The Distribution Characteristics and Influencing Factors of the CO2 Fluxes Across the Sea–Air Interface in the Artificial Reefs Area of Sanheng Island
Abstract
1. Introduction
2. Materials and Methods
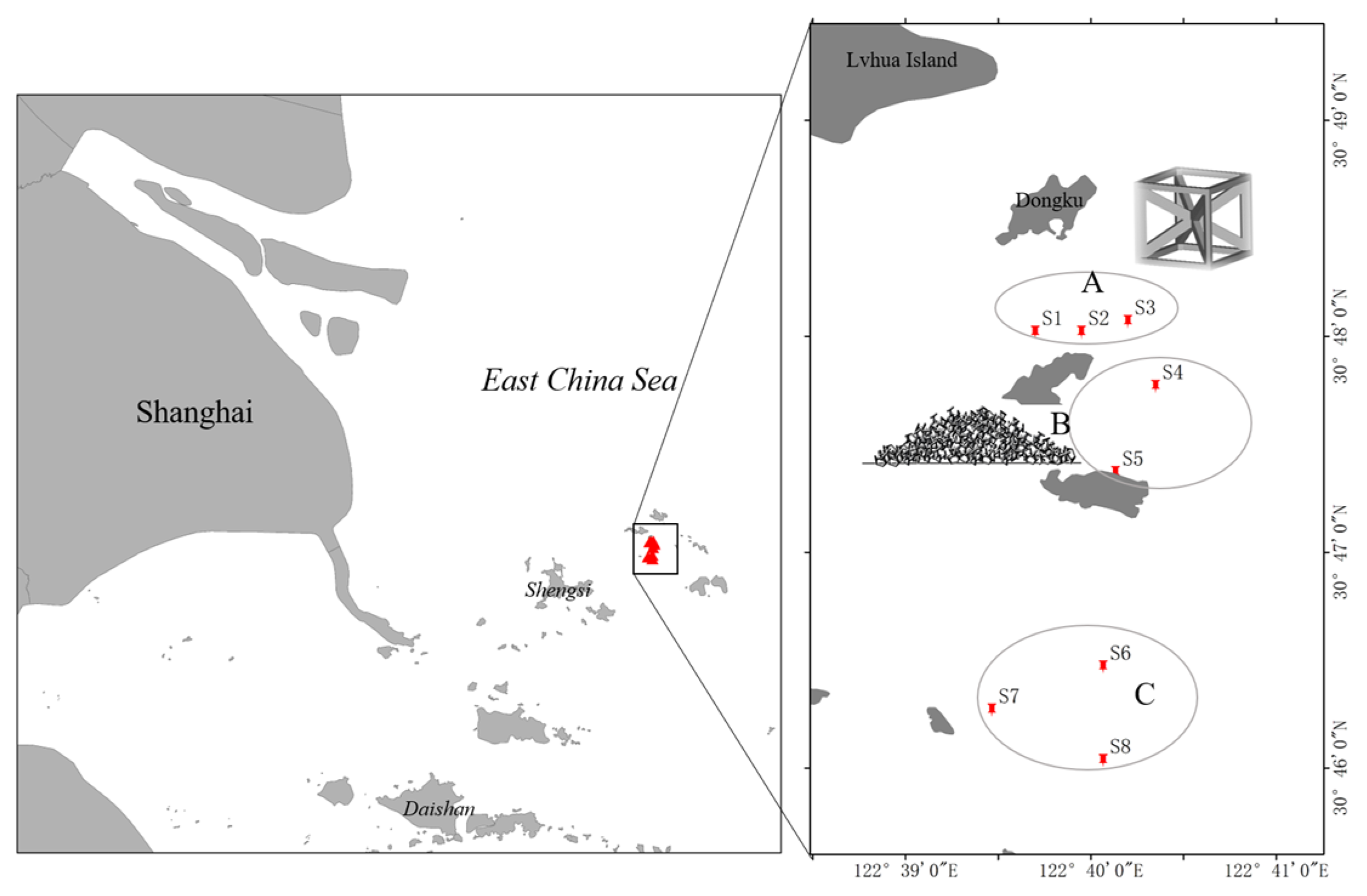
2.1. Study Area and Sampling Sites
2.2. Sample Collection and Processing
2.3. Estimation of Carbon Flux
2.4. Data Analysis
3. Results and Analysis
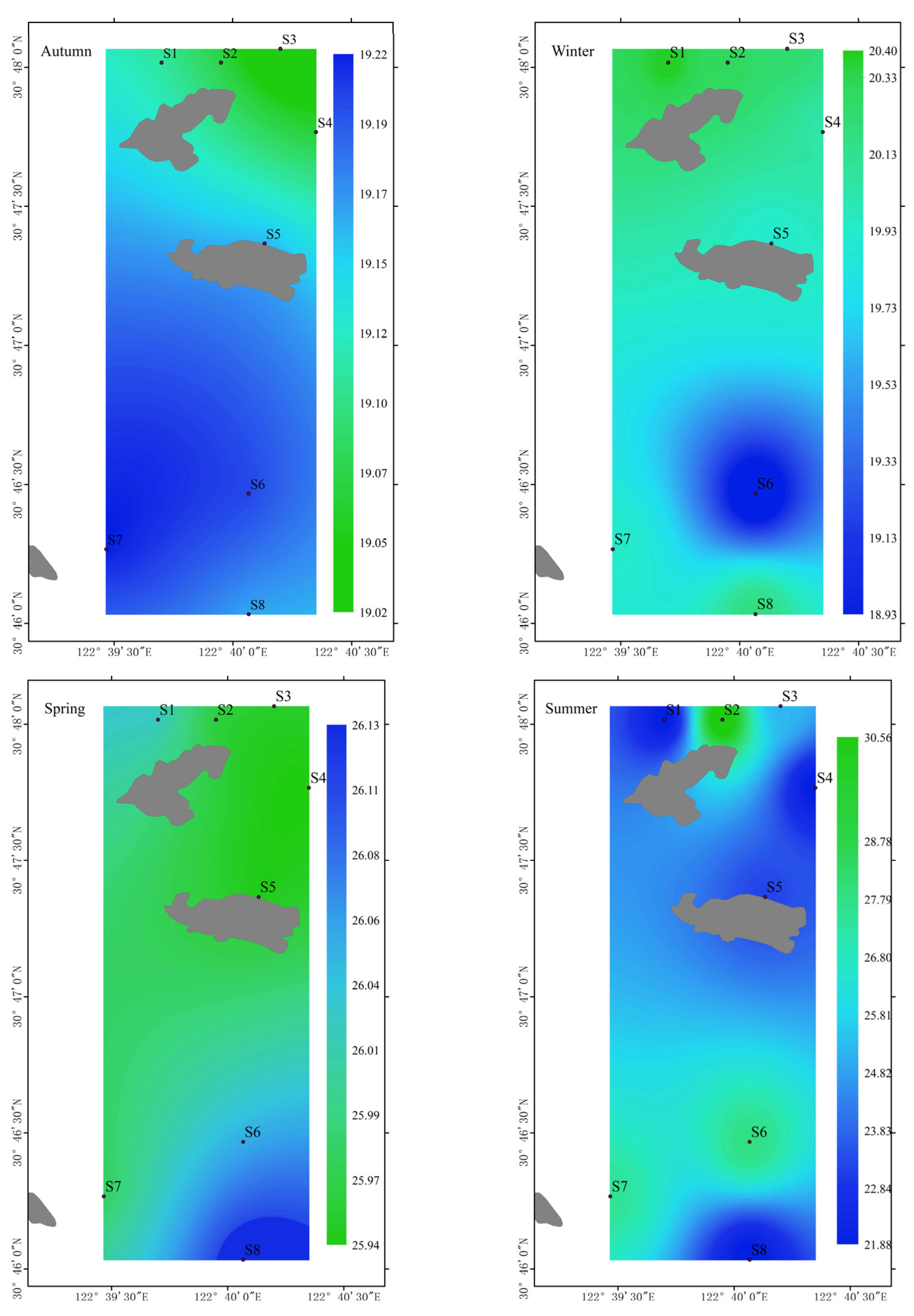
3.1. Surface Seawater Environment Characteristics
3.2. Surface Seawater pCO2w and FCO2
3.3. The Relationship Between Surface Seawater pCO2w and Environmental Factors
4. Discussion
4.1. The Main Environmental Factors of Island Reef Areas Affecting the Surface Seawater pCO2w
4.2. The Carbon Flux and Carbon Sequestration Capacity in Marine Ranching
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; House, J.I. Carbon and Other Biogeochemical Cycles; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Jiao, N. Developing Ocean Negative Carbon Emission Technology to Support National Carbon Neutralization. Bull. Chin. Acad. Sci. 2021, 36, 179–187. [Google Scholar]
- Feng, C.; Ye, G.; Jiang, Q.; Zheng, Y.; Chen, G.; Wu, J.; Feng, X.; Si, Y.; Zeng, J.; Li, P.; et al. The contribution of ocean-based solutions to carbon reduction in China. Sci. Total Environ. 2021, 797, 149168. [Google Scholar] [CrossRef] [PubMed]
- Reeds, K.A.; Smith, J.A.; Suthers, I.M.; Johnston, E.L. An ecological halo surrounding a large offshore artificial reef: Sediments, infauna, and fish foraging. Mar. Environ. Res. 2018, 141, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, X.; Zhang, S.; Lin, J. Marine Ranching Construction and Management in East China Sea: Programs for Sustainable Fishery and Aquaculture. Water 2019, 11, 1237. [Google Scholar] [CrossRef]
- Shi, X.; Wang, T.; Luo, K.; Wang, J.; Gu, X. The mechanism of constructing marine ranching industrial ecosystem based on grounded theory: A case study of Yantai, China. Reg. Stud. Mar. Sci. 2023, 68, 103214. [Google Scholar] [CrossRef]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liang, J.; Wang, Z.H.; Wang, K. Distribution characteristics of benthic algae in intertidal zone of Ma’an Archipelago of ZheJiang Province. Chin. J. Appl. Ecol. 2008, 10, 2299–2307. [Google Scholar]
- Ma, H.; Qin, C.; Chen, P.; Feng, X.; Yuan, R.; Li, X.; Lin, H. Study of biomass carbon storage in Zhelin Bay marine ranch of South China Sea. South China Fish. Sci. 2017, 13, 56–64. [Google Scholar] [CrossRef]
- Duarte, C.M.; Cebrian, J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996, 41, 1758–1766. [Google Scholar] [CrossRef]
- Wang, S.; Yan, D.; Wang, C.; Wu, L.; Huang, Y. A bibliometric analysis of blue carbon (1993–2023): Evolution of research hot topics and trends. Front. Mar. Sci. 2024, 11, 1430545. [Google Scholar] [CrossRef]
- Tang, Q. Fisheries carbon sinks: Faster and better development of modern fisheries. Jiangxi Fish. Sci. Technol. 2011, 5–7. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Z.; Di, Q.; Liang, C. Marine fishery carbon emission reduction and changing factors behind marine fishery eco-efficiency growth in China. Ecol. Inform. 2024, 80, 102478. [Google Scholar] [CrossRef]
- Shen, J.; Liang, R. Study on the blue carbon sink pricing of marine ranch. Resour. Sci. 2018, 40, 1812–1821. [Google Scholar] [CrossRef]
- Shi, C.; Zhi, J.; Yao, X.; Zhang, H.; Yu, Y.; Zeng, Q.; Li, L.; Zhang, Y. How can China achieve the 2030 carbon peak goal—A crossover analysis based on low-carbon economics and deep learning. Energy 2023, 269, 126776. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, A.D.; Da, Y.B. Regional allocation of carbon emission quotas in China: Evidence from the Shapley value method. Energy Policy 2014, 74, 454–464. [Google Scholar] [CrossRef]
- Chen, X.; Di, Q.; Jia, W.; Hou, Z. Spatial correlation network of pollution and carbon emission reductions coupled with high-quality economic development in three Chinese urban agglomerations. Sustain. Cities Soc. 2023, 94, 104552. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H. Spatiotemporal dynamic decoupling states of eco-environmental quality and land-use carbon emissions: A case study of Qingdao City, China. Ecol. Inform. 2023, 75, 101992. [Google Scholar] [CrossRef]
- Na, R.; Rong, Z.; Wang, Z.A.; Liang, S.; Liu, C.; Ringham, M.; Liang, H. Air-sea CO2 fluxes and cross-shelf exchange of inorganic carbon in the East China Sea from a coupled physical-biogeochemical model. Sci. Total Environ. 2024, 906, 167572. [Google Scholar] [CrossRef]
- Liu, K.K.; Peng, T.H.; Shaw, P.T.; Shiah, F.K. Circulation and biogeochemical processes in the East China Sea and the vicinity of Taiwan: An overview and a brief synthesis. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 1055–1064. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, H.; Zhu, Z.; Liu, L.; Zhou, S. Distribution characteristics of p(CO2) in the sea-atmosphere interface in the East China Sea. Environ. Sci. Technol. 2019, 42, 121–127. [Google Scholar]
- Peng, P.; Ma, Y.; Shi, R.; Wang, D.; Xu, X.; Yan, B. Temporal and spatial variation in the sea–air CO2 flux in the oyster aquaculture area of Kaozhou Bay. Mar. Sci. 2022, 46, 140–149. [Google Scholar]
- Fodrie, F.J.; Rodriguez, A.B.; Gittman, R.K.; Grabowski, J.H.; Lindquist, N.L.; Peterson, C.H.; Piehler, M.F.; Ridge, J.T. Oyster reefs as carbon sources and sinks. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Zhao, Y.; Zhou, S.; Wu, Y. Prediction and assessment of marine fisheries carbon sink in China based on a novel nonlinear grey Bernoulli model with multiple optimizations. Sci. Total Environ. 2024, 914, 169769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Hu, X. The evolution and effectiveness of China’s marine carbon sink fishery policies. Ocean Coast. Manag. 2024, 259, 107470. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; PICES Special Publication; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007. [Google Scholar]
- Zhou, J.H.; Guo, Y.Z.; Zhao, X.; Yu, J.C.; Zhou, J.; Lin, J.; Huang, H. Impacts of shellfish and macroalgae mariculture on the seawater carbonate system and air-sea CO2 flux in Haizhou Bay, China. Mar. Environ. Res. 2024, 202, 106774. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.G.; Miller, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Cai, W.J.; Dai, M.; Wang, Y.; Zhai, W.; Huang, T.; Chen, S.; Zhang, F.; Chen, Z.; Wang, Z. The biogeochemistry of inorganic carbon and nutrients in the Pearl River estuary and the adjacent Northern South China Sea. Cont. Shelf Res. 2004, 12, 1301–1319. [Google Scholar] [CrossRef]
- Dickson, A.G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 5, 755–766. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods 2014, 6, 351–362. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, B.; Cui, Z.; Lin, Z.; Xie, Z.; Zheng, H.; Liao, B.; Xiao, B.; Zhu, C. Distribution characteristics of pCO2 and source-sink conversion mechanism of CO2 in a subtropical coral reef water during the dry season: A case study of the Yangmeikeng Sea area in Shenzhen, China. Mar. Sci. 2023, 47, 45–56. [Google Scholar]
- Song, J.M.; Li, X.G.; Yuan, H.M.; Zheng, G.X.; Yang, Y.F. Carbon Fixed by Phytoplankton and Cultured algae in China Coastal Seas. Acta Ecol. Sin. 2008, 2, 551–559. [Google Scholar]
- Craig, S.E.; Thomas, H.; Jones, C.T.; Li, W.K.; Greenan, B.J.; Shadwick, E.H.; Burt, W.J. The effect of seasonality in phytoplankton community composition on CO2 uptake on the Scotian Shelf. J. Mar. Syst. 2015, 147, 52–60. [Google Scholar] [CrossRef]
- Kako, S.I.; Nakagawa, T.; Takayama, K.; Hirose, N.; Isobe, A. Impact of Changjiang River Discharge on Sea Surface Temperature in the East China Sea. J. Phys. Oceanogr. 2016, 46, 1735–1750. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, M. On the seasonal variation of air-sea CO2 fluxes in the outer Changjiang (Yangtze River) Estuary, East China Sea. Mar. Chem. 2009, 117, 2–10. [Google Scholar] [CrossRef]
- Hu, D.; Gao, G.; Zhai, H.; Zhang, C. Seasonal Variability of Shipboard Air-Sea CO2 Flux in the East China Sea and South Yellow Sea. Adv. Mar. Sci. 2016, 34, 474–485. [Google Scholar]
- Li, G.; Qiao, L.; Dong, P.; Ma, Y.; Xu, J.; Liu, S.; Liu, Y.; Li, J.; Li, P.; Ding, D.; et al. Hydrodynamic condition and suspended sediment diffusion in the Yellow Sea and East China Sea. J. Geophys. Res. Ocean 2016, 121, 6204–6222. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, L.; Li, G.; Li, J.; Wang, N.; Yang, J. Distribution and cross-front transport of suspended particulate matter over the inner shelf of the East China Sea. Cont. Shelf Res. 2015, 107, 92–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, M.; Zhong, Y.; Zhang, G.; Jiang, S.; Gao, Y.; Zhang, R.; Smith, W.O., Jr. Spatial Variations of Phytoplankton Biomass Controlled by River Plume Dynamics Over the Lower Changjiang Estuary and Adjacent Shelf Based on High-Resolution Observations. Front. Mar. Sci. 2020, 7, 587539. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, W.; Zhao, Y.; Guo, X.; Yin, Z.; Du, C.; Zhao, H.; Dai, M. Diagnosis of CO2 dynamics and fluxes in global coastal oceans. Natl. Sci. Rev. 2020, 7, 786–797. [Google Scholar] [CrossRef]
- Qu, B.; Song, J.; Yuan, H.; Li, X. Advances of seasonal variations and controlling factors of the sea-air CO2 flux in the East China Sea. Adv. Earth Sci. 2013, 28, 783–793. [Google Scholar]
- Koike, S.; Otake, S. The Effect to the Flow around the Artificial Reef in the Different Arrangement in Situ. Fish. Eng. 2017, 53, 139–147. [Google Scholar]
- Gong, P.; Li, J.; Guan, C.; Li, M. Estimation and experiment of carbon sequestration by oysters attached to the enhancement artificial. Chin. J. Appl. Ecol. 2014, 25, 3032–3038. [Google Scholar]
- Fang, L.; Chen, P.; Chen, G.; Tang, Y.; Yuan, H.; Feng, X. Preliminary Evaluation on Resources Enhancement of Artificial Reef in the East Corner of Zhelang Shanwei. Asian Agric. Res. 2013, 5, 111–115. [Google Scholar]
- Chen, Y. Analysis of Ecological Effects of Southern Dalajia Island Artificial Area in Daya Bay, Guang Dong, China. Ph.D. Thesis, Jinan University, Guangzhou, China, 2009. [Google Scholar]
- Jiao, J.J.; Pan, Y.X.; Sun, L.Y.; Yang, B.Q.; Qiu, S.Y. Effect of Artificial reefs on Fish Multiplication. Fish. Sci. 2011, 2, 79–82. [Google Scholar]
- Feng, X.; Fan, J.T.; Sun, X.; Hong, J.Z.; Chen, P.M. The stock enhancement effect evaluation of artificial reef in Wailingding, Zhuhai. J. South. Agric. 2021, 52, 3228–3236. [Google Scholar]
- Zhang, H.; Zhu, K.W.; Tang, J.H. The effect of fishery resource enhancement in the artificial reef area in the Haizhou Gulf. Mar. Fishries 2005, 1, 38–43. [Google Scholar]
- Wang, Z.; Zhang, S.; Wang, K. Fish and macroinvertebrates community structure in artificial habitat around Sanheng Island, Shengsi, China. Acta Ecol. Sin. 2010, 30, 2026–2935. [Google Scholar]
- Champion, C.; Suthers, I.M.; Smith, J.A. Zoo planktivory is a key process for fish production on a coastal artificial reef. Mar. Ecol. Prog. Ser. 2015, 541, 1–14. [Google Scholar] [CrossRef]
- Badalamenti, F.; Chemello, R.; D’anna, G.; Ramos, P.H.; Riggio, S. Are artificial reefs comparable to neighboring natural rocky areas? A mollusk case study in the Gulf of Castellammare (NW Sicily). ICES J. Mar. Sci. 2002, 59, S127–S131. [Google Scholar] [CrossRef]
- Crawford, C.M.; Edgar, G.J.; Gillies, C.L.; Heller-Wagner, G. Relationship of biological communities to habitat structure on the largest remnant flat oyster reef (Ostrea angasi) in Australia. Mar. Freshw. Res. 2020, 71, 972–983. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G.; Opaluch, J.J.; Peterson, C.H.; Piehler, M.F.; Powers, S.P.; Smyth, A.R. Economic Valuation of Ecosystem Services Provided by Oyster Reefs. Bioscience 2012, 62, 900–909. [Google Scholar] [CrossRef]
- Xiao, X.; Agustí, S.; Yu, Y.; Huang, Y.; Chen, W.; Hu, J.; Li, C.; Li, K.; Wei, F.; Lu, Y.; et al. Seaweed farms provide refugia from ocean acidification. Sci. Total Environ. 2021, 776, 145192. [Google Scholar] [CrossRef] [PubMed]

| Season | Region | SST (°C) | Salinity (pSU) | pH | TA (mg/L) | DIC (mg/L) |
|---|---|---|---|---|---|---|
| Autumn | A | 20.78 | 32.20 | 8.33 | 134.59 | 19.05 |
| B | 20.98 | 32.75 | 8.24 | 132.97 | 19.13 | |
| C | 20.37 | 33.3 | 8.07 | 146.98 | 19.21 | |
| Winter | A | 11.31 | 32.13 | 7.82 | 153.77 | 20.33 |
| B | 11.15 | 32.15 | 7.81 | 152.95 | 20.01 | |
| C | 11.26 | 31.50 | 7.81 | 146.36 | 19.37 | |
| Spring | A | 19.93 | 28.80 | 8.06 | 133.87 | 26.10 |
| B | 19.35 | 29.10 | 8.04 | 136.52 | 22.00 | |
| C | 18.97 | 29.5 | 8.07 | 132.29 | 26.49 | |
| Summer | A | 27.18 | 27.54 | 8.27 | 97.65 | 25.88 |
| B | 27.48 | 28.84 | 8.25 | 87.94 | 22.55 | |
| C | 27.41 | 27.93 | 8.36 | 80.03 | 25.75 |
| Item | Area | Autumn | Winter | Spring | Summer |
|---|---|---|---|---|---|
| pCO2w | Total area | 159.32 ± 67.13 | 477.36 ± 21.74 | 228.55 ± 17.03 | 73.33 ± 30.23 |
| united artificial reefs | 130.50 ± 73.31 | 487.12 ± 25.51 | 230.57 ± 8.54 | 91.65 ± 32.22 | |
| artificial seamount | 109.22 ± 11.79 | 486.31 ± 21.53 | 243.18 ± 18.83 | 79.19 ± 8.79 | |
| control area | 221.54 ± 64.34 | 461.63 ± 13.31 | 216.79 ± 17.03 | 51.10 ± 18.49 | |
| FCO2 | Total area | −86.3 ± 21.99 | 25.89 ± 9.62 | −66.86 ± 6.59 | −98.61 ± 8.22 |
| united artificial reefs | −95.94 ± 24.39 | 30.16 ± 11.24 | −65.45 ± 6.07 | −93.95 ± 10.69 | |
| artificial seamount | −102.22 ± 4.38 | 29.93 ± 9.68 | −61.79 ± 4.00 | −95.26 ± 1.43 | |
| control area | −66.06 ± 20.96 | 18.94 ± 9.62 | −71.67 ± 6.61 | −104.83 ± 5.00 |
| SST | Salinity | pH | DIC | TA | pCO2w | FCO2 | |
|---|---|---|---|---|---|---|---|
| SST | 1 | ||||||
| Salinity | −0.60 ** | 1 | |||||
| pH | 0.89 ** | −0.39 * | 1 | ||||
| DIC | 0.44 * | −0.73 ** | 0.29 | 1 | |||
| TA | −0.83 ** | 0.68 ** | −0.70 ** | −0.36 * | 1 | ||
| pCO2w | −0.95 ** | 0.47 ** | −0.97 ** | −0.34 | 0.75 ** | 1 | |
| FCO2 | −0.91 ** | 0.42 * | −0.95 ** | −0.32 | 0.67 ** | 0.99 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Wang, K.; Zhao, X.; Dai, L.; Chen, L.; Zhou, H. The Distribution Characteristics and Influencing Factors of the CO2 Fluxes Across the Sea–Air Interface in the Artificial Reefs Area of Sanheng Island. J. Mar. Sci. Eng. 2024, 12, 2272. https://doi.org/10.3390/jmse12122272
Zhu M, Wang K, Zhao X, Dai L, Chen L, Zhou H. The Distribution Characteristics and Influencing Factors of the CO2 Fluxes Across the Sea–Air Interface in the Artificial Reefs Area of Sanheng Island. Journal of Marine Science and Engineering. 2024; 12(12):2272. https://doi.org/10.3390/jmse12122272
Chicago/Turabian StyleZhu, Mingdong, Kai Wang, Xu Zhao, Linwei Dai, Lei Chen, and Hanyu Zhou. 2024. "The Distribution Characteristics and Influencing Factors of the CO2 Fluxes Across the Sea–Air Interface in the Artificial Reefs Area of Sanheng Island" Journal of Marine Science and Engineering 12, no. 12: 2272. https://doi.org/10.3390/jmse12122272
APA StyleZhu, M., Wang, K., Zhao, X., Dai, L., Chen, L., & Zhou, H. (2024). The Distribution Characteristics and Influencing Factors of the CO2 Fluxes Across the Sea–Air Interface in the Artificial Reefs Area of Sanheng Island. Journal of Marine Science and Engineering, 12(12), 2272. https://doi.org/10.3390/jmse12122272




