Abstract
Suspended kelp canopies have the potential to provide a coastal protection service in addition to their primary function of generating a sustainable resource. In this study, the attenuation of incident waves by kelp suspended from the surface was quantified. We adapted an analytical 1D cross-shore wave attenuation model and tested the effect of (1) water depth, (2) vegetation density, and (3) longline density. The results show that as the percentage of vegetation in the water column increases, wave attenuation by the canopy also increases. However, this attenuation is affected by seasonal variations in kelp growth as well as harvesting strategies. Careful choice of the adopted harvesting strategy was found to be important to maintain optimal wave attenuation by kelp aquaculture farms throughout the year. Partial and targeted removal of the vegetation along longlines is preferred to harvesting all laterals on longlines. This study demonstrates that there is an opportunity for the emerging global kelp aquaculture industry to provide a coastal protection service in addition to resource production, which will help to affect how coastal protection is realized and scaled globally.
1. Introduction
Nature-based solutions are increasingly used as important tools to tackle socio-environmental challenges such as climate change, water pollution, food security, biodiversity loss, and disaster risk management [1]. Long-term coastal protection and resilience strategies are critical to address the risk of erosion and flooding for coastal communities, which could increase by up to 48% by 2100 due to climate-induced changes in hazard drivers (e.g., sea level rise and greater storminess) [2]. Conventional engineering approaches (e.g., seawalls) have significant ecological consequences through shoreline hardening [3]. They also need additional capital and operational investment for their ongoing maintenance, upgrade, and eventual replacement. With the realization that large-scale adoption of these hard structures for hazard management may not be sustainable both for existing and projected hazards due to climate change, there has been increasing interest in adaptive nature-based solutions that offer multiple economic, social, and environmental benefits to increase coastal resilience [4]. Examples of these are ‘living shorelines’ or ‘nature-based coastal defenses’ [5,6] that restore coastal habitats such as dunes, mangroves, saltmarsh, seagrass, and shellfish and coral reefs either without (soft approach) or with (hybrid approach) an engineered structural component.
Natural habitats, and those that are restored through nature-based approaches, increase coastal resilience through the attenuation of waves and currents by depth-induced wave breaking and drag dissipation, as well as promoting sediment accumulation and stabilization [7]. Living shorelines, a subset of nature-based solutions, are bottom-attached canopies that extend into the water column and are typically created by vegetation or reef-building organisms [8]. Living shorelines often have a goal of habitat restoration or at least regaining some ecological functions, as well as providing coastal protection or resilience. The many co-benefits of living shorelines include the support of biodiversity, fisheries, water filtration, and carbon sequestration [9]. For this reason, they are increasingly favoured over conventional structures where appropriate. Several factors influence the effectiveness of both conventional engineered structures and living shorelines at reducing hazards. These include the freeboard, meaning the height of the structure or vegetation relative to the water level, the width of the structure or canopy, and the wave conditions [10,11]. Other factors that are also likely to be important for the efficacy of nature-based methods but have been less well characterized include organism density and species, which can affect the shape, frontal area, and flexibility for vegetation, as well as seasonal differences [12]. While most of the research that has been undertaken to quantify the impact of natural ecosystems on waves has focused on bottom-attached canopies such as a coral reefs [13], seagrass meadows [14], or kelp forests [15], the global expansion of aquaculture production may yield opportunities to provide an ecosystem-based coastal protection strategy that takes advantage of the marine infrastructure used to support aquaculture production.
Global aquaculture production has increased by 6.7 percent per year on average in the period 1990–2020 and has been the main driver of the growth of total fisheries and aquaculture production since the 1980s [16]. Much of this growth has been associated with the need to support global food security and reduce pressure on declining wild fishery catches. Mariculture contributed 55.6% to total aquaculture production in 2020, with the majority of aquatic animals produced by mariculture being shelled molluscs (56.2%). The global seaweed production in 2019 was 35.8 million tonnes and was predominantly generated through cultivation (97%) [17]. The Asian seaweed aquaculture industry is the primary contributor to global seaweed production, with a 97.4% share of world seaweed production [17]. This global imbalance in production highlights the opportunity for the development of seaweed and microalgae cultivation outside of Asia, and while slow growth in production has recently been experienced in the tropical seaweed industry, farming of temperate and cold-water species is on the rise [16]. While food provision has been the primary objective of aquaculture generally, there is increasing interest in the co-benefits that may be provided by mariculture [18], with an extension to ecologically beneficial aquaculture that has intended net environmental benefits [19]. Overton et al. [19] identified 12 ecologically beneficial outcomes that are achievable by aquaculture, with one being coastal protection.
Mariculture typically has spatially extensive coastal infrastructure such as longlines or baskets for growing algae and shellfish, which is often located in shallow waters along coastlines. Consequently, there is increasing interest in understanding how mariculture infrastructure interacts with waves and if this infrastructure can reduce shoreline impacts or increase coastal resilience. Most mariculture utilizes suspended infrastructure that is usually located higher in the water column near the surface. Much of the research to date has focused on how finfish mariculture infrastructure affects coastal processes (typically currents) and has been undertaken to understand the environmental impact (e.g., waste deposition, nutrient dispersal) of these operations [20] or for the design of the infrastructure itself (e.g., forces imposed on the mooring systems). Thus, while numerous studies have quantified the interaction between aquaculture infrastructure and the coastal environment, the opportunity for suspended aquaculture to make a meaningful contribution to coastal protection remains poorly understood.
The most analogous aquaculture infrastructure to kelp production is the approach used for shellfish droppers mounted on longlines [21]. Conventional approaches to model these dropper canopies have considered these droppers as an array of suspended vertical cylinders [22]. However, because these approaches typically neglect the motion of these droppers, their wave attenuation potential may be overestimated. Furthermore, recent studies have shown that the morphological and mechanical properties of the kelp species are important to describe the motion of a suspended canopy as well as the canopy’s attenuation of incident waves [23,24]. Factors that affect production such as infrastructure and dropper density, kelp growth rates, and harvesting strategies have also been shown to affect the attenuation of incident waves [23,25]. It is important to note that much of this work has been based on S. latissimi (sugar kelp), which is also the species considered in numerical solutions that have been developed to describe blade motions [24] as well as the analytical solutions used to describe wave attenuation [25]. Globally however, there are many other species that are likely to be considered for production.
In Australia, golden kelp Ecklonia radiata is a dominant habitat-forming species on the temperate Great Southern Reef (GSR), a series of reefs spanning 71,000 km2 of Australia’s southern coastline [26]. Kelp forests support diverse ecological communities, nutrient cycling, fishing, and tourism, which, in the GSR, generates at least AUD 10 billion per year [26]. Ecklonia radiata is the subject of increasing restoration efforts due to habitat loss caused by climate change, urbanization, and herbivore overgrazing [27]. It is also a target species for the growth of the Australian seaweed aquaculture industry, with a national plan of action to enable an AUD 10 million gross value production (GVP) per annum industry by 2025, growing to an AUD 1 billion industry by 2040 [28]. Preliminary trials in south-eastern Australia have shown that seeding E. radiata onto rope for longline cultivation is a successful method for aquaculture [29]. However, there are notable morphological and mechanical differences to S. latissimi, which are likely to affect the wave attenuation performance of E. radiata.
The aim of this study was to investigate the extent to which suspended kelp canopies in aquaculture may attenuate wave energy. The study focuses on E. radiata and the environmental conditions in Victoria, Australia, as a case study typical of a shallow coastal bay. Adapting an analytical 1D cross-shore wave attenuation model [25], we tested the effect of (1) water depth, (2) vegetation density, and (3) longline density on wave attenuation (Figure 1). Additionally, the impact of seasonal variation and harvesting strategies on wave dissipation capacity was considered to understand the limitations of the canopy in periods of early growth and the ideal harvesting techniques to allow the canopy to maintain a coastal protection value throughout the production cycle.
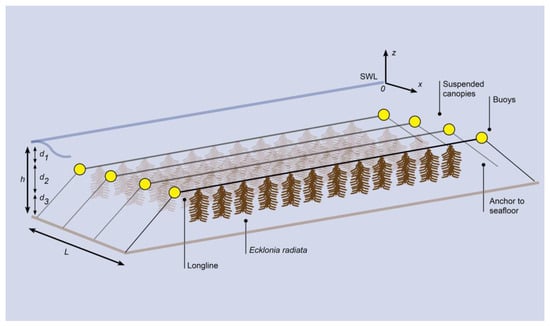
Figure 1.
Sketch of the model for wave attenuation by a suspended E. radiata canopy involving a series of longlines attached to buoys anchored to the seafloor in a shallow coastal bay. The coordinate (x, z) originates at the leading edge of the canopy (x = 0) and the still water level (SWL, z = 0). The horizontal coordinate (x) is positive in the direction of wave propagation. The canopy length is L, the water depth is h, the canopy height is d2, the distance between the top of the canopy and the SWL is d1, and the distance between the bottom of the canopy and sea floor is d3.
2. Materials and Methods
2.1. Model Overview
In this study, the attenuation of incident waves by kelp suspended from the surface was quantified. We adapted the model developed by Zhu et al. [25], which has previously been validated against several laboratory studies (Figure 1). A full description of the model can be found in Zhu et al. [25]; only a brief summary is provided here. This model builds on theoretical models [30,31], which assume vegetation acts as a rigid canopy and that wave energy dissipation is principally due to the drag of vegetation. The analytical model developed by Zhu et al. [25] differs by considering the motion of suspended kelp canopies and was derived by simplifying and linearizing kelp blade motion based on the vegetation morphology and its mechanical properties, assuming negligible vertical forces (net buoyancy and friction).
The model relies on several input parameters that include hydrodynamic bulk parameters (water depth, wave period, and wave height), canopy parameters (height, length in the direction of wave propagation, and density described by number of blades per unit area), and vegetation parameters (mass density, flexural rigidity, and morphological measurements). Vegetation motion within the canopy is modelled using the cantilever beam model, which is suitable for slender blades of a kelp species suspended in a canopy [23]. The approach used here simplifies the blade motion to a balance of drag force and blade bending resistance and considers frequency-dependent analytical solutions for blade displacements in random waves, considering the effects of inertial forces. This approach is suitable for small-amplitude blade motion.
A structural dynamics model was used to obtain the horizontal displacement of the blade, resolving the motion of individual blades within the canopy. The key outputs of the model include the wave height along the canopy, wave decay coefficient, bulk drag coefficient for wave attenuation by the canopy with linear motion, and the effective blade length ratio. This approach has been validated with laboratory and field experiments for submerged seagrass vegetation by Luhar et al. [32] and Lei and Nepf [33], and for suspended kelp canopies by Zhu et al. [24]. The analytical solutions for bulk drag coefficient and effective blade length were compared with the values from the laboratory and field experiments and have also been used to investigate the wave attenuation performance of canopies under seasonal variation [25].
2.2. Model Adjustments
The original model by Zhu et al. [25] was based on the morphological properties of Saccharina latissima. To apply the model to the kelp species Ecklonia radiata, we adjusted the model parameters as well as the model structure to better represent its morphological and mechanical properties. E radiata is a small, stipitate kelp, reaching a maximum length of 2 m (Figure S1), although these characteristics vary with spatial distribution and in response to environmental conditions [34,35]. The morphological and mechanical characteristics were defined based on previous measurements of E. radiata at five sites in Port Phillip Bay, Victoria (Supplementary Materials; [15]). These characteristics included the length of the stipe, lamina, and laterals. The flexural rigidity of E. radiata lamina and laterals was measured from kelp samples collected from Port Phillip Bay in May 2022 (Supplementary Materials).
Previous applications of the model have characterized kelp with two components: a rigid stem element and a flexible blade component. Due to the different morphological and mechanical properties of E. radiata, which consists of a stipe, central lamina, and laterals, we adjusted the modelling approach to include three components. The physics of the rigid and flexible models as proposed by [24,25] was not altered. The stipe was simulated as a rigid component due to its short and more rigid form compared to the lamina and laterals. The lamina and laterals were simulated as flexible components. Consistent with other applications of this model, we calculated the wave attenuation by each component separately using the rigid [24] and flexible [25] analytical models, and then calculated an overall canopy attenuation parameter simply as the sum of the attenuation parameters for each component. Like in other applications of the modelling approach, the impact of the whole canopy on wave attenuation was determined by summing the wave decay coefficients (kD, as defined in [25]) of each component. This kD was then used to estimate the wave attenuation over the entire canopy.
The length of the canopy was defined by the number of longlines and was positive in the direction of wave propagation. The canopy height was defined by the length of the combined stipe and lamina lengths, and the gap below the canopy was defined by the water depth minus the canopy height. The number of laterals on individual E. radiata was defined as the number of blades per square meter. This was represented along the longlines as a lateral density. The overall vegetation density was defined as the combined number of stipe, lamina, and laterals per square meter. We neglected the sheltering that may occur from kelp individuals being in close proximity, which was consistent with the approach used by Zhu et al. [25]. We note that to accurately define this, a comprehensive investigation of the blade-to-blade interaction would be required to measure the horizontal force on rows of E. radiata in a suspended canopy. Finally, the mass density of E. radiata was based on the mass density of the kelp species identified by Zhu et al. [25] as this information was not available for E. radiata. Whilst there are likely to be differences between the two species, these were considered to be of second-order importance in this particular study.
2.3. Modelled Scenarios (Parameters)
Several parameters were investigated to quantify how the attenuation of waves by a suspended canopy varies with water depth and different canopy configurations under the same incident wave conditions (Table 1). In addition, the attenuation was also investigated for different seasonal conditions by adjusting the morphological characteristics, which are associated with varying degrees of growth. Lateral harvesting and longline harvesting techniques were assessed by adjusting lateral density and canopy density parameters. The wave attenuation performance of canopy design configurations was quantified using the wave energy dissipation rate (EDR):
where H(0) is the incident wave height at the offshore edge of the canopy, Lv is the length of the canopy in the direction of wave propagation, and kD is the wave decay coefficient as defined in [25]. We note that whilst this equation does not explicitly account for the influence of water depth, this effect is accounted for in the calculation of kD for each component of the kelp morphology [24,25].

Table 1.
Parameters considered in the model scenarios.
The base case was set with wave conditions typical for a shallow coastal bay (0.5 m height; 6 s wave period [15]). This enabled a comparison to be made between canopy configurations and the influence of seasonal variation and harvesting strategies on wave attenuation performance of the canopy. To investigate the impact of the geometric configuration of suspended canopies on wave attenuation, the model was run for a series of scenarios with these base wave conditions and with adjusted water depth and canopy configurations. The impact of water depth on wave attenuation performance was assessed for several water depth scenarios (2, 3, and 4 m) with a canopy configuration of 50 longlines over 100 m in the direction of wave propagation and canopy density of 5 individual E. radiata per m2. The configuration was based on that used for the S. Latissimi modelled scenarios [25] and common methods identified by the Australian Seaweed Institute in their Marine Seaweed Aquaculture Risk Assessment [36]. To understand the effect of vegetation density and area of exposure on wave attenuation performance, various suspended canopy design configurations (but with constant water depth and wave conditions) were considered. Canopy parameters were adjusted to compare longlines spaced 2 m, 3 m, and 4 m apart in the direction of wave propagation over a 100 m distance to test the effect of density, and to test the effect of area over a distance of 100 m, 150 m, and 200 m, respectively, in the direction of wave propagation.
Seasonal (temporal) variations in the growth rates of E. radiata were expected to affect the wave attenuation performance of suspended canopies. The wave attenuation performance by the canopy at different stages of vegetation growth was investigated by adjusting E. radiata morphological parameters. These parameters were the lamina length, width, and thickness, stipe length and diameter, and lateral length, width, and number. Input parameters for a full-growth canopy were obtained from field data, with half-growth and quarter-growth canopies calculated from these data. To understand the impact of harvesting techniques on wave attenuation performance, we adjusted the kelp morphology parameters and canopy parameters based on growth rates and typical harvesting periods used in industry. Both hand and mechanical harvesting methods are used in longline kelp aquaculture farms [37]. For smaller farms, harvesting kelp from longlines may be carried out manually with a sharp knife from harvesting platforms such as a small barge, while for larger farms, power lifting equipment, which assists in lifting sections of longline onto the barge for processing, is often used [38]. The two harvesting techniques compared here are based on techniques practiced on smaller farms [38,39] and rely on a hand-cutting process. The lateral harvesting technique involves removal of only E. radiata laterals from all vegetation, while a longline harvesting technique involves removal of half of the vegetation along longlines. To compare harvesting strategies, the model was run with the adjusted vegetation parameters under wave conditions, water depth, and canopy configuration parameters of the base case.
3. Results
3.1. Water Depth
The model results indicate that the wave attenuation by a canopy increases as the water depth decreases as well as with distance across the canopy array (Figure 2a). For the cases considered in this study, the wave energy dissipation rate (EDR) was as much as 94% for the 2 m water depth. However, this decreased to 81% and 67% for canopies in 3 m and 4 m water depths, respectively. Notably for shallower water depths, greater attenuation occurs near the outer edge of the canopy, and this effect reduces with increased water depth; a larger array is required to achieve the same wave attenuation.
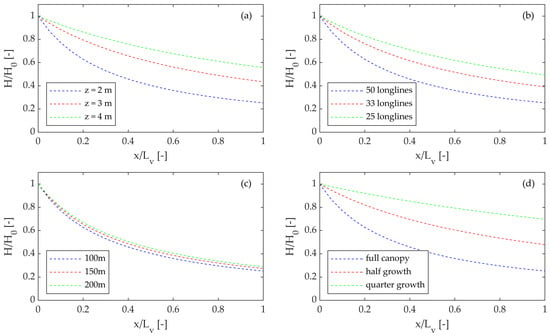
Figure 2.
(a–d): Wave height decay over suspended E. radiata canopy configurations testing the effect of (a) water depth, (b) vegetation density (number of longlines per 100 m), (c) area of vegetation (spacing of 50 longlines per length), and (d) vegetation growth.
3.2. Vegetation Density
Vegetation attenuation of incident waves decreases with vegetation density. For the cases considered in this study, as the number of longlines within a 100 m cross-shore distance decreased, there was a substantial reduction in the EDR of the kelp. This reduction was also non-linear. For example, a kelp farm with longlines spaced at 2 m exhibited an EDR of 94%, whereas for 3 m and 4 m spacing, the EDR was 85% and 76%, respectively (Figure 2b). For a very dense canopy (2 m spacing, the likely maximum that is practically achievable from an operations perspective), the overall attenuation is similar to shallow water conditions except that this attenuation occurs at a slower (spatial) rate.
3.3. Area of Vegetation (Number of Longlines)
The spatial arrangement of the longlines had little impact on the EDR. For the cases considered in this study, the EDR was similar irrespective of how 50 longlines were spaced across a distance of 100, 150, or 200 m in the direction of wave propagation (Figure 2c). This suggests that the overall number of longlines is more important (i.e., the density) than the spatial arrangement of these longlines. Consequently, a narrow, denser arrangement is likely to offer similar attenuation to a less dense but spatially larger arrangement.
3.4. Seasonal Patterns
The wave attenuation performance of kelp increases over time (Figure 2d). The wave attenuation performance for early-stage growth can be as low as 51% for canopies at quarter growth; however, over time as the kelp grows, the attenuation increases substantially. For half growth, the EDR increases to 75%, and it reaches as much as 94% for full growth. This demonstrates that the attenuation performance of kelp longlines will require measurement over time and its performance can be expected to increase.
3.5. Harvesting Periods
After a period of growth, and in recognition of the resource production function of many of these aquaculture operations, there is a practical need for kelp harvesting. The analysis indicated that the lateral harvesting technique results in a substantial reduction in wave attenuation performance, with the EDR decreasing to <7% (Figure 3). However, if a longline harvesting technique is adopted, the reduction in EDR is much less (76%). This indicates that in order for kelp aquaculture to be a reliable and effective wave attenuation device, careful consideration of the method and timing of harvesting will be required.
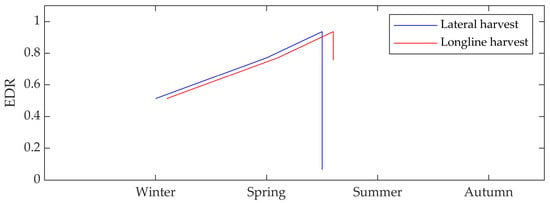
Figure 3.
Wave energy dissipation rate (EDR) for suspended E. radiata canopies of 50 longlines over 100 m at 2 m water depth comparing a lateral harvesting technique (removing all laterals; blue) and longline harvesting technique (removing half of the vegetation along each longline; red).
4. Discussion
In this study, we showed that suspended E. radiata aquaculture canopies attenuate wave energy and that this attenuation is influenced by both canopy configuration and water depth. For a typical aquaculture operation (full-growth kelp, 50 longlines over a 100 m distance, 2 m water depth with 0.5 m wave height and 6 s wave period), these suspended kelp canopies may have an energy dissipation rate of up to 94%. However, this dissipation rate decreases with an increase in water depth or a decrease in vegetation or longline density. Furthermore, seasonal variation and different harvesting strategies impact the vegetation density and thus the wave attenuation. The results of this study provide important insights into the design and management of kelp aquaculture farms to maximize multi-functional benefits that include coastal protection as well as resource production.
4.1. Water Depth
Vegetation that is suspended from the surface or is bottom-mounted attenuates wave energy by imposing a drag force on (the horizontal component of) incident waves. In shallow water depths, the horizontal component of the wave orbital velocities associated with these incident waves is approximately uniform in terms of depth. For this case, the imposed drag force becomes a function of the wave length, percent of the water column occupied by vegetation, and characteristics of both suspended and bottom-mounted vegetation [10].
For bottom-attached vegetation (e.g., seagrass and saltmarsh) the height of the vegetation relative to the depth of the water column has been shown to be a key parameter for wave (and flow) attenuation [10]. Similar observations have been made for artificial or biogenic (e.g., oysters or corals) canopies where the depth of water above the crest of the reef (‘freeboard’) has been shown to control wave transmission by imposing drag as well as initiating wave breaking [13]. Thus, as the water depth increases, more wave energy can pass over a bottom-attached canopy or reef relative to the same canopy in shallower water depths. Our investigation of suspended E. radiata found that it also dampens wave energy for short-period, wind-driven waves, and that this attenuation decreases as the fraction of the water column occupied by vegetation decreases. While this study did not make a direct comparison between the wave attenuation of bottom-mounted and suspended vegetation canopies, it would be reasonable to expect that the attenuation effect may be greater when compared to bottom-attached vegetation of similar characteristics, particularly as the water depth increases. This is because the attenuation of wave energy by suspended vegetation is due to the interaction of vegetation with wave orbital velocities [25] as the water depth increase is greater at the surface, while on the seafloor, wave energy decreases and vertical orbital velocity approaches zero [40].
4.2. Vegetation Density
The effect of vegetation density was simulated in this study by varying the number of longlines within a set distance (i.e., 100 m). Decreasing the density of longlines (i.e., vegetation) decreased wave attenuation; however, there was no effect of increasing the spacing of the same number of longlines over greater distances (i.e., 50 longlines over 100–200 m showed the same EDR performance). The number of longlines, and therefore overall mass of vegetation in the canopy, thus has a greater influence on the wave attenuation performance compared to the distance of the canopy in the direction of wave propagation. This is consistent with studies on optimizing the configuration of artificial reef modules for wave attenuation, where a greater spacing between rows of modules had no, or even a reduced, effect on wave transmission [41]. The exact mechanism for the effect of spacing needs further investigation but could result from increased drag forces as the waves interact with greater-spaced infrastructure. This suggests that in the design of a kelp aquaculture farm for wave attenuation, there is a minimum number of longlines required for a specific EDR; however, the spacing of these can be adjusted according to space available or other factors that may be important for maximizing kelp growth or other co-benefits. For example, established kelp farms in the North Atlantic suggest a long and narrow farm allows for a higher yield for a given amount of longline due to improved nutrient availability for the kelp in the centre of the farm [38]. Additionally, Flavin et al. [38] identify the separation distance between longlines as an important consideration in farm design. A comparison of sections of farms with different spacing between longlines showed that longlines spaced 1.5 m apart resulted in crossed lines impacting harvest as well as biomass loss, while a 4.5 m separation distance resulted in no crossed or tangled lines. Further, harvesting kelp from longlines at smaller farms may be carried out by hand from a small boat, or by using harvesting platforms such as a barge and powerlifting equipment for larger farms [38]. The distance between longlines may be determined by the space required to access longlines and manoeuvre a small boat or larger barge that is required for harvesting.
4.3. Vegetation Morphology
Comparison of E. radiata canopies to preliminary work in this area by Zhu et al. [25] simulating Laminaria saccharina indicates that morphological differences between each kelp species impact wave attenuation performance by the canopy. With multiple flexible laterals off a central lamina, E. radiata has a greater density per m2 compared to L. saccharina, which consists of a single blade. The greater canopy density due to the morphological difference may therefore be more effective for wave attenuation compared to L. saccharina as a series of single flexible blades in a suspended canopy. The effect of this difference was shown across modelled scenarios. The wave height modelled scenario of a suspended L. saccharina canopy 1.2 m below the still water level in 5 m deep water with a configuration of 50 longlines and wave conditions of a 1 m wave height and 6 s period resulted in an EDR value of 43% [25], while a comparable E. radiata canopy configuration of 50 longlines suspended in 4 m water resulted in an EDR of 67%.
For economic benefits, kelp is often seeded close together on longlines, increasing density [29], and thus this would also increase wave attenuation performance. With greater density comes greater interaction of vegetation within the canopy. A limitation of this study was neglecting the sheltering factor, which accounts for interactions between the lamina and lateral blades. The sheltering effects of kelp seeded close together can reduce drag, resulting in reduced wave attenuation [25]. This study used the default sheltering factor of 1 for no sheltering, which may overestimate wave attenuation by the canopy. Laboratory experiments are required to understand blade-to-blade interactions to calculate the sheltering factor using the measured horizontal force on rows of E. radiata in a suspended canopy. Further research is also required to determine the correct mass density of E. radiata at different growth stages as well as the flexural rigidity and if the blade motion within the canopy is similar or different to that of L. saccharina.
4.4. The Impact of Seasonality
Ecklonia radiata experiences seasonal growth and senescence. This small, stipitate kelp reaches a maximum length of 2 m, although this varies considerably along its distribution and in response to environmental conditions [15,35]. Seasonal growth patterns presented by Bearham et al. [42] indicate a maximum growth rate in the austral spring, with a rapid reduction to a minimum during summer for locations in south-western Australia. Seeding of Ecklonia radiata onto longlines has been explored by small farms in Australia and New Zealand [28] following the typical method for kelp farming [29]. This method (based on kelp farm operations in the North Atlantic [38]) involves multiple planting seasons in autumn, winter, and spring, followed by harvest periods in late autumn and spring. The upkeep of the site typically involves weekly or bi-monthly sampling, data capture and maintenance visits. Based on these findings, the development of suspended kelp farms in Australia may involve multiple seeding and harvesting periods annually, with fluctuations in biomass production and therefore wave attenuation capacity throughout the year.
Our analysis shows that the gradual growth from winter to spring affects the EDR and thus the contribution the canopy may make to coastal wave attenuations. Initial canopy growth for the base case resulted in an EDR of 51%, which increased to 94% at full growth prior to a spring harvest. Upon harvesting, there was a sharp drop to <7% following a lateral harvest and 76% following a longline harvest. This suggests that partial harvesting of longlines will maintain a degree of wave attenuation by the canopy throughout the year compared to a lateral harvest. Careful consideration of harvesting practices versus the seasonal wave attenuation performance requirements will be a critical factor to be consider.
5. Conclusions
Nature-based solutions for coastal protection offer an alternative to conventional engineered structures [7]. Seaweed aquaculture is an emerging industry in Australia, offering economic, social, and environmental benefits, including to First Nations people whose knowledge of native seaweeds and their diverse applications dates back over 65,000 years [43]. This study has demonstrated the potential of multi-functional suspended kelp aquaculture in a shallow coastal bay. The results of the modelled scenarios for different canopy configurations can be used to inform kelp aquaculture farms on strategic placement of longlines to maximize the reduction in incident wave energy while balancing other factors. There will be a minimum number of longlines in the direction of incident waves required for a particular wave energy reduction, but the spacing between the longlines can be varied. The density of vegetation on the longlines impacts wave attenuation, and thus a partial kelp harvest is a preferable method for maintaining some degree of wave attenuation by the canopy for kelp farms that seek to provide a coastal protection service. Additional modelling will be useful to further understand the impact of suspended kelp aquaculture farms on sediment transport and how this can mitigate coastal erosion; 3D modelling may be employed, as in previous studies using the SWAN model to improve wave attenuation prediction under time-varying wave conditions [44]. Additionally, in laboratory studies, a wave flume for a 1:10 scale model has been used to assist the investigation of blade dynamics and understanding of wave attenuation capacity by the whole canopy [24]. The initial results from this study can help identify appropriate locations for field trials to further develop an understanding of the impact of kelp aquaculture on coastal protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse11091822/s1, Figure S1: Typical example of an E. radiata sample collected within Port Phillp Bay, Victoria, Australia; Figure S2: An example of an E. radiata sample with the holdfast, stipe, lamina, and lateral samples separated and prepared for tensile testing; Figure S3: Tensile testing of E. radiata samples; Table S1: E. Radiata morphological measurements; Table S2: E. radiata mechanical properties.
Author Contributions
Conceptualization, R.B., A.W.M.P. and R.L.M.; methodology, R.B., A.W.M.P. and R.L.M.; formal analysis, R.B. and A.W.M.P.; investigation, R.B.; writing—original draft preparation, R.B.; writing—review and editing, A.W.M.P. and R.L.M.; supervision, A.W.M.P. and R.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
This study was completed as a master’s thesis at the University of Melbourne. The authors gratefully acknowledge the support of course tutor Glen Currie and course coordinator Murray Peel, the assistance of the laboratory technician Steve Adams for material testing, and the diving team for supplying the morphological measurements of E. radiata. The authors also gratefully acknowledge the assistance of Longhuan Zhu for supplying the analytical model used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. Nature-based solutions to address global societal challenges. IUCN Gland Switz. 2016, 97, 2016–2036. [Google Scholar]
- Kirezci, E.; Young, I.R.; Ranasinghe, R.; Muis, S.; Nicholls, R.J.; Lincke, D.; Hinkel, J. Projections of global-scale extreme sea levels and resulting episodic coastal flooding over the 21st Century. Sci. Rep. 2020, 10, 11629. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Beck, M.W.; Reguero, B.G.; Losada, I.J.; van Wesenbeeck, B.; Pontee, N.; Burks-Copes, K.A. The Effectiveness, Costs and Coastal Protection Benefits of Natural and Nature-Based Defences. PLoS ONE 2016, 11, e0154735. [Google Scholar] [CrossRef]
- Chowdhury, M.S.N.; La Peyre, M.; Coen, L.D.; Morris, R.L.; Luckenbach, M.W.; Ysebaert, T.; Smaal, A.C. Ecological engineering with oysters enhances coastal resilience efforts. Ecol. Eng. 2021, 169, 106320. [Google Scholar] [CrossRef]
- Bilkovic, D.M.; Mitchell, M.M.; La Peyre, M.K.; Toft, J.D. (Eds.) Living Shorelines: The Science and Management of Nature-Based Coastal Protection; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Morris, R.L.; Konlechner, T.M.; Ghisalberti, M.; Swearer, S.E. From grey to green: Efficacy of eco-engineering solutions for nature-based coastal defence. Glob. Change Biol. 2018, 24, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Möller, I. Applying Uncertain Science to Nature-Based Coastal Protection: Lessons from Shallow Wetland-Dominated Shores. Front. Environ. Sci. 2019, 7, 49. [Google Scholar] [CrossRef]
- Smith, C.S.; Rudd, M.E.; Gittman, R.K.; Melvin, E.C.; Patterson, V.S.; Renzi, J.J.; Silliman, B.R. Coming to Terms with Living Shorelines: A Scoping Review of Novel Restoration Strategies for Shoreline Protection. Front. Mar. Sci. 2020, 7, 434. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- Möller, I. Quantifying saltmarsh vegetation and its effect on wave height dissipation: Results from a UK East coast saltmarsh. Estuar. Coast. Shelf Sci. 2006, 69, 337–351. [Google Scholar] [CrossRef]
- Van der Meer, J.W.; Briganti, R.; Zanuttigh, B.; Wang, B. Wave transmission and reflection at low-crested structures: Design formulae, oblique wave attack and spectral change. Coast. Eng. 2005, 52, 915–929. [Google Scholar] [CrossRef]
- Morris, R.L.; Bilkovic, D.M.; Walles, B.; Strain, E. Nature-based coastal defence: Developing the knowledge needed for wider implementation of living shorelines. Ecol. Eng. 2022, 185, 106798. [Google Scholar] [CrossRef]
- Ferrario, F.; Beck, M.W.; Storlazzi, C.D.; Micheli, F.; Shepard, C.C.; Airoldi, L. The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 2014, 5, 3794. [Google Scholar] [CrossRef]
- Reidenbach, M.A.; Thomas, E.L. Influence of the Seagrass, Zostera marina, on Wave Attenuation and Bed Shear Stress Within a Shallow Coastal Bay. Front. Mar. Sci. 2018, 5, 397. [Google Scholar] [CrossRef]
- Morris, R.L.; Graham, T.D.J.; Kelvin, J.; Ghisalberti, M.; Swearer, S.E. Kelp beds as coastal protection: Wave attenuation of Ecklonia radiata in a shallow coastal bay. Ann. Bot. 2019, 125, 235–246. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Garrido Gamarro, E.; Geehan, J.; Lucente, D.; Mair, G.; Roubach, R. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021. [Google Scholar]
- Spillias, S.; Valin, H.; Batka, M.; Sperling, F.; Havlík, P.; Leclère, D.; McDonald-Madden, E. Reducing global land-use pressures with seaweed farming. Nat. Sustain. 2023, 6, 380–390. [Google Scholar] [CrossRef]
- Overton, K.; Dempster, T.; Swearer, S.E.; Morris, R.L.; Barrett, L.T. Achieving conservation and restoration outcomes through ecologically beneficial aquaculture. Conserv. Biol. 2023, 2023, e14065. [Google Scholar] [CrossRef] [PubMed]
- Carballeira Braña, C.B.; Cerbule, K.; Senff, P.; Stolz, I.K. Towards environmental sustainability in marine finfish aquaculture. Front. Mar. Sci. 2021, 8, 343. [Google Scholar] [CrossRef]
- Stevens, C.; Plew, D.; Hartstein, N.; Fredriksson, D. The physics of open-water shellfish aquaculture. Aquac. Eng. 2008, 38, 145–160. [Google Scholar] [CrossRef]
- Stevens, C.; Plew, D. Bridging the Separation Between Studies of the Biophysics of Natural and Built Marine Canopies. Front. Mar. Sci. 2019, 6, 217. [Google Scholar] [CrossRef]
- Zhu, L.; Huguenard, K.; Zou, Q.-P.; Fredriksson, D.W.; Xie, D. Aquaculture farms as nature-based coastal protection: Random wave attenuation by suspended and submerged canopies. Coast. Eng. 2020, 160, 103737. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, J.; Huguenard, K.; Fredriksson, D.W. Wave attenuation by suspended canopies with cultivated kelp (Saccharina latissima). Coast. Eng. 2021, 168, 103947. [Google Scholar] [CrossRef]
- Zhu, L.; Huguenard, K.; Fredriksson, D.W.; Lei, J. Wave attenuation by flexible vegetation (and suspended kelp) with blade motion: Analytical solutions. Adv. Water Resour. 2022, 162, 104148. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Connell, S.D.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 2015, 67, 47–56. [Google Scholar] [CrossRef]
- Carnell, P.E.; Keough, M.J. Reconstructing Historical Marine Populations Reveals Major Decline of a Kelp Forest Ecosystem in Australia. Estuaries Coasts 2019, 42, 765–778. [Google Scholar] [CrossRef]
- Australian Seaweed Industry. Australian Seaweed Industry Blueprint—A Blueprint for Growth (Publication No. 20-072). AgriFutures Australia. 2020. Available online: https://www.australianseaweedinstitute.com.au/australian-national-seaweed-industry-blueprint-report-agrifutures (accessed on 10 May 2022).
- Wiltshire, K.H. Application of Seaweeds to Integrated Multi-Trophic Aquaculture in Southern Australia: Identifying and Investigating Suitable Native Species. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2020. [Google Scholar]
- Dalrymple, R.A.; Kirby, J.T.; Hwang, P.A. Wave Diffraction Due to Areas of Energy Dissipation. J. Waterw. Port Coast. Ocean. Eng. 1984, 110, 67–79. [Google Scholar] [CrossRef]
- Kobayashi, N.; Raichle, A.W.; Asano, T. Wave Attenuation by Vegetation. J. Waterw. Port Coast. Ocean. Eng. 1993, 119, 30–48. [Google Scholar] [CrossRef]
- Luhar, M.; Infantes, E.; Nepf, H. Seagrass blade motion under waves and its impact on wave decay. J. Geophys. Res. Ocean. 2017, 122, 3736–3752. [Google Scholar] [CrossRef]
- Lei, J.; Nepf, H. Wave damping by flexible vegetation: Connecting individual blade dynamics to the meadow scale. Coast. Eng. 2019, 147, 138–148. [Google Scholar] [CrossRef]
- Wernberg, T.; Coleman, M.; Fairhead, A.; Miller, S.; Thomsen, M. Morphology of Ecklonia radiata (Phaophyta: Laminarales) along its geographic distribution in south-western Australia and Australasia. Mar. Biol. 2003, 143, 47–55. [Google Scholar] [CrossRef]
- Fowler-Walker, M.J.; Wernberg, T.; Connell, S.D. Differences in kelp morphology between wave sheltered and exposed localities: Morphologically plastic or fixed traits? Mar. Biol. 2006, 148, 755–767. [Google Scholar] [CrossRef]
- Australian Seaweed Industry. Marine Seaweed Aquaculture Risk Assessment (Publication No. 23-024). AgriFutures Australia. 2023. Available online: https://agrifutures.com.au/wp-content/uploads/2023/02/23-024.pdf (accessed on 30 March 2023).
- Wilding, C.; Tillin, H.M.; Corrigan, S.E.; Stuart, E.; Ashton, I.A.; Felstead, P.; Smale, D.A. Seaweed Aquaculture and Mechanical Harvesting: An Evidence Review to Support Sustainable Management; Natural England: Bristol, UK, 2021.
- Flavin, K.; Flavin, N.; Flahive, B. Kelp Farming Manual. 2013. Available online: https://algaefoundationatec.org/aces/library/Kelp%20Farming%20Manual.pdf (accessed on 10 April 2023).
- Juanich, G. Manual of running water fish culture 1 Eucheuma spp. In Projected Reports Cebu, Philippines, FAO; Marine Agronomy Group: Hilo, HI, USA, 1988. [Google Scholar]
- Hansen, J.C.R.; Reidenbach, M.A. Wave and tidally driven flows in eelgrass beds and their effect on sediment suspension. Mar. Ecol. Prog. Ser. 2012, 448, 271–287. [Google Scholar] [CrossRef]
- Geldard, J.; Lowe, R.; Draper, S.; Ellwood, G.; Wood, A.; Roe, T.; Allen, M. Performance of engineered wave attenuating structures. In Proceedings of the Australasian Coast and Ports 2021 Conference, Christchurch, New Zealand, 30 November–3 December 2021. [Google Scholar]
- Bearham, D.; Vanderklift, M.A.; Gunson, J.R. Temperature and light explain spatial variation in growth and productivity of the kelp Ecklonia radiata. Mar. Ecol. Prog. Ser. 2013, 476, 59–70. [Google Scholar] [CrossRef]
- Thurstan, R.; Brittain, Z.; Jones, D.; Cameron, E.; Dearnaley, J.; Bellgrove, A. Aboriginal uses of seaweeds in temperate Australia: An archival assessment. J. Appl. Phycol. 2018, 30, 1821–1832. [Google Scholar] [CrossRef]
- Christie, E.; Möller, I.; Spencer, T.; Yates, M. Modelling wave attenuation due to saltmarsh vegetation using a modified SWAN model. Coast. Eng. Proc. 2018, 1, 73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).