1. Introduction
Water is one of the fundamental necessities required for life. Water scarcity and the growing demand for water are among the most pressing problems facing development, industry, and urbanization [
1,
2,
3]. Around 1.2 billion people around the globe are affected by extreme water scarcity [
4]. Much of the world’s water cannot be utilized directly for freshwater consumption, even in the small amounts in which it is primarily used as potable water [
5,
6]. Therefore, water desalinization may be the solution. One of the significant issues affecting current methods for generating freshwater is the high level of investment required for water treatment.
On the other hand, most traditional desalination systems rely on fossil fuels to power their operations. There is a necessity to develop more sustainable methods due to the environmental effect of these energy sources and their inherent unsustainability. In terms of performance and commercial viability, conventional systems remain superior to sustainable desalination alternatives. However, there is a significant potential that renewable energy-based desalination systems will surpass conventional plants over time [
7]. High energy consumption is a major barrier to expanding the scale of the desalination systems used in industrial and commercial applications. This revelation gave rise to the integration of renewable energy sources with desalination techniques.
Thermal and membrane desalination technologies account for the vast majority of what is now available. The former technologies suffer from high costs, corrosion, and complicated installation procedures (e.g., multi-stage flash (MSF)). The latter are affected by membrane fouling, which necessitates the pre- and post-treatment of the water before it can be delivered to the desalination modules. There are various other less-well-known desalination methods, such as pervaporation, forward osmosis, and membrane distillation, each with benefits and drawbacks.
Many pilot desalination plants that use renewable energy sources have been established, and most have been running efficiently for years. Most of them use solar or geothermal energy to produce potable water, and they are each suited to their specific location [
8]. Renewable-energy-powered desalination systems are not yet cost-competitive with conventional desalination systems. However, they are suitable in certain locations, and they are expected to become more widely viable soon. El-Ghonemy [
9] examined renewable-energy-powered water desalination systems, focusing on recent developments in this field from both a technological and a financial perspective.
Different desalination technologies—mostly reverse osmosis (RO) and humidification–dehumidification (HDH) systems—have been integrated with solar photovoltaic (PV) systems to increase their sustainability. Shalaby [
10] discussed RO desalination systems powered by solar energy and reported that photovoltaic (PV) energy is the renewable energy source most commonly utilized to power RO systems because it is more cost-effective than using solar organic Rankine cycles (ORCs). Herold and Neskakis [
11] experimented with a PV–RO battery-based system that could produce 0.3 to 0.8 m
3/day. With a feed pressure of 63 bar, the SEC was 15 kWh/m
3. Additionally, an investigation by Mohamed et al. [
12] examined the financial viability of a PV–RO system in two scenarios: with and without batteries. Even though the SEC dropped when the system with batteries was examined, the price rose from EUR 7.5 to EUR 8.3 per cubic meter owing to the high level of investment and expense required for replacement. Kettani and Bandelier [
13] presented a techno-economic analysis of a PV-RO desalination system on a major scale. For a plant with a capacity of 275,000 m
3/day, they estimated a freshwater cost of around USD 1 per cubic meter.
Conversely, a few research studies involving HDH systems with integrated solar PV systems were conducted. Wang et al. [
14] demonstrated that a PV-powered HDD in which the power is supplied directly and indirectly by solar radiation is an economically feasible choice for desalination in remote areas. In addition, they discovered that forced convection improved the performance of this setup under the same operational conditions. Wang et al. [
15] studied the effects of several variables on the rates of evaporation and condensation. The highest freshwater output was around 0.873 L/m
2/d with forced convection and an evaporator temperature of 64.3 °C; this was higher than the 0.789 kg/m
2/d obtained using a free convection process. From this study, it can be concluded that the integration of solar PV systems is more appropriate for small-scale HDH systems and is favorable for remote areas away from electric power supplies. Using thermal energy recovery (TES) technology, Giwa et al. [
16] studied the various environmental circumstances that affected a PV–HDH system’s productivity. Using an HDH desalination unit that was combined with PV/T modules, Gabrielli et al. [
17] investigated the effect of the design and the working environment on the performance of the system. In a theoretical study, Rafiei et al. [
18] investigated how the operating circumstances affected the amount of freshwater produced by an HDH desalination system that used a solar dish concentrator and a PV/T system. Mahmoud et al. [
19] hypothetically evaluated the performance of a hybrid solar distiller/HDH desalination system with incorporated PV panels and solar concentrators.
Many researchers have conducted such hybridization techniques. Narayan et al. [
20] presented a hybrid HDH and reverse osmosis system to minimize energy consumption. Compared to conventional HDH systems, the GOR for this hybrid system was significantly increased to reach 20. Similarly, Kabeel and El-Said [
21] examined a system that combined air heated HDH with a flash desalination system in a single stage. Solar collectors were used to heat both air and water at a laboratory scale [
22]. In a separate study performed by the same authors [
23], the system’s economic viability was examined. The hybrid system was more productive and cost-effective than the independent units. Also, from a financial perspective, Eslamimanesh et al. [
24] compared a pilot HDH system to an RO system. They recommended integrating an HDH system with an RO for the highest potential. A PV-powered HDH–RO system including thermal recovery (PV cooling) units, solar collectors (air and water), and a pressure exchanger was simulated by Abdelgaied et al. [
25]. The collectors were employed to improve the HDH system’s efficiency, while the PV cooling units served a dual purpose: they both preheated the HDH system’s water and increased the PV system’s power output. With SEC values between 1.22 and 1.24 kWh/m
3, the suggested system achieved a maximum hourly production of 192–200 L.
On the other hand, other researchers have investigated the hybridization of the HDH with the RO system using the second law of thermodynamics. The second law of thermodynamics, widely referred to as exergy, attempted to enhance the quality of the energy released through its exergy in a dead state or, in particular, with regard to the ambient environment. Al-Sulaiman et al. [
26] examined a hybrid HDH–RO system theoretically, where they modeled the whole system thermodynamically and calculated exergy for all components, ignoring concentration change in HDH. They treated water as a real solution and analyzed its characteristics using Sharqawy et al. [
27]. The entire unit exergy efficiency was stated to be 10–11.9%. Likewise, Jamil et al. [
28] compared two HDH desalination systems, one using the OAOW process and the other using an OACW process. The combination of an RO and HDH unit was also investigated. According to the study, the HDH–RO with PX has the highest exergy efficiency at 3%. Furthermore, Ameri and Eshaghi [
29] introduced a water desalination system combining RO with an HDH system, in which chemical exergy was considered. The stand-alone RO, HDH, and RO–HDH had exergy efficiencies of 0.064–0.724%, 7.95–12.66%, and 15.90–20.60%, respectively.
Many HDH and RO exergy studies have been found in the literature, but few examine the chemical exergy of flows or salt propagation into freshwater produced by HDH systems. In addition, few studies have examined the usage of solar PV systems with such hybrid RO–HDH and the effect of the PV integration on the hybrid system from an exergy analysis perspective. Consequently, this study aimed to propose a hybrid desalination system integrating two methods of desalination, RO and HDH. The novelty can be considered in the integration of the RO system with the HDH, which is driven by a heat pump. Moreover, the hybrid system is powered by a solar PV system, which is adequate for such a small-scale desalination system, as indicated by the Wang et al. [
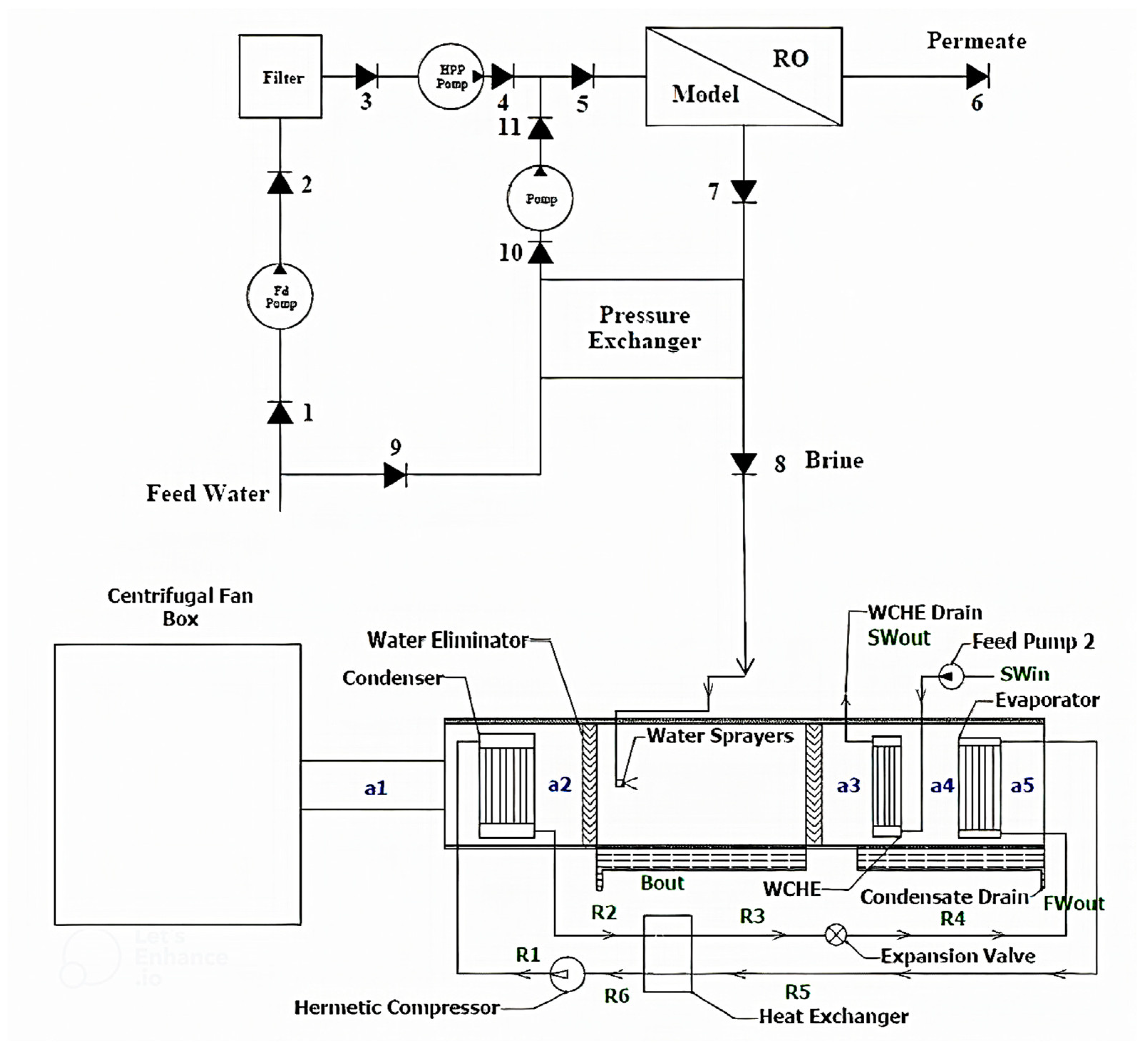
14] study. Using a pressure exchanger as an ERD, the RO system’s energy consumption was improved. Energy and exergy assessments are examined for the RO–HDH–PX–PV system. In addition, an advanced exergy model is used to evaluate system components through an appropriate exergy efficiency definition.
3. Exergy Analysis
The exergy of a material stream consists mainly of a thermo-mechanical (physical) exergy part and a chemical exergy part. The thermo-mechanical part is defined as the maximum work produced when the temperature and pressure of the system are varied to the temperature and pressure of the surrounding environment (
T0,
P0) while maintaining the same concentration of all system elements. Therefore, a thermo-mechanical equilibrium with the environment is achieved. Chemical exergy is the amount of work that can be conducted when the concentration of each substance in the system varies to the concentration in the environment at the same pressure and temperature as the environment (
T0,
P0). As a result, a state of chemical equilibrium arises. The mathematical description of the flow exergy may be stated as considering the physical and chemical exergy as follows [
40,
41]:
where the properties denoted by “
0” are determined at the dead state temperature and pressure (
T0,
P0) but the initiation composition or concentration of the flow stream.
is the chemical potential of “
i” at
T0 and
P0 when the composition is that of the state under consideration and
represents the chemical potential of “
i” when, at
T0 and
P0, the system reaches chemical equilibrium with the environment.
is the mass fraction of component “
i”.
Exergy is not a conservative quantity in any existing system since exergy destruction (due to irreversibilities) occurs in every real system. It is necessary to determine the flow exergy at each state in the desalination plant before studying the system. The system is examined under steady-state conditions while disregarding the kinetic and potential energy of the fluid streams. So, the exergy balance is represented as follows:
It is critical to clarify that the amount of energy consumed by any system or component should be positive. A negative exergy destroyed value indicates a negative entropy generation, which violates the second law of thermodynamics.
In calculating the specific exergy, the specific enthalpy, specific entropy, and chemical potential have been estimated using the correlations presented by Sharqawy et al. [
42].
On the other hand, the specific exergy of the moist air can be determined using Equation (22) presented by Wepfer et al. [
43]. Therefore, it may be defined as follows:
In this equation, the physical exergy is referred to by the first two terms and the chemical exergy by the last term. The following parameter values were employed in this investigation: = 1.003 kJ/kg.K, = 1.872 kJ/kg.K, and = 0.287 kJ/kg.K.
In this work, the values of the properties of the environment are as follows: T0 = 25 °C, P0 = 101.325 kPa, 0 = 0.0099 kg vapor/kg dry air, and the concentration of salted feed water: TDS0 = 35,000 ppm.
It is possible to define the exergy balance in terms of output exergetic effect and the required input or driven exergy expenditure, known as product and fuel exergy, respectively.
Finally, the exergy efficiency may be represented as follows:
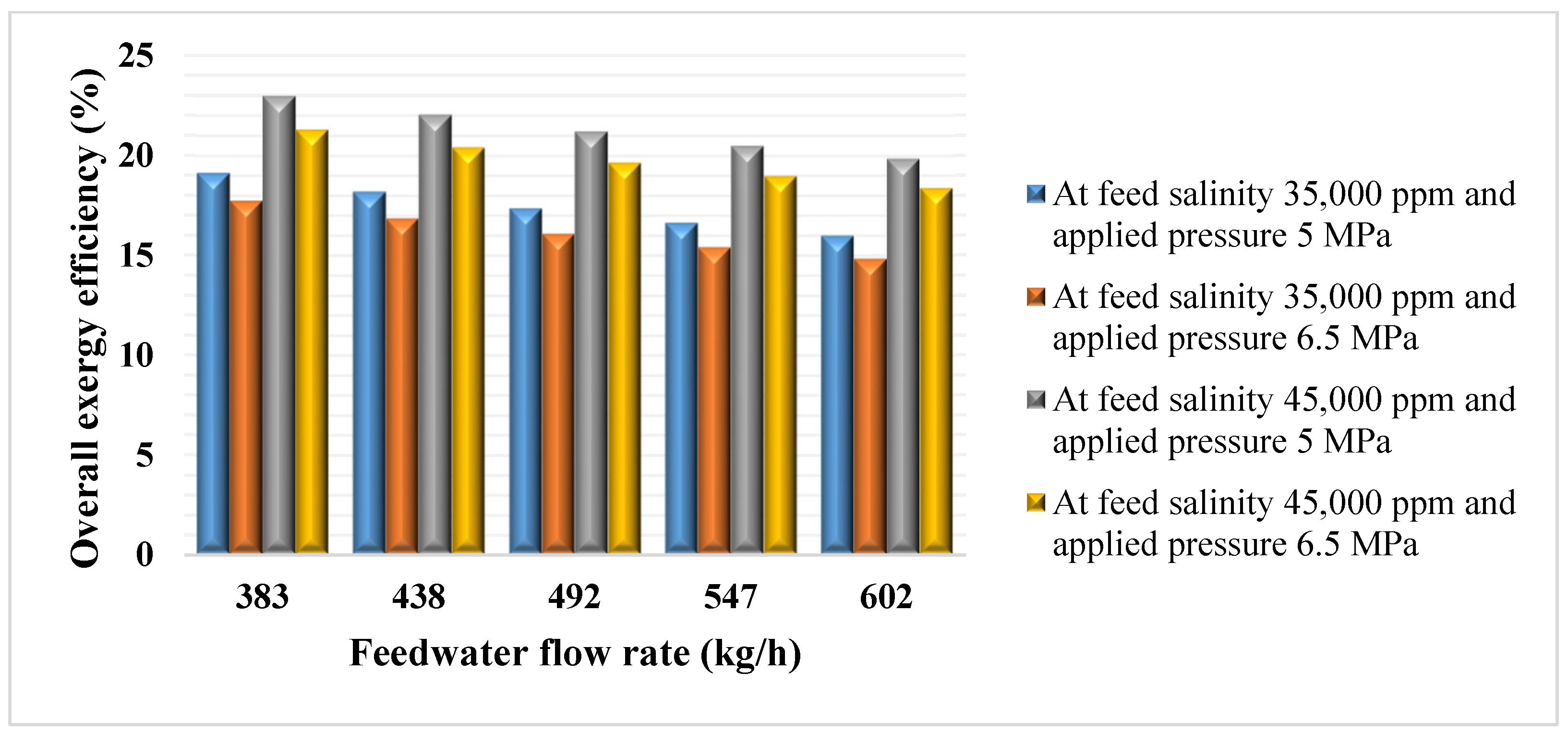
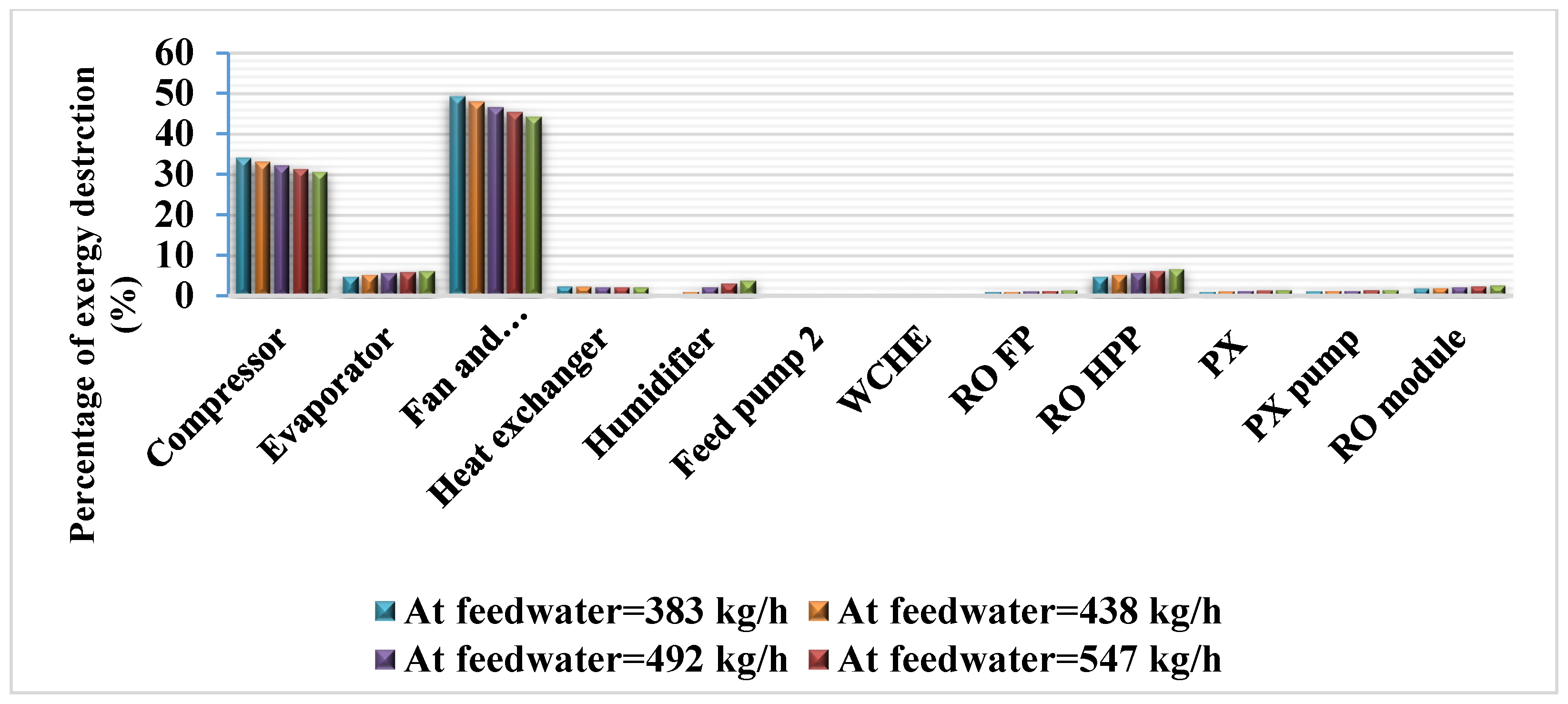
3.1. Exergy Balance of the Desalination Systems
The exergy destroyed at the different components of the desalination systems is compiled in
Table 13.
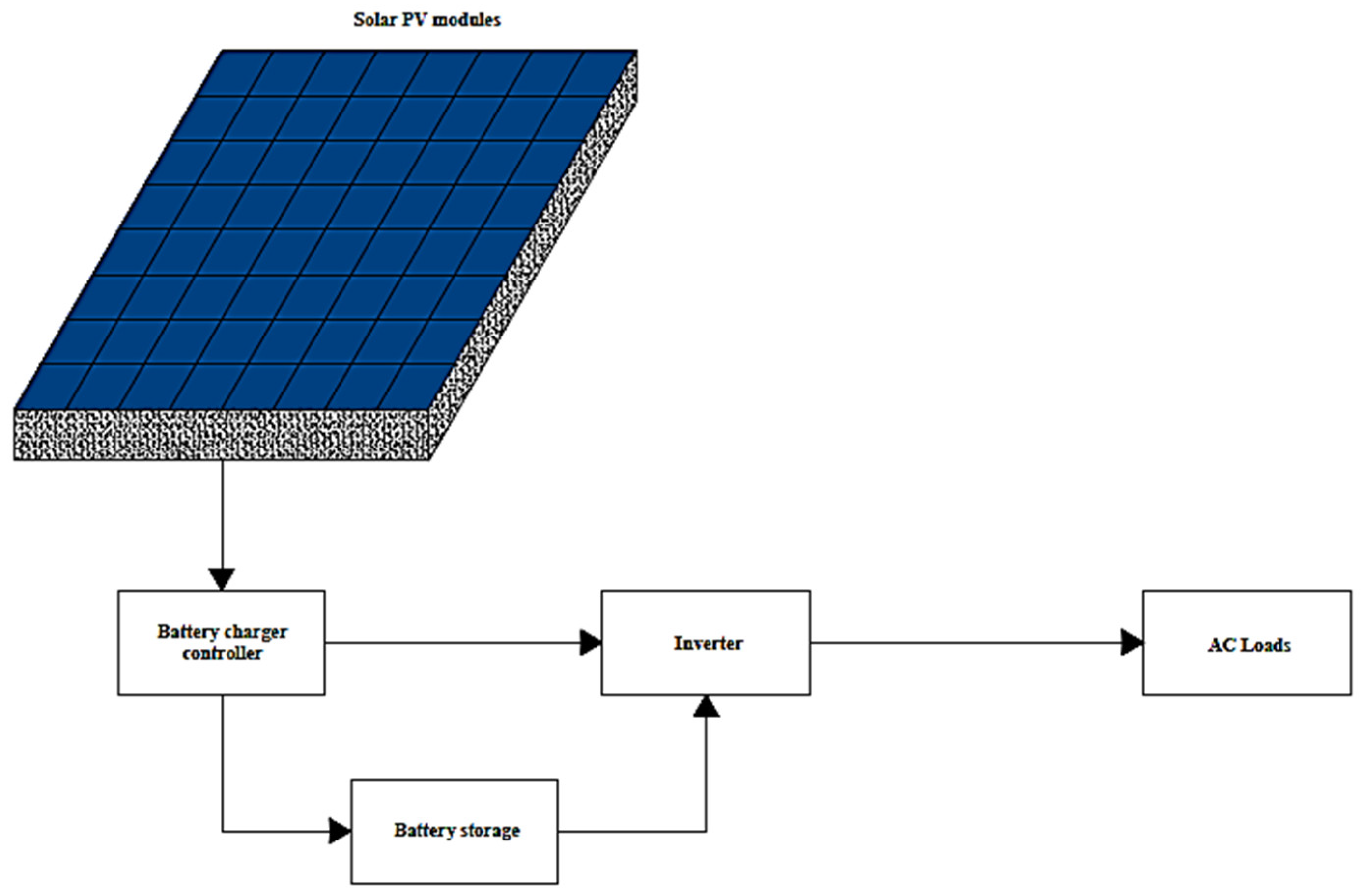
3.2. Exergy Analysis of the Solar PV Panel
The exergetic assessment of the solar PV system, assuming a steady-state flow process, can be obtained using Equation (23). Electrical and thermal energy are the two energy forms produced from solar radiation converted by solar cells. The potential of electrical energy is the main target. In contrast, thermal energy is wasted in the surrounding environment in the form of heat, causing exergy destruction.
The exergy efficiency of the PV module can be calculated using Equation (24).
The fuel exergy of a PV system includes only solar radiation exergy from the sun. The PV fuel exergy can be described as follows [
44]:
where the sun temperature
is assumed to be 6000 K.
is the PV module area (m
2) and
G is the solar radiation intensity (W/m
2) defined based on the chosen location.
The product exergy of the PV system can be calculated as [
45]:
The electrical exergy in the output electrical power of the PV module is given as follows [
46]:
FF denotes the fill factor, which can be determined using Equation (28). It indicates the most influential power transformation achieved by the PV module [
47]. Meanwhile,
and
are the open-circuit voltage and short-circuit current of the PV module, respectively.
where
and
are the maximum current and voltage delivered by the PV module, respectively.
However, the thermal exergy can be considered an exergy loss
, which is given as follows:
where
denotes the heat losses from the PV cells, which can be estimated by:
where
is the overall heat transfer coefficient of the PV module and
the solar PV module temperature. The overall heat transfer coefficient
of a PV module includes convection and radiation losses that can be calculated by:
The convective heat transfer coefficient is defined by [
33], as follows:
where
is the wind speed [
38].
The radiative heat transfer coefficient, as described by Watmuff et al. [
48], is defined as follows:
where the emissivity of the panel
is assumed to be 0.9, while
is Stefan Boltzmann’s Constant 5.67 × 10
−8 W/m
2K
4 and
is the effective temperature of the sky defined by Watmuff et al. [
48], as follows:
The solar PV module temperature,
Tpv,m, can be determined using the nominal operating cell temperature,
NOCT, which is defined as the temperature reached by open-circuited cells in a module under the conditions G = 800 W/m
2,
= 20 °C, and
= 1 m/s [
47].
Thus, the overall system exergy efficiency of the RO–HDH–PX system combined with the solar PV system can be defined as:
And, hence, the total exergy loss of the RO–HDH–PX system combined with the solar PV system is: