Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Total Lipid Content, Oil Yield Extraction, and Fatty Acid Profiles of Sargassum spp.
2.2. Kinematic Viscosity and Viscosity Index (VI) of the Oil Formulations
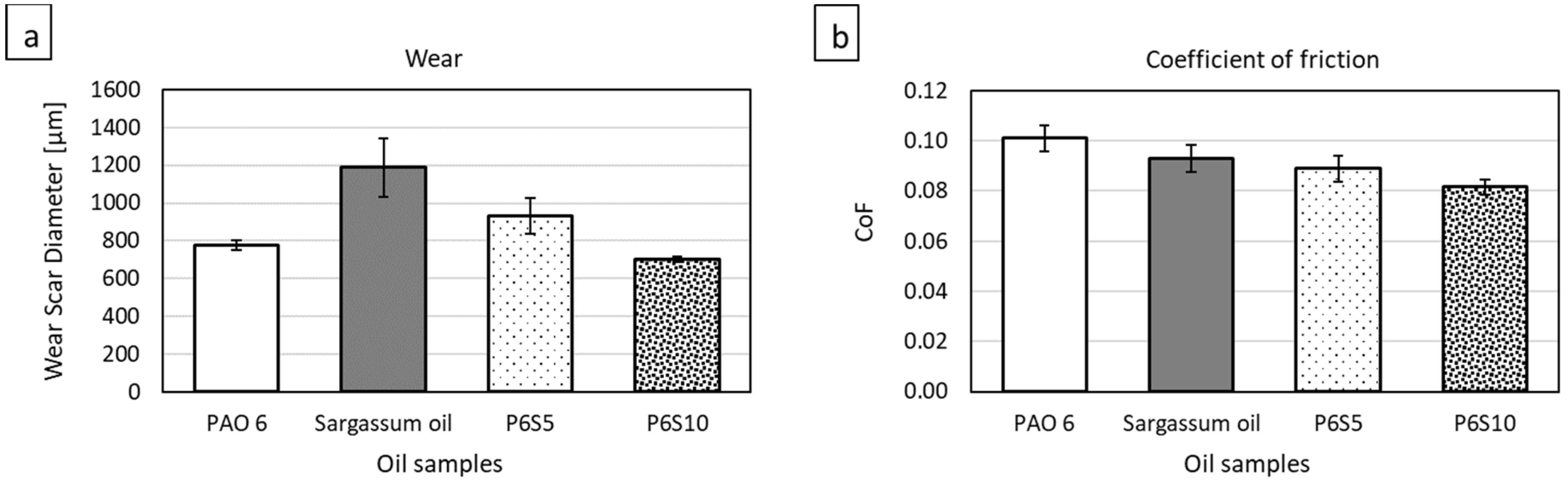
2.3. Tribological Behavior
3. Materials and Methods
3.1. Macroalgae Biomass Collection, Pre-Treatment, and Oil Extraction
3.2. Evaluation of the Total Lipid Content and FAME Profile of Sargassum spp.
3.3. Lubricant Sample Preparation
3.4. Determination of Density and Viscosity of the Oils
3.5. Tribological Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Khourdajie, A.A.; House, J.; et al. A Review of Trends and Drivers of Greenhouse Gas Emissions by Sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Wang, Z.; Stout, S.A. Chemical fingerprinting methods and factors affecting petroleum fingerprints in the environment. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed; Academic Press: Cambridge, MA, USA, 2016; Chapter 3; pp. 61–129. [Google Scholar]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and Health Effects of Lubricant Oils Emitted into the Environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xia, C.; Alqahtani, A.; Sharma, A.; Pugazhendhi, A. A Review on Optimistic Biorefinery Products: Biofuel and Bioproducts from Algae Biomass. Fuel 2023, 338, 127378. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, S.; Angelidaki, I.; Holdt, S.L.; Karakashev, D.B.; Morales, M.A.; Das, D. Recent Developments on Biofuels Production from Microalgae and Macroalgae. Renew. Sustain. Energy Rev. 2016, 65, 235–249. [Google Scholar] [CrossRef]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; de Nys, R. Total Lipid and Fatty Acid Composition of Seaweeds for the Selection of Species for Oil-based Biofuel and Bioproducts. GCB Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef]
- Russell, C.; Rodriguez, C.; Yaseen, M. Microalgae for Lipid Production: Cultivation, Extraction & Detection. Algal Res. 2022, 66, 102765. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for Biofuels: A Review of Thermochemical Conversion Processes and Associated Opportunities and Challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.J.; Guo, H.W.; Wang, Y.J.; Ji, R.; Kang, L.L.; Wang, Y.T.; Guo, X.; Li, J.G.; Jiang, L.Q.; et al. A Review of the Strategy to Promote Microalgae Value in CO2 Conversion-Lipid Enrichment-Biodiesel Production. J. Clean. Prod. 2024, 436, 140538. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of Art Review on Conventional and Advanced Pyrolysis of Macroalgae and Microalgae for Biochar, Bio-Oil and Bio-Syngas Production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Lim, J.H.K.; Gan, Y.Y.; Ong, H.C.; Lau, B.F.; Chen, W.H.; Chong, C.T.; Ling, T.C.; Klemeš, J.J. Utilization of Microalgae for Bio-Jet Fuel Production in the Aviation Sector: Challenges and Perspective. Renew. Sustain. Energy Rev. 2021, 149, 111396. [Google Scholar] [CrossRef]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal Biorefinery Concepts for the Circular Bioeconomy: A Review on Biotechnological Developments and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Jaworowska, A.; Murtaza, A. Seaweed derived lipids are a potential anti-inflammatory agent: A Review. Int. J. Environ. Res. Public Health 2022, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Chen, M.; Wang, J. Microwave-assisted pyrolysis of seaweed biomass for aromatics-containing bio-oil production. E3S Web Conf. 2021, 261, 02045. [Google Scholar] [CrossRef]
- Choi, J.H.; Woo, H.C.; Suh, D.J. Pyrolysis of Seaweeds for Bio-oil and Bio-char Production. Chem. Eng. Trans. 2014, 37, 121–126. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ly, H.V.; Choi, G.-H.; Kim, J.; Woo, H.C. Pyrolysis characteristics and kinetics of the alga Saccharina japonica. Bioresour. Technol. 2012, 123, 445–451. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Jordán-Dahlgren, E. Pelagic Sargassum Cleanup Cost in Mexico. Ocean. Coast. Manag. 2023, 237, 106542. [Google Scholar] [CrossRef]
- Rosellón-Druker, J.; Calixto-Pérez, E.; Escobar-Briones, E.; González-Cano, J.; Masiá-Nebot, L.; Córdova-Tapia, F. A Review of a Decade of Local Projects, Studies and Initiatives of Atypical Influxes of Pelagic Sargassum on Mexican Caribbean Coasts. Phycology 2022, 2, 254–279. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum Linearifolium and Cystoseira Crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef]
- Belattmania, Z.; El Atouani, S.; Bentiss, F.; Jama, C.; Falace, A.; Chaouti, A.; Reani, A.; Sabour, B. Seasonal Patterns of Growth, Alginate Content and Block Structure of the Alien Invader Sargassum Muticum (Fucales, Ochrophyta) from the Atlantic Coast of Morocco. Bot. Mar. 2022, 65, 69–78. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Bressi, V.; Chiofalo, M.T.; Morabito, M.; Espro, C.; Genovese, G.; Iannazzo, D.; Trifilò, P. Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus Vulgaris. Plants 2023, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Soekamto, N.H.; Firdaus, B. Total Phenolics and Flavonoids Level of N-Hexane, Ethyl Acetate and Methanol Extracts of Sargassum Sp. Along with Their Antioxidant Activity by DPPH Method; AIP Publishing: Melville, NY, USA, 2022; p. 060009. [Google Scholar]
- Farfan-Cabrera, L.I.; Rojo-Valerio, A.; Calderon-Najera, J.d.D.; Coronado-Apodaca, K.G.; Iqbal, H.M.N.; Parra-Saldivar, R.; Franco-Morgado, M.; Elias-Zuñiga, A. Microalgae Oil-Based Metal Working Fluids for Sustainable Minimum Quantity Lubrication (MQL) Operations—A Perspective. Lubricants 2023, 11, 215. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Franco-Morgado, M.; González-Sánchez, A.; Pérez-González, J.; Marín-Santibáñez, B.M. Microalgae Biomass as a New Potential Source of Sustainable Green Lubricants. Molecules 2022, 27, 1205. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Uppar, R.; Dinesha, P.; Kumar, S. A Critical Review on Vegetable Oil-Based Bio-Lubricants: Preparation, Characterization, and Challenges. Environ. Dev. Sustain. 2023, 25, 9011–9046. [Google Scholar] [CrossRef]
- Hamnas, A.; Unnikrishnan, G. Bio-Lubricants from Vegetable Oils: Characterization, Modifications, Applications and Challenges—Review. Renew. Sustain. Energy Rev. 2023, 182, 113413. [Google Scholar] [CrossRef]
- Perera, M.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Bioprocess Development for Biolubricant Production Using Non-Edible Oils, Agro-Industrial Byproducts and Wastes. J. Clean. Prod. 2022, 357, 131956. [Google Scholar] [CrossRef]
- Gul, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Mujtaba, M.A. A Review: Role of Fatty Acids Composition in Characterizing Potential Feedstock for Sustainable Green Lubricants by Advance Transesterification Process and Its Global as Well as Pakistani Prospective. Bioenergy Res. 2020, 13, 1–22. [Google Scholar] [CrossRef]
- Wedler, C.; Trusler, J.P.M. Review of Density and Viscosity Data of Pure Fatty Acid Methyl Ester, Ethyl Ester and Butyl Ester. Fuel 2023, 339, 127466. [Google Scholar] [CrossRef]
- Cheah, M.Y.; Ong, H.C.; Zulkifli, N.W.M.; Masjuki, H.H.; Salleh, A. Physicochemical and Tribological Properties of Microalgae Oil as Biolubricant for Hydrogen-Powered Engine. Int. J. Hydrogen Energy 2020, 45, 22364–22381. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A.E.F. Screening of Seaweeds for Sustainable Biofuel Recovery through Sequential Biodiesel and Bioethanol Production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493. [Google Scholar] [CrossRef] [PubMed]
- Jeliani, Z.Z.; Fazelian, N.; Yousefzadi, M. Introduction of Macroalgae as a Source of Biodiesel in Iran: Analysis of Total Lipid Content, Fatty Acid and Biodiesel Indices. J. Mar. Biol. Assoc. U. K. 2021, 101, 527–534. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Reyes, A.G.; Morreeuw, Z.P.; Flores-Contreras, E.A.; Araújo, R.G.; Ramírez-Gamboa, D.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; González-Meza, G.M.; Bonaccorso, A.D.; et al. Comparative Study on the Valorization of Sargassum from the Mexican Caribbean Coast and Gulf of California as an Ingredient on Healthy Diets for Shrimp Farming. Aquac. Rep. 2023, 32, 101709. [Google Scholar] [CrossRef]
- de Melo, N.S.M.; Cardoso, L.G.; de Castro Nunes, J.M.; Brito, G.B.; Caires, T.A.; de Souza, C.O.; Portz, L.; Druzian, J.I. Effects of Dry and Rainy Seasons on the Chemical Composition of Ulva Fasciata, Crassiphycus Corneus, and Sargassum Vulgare Seaweeds in Tropical Environment. Braz. J. Bot. 2021, 44, 331–344. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Melchor-Martínez, E.M.; Carrillo-Nieves, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Seasonal Characterization and Quantification of Biomolecules from Sargassum Collected from Mexican Caribbean Coast—A Preliminary Study as a Step Forward to Blue Economy. J. Environ. Manag. 2021, 298, 113507. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An Overview of Extraction and Purification Techniques of Seaweed Dietary Fibers for Immunomodulation on Gut Microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal Polyunsaturated Fatty Acids: Hotspots and Production Techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Liu, T.; Zhang, L.; Liu, H.; Guan, H. Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Mar. Drugs 2016, 14, 68. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Milledge, J.J.; Maneein, S.; Arribas-López, E.; Bartlett, D. Sargassum Inundations in Turks and Caicos: Methane Potential and Proximate, Ultimate, Lipid, Amino Acid, Metal and Metalloid Analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef]
- Prasannakumar, P.; Edla, S.; Thampi, A.D.; Arif, M.; Santhakumari, R. A Comparative Study on the Lubricant Properties of Chemically Modified Calophyllum Inophyllum Oils for Bio-Lubricant Applications. J. Clean. Prod. 2022, 339, 130733. [Google Scholar] [CrossRef]
- Nishimura, K.; Maeda, K.; Kuramochi, H.; Nakagawa, K.; Asakuma, Y.; Fukui, K.; Osako, M.; Sakai, S. Solid−Liquid Equilibria in Fatty Acid/Triglycerol Systems. J. Chem. Eng. Data 2011, 56, 1613–1616. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Kargın, H.; Bilgüven, M. Microalgae—Macroalgae based nutraceuticals and their benefits. Curr. Trends Nat. Sci. 2022, 11, 232–246. [Google Scholar] [CrossRef]
- Pardilhó, S.; Costa, E.; Melo, D.; Machado, S.; Espírito-Santo, L.; Oliveira, M.B.; Maia-Dias, J. Comprehensive Characterization of Marine Macroalgae Waste and Impact of Oil Extraction, Focusing on the Biomass Recovery Potential. Algal Res. 2021, 58, 102416. [Google Scholar] [CrossRef]
- Berneira, L.M.; de Santi, I.I.; da Silva, C.C.; Venzke, D.; Colepicolo, P.; Vaucher, R.d.A.; dos Santos, M.A.Z.; de Pereira, C.M.P. Bioactivity and Composition of Lipophilic Metabolites Extracted from Antarctic Macroalgae. Braz. J. Microbiol. 2021, 52, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Tabakaev, A.V.; Tabakaeva, O.V. Fatty Acids from the Brown Seaweed Sargassum Miyabei. Chem. Nat. Compd. 2021, 57, 911–913. [Google Scholar] [CrossRef]
- Santos, D.C.; Lima, E.R.A.; Paredes, M.L.L. Solubility Parameter of Narrow Oil Cuts by Several Models: Quantifying the Discrepancy among Predictions for Heavy Cuts. Braz. J. Chem. Eng. 2021, 38, 967–976. [Google Scholar] [CrossRef]
- Rac, A.; Vencl, A. Performance investigation of chain saw lubricants based on new sunflower oil (NSO). Tribol. Schmier. 2009, 56, 51–54. [Google Scholar]
- Farfan-Cabrera, L.I.; Gallardo-Hernández, E.A.; Gómez-Guarneros, M.; Pérez-González, J.; Godínez-Salcedo, J.G. Alteration of lubricity of jatropha oil used as bio-lubricant for engines due to thermal ageing. Renew. Energy 2020, 149, 1197–1204. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Honary, L.A.T. An investigation of the use of soybean oil in hydraulic systems. Bioresour. Technol. 1996, 56, 41–47. [Google Scholar] [CrossRef]
- Zulkifli, N.; Masjuki, H.; Kalam, M.; Yunus, R.; Azman, S. Lubricity of bio-based lubricant derived from chemically modified jatropha methyl ester. J. Tribol. 2014, 1, 18–39. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Aguilar-Rosas, O.A.; Farfan-Cabrera, L.I.; Erdemir, A.; Cao-Romero-Gallegos, J.A. Electrified four-ball testing—A potential alternative for assessing lubricants (E-fluids) for electric vehicles. Wear 2023, 522, 204676. [Google Scholar] [CrossRef]

| Compound Name | Carbon Number | Percent (%) of Total Fatty Acids |
|---|---|---|
| Myristic acid | C14:0 | 1.64 ± 0.01 |
| Palmitic acid | C16:0 | 29.72 ± 0.45 |
| Palmitoleic acid | C16:1 | 5.86 ± 0.11 |
| Stearic acid | C18:0 | 3.37 ± 0.14 |
| Oleic acid | C18:1n9 | 25.45 ± 0.54 |
| Linoleic acid | C18:2n6 | 2.63 ± 0.05 |
| Alpha-linolenic acid | C18:3n3 | 12.19 ± 0.33 |
| Arachidonic acid | C20:4n6 | 0.73 ± 0.01 |
| Eicosapentaenoic acid | C20:5n3 | 12.04 ± 1.70 |
| Erucic acid | C22:1n9 | 2.03 ± 0.04 |
| Docosahexaenoic acid | C22:6n3 | 4.31 ± 0.20 |
| ∑SFAs | 34.73 ± 0.59 | |
| ∑MUFAs | 33.34 ± 0.69 | |
| ∑PUFAs | 31.91 ± 1.81 |
| Oil | Viscosity Index (VI) | Reference |
|---|---|---|
| Sunflower oil | 218 | [52] |
| Jatropha oil | 262 | [53] |
| Rapeseed oil | 218 | [54] |
| Soybean oil | 246 | [55] |
| Test Parameter | Value |
|---|---|
| Load [N] | 392 |
| Maximum Hertzian contact pressure [GPa] | 4.5 |
| Speed [rpm] | 1200 |
| Test duration [min] | 60 |
| Temperature [°C] | 75 ± 2 |
| Humidity [%] | 32 |
| Amount of oil per test [mL] | 15 |
| Repeats using new samples | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Meza, G.M.; Rubio-Hernández, C.C.; López-Pacheco, I.Y.; López-Pacheco, L.D.; Marín-Santibáñez, B.M.; Medina-Bañuelos, E.F.; Melchor-Martínez, E.M.; Farfan-Cabrera, L.I. Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations. J. Mar. Sci. Eng. 2024, 12, 2242. https://doi.org/10.3390/jmse12122242
González-Meza GM, Rubio-Hernández CC, López-Pacheco IY, López-Pacheco LD, Marín-Santibáñez BM, Medina-Bañuelos EF, Melchor-Martínez EM, Farfan-Cabrera LI. Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations. Journal of Marine Science and Engineering. 2024; 12(12):2242. https://doi.org/10.3390/jmse12122242
Chicago/Turabian StyleGonzález-Meza, Georgia M., Carlos C. Rubio-Hernández, Itzel Y. López-Pacheco, Lizbeth D. López-Pacheco, Benjamín M. Marín-Santibáñez, Esteban F. Medina-Bañuelos, Elda M. Melchor-Martínez, and Leonardo I. Farfan-Cabrera. 2024. "Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations" Journal of Marine Science and Engineering 12, no. 12: 2242. https://doi.org/10.3390/jmse12122242
APA StyleGonzález-Meza, G. M., Rubio-Hernández, C. C., López-Pacheco, I. Y., López-Pacheco, L. D., Marín-Santibáñez, B. M., Medina-Bañuelos, E. F., Melchor-Martínez, E. M., & Farfan-Cabrera, L. I. (2024). Evaluation of Sargassum spp. Oil as a Potential Additive for Biolubricant Formulations. Journal of Marine Science and Engineering, 12(12), 2242. https://doi.org/10.3390/jmse12122242







