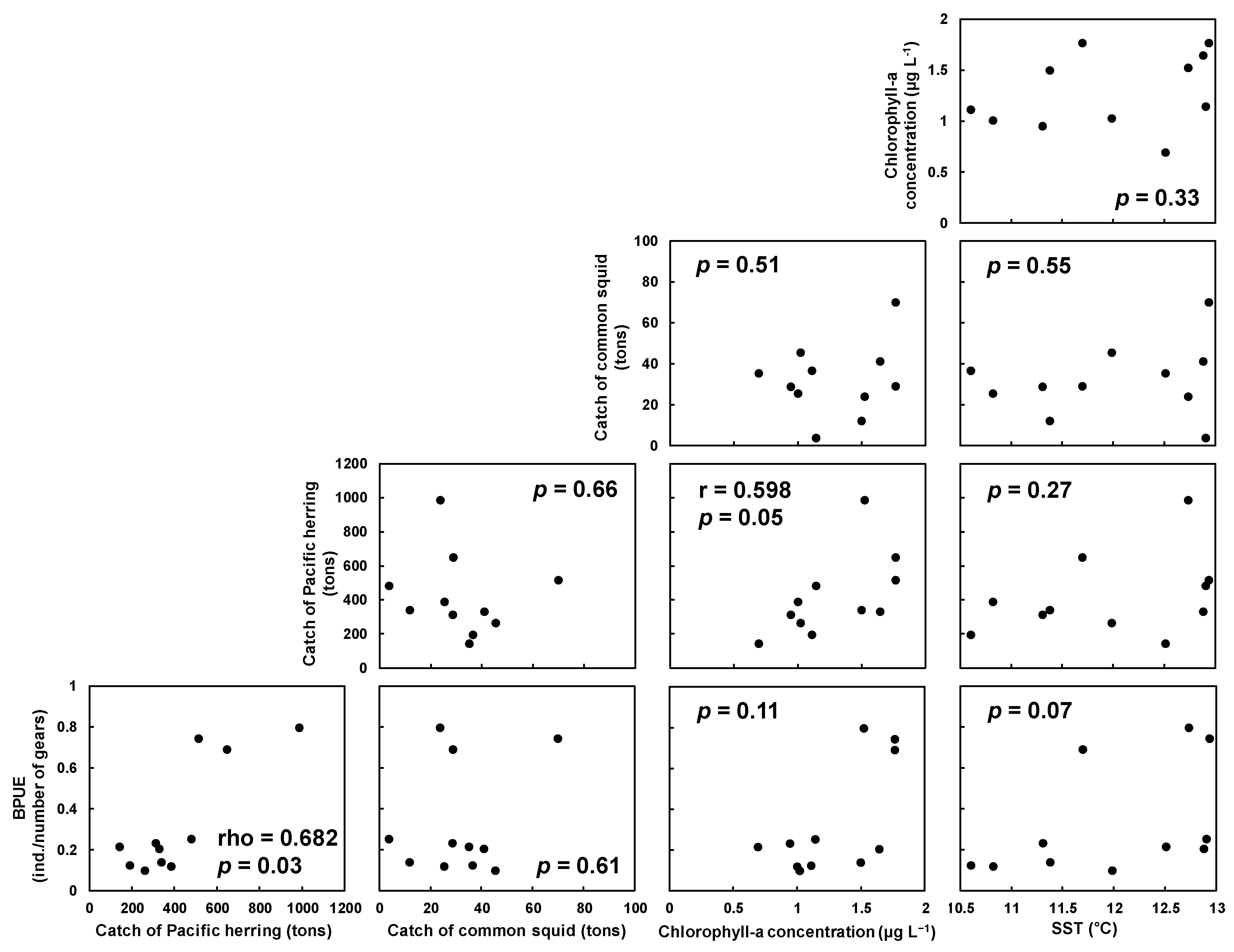
3.1. Characteristics of Temporal Changes in BPUE
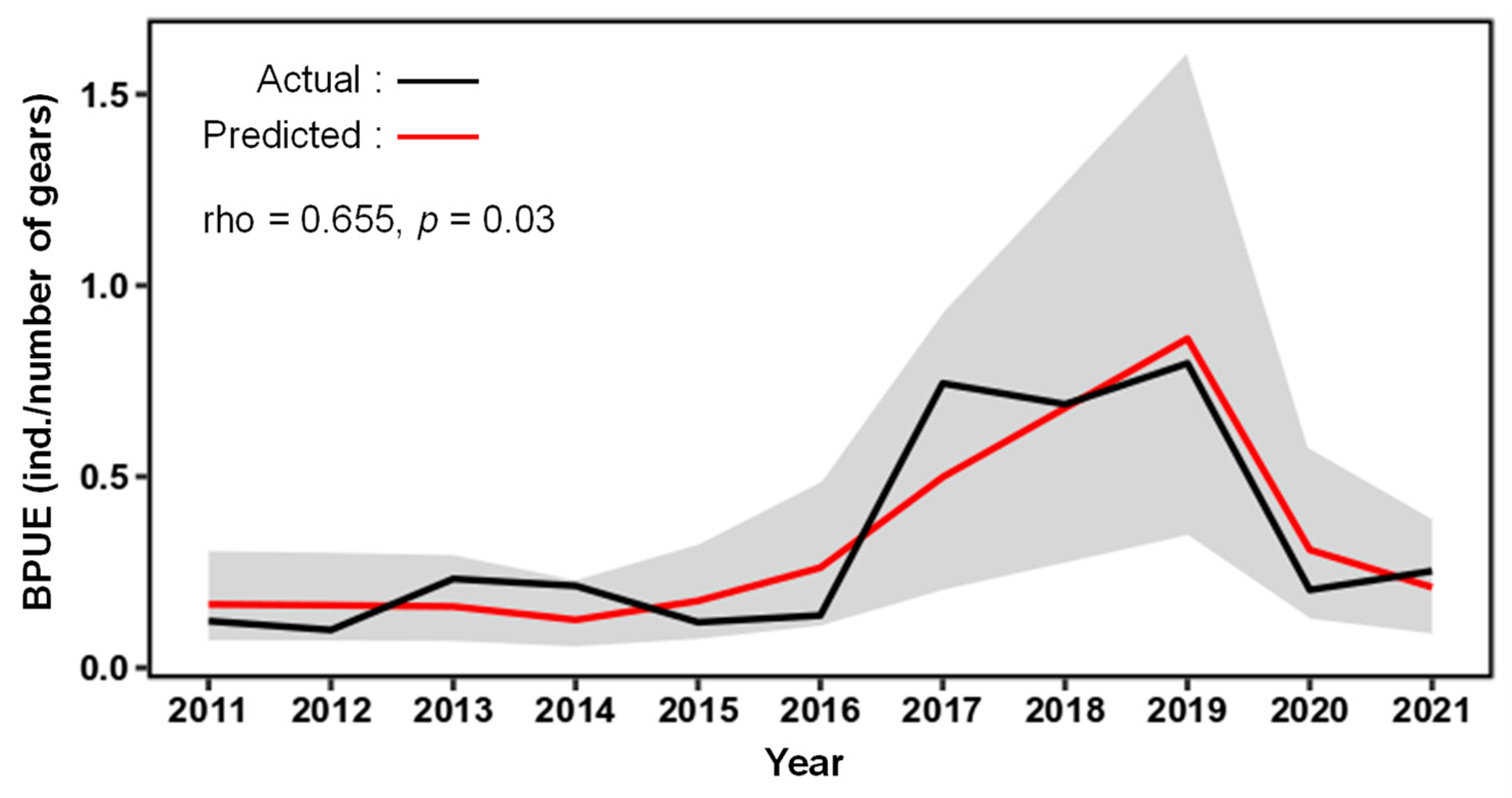
3.2. Statistical Habitat Modeling
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jefferson, T.A.; Webber, M.A.; Pitman, R.L. Marine Mammals of the World: A Comprehensive Guide to Their Identification; Academic Press: San Diego, CA, USA, 2015; pp. 268–272. [Google Scholar]
- Perrin, W.F. Common dolphins—Delphinus delphis and D. Capensis. In Encyclopedia of Marine Mammals; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 255–259. [Google Scholar]
- Ferrero, R.C.; Walker, W.A. Growth and reproduction of the common dolphin, Delphinus delphis Linnaeus, in the offshore waters of the North Pacific Ocean. Fish. Bull. 1995, 93, 483–494. [Google Scholar]
- Yoo, J.T.; Park, K.J.; Lee, K.; Lee, D. A first study on distribution characteristics of common dolphin in Korean waters: A study using data collected during the past 20 years. J. Mar. Sci. Eng. 2023, 11, 1635. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, H.W.; Kim, S.; Lee, Y.R.; Park, K.J.; Kim, D.N.; An, D.H. Diet of long-beaked common dolphin (Delphinus capensis) in the East Sea, Korea. Anim. Cells Syst. 2014, 18, 340–350. [Google Scholar] [CrossRef]
- Kim, D.N.; Sohn, H.; An, Y.R.; Park, K.J.; Kim, H.W.; Ahn, S.E.; An, D.H. Status of the cetacean bycatch near Korean waters. Korean J. Fish. Aquat. Sci. 2013, 46, 892–900. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.H.; Lee, K.; Park, K.J.; An, Y.R.; Kim, H.W. Occurrence and spatial distribution of marine mammals by sighting surveys in Korean waters during 2011-2020. Korean J. Fish. Aquat. Sci. 2022, 55, 938–945. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Kim, J.H.; Kim, H.W.; Sohn, H. Characteristics of the cetacean bycatch in Korean coastal waters from 2011 to 2017. Korean J. Fish. Aquat. Sci. 2018, 51, 704–713. [Google Scholar] [CrossRef]
- Harcourt, A.H. Coincidence and mismatch of biodiversity hotspots: A global survey for the order, primates. Biol. Conserv. 2000, 93, 163–175. [Google Scholar] [CrossRef]
- Nelson, T.A.; Boots, B. Detecting spatial hot spots in landscape ecology. Ecography 2008, 31, 556–566. [Google Scholar] [CrossRef]
- Brough, T.E.; Rayment, W.J.; Slooten, L.; Dawson, S. Prey and habitat characteristic contribute to hotspots of distribution for an endangered coastal dolphin. Front. Mar. Sci. 2023, 10, 1204943. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R. Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl. Acad. Sci. USA 2006, 103, 19374–19379. [Google Scholar] [CrossRef]
- Brough, T.; Rayment, W.; Slooten, E.; Dawson, S. Fine scale distribution for a population of New Zealand’s only endemic dolphin (Cepbalorbyncbus bectori) shows long-term stability of coastal hotspots. Mar. Mamm. Sci. 2018, 35, 1–24. [Google Scholar] [CrossRef]
- Hamilton, C.D.; Lydersen, C.; Aars, J.; Biuw, M.; Boltunov, A.N.; Born, E.W.; Dietz, R.; Folkow, L.P.; Glazov, D.M.; Haug, T.; et al. Marine mammal hotspots in the Greenland and Barents Seas. Mar. Ecol. Prog. Ser. 2021, 659, 3–28. [Google Scholar] [CrossRef]
- Santora, J.A.; Veit, R.R. Spatio-temporal persistence of top predator hotspots near the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2013, 487, 287–304. [Google Scholar] [CrossRef]
- Davidson, A.D.; Boyer, A.G.; Kim, H.; Pompa-Mansilla, S.; Hamilton, M.J.; Costa, D.P.; Ceballos, G.; Brown, J.H. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. USA 2012, 109, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.E.; Sharples, J.; Ross, O.N.; Wang, J.; Pierce, G.J.; Camphuysen, C.J. Sub-surface hotspots in shallow seas: Fine-scale limited locations of top predator foraging habitat indicated by tidal mixing and sub-surface chlorophyll. Mar. Ecol. Prog. Ser. 2010, 408, 207–226. [Google Scholar] [CrossRef]
- Bennington, S.; Rayment, W.; Currey, R.; Oldridge, L.; Henderson, S.; Guerra, M.; Brough, T.; Johnston, D.; Corne, C.; Johnson, D.; et al. Long-term stability in core habitat of an endangered population of bottlenose dolphins (Tursiops truncatus): Implications for spatial management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 31, 665–676. [Google Scholar] [CrossRef]
- Avila, I.C.; Kaschner, K.; Dormann, C.F. Current global risks to marine mammals: Taking stock of the threats. Biol. Conserv. 2018, 211, 44–58. [Google Scholar] [CrossRef]
- Brownell, R.L., Jr.; Reeves, R.R.; Read, A.J.; Smith, B.D.; Thomas, P.O.; Ralls, K.; Amano, M.; Berggren, P.; Chit, A.M.; Collins, T.; et al. Bycatch in gillnet fisheries threatens critically endangered small cetaceans and other aquatic megafauna. Endanger. Species Res. 2019, 40, 285–296. [Google Scholar] [CrossRef]
- Cañadas, A.; Vázquez, J.A. Common dolphins in the Alboran Sea: Facing a reduction in their suitable habitat due to an increase in sea surface temperature. Deep-Sea Res. II 2017, 141, 306–318. [Google Scholar] [CrossRef]
- Cotton Rockwood, R.; Elliott, M.L.; Saenz, B.; Nur, N.; Jahncke, J. Modeling predator and prey hotspots: Management implications of baleen whale co-occurrence with krill in Central California. PLoS ONE 2020, 15, e0235603. [Google Scholar] [CrossRef]
- Weelden, C.V.; Towers, J.R.; Bosker, T. Impacts of climate change on cetacean distribution, habitat and migration. Clim. Chang. Ecol. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Azzellino, A.; Gaspari, S.A.; Airoldi, S.; Lanfredi, C. Biological consequences of global warming: Does sea surface temperature affect cetacean distribution in the western Ligurian Sea? J. Mar. Biol. Assoc. UK 2008, 88, 1145–1152. [Google Scholar] [CrossRef]
- Ferrero, R.C.; Hobbs, R.C.; VanBlaricom, G.R. Indications of habitat use patterns among small cetaceans in the central North Pacific based on fisheries observer data. J. Cetacean Res. Manag. 2002, 4, 311–321. [Google Scholar] [CrossRef]
- Citta, J.J.; Okkonen, S.R.; Quakenbush, L.T.; Maslowski, W.; Osinski, R.; George, J.C.; Small, R.J.; Brower Jr, H.; Heide-Jørgensen, M.P.; Harwood, L.A. Oceanographic characteristics associated with autumn movements of bowhead whales in the Chukchi Sea. Deep Sea Res. II 2018, 152, 121–131. [Google Scholar] [CrossRef]
- Cox, S.L.; Witt, M.J.; Embling, C.B.; Godley, B.J.; Hosegood, P.J.; Miller, P.I.; Votier, S.C.; Ingram, S.N. Temporal patterns in habitat use by small cetaceans at an oceanographically dynamic marine renewable energy test site in the Celtic Sea. Deep Sea Res. II 2017, 141, 178–190. [Google Scholar] [CrossRef]
- Cañadas, A.; Hammond, P.S. Abundance and habitat preferences of the short-beaked common dolphin Delphinus delphis in the southwestern Mediterranean: Implications for conservation. Endanger. Species Res. 2008, 4, 309–331. [Google Scholar] [CrossRef]
- Pietroluongo, G.; Cipriano, G.; Ashok, K.; Antichi, S.; Carlier, H.; Miliou, A.; Maglietta, R.; Fanizza, C.; Carlucci, R. Density and abundance of Delphinus delphis in waters south of Samos Island, Greece (Eastern Mediterranean Sea). J. Mar. Sci. Eng. 2020, 8, 218. [Google Scholar] [CrossRef]
- Meyer-Gutbrod, E.L.; Davies, K.T.A.; Johnson, C.L.; Plourde, S.; Sorochan, K.A.; Kenney, R.D.; Ramp, C.; Gosselin, J.-F.; Lawson, J.W.; Greene, C.H. Redefining North Atlantic right whale habitat-use patterns under climate change. Limnol. Oceanogr. 2023, 68, S71–S86. [Google Scholar] [CrossRef]
- La Manna, G.; Ronchetti, R.; Sarà, G. Predicting common bottlenose dolphin habitat preference to dynamically adapt management measures from a marine spatial planning perspective. Ocean Coast. Manag. 2016, 130, 317–327. [Google Scholar] [CrossRef]
- Torres, L.G.; Read, A.J.; Halpin, P. Fine-scale habitat modeling of a top marine predator: Do prey data improve predictive capacity? Ecol. Appl. 2008, 18, 1702–1717. [Google Scholar] [CrossRef]
- Raudino, H.C.; Bouchet, P.J.; Douglas, C.; Douglas, R.; Waples, K. Aerial abundance estimates for two sympatric dolphin species at a regional scale using distance sampling and density surface modeling. Front. Ecol. Evol. 2023, 10, 1086686. [Google Scholar] [CrossRef]
- Barlow, J. Inferring trackline detection probabilities, g(0), for cetaceans from apparent densities in different survey conditions. Mar. Mammal Sci. 2015, 31, 923–943. [Google Scholar] [CrossRef]
- Sahri, A.; Mustika, P.L.K.; Purwanto, P.; Murk, A.J.; Scheidat, M. Using cost-effective surveys from platforms of opportunity to assess cetacean occurrence patterns for marine park management in the heart of the coral triangle. Front. Mar. Sci. 2020, 7, 569936. [Google Scholar] [CrossRef]
- Thomas, L.; Williams, R.; Sandilands, D. Designing line transect surveys for complex survey regions. J. Cetacean Res. Manag. 2007, 9, 1–13. [Google Scholar] [CrossRef]
- Yoo, J.T.; Park, K.J. Can the annual bycatch per unit effort of common dolphins (Delphinus delphis) be used to index the species’ relative abundance in the East Sea of Korea? Mar. Mam. Sci. 2023, 1–10. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Company: New York, NY, USA, 1995; pp. 593–608. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.; Pius, R. R for Health Data Science; Chapman and Hall/CRC Press: New York, NY, USA, 2020; pp. 213–251. [Google Scholar] [CrossRef]
- Ingman, K.; Hines, E.; Mazzini, P.L.F.; Cotton Rockwood, R.; Nur, N.; Jahncke, J. Modeling changes in baleen whale seasonal abundance, timing of migration, and environmental variables to explain the sudden rise in entanglements in California. PLoS ONE 2021, 16, e0248557. [Google Scholar] [CrossRef] [PubMed]
- Lechwar, A.; Rasmussen, M.; Basran, C.; Collins, S. Habitat preference and trend in relative abundance of common minke whales (Balaenoptera acutorostrata) in Skjálfandi Bay, Iceland. J. Cetacean Res. Manag. 2023, 24, 29–46. [Google Scholar] [CrossRef]
- Anderson, C.J.; Verkuilen, J.; Johnson, T. Applied Generalized Linear Mixed Models: Continuous and Discrete Data; Springer: New York, NY, USA, 2012; pp. 7–59. [Google Scholar]
- Redfern, J.V.; Ferguson, M.C.; Becker, E.A.; Hyrenbach, K.D.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.F.; Forney, K.A.; Ballance, L.T.; et al. Techniques for cetacean-habitat modeling. Mar. Ecol. Prog. Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Gailey, G.; Sychenko, O.; Zykov, M.; Rutenko, A.; Blanchard, A.; Melton, R.H. Western gray whale behavioral response to seismic surveys during their foraging season. Environ. Monit. Assess. 2022, 194, 740. [Google Scholar] [CrossRef] [PubMed]
- Ausen, E.; Barber, D.G.; Basu, A.; Ehn, J.; Walker, D.; Dalman, L.; Marcoux, M. River-influenced beluga (Delphinapterus leucas) summer habitat use in western Hudson Bay, Canada. Arct. Sci. 2023, 9, 869–885. [Google Scholar] [CrossRef]
- Derville, S.; Torres, L.G.; Garrigue, C. Social segregation of humpback whales in contrasted coastal and oceanic breeding habitats. J. Mammal. 2018, 99, 41–54. [Google Scholar] [CrossRef]
- Anganuzzi, A.A.; Buckland, S.T. Reducing bias in trends in dolphin relative abundance, estimated form tuna vessel data. Rep. Int. Whal. Commn. 1989, 39, 323–334. [Google Scholar]
- Campbell, G.S.; Thomas, L.; Whitaker, K.; Douglas, A.B.; Calambokidis, J.; Hildebrand, J.A. Inter-annual and seasonal trends in cetacean distribution, density and abundance off southern California. Deep-Sea Res. II 2015, 112, 143–157. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, J.M.; Park, J.H.; Baeck, G.W. Feeding habits of larval Clupea pallasii in the eastern Jinhae Bay, Korea. J. Korean Soc. Fish. Technol. 2017, 53, 107–113. [Google Scholar] [CrossRef]
- Son, S.H.; Jeong, J.M.; Kim, H.J.; Kim, K.R.; Baeck, G.W. Feeding habits of the Pacific herring Clupea pallasii in the coastal waters of the East Sea, Korea. Korean J. Fish. Aquat. Sci. 2022, 55, 224–228. [Google Scholar] [CrossRef]
- Yoo, S.; Park, J. Why is the southwest the most productive region of the East Sea/Sea of Japan? J. Mar. syst. 2009, 78, 301–315. [Google Scholar] [CrossRef]
- Joo, H.T.; Son, S.H.; Park, J.W.; Kang, J.J.; Jeong, J.Y.; Lee, C.I.; Kang, C.K.; Lee, S.H. Long-term pattern of primary productivity in the East/Japan Sea based on ocean color data derived from MODIS-Aqua. Remote Sens. 2016, 8, 25. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J. Primary production by phytoplankton in the territorial seas of the Republic of Korea. Algae 2022, 37, 265–279. [Google Scholar] [CrossRef]
- Mills, E.L.; Fournier, R.O. Fish production and the marine ecosystems of the Scotian shelf, Eastern Canada. Mar. Biol. 1979, 54, 101–108. [Google Scholar] [CrossRef]
- Shannon, L.V.; Field, J.G. Are fish stocks food-limited in the southern Benguela pelagic ecosystem? Mar. Ecol. Prog. Ser. 1985, 22, 7–19. [Google Scholar] [CrossRef]
- Moura, A.E.; Sillero, N.; Rodrigues, A. Common dolphin (Delphinus delphis) habitat preferences using data from two platforms of opportunity. Acta Oecologica 2012, 38, 24–32. [Google Scholar] [CrossRef]
- Yoo, J.T.; Kim, J.J. Fishing characteristics of Pacific herring Clupea pallasii and relationship between its catch and sea temperature during the past 50 years in Korean waters. Korean J. Fish. Aquat. Sci. 2021, 54, 208–217. [Google Scholar] [CrossRef]
- Hwang, K.; Kang, S.; Oh, T.Y.; Choi, K.H.; Lee, D.W. Change in the fishing grounds and the relationship between the abundance of the common squid Todarodes pacificus and the distribution of zooplankton in the East Sea. Korean J. Fish. Aquat. Sci. 2012, 45, 173–179. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, K.; Lee, C.I. Migration and distribution of the common squid (Todarodes pacificus) in Korean waters. J. Environ. Sci. Int. 2017, 26, 173–181. [Google Scholar] [CrossRef]
- Murata, M. Oceanic resources of squids. Mar. Behav. Physiol. 1990, 18, 19–71. [Google Scholar] [CrossRef]
- Choi, K.; Lee, C.I.; Hwang, K.; Kim, S.W.; Park, J.H.; Gong, Y. Distribution and migration of Japanese common squid, Todarodes pacificus, in the southwestern part of the East (Japan) Sea. Fish. Res. 2008, 91, 281–290. [Google Scholar] [CrossRef]
- Peters, K.J.; Stockin, K.A.; Saltré, F. On the rise: Climate change in New Zealand will cause sperm and blue whales to seek higher latitudes. Ecol. Indicat. 2022, 142, 109235. [Google Scholar] [CrossRef]
- Bräger, S.; Harraway, J.A.; Manly, B.F.J. Habitat selection in a coastal dolphin species (Cephalorhynchus hectori). Mar. Biol. 2003, 143, 233–244. [Google Scholar] [CrossRef]
- Adams, L. Species distribution model of cetaceans in relation to environmental factors at two locations in the north Atlantic. Plymouth Stud. Sci. 2019, 12, 3–24. [Google Scholar]
- Jackson-Ricketts, J.; Junchompoo, C.; Hines, E.M.; Hazen, E.L.; Ponnampalam, L.S.; IIangakoon, A.; Monanunsap, S. Habitat modeling of Irrawaddy dolphins (Orcaella brevirostris) in the eastern Gulf of Thailand. Ecol. Evol. 2020, 10, 2778–2792. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yoo, J.T. Spatial relationship between distribution of common minke whale (Balaenoptera acutorostrata) and satellite sea surface temperature observed in the East Sea, Korea in May from 2023 to 2020. J. Korean Soc. Fish. Ocean Technol. 2022, 58, 281–287. [Google Scholar] [CrossRef]
- Gauger, M.F.W.; Romero-Vivas, E.; Peck, M.A.; Balart, E.F.; Caraveo-Patiño, J. Seasonal and diel influences on bottlenose dolphin acoustic detection determined by whistles in a coastal lagoon in the southwestern Gulf of California. PeeJ 2022, 10, e13246. [Google Scholar] [CrossRef] [PubMed]
- Slooten, E. Effectiveness of area-based management in reducing bycatch of the New Zealand dolphin. Endanger. Species Res. 2013, 20, 121–130. [Google Scholar] [CrossRef]
| Model (Variables) | BIC |
|---|---|
| Herring catch + Chlorophyll-a concentration | −10.54 |
| Herring catch + Squid catch + Chlorophyll-a concentration | −10.15 |
| Herring catch + Squid catch + Chlorophyll-a concentration + SST | −7.95 |
| Variable | Coefficient | p-Value | 95% Confidence Interval [46,47] |
|---|---|---|---|
| Intercept | 11.462 | <0.01 | [7.454, 16.660] |
| Herring catch | −0.004 | <0.05 | [−0.006, −0.001] |
| Chlorophyll-a concentration | −4.209 | <0.05 | [−7.262, −1.422] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-T.; Lee, M.K.; Joo, H. Environmental Factors Influencing Annual Changes in Bycatch per Unit Effort of Delphinus delphis around Their Main Hotspot in Korean Waters. J. Mar. Sci. Eng. 2024, 12, 525. https://doi.org/10.3390/jmse12040525
Yoo J-T, Lee MK, Joo H. Environmental Factors Influencing Annual Changes in Bycatch per Unit Effort of Delphinus delphis around Their Main Hotspot in Korean Waters. Journal of Marine Science and Engineering. 2024; 12(4):525. https://doi.org/10.3390/jmse12040525
Chicago/Turabian StyleYoo, Joon-Taek, Mi Kyung Lee, and Huitae Joo. 2024. "Environmental Factors Influencing Annual Changes in Bycatch per Unit Effort of Delphinus delphis around Their Main Hotspot in Korean Waters" Journal of Marine Science and Engineering 12, no. 4: 525. https://doi.org/10.3390/jmse12040525
APA StyleYoo, J.-T., Lee, M. K., & Joo, H. (2024). Environmental Factors Influencing Annual Changes in Bycatch per Unit Effort of Delphinus delphis around Their Main Hotspot in Korean Waters. Journal of Marine Science and Engineering, 12(4), 525. https://doi.org/10.3390/jmse12040525





