1. Introduction
The maritime industry is part of the decarbonisation strategy because, despite technological advances in recent years, the maritime sector is almost entirely dependent on fossil fuels. In recent years, several measures have been adopted to ensure that the International Maritime Organization (IMO) targets to reduce greenhouse gas (GHG) emissions will be achieved. Compared to 2008 levels, the IMO targets include a 20% reduction in GHG by 2030 and a 70% reduction by 2040 [
1].
The measures taken by international organisations reflect on financial liabilities, wherein vessel operators following the adopted European Union Emission Trading System (EU ETS) regulation will be obligated to pay taxes for the emitted CO
2 emissions during the voyages that begin or end at a port under the jurisdiction of an EU member state. According to the adopted EU ETS (Directive 2003/87/EC), it will cover maritime transport emissions from 1 January 2024 [
2]. The regulation principle for maritime transport will remain the same as for other ETS sectors. Shipping companies will have to monitor their own emissions and then purchase and surrender ETS emission allowances for each tonne of emitted GHG at the end of the year. It must be highlighted that the complete EU ETS implementation into the maritime transport sector will be achieved through several stages: from 2024, shipping companies will have to cover 40% of emission generated throughout 2024 and 70% of GHG emission generated in 2025 and then from 2026, must cover 100% of surrendered emissions. In addition, implementation will be scaled by type and size of ship: ships over 5000 gross tonnage (GT) carrying cargo or passengers on commercial voyages will be covered by the EU ETS from 2024; then, from 2025, the EU Monitoring, Reporting and Verification (MRV) will apply to general cargo ships between 400 and 5000 GT on commercial voyages. Following the established European climate law and the FuelEU maritime initiative, the financial EU ETS model is expected to be one of the key factors in reducing EU emissions by up to 80% by 2050 to encourage the uptake of renewable and low-carbon fuels in shipping. In general terms, since the EU ETS was introduced in 2005, EU emissions have fallen by a solid 41%, according to the European Council of the European Union [
3]. According to the long-term forecast of the European price of carbon allowances, the price of a unit could potentially reach EUR 150 per tonne of CO
2 by the year 2030.
To achieve the objectives of the strategy, the IMO has introduced the measures through requirements under MARPOL Annex VI: Energy Efficiency Design Index (EEDI)—applicable to new ships only; Energy Efficiency Existing Ships Index (EEXI)—applicable to all ships; Carbon Intensity Indicator (CII)—applicable to all ships. Moreover, in 2022, during the 79th session of the IMO’s Marine Environmental Protection Committee (MEPC 79) confirmed the adoption of a Sulphur Emission Control Area (SECA) in the Mediterranean Sea from 1 May 2023. In such a zone, the limit for sulphur in fuel oil on board ships is 0.10% mass by mass [
4].
The Energy Efficiency Design Index (EEDI) quantifies the energy efficiency per capacity mile for various ship types and sizes (in grams of CO
2 per tonne-mile). This regulation, introduced in 2011, aimed to expedite the adoption of energy-efficient and pioneering technologies in ships, including propulsion optimisation, engine enhancement, energy efficiency technologies, and carbon capture implementation. Starting 1 April 2022, the EEDI Phase 3 took effect with the regulation that ships built from April 2022 onward exhibit a carbon intensity at least 30–50% lower than the established baseline [
5].
On 1 November 2022, the amendments to Annex VI of the International Convention for the Prevention of Pollution from Ships came into force. The amendment delegates ships to enhance their energy efficiency, aiming to reduce CO
2 emissions. Following the amendment, from 1 January 2023, it will be mandatory for all ships to calculate their EEXI and, at the same time, initiate the collection of data for the reporting of the annual operational carbon intensity indicator (CII) and rating. The EEXI represents the minimum energy efficiency standards for ships already in service and applies to all cargo and passenger ships over 400 GT falling under MARPOL Annex VI, with the exception that the EEXI does not apply to ships built to EEDI Phase 2 or Phase 3 requirements [
6]. As part of the IMO regulations, the EEXI stands out as a key initiative to encourage the adoption of environmentally friendly technologies to reduce the carbon footprint of the maritime sector. Similar to the EEDI, the EEXI assessment is based on ship design parameters, including equipment specifications and technical data. Neither index calculation method included carbon capture technologies as an index minimising factor, which is why the International Chamber of Shipping submitted a formal request to the IMO’s Marine Environment Protection Committee in September 2022 to include carbon capture technologies in the IMO’s regulatory framework to reduce greenhouse gas emissions from ships. The proposed amendment to the EEDI formula is presented in Equation (1) [
7].
where
is the quantity in tonnes of CO
2 captured and stored onboard per hour of operation at the shaft power of the engine.
where
is the total available storage volume of CO
2 in tonnes;
Fuelonboard is 50% of fuel’s total storage tank capacity in tonnes.
Another measure introduced by the IMO is the CII, which measures emissions in grams of CO
2 emitted per cargo-carrying capacity and nautical mile. Based on the CII assessment, ships are classified as A, B, C, D or E. If a ship has a D rating for three consecutive years or an E rating for one year, it must provide a reduction strategy in the Ship Energy Efficiency Management Plan (SEEMP Part III) [
8]. This plan should include steps to achieve the required C-rating or better. Due to the characteristics of LNG fuel and the advantages over Low Sulphur Marine Diesel Oil (LS-MDO) in the CII classification, LNG-fuelled ships are classified above Class A. The measures implemented are in line with decarbonisation strategies and are seen as an acceleration point for the integration of LNG fuel in the maritime sector, considering LNG as a transition fuel on the way to clean energy with the capability to reduce CO
2 emissions by 25% compared to low-sulphur marine diesel oil. In DNV’s 2023 Maritime Forecast to 2050 report, it was highlighted that new technologies and fuel production will need to be developed for shipping to meet its decarbonisation targets. DNV’s observation can be seen through the CII measure, where it is foreseen that around 2030, the LNG-fuelled ships will need to introduce additional technologies to remain compliant with the carbon intensity standards [
9].
Figure 1 illustrates the comparison of LNG-fuelled ships according to the CII classification and the decarbonisation target towards 2050, where the Carbon Capture, Storage, and Utilization (CCSU) could serve as a potential solution. According to the National Energy Technology Laboratory’s database, 397 carbon capture and storage projects are registered worldwide as of 2023. In relation to the growing potential of CCS, in September 2023, the DNV released class guideline No. DNV-CG-0667 for CCS implementation onboard ships [
10].
Carbon capture technologies encompass various methods aimed at capturing carbon dioxide (CO
2) emissions from industrial processes or directly from the atmosphere. The field of carbon capture and storage (CCS) technology has become a more discussed topic. The principle of this technology is to eliminate CO
2 before it is emitted into the atmosphere. The CO
2 capture technologies can be grouped into three main types: pre-combustion capture, oxy-fuel combustion capture and post-combustion capture [
12].
Pre-combustion capture is based on a method of removing CO
2 before the fuel combustion cycle. In the pre-combustion process, fossil fuels are gasified and reformed by air and water vapour through steam reforming reactions and water-gas shift reactions prior to combustion. Finally, the CO
2 and H
2 are separated in a gas separator, where the H
2 is used as hydrogen fuel, and the CO
2 is captured for storage and further utilisation. This technology allows for low energy consumption and a CO
2 capture efficiency of close to 90% [
13]. However, the implementation of pre-combustion technology on existing ships would require the retrofitting of engines with hydrogen-fuelled engines, which would be too costly, and therefore, this technology is not considered compatible with ships in service. The schematical layout of pre-combustion technology is presented in
Figure 2.
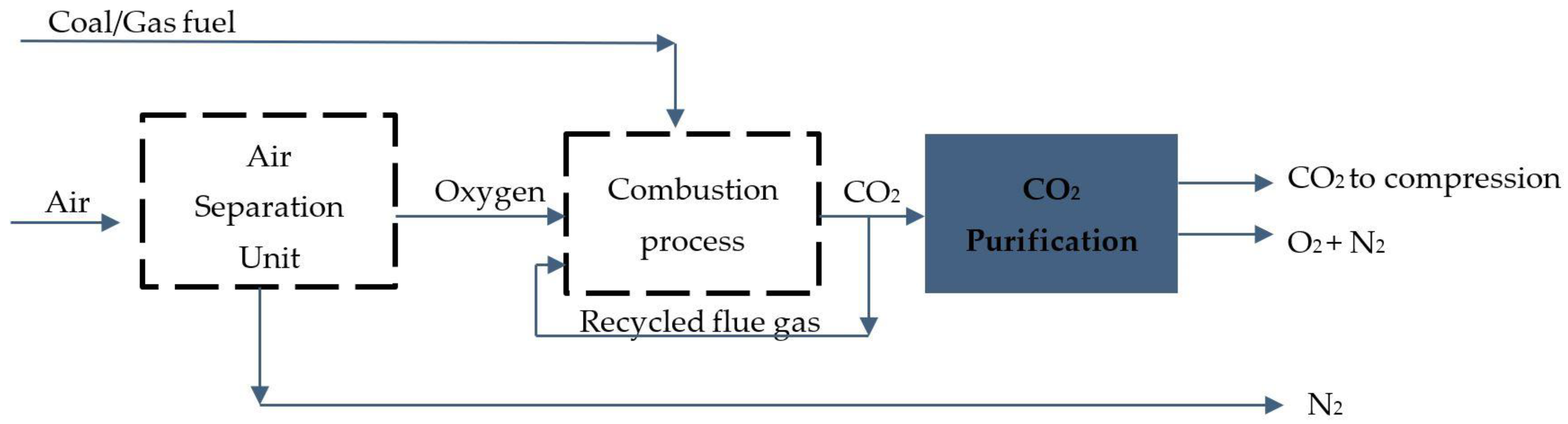
Oxy-fuel combustion carbon capture is based on the fossil fuel combustion cycle where pure oxygen is added to the combustion process instead of air, resulting in CO
2 and water vapour after combustion, which means fuel is burnt to have zero carbon emission. The CO
2 and H
2O vapours are then separated by a condensation cycle. The implementation of oxy-fuel combustion capture on the ship would require a huge amount of pure oxygen supply, so ship technology would require extensive modification and investment in an air separation unit and additional power supply to produce pure oxygen in the first stage and after the combustion cycle to perform H
2O separation in the CO
2 drying process. For these reasons, the technology is not considered to be an effective solution for reducing GHG emissions from ships [
15]. The schematical layout of oxy-combustion technology is presented in
Figure 3.
Post-combustion capture is based on the method of capturing CO
2 from the flue gas after the combustion cycle of fossil fuel in exhaust flow [
16]. This technology stands out from others due to its installation possibility, which does not require wide modification and compatibility with existing equipment. The main technological intervention relates to modifying exhaust gas and integrating additional equipment into it. Due to this main advantage, compared to other types of carbon capture technologies, post-combustion capture is the technology most compatible with maritime transport. Moreover, the post-combustion technologies are even more compatible with LNG plants where the LNG cryogenic cold potential could be combined with carbon capture technology without major modification to the existing facility. Moreover, there is a good amount of experience with post-combustion capture technologies in power plans that provide confidence in the effectiveness of the technology and in the event of failure of the carbon capture facility, the performance of the ship’s engines or associated essential equipment will not be affected as the exhaust gas can be diverted to the atmosphere. [
17]. The schematical layout of post-combustion technology is presented in
Figure 4.
In 2021, the Oil and Gas Climate Initiative (OGCI), together with Sena Bulk and the Netherlands Organisation for Applied Scientific Research (TNO), conducted a feasibility study to assess the feasibility of carbon capture on ships. A published report compared carbon capture technologies, with post-combustion cryogenic carbon capture and chemical absorption technologies being the most compatible with ships. The chemical absorption process based on liquid amine solution was selected as the most compatible solution for LSMDO-fuelled Suezmax-type vessels due to its high level of maturity. The cryogenic capture approach was highlighted as the most compatible solution for LNG-fuelled vessels due to the availability of LNG cold temperature potential of −160 °C in cogeneration cycles to bring the exhaust gas down to temperature condition equal to the liquid phase of CO
2 at a relatively low 4% concentration. The advantage of cryogenic carbon capture technology against the other towards CO
2 capture is made considering the amount of energy potential from the LNG vapourisation process of LNG supply as fuel to engines; therefore, it is necessary to highlight that such an amount of energy is negligible compared to available energy potential on the Floating Storage Regasification Unit (FSRU) [
18].
Table 1 contains the assessment criteria of technology that has the highest effectiveness towards carbon capture. One of the main criteria is the carbon capture ratio, where the chemical absorption method and cryogenic separation method stand as leading options against the other two. The cryogenic separation requires cryogenic temperature when the exhaust gas is being cooled down to the CO
2 liquefaction phase. Meanwhile, membrane separation is based on the filtration method. Khalilpour et al. [
19] explain that the membrane separation method has selective permeability, allowing CO
2 molecules to pass through more easily than other gases due to their size and chemical properties. This allows the CO
2 to separate from the rest of the gases. The absorption is different as well, where the method engages sorbents in the process of separation. Heidari et al. [
20] performed a study of the practical application of CaO-based sorbents practical application which is considered the most advantageous type of sorbent due to various benefits, such as high theoretical sorption capacity and ability to capture CO
2 at temperatures above 500 °C.
The FSRUs carry out the regasification process of LNG, which is a natural gas whose main composition is methane with some mixture of ethane, which has been cooled to the liquid phase and has a temperature of −160 °C. The FSRU is classified as a tanker-class vessel designed for regasification operations. Although the FSRU vessel is considered a convenient alternative to a land-based LNG terminal, it is not exempt from the IMO’s decarbonisation targets [
22]. Regardless of the propulsion complex type, the LNG regasification cycle is theoretically identical when the cargo is transferred from the LNG tanks to the high-pressure booster pump, whose purpose is to increase the LNG pressure before entering the LNG vapouriser where the LNG is heated and sent to the grid [
23]. The thermal energy for LNG regasification is extracted from the seawater, which is used as a heat source to raise the temperature of the refrigerant, which is then in direct contact with the LNG in the vapouriser. The regasification of FSRU vessels can be operated in one of three ways:
Closed-loop mode, in which the seawater in a closed system is heated by the steam produced from FSRU regasification boilers;
In open-loop mode, the warm water is drawn in through the FSRU’s sea chest. The warm seawater is used as a heat source to heat the regasification agent;
A combined mode is used in which the colder water is drawn in and then heated by steam from FSRU regasification boilers to provide sufficient heat for the regasification of the LNG [
23].
Due to the heat exchange operation on the FSRU vessel, the cryogenic carbon capture technology can be technically compatible with LNG regasification methods by strategically integrating processes to mitigate energy demands. Considering various regasification modes, such as combined and closed-loop configurations, the higher energy consumption necessitates a balance that incorporates additional steam production. The introduction of LNG carbon capture technology can be facilitated through meticulous heat exchange operations, ensuring minimal energy input and maintaining the efficiency of the LNG infrastructure and compliance with environmental regulations [
24].
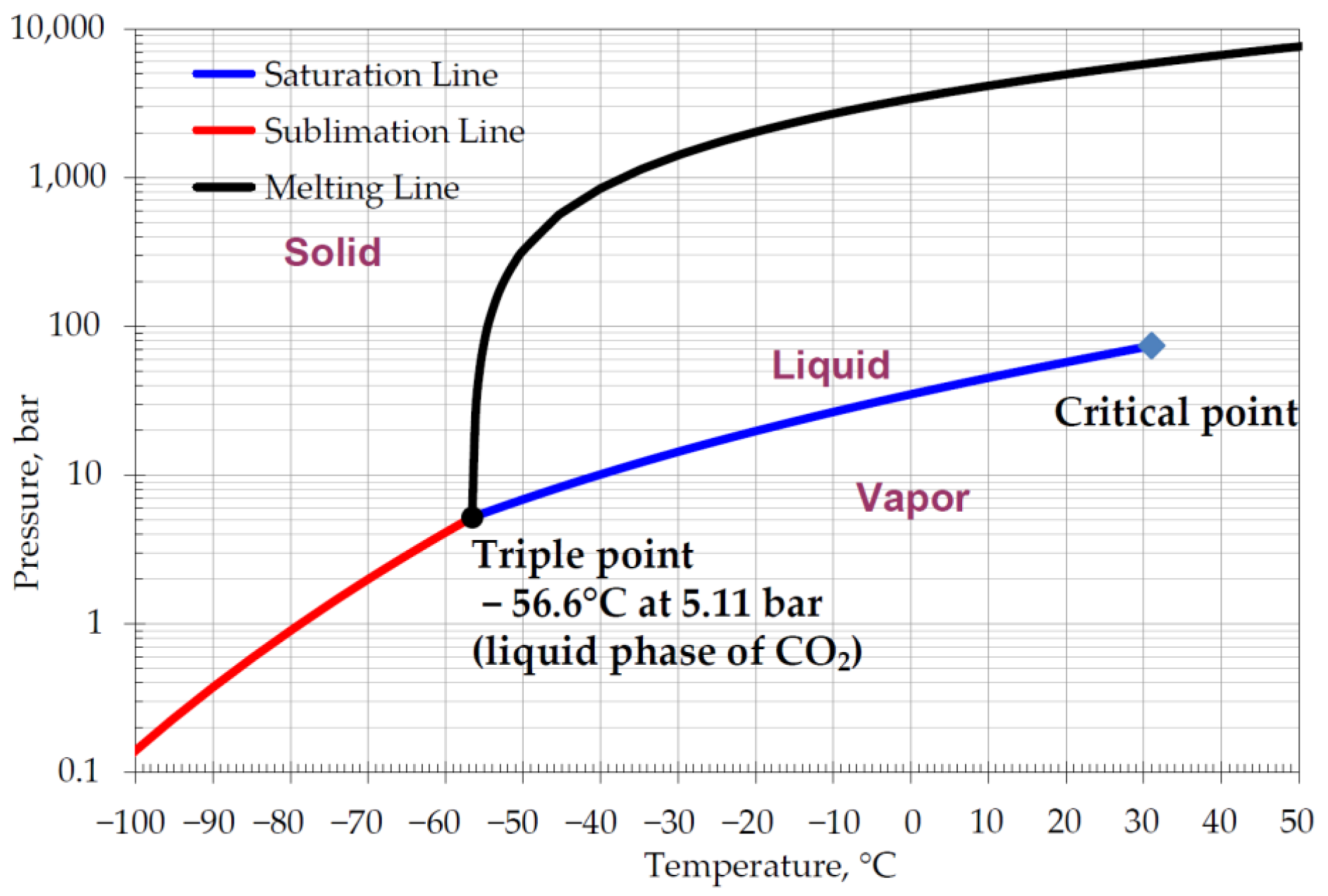
The concept of cryogenic carbon capture is based on the cold potential of LNG as a kind of energy source to reduce the exhaust gas temperature while at the same time absorbing heat for the regasification processes so that the cold temperature acts as a separating force for the separation of carbon dioxide. The separated CO
2 is typically compressed and align-cooled to −56.6 °C at 5.11 bar pressure, at which point CO
2 converts into the liquid phase for further utilisation in carbon capture and storage (CCS) projects for injection and storage in a depleted offshore oil and gas field or underground reservoir. The liquid CO
2 can also be converted into a solid phase called “dry ice”; in order to reach the liquid phase of carbon dioxide, it must be cooled to −78.5 °C at temperature and 1 bar pressure. The density of the liquid phase of CO
2 is 1156 kg/m
3, and the solid phase is 1562 kg/m
3 [
25]; hence, the high-density potential allows for CO
2 to be stored onboard before disposal, contributing to the chain of CCS logistic projects. The cryogenic carbon capture cycle follows four main stages [
9]:
- (1)
First stage—Exhaust gas drying. In this stage, the H2O has to be removed from the exhaust gas in the condensate phase. Meanwhile, the exhaust gas is cooled down from 350 °C (the average exhaust gas temperature from the engines) to 0 °C, reaching a condensation temperature of H2O;
- (2)
Second stage—Separation of H2O;
- (3)
Third stage—Cooling down of oxygen and nitrogen remaining in exhaust gas;
- (4)
Fourth stage—Liquefaction of CO2. At this stage, dry exhaust gas is cooled down to −56.6 °C and 5.2 bar pressure;
- (5)
Fifth stage—Capture of CO2.
In 2016, Bezyukov et al. [
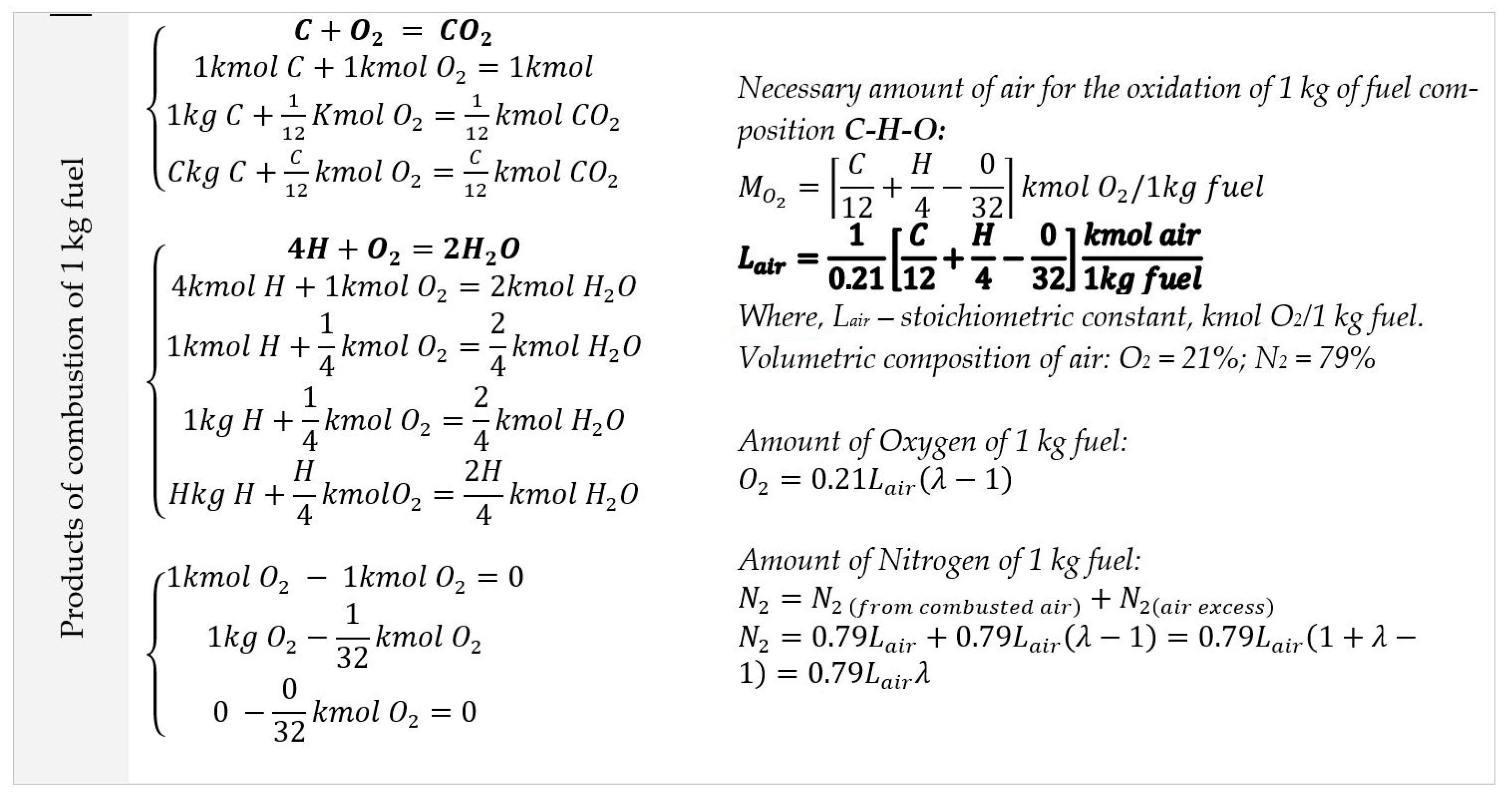
26] proposed a carbon dioxide separation method by utilising LNG cryogenic cold potential to convert carbon dioxide into a solid phase of dry ice. Since natural gas consists of more than 95% methane (CH
4), the methane properties are considered to be LNG properties in the study. The following fuel combustions are considered in the study: excess air ratio (α) for boiler plans is applicable at a 1.1 ratio, and for diesel engines 1.8–2.1, specific heat C
p = 1. According to the study results, LNG regasification requires about 750–800 kJ/kg of thermal energy to achieve a gas temperature for combustion injection. During combustion, 2.75 kg of CO
2 is produced from 1 kg of burnt LNG fuel. Converting CO
2 into a solid phase requires 650 kJ/kg energy or 1787.5 kJ/1 kg LNG fuel, which allows it to achieve a 40–44% CO
2 capture ratio. However, the authors analysed the general gas of the diesel combustion cycle, where the air and nitrogen ratio were not considered as products of combusted gas.
The evaluation studies of the implementation of carbon capture technologies in ships can be observed in the literature. In 2022, Jasper et al. [
27] released the study results of the implementation of ship-based carbon capture technology onboard LNG-fuelled ships. The technology is based on solvent-based CO
2 capture in a post-combustion cycle through the exhaust gas heat integration into the LNG vapourisation cycle. The study’s results showed that a 50% carbon capture ratio can be achieved at an exhaust gas temperature of 250 °C before treatment. An alternative carbon capture technology method was analysed by Awoyomi et al. [
28], who performed a study of ship-based carbon capture (SBCC) technology implementation on a LNG-fueled vessel of a LNG carrier with the propulsion of a Wartsila 9L46 DF marine diesel engine. The technology method is based on post-combustion technology with the introduction of solvent. According to the study authors, the carbon capture ratio can be 60%.
In terms of the implementation of carbon capture technologies on the vessels, it can be stated that it is still in the pioneering stage; in the past, few carbon capture technologies were installed on ships for trials. In the marine sector, a significant milestone was achieved in 2021 when the world’s first CO
2 capture plant was installed onboard a vessel for a six-month voyage. The pioneering system found its home on the bulk carrier Corona Utility (IMO: 9748021). The announcement of the successful trial underscored the system’s ability to demonstrate the feasibility of capturing carbon dioxide from the exhaust gas of marine engines. This groundbreaking achievement signifies that research and development efforts in the realm of implementing CO
2 capture technologies within the marine sector are still in their nascent stages [
29]. In 2021, Wärtsilä Corporation made a statement that Wärtsilä Exhaust Treatment and Solvang ASA made an agreement on a full-scale pilot retrofit installation of a carbon capture and storage system on one of 21,000 m
3 size ethylene carriers—Clipper Eos with the expectation to completed retrofit by 2023 [
30]. The market interest in carbon capture technologies on the vessels can also be noticed in 2023: the TotalEnergies-owned LNG carrier was undergoing maintenance in July and retrofitted with ship-based carbon capture (SBCC) technology prototype for a trial duration of 125 days or 3000 h. For purposes of the trial, the set plan was to collect 10 metric tons of CO
2 in the liquid phase with storage in a pressurized tank [
31].
From a literature perspective, only a limited number of studies have been conducted and published to analyse the integration of cryogenic carbon capture technology and its actual performance efficiency on dual fuel vessels. To bridge this gap, further research is essential to comprehensively evaluate cryogenic carbon capture technology application’s effectiveness, feasibility, and economic viability on dual fuel vessel structures. The necessity for such research is particularly apparent when considering the long-term perspective of IMO environmental targets where, due to tightening regulations on emission norms, even the LNG-fuelled ships will not comply with the CII norms as of 2030; therefore, the additional technologies integration on ship structures align the LNG fuel is foreseen.
In order to contribute to and supplement the research gap, the primary objective of this research is to employ an experimental methodology for analysing the performance of an FSRU-type vessel equipped with a dual-fuel propulsion system under real operational conditions. The core focus lies in evaluating the feasibility of leveraging the cryogenic properties of LNG for a cogeneration process aimed at both exhaust gas treatment and the subsequent separation and liquefaction of CO2 onboard the vessel. This investigation aligns with the vessel’s dual-fuel engine setup, which offers the flexibility to run on either diesel or LNG fuel, prompting a comparative analysis of the carbon dioxide capture efficiency associated with each fuel type.
In the framework of the study, an energy balance equation model has been integrated for the examination of energy exchanges existing at individual heat exchangers through performance efficiency. In addition, the study is based on incorporating a suite of thermodynamic equations into research. These equations serve the crucial purpose of estimating energy exchange volumes, which is crucial for the separation of CO2 from exhaust gases and its subsequent conversion into a liquid state. Importantly, the analysis takes into account not only the fuel types but also their distinct chemical compositions, crucial in determining the necessary energy volumes, exhaust gas temperatures at different FSRU operational conditions and air-to-fuel ratios based on the equipment where fuel is combusted.
4. Discussion/Conclusions
In the past several years, international organisations have adopted strict environmental regulations as a measure to achieve the goal of decarbonising the shipping industry by 2050. In the timeframe towards the goal, LNG fuel is being identified as a transition fuel upon the infrastructure of green fuel technologies being established in the maritime field. Considering that the CII regulation applicable for the vessels, even with the LNG-fuelled propulsion complex, will require the adoption of additional technologies to comply with regulation, as of the performed analysis, the cryogenic carbon capture technology can be accepted as the most convenient and compatible option to be introduced on dual-fueled vessels. From the performed literature analysis, it can be observed that the minimum evaluation studies of CCC technology implementation on the FSRU-type vessel have been performed, even when the cryogenic capture approach was highlighted as the most compatible solution for LNG-fueled vessels due to the availability of LNG cold temperature potential. On the LNG-fueled vessel, the amount of LNG energy is negligible compared to the available energy potential on the FSRU. Hence, the aim of the research became to perform the experimental study on the FSRU-type vessel to monitor its actual performance related to the heat exchanger operations in the regasification cycle and, furthermore, to provide an overview of the potential possibilities to combine exhaust gas heat and LNG cold temperature potentials over cogeneration cycle in order to utilise the cold potential of the LNG through cooling down stages of the exhaust gases and in parallel to absorb/transfer the exhaust gas heat to LNG for regasification purpose and achieve common advantage: optimisation of the LNG regasification cycle while minimizing the CO2 emissions.
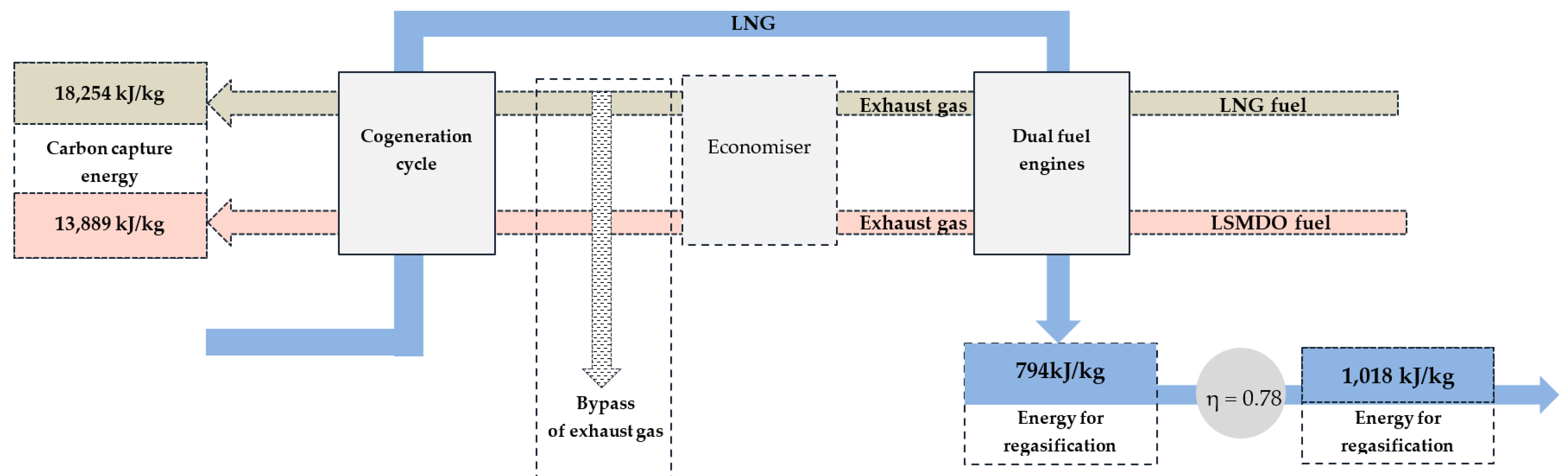
During the research, it was estimated that on the FSRU-type vessel, when exhaust gas is discharged to the atmosphere at the average temperature of 263 °C, the LNG fuel combustion requires 18,254 kJ/kg of energy to separate and capture CO2 in the liquid phase from the exhaust gas. However, despite LSMDO fuel containing a higher CO2 concentration in its exhaust gas, it requires less energy to separate CO2 in the liquid phase, which is 13,889 kJ/kg. Apparently, regardless of the CO2 concentration in the exhaust gas, the H2O removal stage requires most of the energy to dry the exhaust gas. At the time when 1 kg of LNG required 1018 kJ/kg of energy for regasification into its gas phase, considering the estimated LNG vaporizer performance efficiency coefficient of 0.78, the cryogenic carbon capture cycle through LNG cold potential utilisation would allow for the achievement of a carbon capture ratio of 5–6% using LNG as a fuel and 7–8% using LSMDO as a fuel.
Considering the results obtained, an interpolation was made to estimate the carbon capture rate at different exhaust gas temperatures, as shown in
Table 5. The interpolation result showed that within range of reduced exhaust gas temperature, the capture rate is increasing and eventually, when exhaust gas is treated before the cryogenic carbon capture unit, considering that an economizer or alternative machinery such as exhaust gas scrubber could reduce the temperature of exhaust gas down to 20 °C, the CO
2 capture rate to be increased up to 57% (for LNG fuel) and 49% (for LSMDO fuel). This major efficiency rate could be achieved because the exhaust gas drying stage at the cryogenic carbon capture unit is being eliminated. During this stage, the exhaust gas is cooled down through the wet drying” method. The exhaust gas passes through the scrubber-type machinery, where the exhaust gas comes into contact with the sprayed water. The exhaust gas is washed, and the H
2O composition from the exhaust gas is removed in the liquid phase at the outlet of injected water. This method offers an advantage for the cryogenic carbon capture process as wet drying decreases the need for the LNG cold potential, thereby enabling a higher carbon capture rate. In general, the cryogenic carbon capture method is supplemented with additional cold energy taken from the water. Furthermore, from the benchmark perspective, comparing the achieved results to results in other studies where the average carbon capture rate fluctuates between 40–60%, we can state that the competitive cryogenic carbon capture separation requires exhaust gas temperature treatment before it is injected into the separation unit.
Another potential enhancement could be achieved in FSRU-type vessels by introducing exhaust gas treatment into LNG regasification cycles. Given the available cold potential of LNG, where the mass flow rate of LNG to the vaporizer reaches 122,573 kg/h, that requires 23,118 kW or 83,224,800 kJ of heat energy to regasify LNG from the liquid phase at −150.1 °C to gas phase prior trip heater at −37.5 °C. Meanwhile, the cryogenic carbon capture method requires 18,254 kJ of energy per 1 kg combusted LNG fuel. The tremendous cryogenic potential of LNG on the FSRU-type vessel led to the statement that 100% of CO2 could be captured due to proportional higher LNG volume and the heat demand that the regasification process requires.
In the next stage of the study on the application of cryogenic carbon capture technology for a dual-fuel ship by utilising LNG cold potential, the authors intend to simulate the attained results using Thermoflow version No. 31 software. This simulation aims to facilitate a comprehensive thermal analysis of exhaust gas and LNG regasification configurations and evaluate potential possibilities to perform optimisation of existing energy management systems towards decarbonisation goals by implementing cryogenic carbon capture systems on dual fuel vessels.