Abundance and Diversity of Several Bacterial Genera in the Mariculture Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Illumina MiSeq Sequencing of 16S rRNA
2.3. Data Analysis of High-Throughput Sequencing
2.4. 16S rRNA Quantiative PCR (qPCR)
3. Results
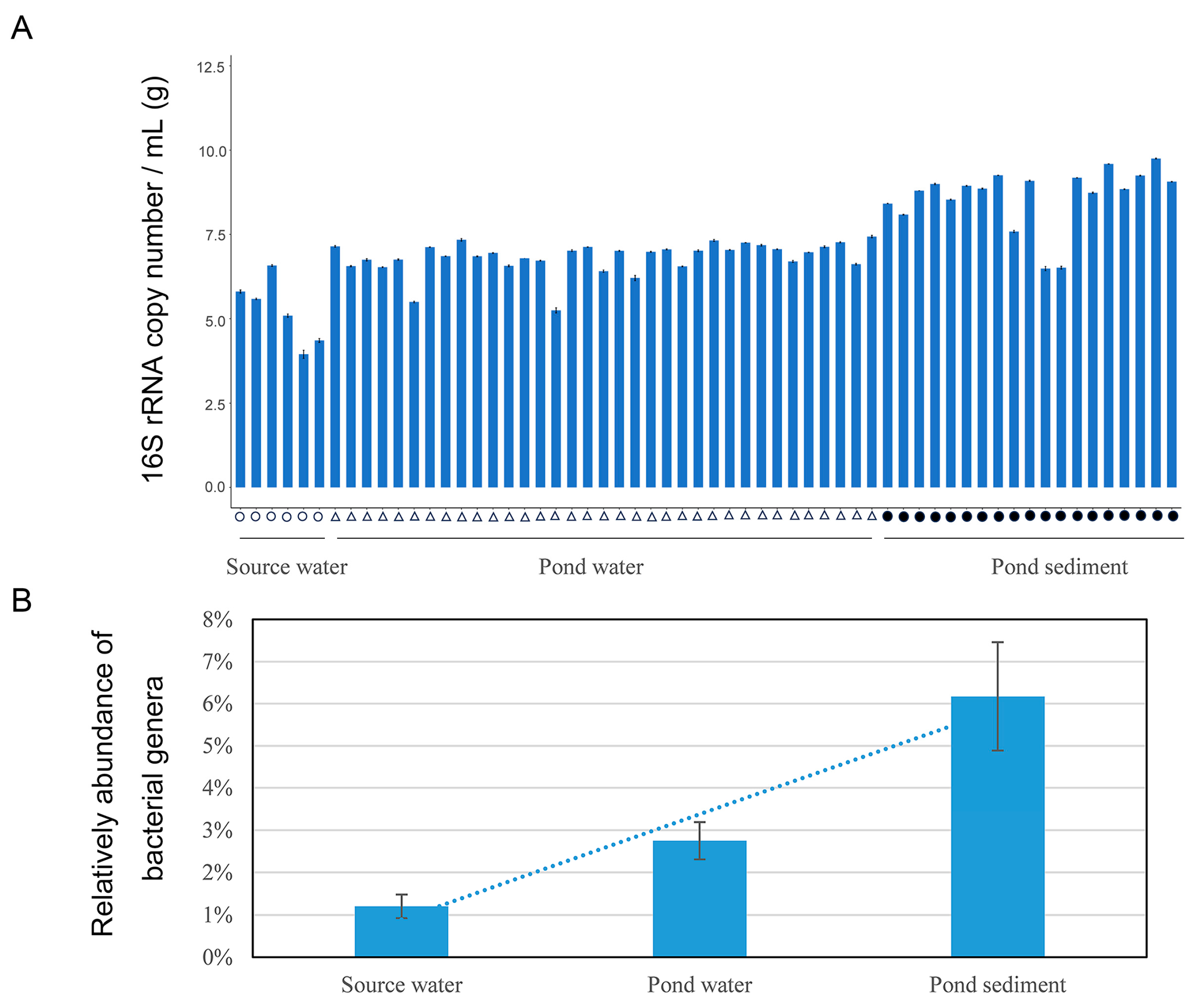
3.1. Abundance of 16S rRNA Genes in Culture Environment Identified through qPCR
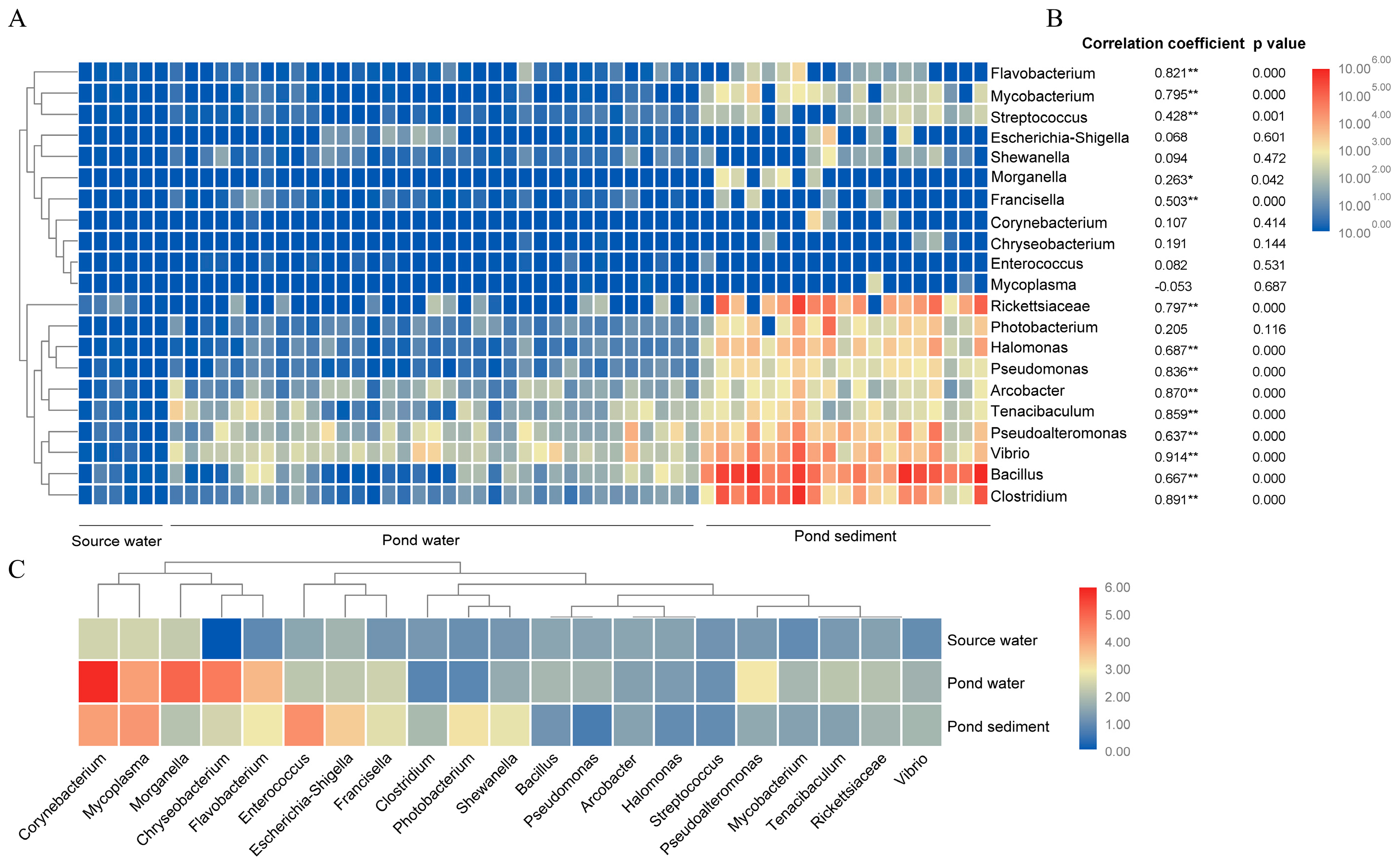
3.2. Diversity and Abundance of 21 Identified Bacteria Genera by Combining qPCR and High throughout Sequencing Analysis
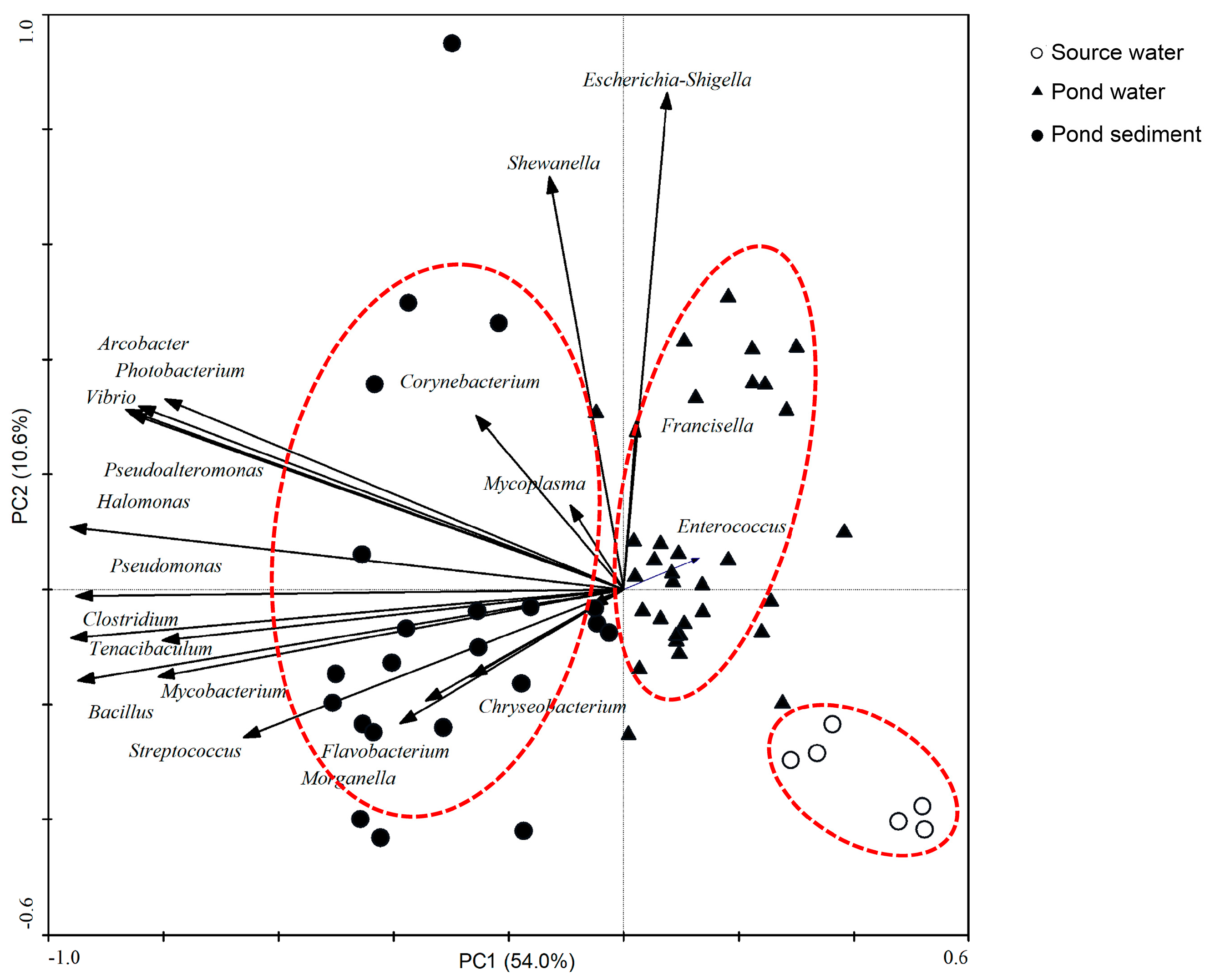
3.3. Principal Component Analysis (PCA) of the Distribution of Bacterial Genera between Different Environments
3.4. Bacterial Genera in the Intestines of Animals in Source Water and Pond Water Based on Illumina Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. How to Feed the World: 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Burge, C.A.; Eakin, C.M.; Friedman, C.S.; Froelich, B.; Hershberger, P.K.; Hofmann, E.E.; Petes, L.E.; Prager, K.C.; Weil, E.; Willis, B.L.; et al. Climate Change Influences on Marine Infectious Diseases: Implications for Management and Society. Annu. Rev. Mar. Sci. 2014, 6, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.R.; Lafferty, K.D. The Elusive Baseline of Marine Disease: Are Diseases in Ocean Ecosystems Increasing? PLoS Biol. 2004, 2, e120. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- El-Sayed, M.R.; Emam, A.M.; Osman, A.E.; Abd El-Galil, M.A.E.-A.A.; Sayed, H.H. Detection and description of a novel Psychrobacter glacincola infection in some Red Sea marine fishes in Hurghada, Egypt. BMC Vet. Res. 2023, 19, 23. [Google Scholar] [CrossRef]
- Duman, M.; Mulet, M.; Altun, S.; Saticioglu, I.B.; Ozdemir, B.; Ajmi, N.; Lalucat, J.; García-Valdés, E. The diversity of Pseudomonas species isolated from fish farms in Turkey. Aquaculture 2021, 535, 736369. [Google Scholar] [CrossRef]
- Umasuthan, N.; Valderrama, K.; Vasquez, I.; Segovia, C.; Hossain, A.; Cao, T.; Gnanagobal, H.; Monk, J.; Boyce, D.; Santander, J. A novel marine pathogen isolated from wild cunners (Tautogolabrus adspersus): Comparative genomics and transcriptome profiling of Pseudomonas sp. Strain J380. Microorganisms 2021, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, I.; Liu, Y.; Wiegertjes, G.F.; Raaijmakers, J.M. Exploring fish microbial communities to mitigate emerging diseases in aquaculture. FEMS Microbiol. Ecol. 2017, 94, fix161. [Google Scholar] [CrossRef]
- Sun, F.; Wang, C.; Chen, L.; Weng, G.; Zheng, Z. The intestinal bacterial community of healthy and diseased animals and its association with the aquaculture environment. Appl. Microbiol. Biot. 2020, 104, 775–783. [Google Scholar] [CrossRef]
- Sun, F.; Wang, C.; Chen, X. Bacterial community in Sinonovacula constricta intestine and its relationship with culture environment. Appl. Microbiol. Biotechnol. 2022, 106, 5211–5220. [Google Scholar] [CrossRef]
- Sun, F.; Xu, Z. Significant differences in intestinal microbial communities in aquatic animals from an aquaculture area. J. Mar. Sci. Eng. 2021, 9, 104. [Google Scholar] [CrossRef]
- Romalde, J.L. Photobacterium damselae subsp. piscicida: An integrated view of a bacterial fish pathogen. Int. Microbiol. 2002, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Austin, B. The bacterial microflora of fish, revised. Sci. World J. 2006, 6, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sullam, K.; Essinger, S.; Lozupone, C.; O’connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Heilig, H.; Benvenuti, G.; Verreth, J.; Verdegem, M. The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci. Rep. 2015, 5, 18206. [Google Scholar] [CrossRef] [PubMed]
- Chaiyapechara, S.; Rungrassamee, W.; Suriyachay, I.; Kuncharin, Y.; Klanchui, A.; Karoonuthaisiri, N.; Jiravanichpaisal, P. Bacterial Community Associated with the Intestinal Tract of P. monodon in Commercial Farms. Microb. Ecol. 2012, 63, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Soonthornchai, W.; Rungrassamee, W.; Karoonuthaisiri, N.; Jarayabhand, P.; Klinbunga, S.; Soderhall, K.; Jiravanichpaisal, P. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 19–28. [Google Scholar] [CrossRef]
- Lavilla-Pitogo, C.R.; Leaño, E.M.; Paner, M.G. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture 1998, 164, 337–349. [Google Scholar] [CrossRef]
- Sung, H.-H.; Hsu, S.-F.; Chen, C.-K.; Ting, Y.-Y.; Chao, W.-L. Relationships between disease outbreak in cultured tiger shrimp (Penaeus monodon) and the composition of Vibrio communities in pond water and shrimp hepatopancreas during cultivation. Aquaculture 2001, 192, 101–110. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Wu, J.; Qiuqian, L.; Yang, K.; Qian, Y.; Zhang, D. Changes in intestinal bacterial communities are closely associated with shrimp disease severity. Appl. Microbiol. Biot. 2015, 99, 6911–6919. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.B.; van de Water, J.A.J.M.; Bourne, D.G.; Altier, C.; Hein, M.Y.; Fiorenza, E.A.; Abu, N.; Jompa, J.; Harvell, C.D. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science 2017, 355, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, A. The occurrence of human pathogenic Vibrio spp. and Salmonella in aquaculture. Int. J. Food Sci. Technol. 1998, 33, 127–138. [Google Scholar] [CrossRef]
- Feldhusen, F. The role of seafood in bacterialfoodborne diseases. Microbes Infect. 2000, 2, 1651–1660. [Google Scholar] [CrossRef]
- Tao, Z.; Larsen, A.M.; Bullard, S.A.; Wright, A.C.; Arias, C.R. Prevalence and population structure of Vibrio vulnificus on fishes from the northern gulf of Mexico. Appl. Environ. Microbiol. 2012, 78, 7611–7618. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Buján, N.; Altun, S.; Romalde, J.L.; Saticioglu, I.B. Population genetic and evolution analysis of Vibrio isolated from Turkish fish farms. Aquaculture 2023, 562, 738728. [Google Scholar] [CrossRef]
- Fleming, L.E.; Broad, K.; Clement, A.; Dewailly, E.; Elmir, S.; Knap, A.; Pomponi, S.A.; Smith, S.; Solo Gabriele, H.; Walsh, P. Oceans and human health: Emerging public health risks in the marine environment. Mar. Pollut. Bull. 2006, 53, 545–560. [Google Scholar] [CrossRef]
- Ganesh, E.A.; Das, S.; Chandrasekar, K.; Arun, G.; Balamurugan, S. Monitoring of total heterotrophic bacteria and Vibrio spp. in an aquaculture pond. Curr. Res. J. Biol. Sci. 2010, 2, 48–52. [Google Scholar]
- Engering, A.; Hogerwerf, L.; Slingenbergh, J. Pathogen–host–environment interplay and disease emergence. Emerg. Microbes Infect. 2013, 2, e5. [Google Scholar] [CrossRef]
- Caruso, G.; Maimone, G.; Mancuso, M.; Modica, A.; Genovese, L. Microbiological controls across the productive cycle of Dicentrarchus labrax L. and Sparus aurata L.: A study from the environment to the final product. Aquac. Res. 2004, 35, 184–193. [Google Scholar] [CrossRef]
- Walters, S.P.; Thebo, A.L.; Boehm, A.B. Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res. 2011, 45, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, C.W.; Tamplin, M.L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 1993, 59, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Höfle, M.; Pruzzo, C. Effects of global Warming on Vibrio ecology. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.E.; Riquelme, C.E. Production of a diatom-bacteria biofilm in a photobioreactor for aquaculture applications. Aquac. Eng. 2007, 36, 97–104. [Google Scholar] [CrossRef]
- Doménech, A.; Fernández-Garayzábal, J.F.; Lawson, P.; García, J.A.; Cutuli, M.T.; Blanco, M.; Gibello, A.; Moreno, M.A.; Collins, M.D.; Domínguez, L. Winter disease outbreak in sea-bream (Sparus aurata) associated with Pseudomonas anguilliseptica infection. Aquaculture 1997, 156, 317–326. [Google Scholar] [CrossRef]
- Vandenberg, O.; Dediste, A.; Houf, K.; Ibekwem, S.; Souayah, H.; Cadranel, S.; Douat, N.; Zissis, G.; Butzler, J.P.; Vandamme, P. Arcobacter species in humans. Emerg. Infect. Dis. 2004, 10, 1863–1867. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Klanchui, A.; Maibunkaew, S.; Chaiyapechara, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Characterization of intestinal bacteria in wild and domesticated adult black tiger shrimp (Penaeus monodon). PLoS ONE 2014, 9, e91853. [Google Scholar] [CrossRef]
- Gainza, O.; Ramírez, C.; Ramos, A.S.; Romero, J. Intestinal microbiota of white shrimp Penaeus vannamei under intensive cultivation conditions in ecuador. Microb. Ecol. 2018, 75, 562–568. [Google Scholar] [CrossRef]
- Ryan, K.J.; Ray, C.G. Medical microbiology. McGraw Hill 2004, 4, 370. [Google Scholar]
- Bernardet, J.-F. Order I. Flavobacteriales ord. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; Krieg, N.R., Staley, J.T., Brown, D.R., Hedlund, B.P., Paster, B.J., Ward, N.L., Ludwig, W., Whitman, W.B., Eds.; Wiley: Hoboken, NJ, USA, 2011; p. 105. [Google Scholar]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Organ. 2006, 71, 255–266. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, U.; Steiropoulos, N.A.; Thompson, K.D.; Adams, A.; Ellis, A.E.; Ferguson, H.W. Confirmation of Piscirickettsia salmonis as a pathogen in European sea bass Dicentrarchus labrax and phylogenetic comparison with salmonid strains. Dis. Aquat. Organ. 2005, 64, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Plante, C.J.; Jumars, P.A.; Baross, J.A. Digestive associations between marine detritivores and bacteria. Annu. Rev. Ecol. Syst. 1990, 21, 93–127. [Google Scholar] [CrossRef]
- Bøgwald, J.; Dalmo, R.A. Gastrointestinal pathogenesis in aquatic animals. In Aquaculture Nutrition; Wiley: Hoboken, NJ, USA, 2014; pp. 53–74. [Google Scholar] [CrossRef]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Hansen, G.H.; Olafsen, J.A. Bacterial interactions in early life stages of marine cold water fish. Microb. Ecol. 1999, 38, 1–26. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Wang, C.; Xu, Z. Abundance and Diversity of Several Bacterial Genera in the Mariculture Environment. J. Mar. Sci. Eng. 2024, 12, 209. https://doi.org/10.3390/jmse12020209
Sun F, Wang C, Xu Z. Abundance and Diversity of Several Bacterial Genera in the Mariculture Environment. Journal of Marine Science and Engineering. 2024; 12(2):209. https://doi.org/10.3390/jmse12020209
Chicago/Turabian StyleSun, Fulin, Chunzhong Wang, and Zhantang Xu. 2024. "Abundance and Diversity of Several Bacterial Genera in the Mariculture Environment" Journal of Marine Science and Engineering 12, no. 2: 209. https://doi.org/10.3390/jmse12020209
APA StyleSun, F., Wang, C., & Xu, Z. (2024). Abundance and Diversity of Several Bacterial Genera in the Mariculture Environment. Journal of Marine Science and Engineering, 12(2), 209. https://doi.org/10.3390/jmse12020209




