Abstract
The impact of temporal factors and physiochemical properties on the quantities and biomass of fish in the Thermaikos Gulf and adjacent habitats around the Pieria artificial reef complex in Northern Greece was evaluated. Data were collected from edge habitats of an artificial reef made of submerged concrete blocks located offshore of Kitros. Between 2015 and 2017, sampling excursions took place in the spring, summer, and fall of each year. The artificial reef complex is positioned 11.5 km offshore from Kitros, near the delta of the Aliakmonas River, and is influenced by freshwater from the Axios, Aliakmonas, Ludias, and Gallikos Rivers. To estimate the biomass and numbers of the primary benthopelagic commercial fish in the region, nine experimental samples were taken from 2015 to 2017, employing a bottom trawl with a mesh size of 10 mm. Each trawl sample lasted for 30 min to reduce the environmental impact of fishing in the areas under study. The sampling schedule was aligned with factors like the breeding times of the dominant species and the seasonal changes in the thermocline zone. Analysis showed seasonal differences in average abundance and biomass values across the 3 years of the study. Mullus barbatus, recognized as the most commercially significant species caught, demonstrated the greatest abundance and biomass during the second sampling season. Conversely, Pagellus erythrinus, the second most vital commercial species caught, exhibited its highest abundance in the first season and reached its peak biomass during the third sampling season. Hierarchical cluster analyses showed that the two major resulting clusters of species proliferated in numbers over the 3-year period, while undergoing a concurrent reduction in their individual sizes.
1. Introduction
Marine coastal ecosystems hold immense significance for fisheries, representing intricate and interconnected environments. These ecosystems play a vital role in supporting fisheries by providing critical habitats for a wide range of fish species. Coastal areas serve as crucial nurseries, offering shelter and sustenance for juvenile fish, and as feeding grounds for adults. The complexity of coastal ecosystems, encompassing diverse habitats like mangroves, seagrass beds, and coral reefs, contributes to the overall productivity and diversity of fish populations [1]. The intricate web of ecological interactions within these ecosystems influences the abundance, distribution, and health of fish stocks, making their understanding essential for effective fisheries management. By comprehending the complexity of marine coastal ecosystems, fisheries managers can evaluate the impacts of environmental changes, habitat degradation, and fishing activities on fish populations. Ensuring the sustainable management of these ecosystems is crucial to safeguarding fisheries, supporting the livelihoods of coastal communities, and preserving the remarkable biodiversity and ecosystem services they provide [2].
Coastal ecosystems boast remarkable levels of fish biodiversity, with a dazzling array of species inhabiting their diverse habitats, ranging from colorful coral reefs to nutrient-rich estuaries and mangrove forests. The intricate interplay of these fish species not only contributes to the ecological balance of coastal ecosystems but also provides crucial resources and sustenance for local communities dependent on the bountiful marine life [3].
The Kitros Pierias marine protected area is affected by two distinct water masses: the Black Sea Water (BSW) and the Levantine Intermediate Waters (LIW). The Levantine waters (S > 38.7 psu) originate from the southeastern Mediterranean’s Levantine Sea and enter the Aegean Sea from the eastern strait situated between Crete and Rhodes. These waters, both surface (LSW) and intermediate (LIW), extend to a depth of 400 m [4]. They flow north, moving parallel to Asia Minor’s western coastline [4,5] until they intersect with the Black Sea’s lower-salinity waters (S = 26–35 psu) in the marine zone between Limnos and Lesvos, exiting through the Dardanelles Pass. This intersection results in a prominent front between the two water masses [6,7,8], owing to salinity differences. The denser Levantine waters are submerged beneath the Black Sea waters. Within this front’s vicinity, the water column is layered: the Black Sea waters occupy the initial 20–80 m, while the higher-salinity LIW control the rest of the water column, reaching a depth of 400 m.
The two distinct water masses, the Black Sea Water (BSW) and the Levantine Intermediate Waters (LIW), flow conjointly, following a counter-clockwise route, moving first from the west and then to the south [4,7,8,9,10,11,12]. Over time, the Black Sea’s surface water mixes vertically with the underlying Levantine waters. The salinity of the Black Sea water reaches approximately 38 psu in the North Sporades area [5]. However, this surface layer movement and the influence of the less saline Black Sea waters are affected by the wind direction, the geographical features of the North Aegean region (including islands and an extensive continental shelf), and the specific properties of the Black Sea water’s leakage from the Dardanelles [5,7]. The marine protected zone of Kitros Pierias is also shaped by the freshwater inflows from the Aliakmonas River Delta as well as the Axios and Loudias Rivers. In the region of Thermaikos in the Aegean Sea, seasonal variability in environmental parameters affects water column stratification and fluctuations in continental-derived river run-off, hence their importance. During spring and heading into summer, a seasonal thermocline forms as the daily photoperiod becomes extended and the solar radiation is increasing. Concurrently, in the marine protected area of Kitros, the freshwater input increases, not only because of high rain in autumn but also because of high flow rates in the rivers due to the ice melting from the mountains of the region [13].
The aim of this study is to analyze and elucidate the temporal variations and environmental influences on fish stocks within the marine protected area surrounding the artificial reef of Kitros, Pieria. This study aims to identify temporal trends in fish populations and correlate fish stock dynamics with pertinent environmental factors, thereby contributing to sustainable fisheries management and conservation efforts in the Mediterranean Sea.
This study seeks to capture the essence of studying temporal (over-time) variations as well as understand how environmental factors may be playing a role in these dynamics
2. Materials and Methods
2.1. Sampling Site
The Thermaikos Gulf, situated in the western quadrant of the North Aegean Sea, is characterized by its micro-tidal nature with bathymetric depths varying between 30 and 200 m. This gulf stands as a pivotal coastal feature of Northern Greece, primarily owing to its encompassment of Thessaloniki, the nation’s second most populous city with an estimated 1.5 million inhabitants. This urban and commercial hub witnesses a myriad of anthropogenic activities, which include industrial operations predominantly to the west, coupled with tourism, maritime transportation, fisheries, agriculture, and aquaculture endeavors extending across both its eastern and western flanks. The hydrological dynamics of the Thermaikos Gulf are notably influenced by freshwater discharges from four primary rivers: Axios, Aliakmonas, Loudias, and Gallikos. Of these, the Axios and Aliakmonas Rivers emerge as particularly significant, giving rise to complex multi-channel deltaic systems, as delineated by [14,15]. It is worth noting that the Axios River has been identified as a source of considerable pollution, channeling elevated nutrient concentrations from its watershed into the Thermaikos Gulf (as documented by [16]). The circulation pattern includes the more saline water entrance from the eastern part, following a northwestern direction, and the exit of lighter water in the form of river plumes with a southward direction, along the western coastline [17]. The Thermaikos Gulf is considered a mesotrophic system; however, extreme eutrophication events often occur under persistent southerly winds [15]. However, overall ecosystem degradation can be observed, with decreasing fish catches and biomass as a result of overfishing and environmental factors [18].
2.2. Seasonal Monitoring
Over a span of three years (2015–2017), three surveys were conducted annually during the spring, summer, and autumn. Each survey comprised three hauls, amounting to a total of 24 hauls conducted at depths ranging from 19 to 62 m, as depicted in Figure 1. The surveys utilized a bottom trawler, navigating specific routes around the artificial reef. This was to ensure that the hauls aligned vertically with predetermined transects and maintained parallelism with the isobaths.
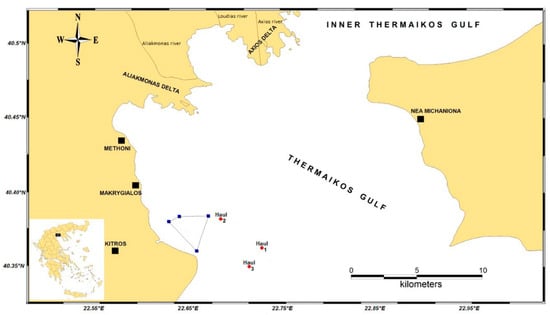
Figure 1.
Haul sites (n = 3) for sampling with bottom trawling in the outer Thermaikos Gulf of the Aegean Sea offshore of the coastal zone of Kitros, in the Pieria region of Greece. Field sites were located on edge habitats of a previously constructed artificial reef complex (polygon with blue edges).
Abbreviations are used to detail the seasonal surveys in Table 1: RTs A1, A2, and A3 (representing the surveys conducted in June 2015) through to RTs I1, I2, and I3 (corresponding to the surveys from September 2017).

Table 1.
Survey routes in the coastal marine protected area of the artificial reef of Kitros Pierias.
The catch was identified in the field at the taxonomical level of species and was measured and weighed. The number of individuals and the biomass of each species were reduced to km2 based on the dredging area of the bottom trawl. For the most abundant commercial species of each area, a length-based population composition per sampling year was calculated as well as length–weight relationships. The estimation of biomass was performed once per year and was calculated in kg per km2 of surface area dredged. Field sites were located in edge habitats along the boundary of a previously installed artificial reef complex (Figure 1).
2.3. Statistical Analyses
Statistical analyses were performed using SPSS version 27 and JASP statistical software version 0.17.3.
2.3.1. Hierarchical Cluster Analysis
To identify assemblages of fish species, a hierarchical cluster analysis was conducted. The cluster analysis procedure was conducted on fish abundance data (Table 2); between-group linkage was used as the method for calculating distances between clusters, and raw values were transformed to z-scale before calculation. Bray–Curtis indexes were calculated between clusters. The decision about the number of clusters to keep was reached based on inspection of the dendrogram and the observation of large gaps between clusters, as well as on the use of the elbow method.

Table 2.
List of taxa collected at field sites in the outer region of the Thermaikos Gulf in the Aegean Sea offshore of the coastal zone of Kitros, in the Pieria region of Greece. Numbers show the mean abundance (N, individual fish/10 km2) and biomass (kg/10 km2).
2.3.2. Abundance and Biomass Factor Scores
After forming the clusters, abundance and biomass factor scores for each cluster were calculated as Anderson–Rubin factor scores of the first principal component extracted from abundances (abundance factor scores) or biomasses (biomass factor scores) of species in each cluster in different samplings. These were used as indicators of cluster-level changes in abundance and biomass across years and seasons.
2.3.3. Statistical Analyses—Length-Based Univariate Analysis of Variance
The lengths of individual fish were standardized and normalized using Blom’s formula. These standardized and normalized length scores were used to calculate average standardized and normalized lengths of the fish within each cluster/assemblage. Univariate analysis of variance was than applied to compare averages of these measures for specimens within each cluster across the studied years and seasons.
2.3.4. Length-Based Univariate Analysis of Variance for Mullus barbatus and Pagellus erythrinus
The same standardization and normalization procedure was performed to transform measures of individual specimen weights of Mullus barbatus and Pagellus erythrinus. Univariate analysis of variance was applied to compare averages of these measures for individual specimens in different years and seasons.
2.3.5. Analysis of Variance
Analysis of variance was then conducted to compare seasonal, yearly, and depth differences in average (standardized and normalized) lengths of specimens from different clusters, as well as for Mullus barbatus and Pagellus erythrinus specifically. For these species, the same analyses were conducted for weight. Along with the results of the analysis of variance, eta squared and partial eta squared were used as measures of effect sizes.
2.3.6. Hydrographic Factor Correlations
Correlations with hydrographic data, such as salinity, chl-a, and temperature, sampled in a previous study in the same area [13] were calculated using the Spearman rank coefficient.
3. Results
3.1. Species Presence, Abundance, and Biomass
In the study area, a rich diversity of 84 species belonging to 24 orders and 44 families was documented: 59 fish, 8 cephalopods, and 12 crustaceans (Table 3), the remaining 5 species belonging to the classes Asteroidea, Holothuroidea, and Malacostraca. A consistent presence of 26 species was seen across all nine samplings. These were Torpedo marmorata, Torpedo nobiliana, Arnoglossus laterna, Trachurus mediterraneus, Spicara flexuosa, Cepola rubescens, Citharus linguatula, Engraulis encrasicolus, Gobius niger, Merluccius merluccius, Mullus barbatus, Scorpaena notata, Scorpaena porcus, Serranus cabrilla, Serranus hepatus, Solea vulgaris, Pagellus erythrinus, Uranoscopus scaber, Loligo vulgaris, Eledone moschata, Octopus vulgaris, Medorippe lanata, Melicertus kerathurus, Parapenaeus longirostris, Liocarcinus depurator, and Squilla mantis.

Table 3.
List of species presences at field sites in the outer region of the Thermaikos Gulf in the Aegean Sea offshore of the coastal zone of Kitros, in the Pieria region of Greece.
As seen in Table 2, the first sampling (RTsA) introduced 45 species, with Diplodus annularis (7887 individuals/km2) and Citharus linguatula (5370 individuals/km2) leading the counts. In terms of biomass, Diplodus annularis (343 kg/km2), Scorpaena notata (275 kg/km2), and Citharus linguatula (228 kg/km2) dominated. In the second sampling (RTsB, 48 species), Citharus linguatula (2852 individuals/km2) and Diplodus annularis (2731 individuals/km2) again led in numbers. The largest biomass was shared between Citharus linguatula (156 kg/km2), Diplodus annularis (136 kg/km2), and Scorpaena notata (94 kg/km2). The third (RTsC, 53 species) and fourth (RTsD, 50 species) samplings saw Mullus barbatus (14,225.5 and 11,964 individuals/km2, respectively) and Citharus linguatula (9761.1 and 6419 individuals/km2, respectively) as the most abundant species. The biomass was highest in Mullus barbatus (642.4 and 505.8 kg/km2, respectively), Citharus linguatula (232.1 and 220.1 kg/km2, respectively), and Pagellus erythrinus (167.9 and 288.0 kg/km2, respectively). The fifth sampling (RTsE, 40 species) was dominated by Citharus linguatula (5165 individuals/km2), Liocarcinus depurator (3606 individuals/km2), and Diplodus annularis (3624 individuals/km2). The largest biomass was found in Citharus linguatula (178.9 kg/km2), Pagellus erythrinus (148.0 kg/km2), and Diplodus annularis (136.7 kg/km2). In the sixth sampling (RTsF, 55 species), Mullus barbatus (22,220 individuals/km2), Diplodus annularis (7768 individuals/km2), and Pagellus erythrinus (5819 individuals/km2) stood out. The heaviest biomass was distributed among Mullus barbatus (1155.2 kg/km2), Pagellus erythrinus (447.8 kg/km2), and Trigla lucerna (248.5 kg/km2). The seventh sampling (RTsG, 50 species) featured Pagellus erythrinus (10,315 individuals/km2) and Mullus barbatus (7979 individuals/km2) as the most abundant. The largest biomass was recorded for Pagellus erythrinus (597.7 kg/km2), Trachurus mediterraneus (287.6 kg/km2), and Mullus barbatus (247.3 kg/km2). In the eighth sampling (RTsH, 52 species), Diplodus annularis (11,026 individuals/km2) and Engraulis encrasicolus (10,826 individuals/km2) led the counts, while the biomass was most substantial in Diplodus annularis (491.53 kg/km2), Pagellus erythrinus (360.09 kg/km2), and Trachurus mediterraneus (180.64 kg/km2). Finally, the ninth sampling (RTsI, 50 species) saw Mullus barbatus (28,109 individuals/km2) leading in abundance, while the heaviest biomass was found in Mullus barbatus (1386.63 kg/km2), Pagellus erythrinus (554.75 kg/km2), and Trigla lucerna (183.16 kg/km2). All species abundances and biomasses are shown in Table 2.
The mean values for the most abundant species per square kilometer over all nine samplings were as follows (Table 4). The species with the greatest abundance was Mullus barbatus, with a remarkable mean of 40,549.7 individuals/km2. Next was Diplodus annularis, demonstrating a healthy population with an average of 22,225.4 individuals/km2. Pagellus erythrinus and Arnoglossus laterna also exhibited strong presence, with respective mean abundances of 22,047.4 and 15,594.4 individuals/km2. Engraulis encrasicolus and Trachurus mediterraneus followed, with means of 13,997.6 and 10,393.4 individuals/km2, respectively.

Table 4.
Species with largest total biomass and abundance rates in all surveys. Species of major commercial interest are in bold.
Next in line were Citharus linguatula with an average of 8569.4 individuals/km2, Spicara flexuosa with 7888.5 individuals/km2, and Serranus hepatus with a mean of 7258.8 individuals/km2. Lastly, Scorpaena notata exhibited a mean abundance of 7225.6 individuals/km2, rounding off the list of the most abundant species in the study area.
In terms of biomass, the mean weight per square kilometer over all nine samplings yielded a different ranking of species, as Mullus barbatus showed an average biomass of 55,444.4 kg/km2. Diplodus annularis was second, with a significant mean biomass of 32,340.6 kg/km2. Citharus linguatula followed closely with an average biomass of 31,400.0 kg/km2, while Pagellus erythrinus and Arnoglossus laterna had mean biomasses of 21,628.2 kg/km2 and 20,373.4 kg/km2, respectively. Trachurus mediterraneus and Scorpaena notata showed consistent populations with respective mean biomasses of 16,182.8 kg/km2 and 14,511.7 kg/km2. Rounding out the top biomass producers were Serranus hepatus with an average biomass of 12,154.9 kg/km2, Liocarcinus depurator with 10,750.6 kg/km2, and Pagellus bogaraveo with 9749.0 kg/km2. Each of these species played a critical role in the ecosystem by contributing substantially to the overall biomass.
3.2. Hierarchical Cluster Analyses
Hierarchical cluster analysis was employed to identify assemblages of marine species, examining the abundances of various fish species across the sampling points. An inspection of the dendrograms and the distances between the clusters uncovered eight distinct assemblages of marine species. These assemblages revealed patterns where the abundances of certain species tended to increase and decrease together (Figure 2).
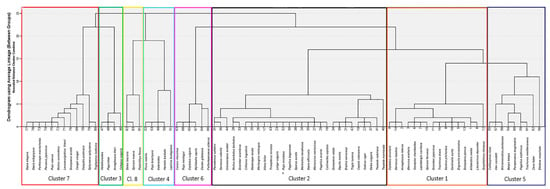
Figure 2.
Dendrogram showing eight clusters of marine species assemblages identified through hierarchical cluster analysis.
After forming the clusters, the first principal component of values of species belonging to that cluster was saved as an Anderson–Rubin factor score. This was performed for abundance and biomass values separately. The purpose of this transformation was to combine values for these variables of different species within each cluster while controlling for differences in scales and variances of these variables between marine species. In this way, these factor scores can serve as central tendency values for the abundance and biomass of each cluster at different sampling points, represented as a deviation from the mean of that cluster for all sampling points. Species clusters along with their respective biomasses are presented in (Table 5).

Table 5.
Species cluster memberships and mean abundances and biomasses for the period 2015–2017.
The data presented in Table 6, Table 7 and Table 8 illustrate diverging trends in the yearly variation across marine species assemblages or clusters. Notably, the largest clusters, clusters 1 and 2, reveal an upward trajectory in organism abundance throughout the observed year. However, this surge in quantity does not correlate with a similar increase in biomass. In these two prominent clusters, peak species abundance was recorded in 2017. Interestingly, the year with the maximum biomass varied across species, and, for only a select few, 2017 marked the highest biomass. This discrepancy suggests that while the species constituting these two clusters have proliferated in numbers over the examined years, there has been a concurrent reduction in their individual sizes.

Table 6.
Changes in relative abundances and biomasses of marine species within each cluster expressed as factor scores in the period 2015–2017.

Table 7.
Changes in biomasses and abundances of different species across years. The numbers of species with the greatest abundance and biomass each year by clusters are shown. Years and clusters with more than 50% of species having the highest abundance or biomass are in bold.

Table 8.
Comparison of average lengths of specimens in clusters standardized and normalized at the species level in the period 2015–2017; one-way ANOVA.
3.3. Length-Based Statistical Analyses
The lengths of individual specimens have been standardized within each species and normalized using Blom’s formula to make sure that the distribution of lengths within each species is close in shape to the theoretical normal distribution. Average normalized standardized lengths of specimens within clusters have been compared between years to note any changes between years in specimen length that might be specific to individual clusters of marine species.
The conclusion that, across the studied years, specimens of the species forming the first two clusters became more numerous but smaller in size is additionally supported by comparing the average lengths of specimens presented in Table 8. These two clusters are the largest, consisting of much larger numbers of marine species than the remaining clusters. Cluster 5 shows a similar but less clear trend. Average abundance factor scores are similar in 2015 and 2016, but visibly higher in 2017. On the other hand, no species from this cluster had the highest biomass in 2017, indicating that marine species from this cluster also became more numerous in 2017, compared to the 2 previous years, but smaller. The survey in 2017 caught larger numbers of smaller specimens of these species. This trend is also visible in Table 8, as the average standardized and normalized lengths of specimens within this cluster increased from 2015 to 2017, and the difference was statistically significant.
The results for clusters 3 and 6 are ambiguous. While the inspection of abundance and biomass values shows that all the species in these clusters had the highest abundance and biomass values in 2016 and this is confirmed by the factor scores, comparison of average standardized and normalized specimen lengths seems to indicate an increase in average length throughout the years. However, it should be noted that the differences between average lengths in cluster 3 are not statistically significant, and it contains a very small number of specimens (27 across four species, of which only one is a species of fish). Cluster 6 has a larger number of specimens (111 across 3 years), but the 2015 catch consisted of only one fairly large specimen of Dentex gibbosus, while 97 of these specimens are from 2016.
Clusters 4, 7, and 8 showed a strong decrease between 2015 and 2016, and then either a smaller decrease, stabilization, or a slight recovery between 2016 and 2017 (Table 6). All of the species in these clusters had the highest abundances, and all save one had the highest biomasses in 2015. The difference in average standardized and normalized lengths between years was statistically significant only for cluster 4. This difference was quite strong, although there were relatively few individual specimens of species from this cluster in the sample. The differences between the average lengths of the specimens in clusters 7 and 8 were not statistically significant. However, it should be noted that the total number of specimens of species belonging to these two clusters strongly decreased through the studied period. Of the species in cluster 7, there were 84 specimens in 2015, 25 in 2016, and only 6 (of all species in total) in 2017. The imbalance was even greater in cluster 8, for which there was only one specimen in 2016 and also in 2017.
3.4. Mullus barbatus and Pagellus erythrinus
Looking at Mullus barbatus and Pagellus erythrinus specifically, it can be noted that Mullus barbatus is a part of cluster 2, while Pagellus erythrinus is a member of cluster 5. Across the studied period, the abundance of Mullus barbatus greatly increased (Table 5). However, its biomass decreased, indicating that the species has become more numerous but that the individual specimens have become smaller in size. Comparisons of the lengths of specimens of this species across the studied period showed a decrease across the years (the raw average lengths of specimens were 154.5 in 2015, 153.99 in 2016, and 149.90 in 2017). The average weight of specimens of this species was the highest in 2016.
Similar to other species in cluster 5, the abundance of Pagellus erythrinus increased over the studied period, while the biomass of the species was the highest in 2016, higher than in the following year (Table 5). The average length of specimens of this species was higher in 2017 than in the 2 previous years, but it was the lowest in 2015 (Figure 3). The average weight steadily increased through the studied years, with the lowest in 2015 (3066) and the highest in 2017 (3207).
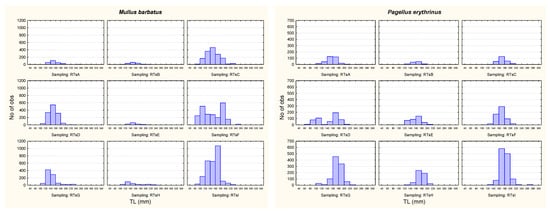
Figure 3.
Length composition of red mullet, Mullus barbatus, and common pandora, Pagellus erythrinus, per survey season.
3.5. Year-Based Statistical Analyses
Next, changes in total species abundance and biomass per year were examined (Table 9 and Table 10; Figure 4). Table 9, Table 10, Table 11 and Table 12 show that abundances, biomasses, and lengths of individual specimens differed across the three studied years. Almost all of the species constituting clusters 2, 6, and 7 had both the largest abundance and the largest biomass in year 3. However, looking at the size of the average specimen from cluster 2 (Table 10), standardized and normalized length scores seem to indicate that the relative length was the lowest in year 3. This indicates that the average size of specimens from species in this cluster was lowest in year 3 but that their numbers more than compensated for this, thus making the biomass values the largest in this year. A similar trend for biomass and abundance was observable for species constituting clusters 6 and 7, with the difference that, in these clusters, the average differences between standardized and normalized average length measures were not statistically significant, nor did they indicate a clear trend. The reason for this non-significance is that most of the specimens from clusters 6 and 7 were caught in year 3. Cluster 6 has only five specimens caught in year 1 and only two specimens caught in year 2. For cluster 7, these numbers are nine specimens in year 1 and four specimens in year 2.

Table 9.
Species cluster memberships and mean abundances and biomasses across the 3 years of sampling. Numbers are mean abundance and biomass values for each year for each species obtained by averaging abundance and biomass values for each year of sampling across the years of the study.

Table 10.
Anderson–Rubin mean factor scores showing changes in relative abundances and biomasses of marine species within each cluster expressed as factor scores across the 3 years.
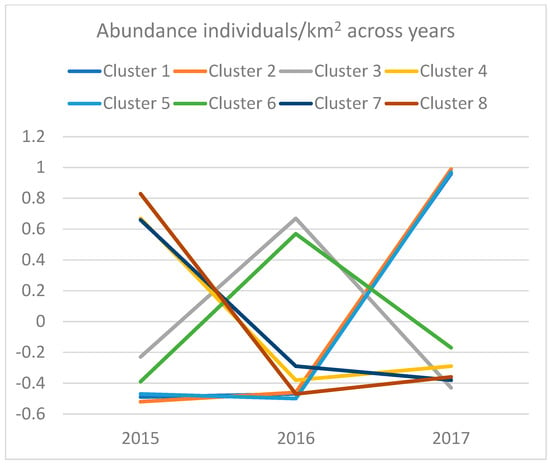
Figure 4.
Changes in relative abundances of marine species within each cluster expressed as factor scores in the period 2015–2017.

Table 11.
Changes in biomass and abundance of different species across years. The numbers of species with the greatest abundance and biomass in each year by clusters are shown.

Table 12.
Comparison of average lengths of specimens in clusters standardized and normalized at the species level across the three years; one-way ANOVA.
The species constituting clusters 3, 5, and 8 all had the largest mean abundances in year 1, but biomass changes across the years were less clear (Table 9). While most species constituting clusters 3 and 8 had the largest biomass also in year 1, this was the case for only half of the species constituting cluster 5. Average factor scores confirm the highest abundances in year 1 for all three clusters but the highest biomasses in this year only for clusters 3 and 8. For the cluster 5 species, the factor scores indicate the largest biomass in year 3.
The average fish length comparison (standardized and normalized scores) is less clear. One-way ANOVA comparisons of differences between annual averages for lengths of species in cluster 3 were very close to reaching the conventional statistical significance level of 0.05, and the size of the differences expressed by eta squared is quite high. They indicate the same average length in years 1 and 2 and a much smaller length in year 3. This indicates that species from cluster 3 tended to be largest and most abundant in year 1. Species from cluster 5 tended to be shorter on average in year 2 compared to year 3, while the average length of specimens in year 1 was in between those in the other 2 years. It can be concluded that species from cluster 5 tended to be the most abundant in year 1 but not necessarily the largest in size. The differences between the yearly averages in the length of specimens from cluster 8 are not statistically significant, although the average values decreased from year 1 to 3, as did the numbers of specimens and abundances, as well as biomasses for the majority of species.
The species constituting cluster 4 tended to have the largest abundances and biomasses in year 2, and comparison of the average lengths of specimens from this cluster across years, although not statistically significant, also indicates the highest average length of specimens in year 2 (Table 9).
All the species constituting cluster 1 had the highest abundance in year 2. However, although numerous, specimens of species from this cluster tended to be shorter on average than in year 1. Biomass comparisons indicate the highest average biomasses of species from this cluster in year 3 (Table 9).
Looking at Mullus barbatus and Pagellus erythrinus specifically, as the two most important commercial fish, it can be noted that both the abundance and biomass of Mullus barbatus were the highest in year 3 but the lowest in year 2 (Table 9). On the other hand, the length of the average specimen was the highest in year 2 (156.4) and lower in the other two years. The average weight of specimens of this species tended to increase through the studied part of a year, being the lowest in year one (2066) and the highest in year 3 (24,522).
Similar to the other species in cluster 5, Pagellus erythrinus showed the highest abundance in year 1, but its biomass was highest in year 3 (Table 9). Both abundance and biomass were the lowest (on average) in year 2. The length of the average specimen tended to increase throughout the studied part of a year, going from an average length of 161.5 in year 1 to 177.6 in year 3. The same trend was seen in average weight, which also increased from year 1 to year 3 from 2950 to 3410.
3.6. Correlation of Abundance and Biomass with Hydrographic Data
Looking at the correlations of temperature, salinity, and density with the abundances and biomasses of marine species, it can be noted that, although many correlations are high, they do not reach conventional statistical significance levels because of the low number of samplings (nine in total). The highest correlations with the examined hydrographic factors were observed for clusters 7 and 8. In these clusters, both the abundances and biomasses of the marine species tended to be lower at higher salinities and higher densities of water, but higher with higher water temperatures. The abundances of cluster 4 species tended to be lower at higher temperatures but higher when the water density was higher. The main correlate of the abundance of species constituting clusters 1, 2, 3, and 5 is salinity. Species from these clusters tended to be more abundant in waters with greater salinity, save for cluster 3, for which this association was reversed. Salinity is also the main correlate of biomass of clusters 2, 5, and 6, with higher salinities being associated with lower biomass for these clusters. None of these correlations, save the ones marked with * for clusters 7 and 8, reach the conventionally accepted levels of statistical significance, although they are very substantial in magnitude.
Table S2 shows particularly pronounced correlations between the lengths of specimens of cluster 4 species and hydrographic factors. Specimens of these species tended to be longer in warmer, less salty, and less dense waters. Correlations of lengths of specimens from clusters 1, 2, 5, and 6 tended to be statistically significant but much lower in size.
Regarding Mullus barbatus and Pagellus erythrinus (Tables S1 and S2), it can be noted that their abundances tended to be higher in saltier waters but their biomasses lower. Also, specimens of these species tended to have higher weights in warmer waters and waters that are less salty and dense. Correlations with length were much lower, although statistically significant due to a large number of individual specimens included in this calculation. Mullus barbatus specimens tended to be a bit longer on average in less salty and less dense waters. Pagellus erythrinus specimens tended to be longer in warmer and saltier waters. Finally, integral models of effects of season, year, and depth on the standardized and normalized lengths of the specimens within each cluster were tested for each cluster separately for Mullus barbatus and Pagellus erythrinus as the two commercially most important species.
3.7. Cluster-Based Effects of Season, Year, and Depth on Species Standardized Lengths
The tests show that models predicting standardized and normalized lengths of specimens constituting clusters 3, 4, 7, and 8 based on the season, year, and depth of the collection do not reach the conventional level of statistical significance of 0.05. The model for cluster 6 is very close to that level. Although effect size measures are often substantial, as in the case of cluster 3, there are not enough specimens across different categories to make the tests of observed mean differences statistically significant.
The models for clusters 1, 2, and 5 are statistically significant, with integral models accounting for 4–5% of the variance of specimen length. In all three clusters, season and year are statistically significant predictors, accounting for between 1% and 3% of variance of specimen standardized and normalized length. Depth (operationalized as a binary variable—up to 30 and more than 30 m in depth) is not a statistically significant predictor in any of these models. However, in each of the three models there is at least one statistically significant interaction with depth. This means that although mean lengths of specimens caught at different depth categories do not differ, when season and year are accounted for, differences in the mean lengths of specimens caught in different seasons or years differ for different depths, depending on the model.
When the lengths and weights of individual specimens of Mullus barbatus and Pagellus erythrinus species are considered (Table S3), the results show that interactions between year and season explain a small part of the variance (5%) of Mullus barbatus specimen lengths. When lengths of individual Pagellus erythrinus specimens are considered, year, season, and depth and their interactions explain 19% of their variance. All three factors (season, year, and depth) are statistically significant in the model along with all save one of their interactions.
While season, year, and depth account for a relatively small part of the variance of length of individual specimens of these two marine species, weight comparisons tell a completely different story. Most of the variance of weight of these two species is accounted for by the three examined factors. Season, depth, and year and their interactions jointly account for 84% of the variance in Mullus barbatus individual specimen weights, while this percentage is 85% for Pagellus erythrinus. While all three factors and their interactions are statistically significant, season is the most important factor for Mullus barbatus weights, accounting alone for 78% of the variance of the specimen weights, followed by a season*year interaction. When the weights of Pagellus erythrinus specimens are considered, season*year interaction turns out to be the best predictor, followed by season. These findings support the great seasonal effects on the weights of individual specimens of these two marine species in the observed period.
4. Discussion
The Mediterranean Sea, although largely oligotrophic, displays environmental characteristics conducive to fish fertility and recruitment during specific periods or in association with certain localized habitat and hydrographic structures [19]. In the Thermaikos region of the Aegean Sea, seasonal environmental parameter changes influence water column stratification and fluctuations in river run-off, emphasizing their significance. The extended daily photoperiod and increasing solar radiation in the spring and summer lead to the formation of a seasonal thermocline. Concurrently, freshwater input surges, not only from autumnal rainfall but also due to increased river flow rates as a result of mountain ice melt in the region.
In the study area, the primary freshwater sources are the Axios and Aliakmonas Rivers, with annual average discharges of 118 m3/s and 31.9 m3/s, respectively [20]. Flowing from the north, these waters move parallel to the western coastline, beyond the Thermaikos Gulf. As [21] point out, significant nutrient loading from the Axios River impacts the coastal zone, but this does not seem excessively high relative to silica loadings, indicating a low potential for coastal zone eutrophication [22]. The combination of nutrient fluxes, short residence times, and high flush rates minimizes eutrophication risks in the coastal area. The Aliakmonas River, however, contributes much fewer nutrient loads than Axios [20].
As [23] noted, fish spawning grounds often align with nutrient-rich freshwater influx areas. The Axios, Aliakmonas, and two other smaller continental water bodies, the Gallikos and Loudias Rivers, serve this role in the study area.
It is well established that temperature and climate change influence fish diversity and geographical distribution. Temperature profoundly impacts fish species’ recruitment, reproduction, growth, and behavior [24], as well as food quality and availability [25,26]. Over recent decades, surface temperatures have increased by 1.1 °C and approximately 0.7 °C at 80 m depth [27], prompting spatial shifts for many fish species. For instance, the round sardinella (Sardinella aurita) has expanded northward along the Mediterranean Spanish coast [28], and numerous exotic species have migrated into the Mediterranean via the Suez Canal and Gibraltar [29,30,31]. The role of rising sea temperature in facilitating the spread of alien species in the Eastern Mediterranean has been a primary concern for scientists since the mid-1950s [32,33]. Moreover, temperature is a key factor influencing the spawning season of thermophilic fish species. The role of temperature in species assemblages is also mentioned in previous studies in the same area [13]. The Kitros marine protected area exhibits the hallmark features of a spawning habitat with elevated temperatures, coupled with the presence of nutrients and chlorophyll-a, accounting for significant biovariability in both adult fish and ichthyoplankton assemblages [13]
Extended spawning seasons have been observed in the Black Sea, where anchovy ichthyoplankton typically collected from June to September were found from May to October between 2011 and 2016, and Sprattus sprattus larvae, typically found in winter, were detected year-round [34]. Another example is the varying spawning season length of the round sardinella due to sea temperature differences between different regions, such as a shorter duration in the northern part of the Western Mediterranean (July–September; ref. [35]) compared to the southern African coast [36]. Finally, it was found that temperature, alongside depth and chl-a values, significantly influenced spawning ground selection for small pelagic species. Given these factors, it can be stated that the study area exhibits all spawning ground characteristics, with high temperature, nutrient availability, and chl-a accounting for the anchovy’s year-round abundance. During the period 2015–2017 in which the samplings took place, several captured species were of high or moderate commercial value. These include Mullus barbatus, Diplodus annularis, Pagellus erythrinus, and Trachurus mediterraneus. The red mullet, Mullus barbatus, was the species with the greatest abundance in comparison to the other commercial species of the area as well as the species with the greatest biomass rate (Table 4). In the marine protected area of Kitros, it appeared in all seasons and showed its greatest abundance in the September (RTsI) survey (Table 2). It is one of the most important commercial species of the Mediterranean Sea and is caught mainly using bottom trawl and coastal nets.
The results show the dominance of Mullus barbatus in abundance and biomass. The Mediterranean Sea’s red mullet, Mullus barbatus, holds substantial economic value [37]. It has been identified that, in many regions, the species is excessively fished, causing a significant decline in its population. Implementing prolonged [38,39] or temporary restrictions [40] on trawl fishing has been proven to significantly boost its biomass. Pipitone and colleagues found that following a 4-year trawling moratorium in the Gulf of Castellammare (Sicily), there was roughly a 25-fold surge in average catch per unit of effort (cpue). The red mullet’s ability to exploit available food resources effectively [41] might be a factor in its population rebound following trawling bans. The species is known to feed extensively on soft sandy/muddy bottoms [42] and exhibit a strong preference for several species of polychaetes [43]. While it is widely recognized that the red mullet population flourishes when protected in areas susceptible to trawling, comprehensive studies on its feeding habits remain sparse, and our understanding of its role in the Mediterranean soft-bottom food webs is limited.
Diplodus annularis, another species that dominated the samples in abundance and biomass, is abundant in the Mediterranean [44], occurring also along all the coast of Africa. It inhabits rocky bottoms and Posidonia beds, from the surface to a depth of 50 m [45]. The muddy and gravelly sea bottom environment of Kitros is evidently attractive for this species, as habitat partitioning appears to be as important as food partitioning in structuring Diplodus assemblages [46,47]. Moreover, in previous studies [48], it was found that Diplodus annularis depended heavily on fauna that belonged to artificial reef communities, such as crustaceans, amphipods, and decapods [49,50].
The second most important commercial fish caught was the common pandora, Pagellus erythrinus. It was the third species in abundance and the fourth in biomass in these samplings (Figure 2). Pagellus erythrinus is a highly valued demersal species distributed in the Mediterranean, the Black Sea, and along the European and African coasts of the Atlantic, from Angola to Norway [51]. It is a protogynous hermaphrodite and a multiple spawner characterized by a protracted late-spring to late-summer spawning period [52,53]. The age and growth of the species have been studied in the Eastern Mediterranean and the Ionian Sea by the authors of [54].
In the Thermaikos Gulf, the predominant benthic substrate is muddy [55], though regions of sandy/muddy and gravelly substrates are also observed. Mullus barbatus, colloquially termed the red mullet, demonstrates a pronounced affinity for certain benthic substrates. Foremost among these is the muddy seabed, which aligns well with the species’ behavioral ecology. Employing their tactile barbels, individuals forage within this substrate, predominantly targeting small invertebrates. Additionally, the pliable characteristics of the muddy substrate facilitate behavioral adaptations, such as burying, which the species utilizes both as an anti-predatory measure and during periods of rest. The evident correlation between Mullus barbatus and muddy environments [56] accentuates the ecological imperative of safeguarding these habitats to ensure the long-term viability and conservation of this marine species. Pagellus erythrinus, known as the common pandora, exhibits a pronounced affinity for specific benthic substrates. Among the variety of seabed types, it appears that gravelly substrates are preferentially selected by this species. The granular nature of such substrates provides both refuge from potential predators and an abundant microhabitat rich in benthic prey, including small crustaceans and other invertebrates. This observed association between Pagellus erythrinus and gravelly environments [57] underscores their ecological relevance and emphasizes the need for focused conservation efforts for habitats of this nature to support the persistence and propagation of the species.
In the last year of this study, species of the first two major clusters showed an increase in abundance but a decrease in size compared to the first year. One of the potential causes of this could be high fishing pressure. Extensive overfishing can lead to an overall decrease in the average size of fish over time. This phenomenon, often referred to as “fishing down the size spectrum”, can result from the faster growth rates of smaller, younger fish and their subsequent increased representation in the population [58]. Other causes of this downward trend in species sizes can be environmental changes. Changes in water temperature, salinity, dissolved oxygen levels, and pH can all influence fish growth rates and overall size. Increases in water temperature due to climate change can lead to increased metabolic rates in fish, which may result in smaller body sizes [59]. Predation pressure can also lead to a decrease in fish size, as larger individuals are more likely to be targeted by predators. Furthermore, in response to predation risk, some species may exhibit “stunted” growth, a survival strategy where energy is invested in reproduction rather than growth [60]. Lastly, some fish species adopt a strategy of producing a large number of offspring that are smaller in size, particularly under conditions of high mortality. This strategy, known as r-selection, prioritizes quantity over the size or quality of offspring [61].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse11091773/s1, Table S1: Spearman correlation coefficients between abundance and biomass factor scores of clusters of marine species as well as two commercial fish species, Mullus barbatus and Pagellus erythrinus; Table S2: Spearman correlation coefficients between standardized and normalized lengths of specimens in clusters of marine species and lengths and weights of the two commercially most important fish species and hydrographic characteristics; Table S3: Predicting standardized, normalized lengths of Mullus barbatus and Pagellus erythrinus, based on season, year, and depth; one-way ANOVA.
Author Contributions
Conceptualization, A.A.K. methodology, A.A.K. and I.E.B.; data curation, A.A.K.; writing—original draft preparation, A.A.K.; writing—review and editing, A.A.K.; supervision, I.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this paper were collected in the context of a research project titled “Monitoring of an artificial reef in Kitros off the coast of Pieria” in Northern Greece conducted by the Fisheries Research Institute of Kavala, Greece. The project was financed by the Greek National Program for the restructuring of fisheries.
Acknowledgments
The authors thank the crew of Ag. Andreas for their help during the sampling trips and the Kavala Artificial Reef Team of the Fisheries Research Institute for their contributions in the sampling, processing, and species identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Douvere, F. The importance of marine spatial planning in advancing ecosystem-based sea use management. Mar. Policy 2008, 32, 762–771. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Zervakis, V.; Georgopoulos, D.; Karageorgis, A.P.; Theocharis, A. On the response of the Aegean Sea to climatic variability: A review. Int. J. Climatol. 2004, 24, 1845–1858. [Google Scholar] [CrossRef]
- Karageorgis, A.; Nikolaidis, N.; Karamanos, H.; Skoulikidis, N. Water and sediment quality assessment of the Axios River and its coastal environment. Cont. Shelf Res. 2003, 23, 1929–1944. [Google Scholar] [CrossRef]
- Zodiatis, G.; Alexandri, S.; Pavlakis, P.; Jonsson, L.; Kallos, G.; Demetropoulos, A.; Georgiou, G.; Theodorou, A.; Balopoulos, E. Tentative study of flow patterns in the North Aegean Sea using NOAA-AVHRR images and 2D model simulation. Ann. Geophys. 1996, 14, 1221–1231. [Google Scholar] [CrossRef]
- Poulos, S.; Drakopoulos, P.; Collins, M. Seasonal variability in sea surface oceanographic conditions in the Aegean Sea (Eastern Mediterranean): An overview. J. Mar. Syst. 1997, 13, 225–244. [Google Scholar] [CrossRef]
- Lykousis, V.; Chronis, G.; Tselepides, A.; Price, N.; Theocharis, A.; Siokou-Frangou, I.; Van Wambeke, F.; Danovaro, R.; Stavrakakis, S.; Duineveld, G.; et al. Major outputs of the recent multidisciplinary biogeochemical researches undertaken in the Aegean Sea. J. Mar. Syst. 2002, 33–34, 313–334. [Google Scholar] [CrossRef]
- Lacombe, H.; Tchernia, P.; Benoist, P. Contribution a l’etude hytrologique de la mer Egee en periode d’ete. Bull. Inf. COECX 1958, 8, 454–468. [Google Scholar]
- Ovchinnikov, I.M. Circulation in the surface and intermediate layers of the Mediterranean. Oceanology 1966, 24, 168–173. [Google Scholar]
- Kourafalou, V.H.; Barbopoulos, K. High resolution simulations on the North Aegean Sea seasonal circulation. Ann. Geophys. 2003, 21, 251–265. [Google Scholar] [CrossRef]
- Sylaios, G.; Stamatis, N.; Kallianiotis, A.; Vidoris, P. Monitoring Water Quality and Assessment of Land-Based Nutrient Loadings and Cycling in Kavala Gulf. Water Resour. Manag. 2005, 19, 713–735. [Google Scholar] [CrossRef]
- Kallianiotis, A.A.; Kamidis, N.; Tselepides, A.; Batjakas, I.E. Spatiotemporal and Environmental Dynamics of Abundances and Diversity of Larval Fish in Artificial Reef Edge Habitats of Kitros, Pieria (Northern Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 40. [Google Scholar]
- Krestenitis, Y.; Androulidakis, Y.; Makris, C.; Kombiadou, K.; Baltikas, V.; Diamanti, P. Evolution of storm surge extreme events in Greek Seas under climate change scenario. In Proceedings of the 11th Symposium of Oceanography and Fisheries, Mytilene, Greece, 13–17 May 2015; pp. 849–852. [Google Scholar]
- Androulidakis, Y.; Kolovoyiannis, V.; Makris, C.; Krestenitis, Y.; Baltikas, V.; Stefanidou, N.; Chatziantoniou, A.; Topouzelis, K.; Moustaka-Gouni, M. Effects of ocean circulation on the eutrophication of a Mediterranean gulf with river inlets: The Northern Thermaikos Gulf. Cont. Shelf Res. 2021, 221, 104416. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Gritzalis, K.C.; Kouvarda, T.; Buffagni, A. The development of an ecological quality assessment and classification system for Greek running waters based on benthic macroinvertebrates. Hydrobiologia 2004, 516, 149–160. [Google Scholar] [CrossRef]
- Poulos, S.E.; Chronis, G.T.; Collins, M.B.; Lykousis, V. Thermaikos Gulf coastal system, NW Aegean Sea: An overview of water/sediment fluxes in relation to air–land–ocean interactions and human activities. J. Mar. Syst. 2000, 25, 47–76. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Makino, M.; Prayoga, M.R.; Zeller, D.; Vianna, G.M.S.; Humphries, A.T. Responses in fisheries catch data to a warming ocean along a latitudinal gradient in the western Pacific Ocean. Environ. Biol. Fishes 2022, 105, 1347–1362. [Google Scholar] [CrossRef]
- Estrada, M. Primary production in the northwestern Mediterranean. Sci. Mar. 1996, 60 (Suppl. 2), 55–64. [Google Scholar]
- Skoulikidis, N.T.; Bertahas, I.; Koussouris, T. The environmental state of freshwater resources in Greece (rivers and lakes). Environ. Geol. 1998, 36, 1–17. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Karageorgis, A.P.; Kapsimalis, V.; Drakopoulou, P.; Skoulikidis, N.; Behrendt, H.; Levkov, Z. Management of nutrient emissions of Axios River catchment: Their effect in the coastal zone of Thermaikos Gulf, Greece. Ecol. Model. 2009, 220, 383–396. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J. River basin nutrient delivery to the coastal sea: Assessing its potential to sustain new production of non-siliceous algae. Mar. Chem. 2007, 106, 148–160. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Iglesias, M.; Tugores, M.; Bonnano, A.; Patti, B.; Felice, A.; Leonori, I.; Bigot, J.L.; Tičina, V.; Pyrounaki, M.; et al. Characterizing the potential habitat of European anchovy Engraulis encrasicolus in the Mediterranean Sea, at different life stages. Fish. Oceanogr. 2013, 22, 69–89. [Google Scholar] [CrossRef]
- Lloret, J.; Rätz, H.J. Condition of cod (Gadus morhua) off Greenland during 1982–1998. Fish. Res. 2000, 48, 79–86. [Google Scholar] [CrossRef]
- Jørgensen, T. Long-term changes in growth of North-east Arctic cod (Gadus morhua) and some environmental influences. ICES J. Mar. Sci. 1992, 49, 263–277. [Google Scholar] [CrossRef]
- Nilssen, E.M.; Pedersen, T.; Hopkins, C.C.E.; Thyholt, K.; Pope, J.G. Recruitment variability and growth of Northeast Arctic cod: Influence of physical environment, demography, and predator-prey energetics. ICES Mar. Sci. Symp. 1994, 198, 449–470. [Google Scholar]
- Salat, J.; Pascual, J. The oceanographic and meteorological station at l’Estartit (NW Mediterranean). In Proceedings of the Tracking Long-Term Hydrological Change in the Mediterranean Sea, Monaco, 22–24 April 2002; CIESM Workshop Series n° 16. pp. 29–32. [Google Scholar]
- Sabates, A.; Olivar, M.P. Variation of larval fish distributions associated with variability in the location of a shelf-slope front. Mar. Ecol. Prog. Ser. 1996, 135, 11–20. [Google Scholar] [CrossRef]
- Golani, D.; Orsi-Relini, L.; Massuti, E.; Quignard, J.P. Atlas of Exotic Species in the Mediterranean; CIESM: Villa Girasole, Monaco, 2002. [Google Scholar]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Ben, T.A. Red Sea fishes recently found in the Mediterranean. Copeia 1966, 2, 254–275. [Google Scholar]
- Ben Yami, M.; Glaser, T. The invasion of Saurida undosquamis (Richardson) into the Levant Basin. Fish. Bull. 1974, 72, 359–373. [Google Scholar]
- Klimova, T.; Podrezova, P. Seasonal distribution of the Black Sea ichthyoplankton near the Crimean Peninsula. Reg. Stud. Mar. Sci. 2018, 24, 260–269. [Google Scholar] [CrossRef]
- Palomera, I.; Sabatés, A. Co-occurrence of Engraulis encrasicolus and Sardinella aurita eggs and larvae in the Northwestern Mediterranean. Sci. Mar. 1990, 54, 63–69. [Google Scholar]
- Quaatey, S.N.K.; Maravelias, C.D. Maturity and spawning pattern of Sardinella aurita in relation to water temperature and zooplankton abundance off Ghana, West Africa. J. Appl. Ichthyol. 1999, 15, 63–69. [Google Scholar] [CrossRef]
- Relini, G.; Bertrand, J.; Zamboni, A. Synthesis of the knowledgeon bottom fishery resources in the central Mediterranean (Italy and Corsica). Biol. Mar. Mediterr. 1999, 6 (Suppl. 1), 314–322. [Google Scholar]
- Potoschi, A.; Cavallaro, G.; Sturiale, P.; Lo Duca, G. Eggs and larvae of swordfish, tuna and albacore caught in the Ionian Sea. Biol. Mar. Mediterr. 1994, 1, 119–124. [Google Scholar]
- Pipitone, C. Divieto di pesca a strascico nel Golfo di Castellammare (Sicilia nord-occidentale) alcune considerazioni. Biol. Mar. Mediterr. 1996, 3, 200–204. [Google Scholar]
- Hilborn, R.; Ovando, D.; Euan, D. Partial closures in fisheries: Lessons from Indigenous practices. Science 2015, 350, 906–907. [Google Scholar]
- Vassilopoulou, V.; Papaconstantinou, C. Feeding habits of red mullet (Mullus barbatus) in a gulf in western Greece. Fish. Res. 1993, 16, 69–83. [Google Scholar] [CrossRef]
- Badalamenti, F.; Pinnegar, J.K.; Polunin, N.V.; D’Anna, G. Estimates of trophic level in the red mullet Mullus barbatus: Comparison between gut-contents and stable-isotope data. In Proceedings of the Fishing down the Mediterranean Food Webs, Kerkyra, Greece, 26–30 July 2000; pp. 19–21. [Google Scholar]
- Machias, A.; Labropoulou, M. Intra-specific Variation in Resource Use by Red Mullet, Mullus barbatus. Estuar. Coast. Shelf Sci. 2002, 55, 565–578. [Google Scholar] [CrossRef]
- Tortonese, E. Osteichthys pesci ossei. In Fauna d’ltalia; Calderini: Bologna, Italy, 1975; Vol XI. [Google Scholar]
- Corbera, J.; Sabates, A.; Garcia-Rubies, A. Peces de Mar de la Peninsula Iberica; Planeta: Barcelona, Spain, 1996. [Google Scholar]
- Sala, E. The Role of Fishes in the Organization of a Mediterranean Sublittoral Community. Ph.D. Thesis, Universite Aix-Marseille, Marseille, France, 1996. [Google Scholar]
- Ballesteros, E. EIS Vegetals i la Zonacio Litoral: Especies, Comunitats i Factors que Influeixen en la Seva Distribucio; Arxius de la Seccio de Ciencies CI; Institut d’Estudis Catalans: Barcelona, Spain, 1992. [Google Scholar]
- Leitão, F.; Santos, M.N.; Monteiro, C.C. Contribution of artificial reefs to the diet of the white sea bream (Diplodus sargus). ICES J. Mar. Sci. 2007, 64, 473–478. [Google Scholar] [CrossRef][Green Version]
- Relini, G.; Relini, M.; Torchia, G.; De Angelis, G. Trophic relationships between fishes and an artificial reef. ICES J. Mar. Sci. 2002, 59, S36–S42. [Google Scholar] [CrossRef][Green Version]
- Pepe, P.; Badalamenti, F.; D’Anna, G. Abitudini alimentari di Diplodus vulgaris sulle strutture artificiali del Golfo di Castellammare (Sicilia Nord-Occidentale). Biol. Mar. Mediterr. 1996, 3, 514–515. [Google Scholar]
- Fischer, W.; Schneider, M.; Bauchot, M.L. Méditerranée et mer Noire, Zone de pêche 37. Volume II. Vertébrés. In Fiches FAO d’Identification des Espèces pour les Besoins de la Pêche; Révision 1; FAO et Commissions des Communautés Européennes (Projet GCP/INT/422/EEC); FAO: Rome, Italy, 1987; pp. 761–1530. [Google Scholar]
- Girardin, M. Pagellus erythrinus (LINNAEUS, 1758) et Boops boops (LINNAEUS, 1758) (Pisces, Sparidae) du Golfe du Lion. Ecobiologie—Prises Commerciales et Modèles de Gestion. Ph.D. Thesis, Université des Sciences et Techniques du Languedoc, Montpellier, France, 1981. [Google Scholar]
- Pajuelo, J.; Lorenzo, J. Population biology of the common pandora Pagellus erythrinus (Pisces: Sparidae) off the Canary Islands. Fish. Res. 1998, 36, 75–86. [Google Scholar] [CrossRef]
- Papaconstantinou, C.; Caragitsou, E.; Mytilineou, C.; Petrakis, G.; Vassilopoulou, V. Dynamics of Demersal Fish Stocks in the Korinthiakos and Patraikos Gulfs and in the Ionian Sea. Part II; Thalassographica Special Publications No. 16; National Centre for Marine Research: Athens, Greece, 1988. [Google Scholar]
- Kapsimalis, V.; Panagiotopoulos, I.; Kanellopoulos, T.; Hatzianestis, I.; Antoniou, P.; Anagnostou, C. A multi-criteria approach for the dumping of dredged material in the Thermaikos Gulf, Northern Greece. J. Environ. Manag. 2010, 91, 2455–2465. [Google Scholar] [CrossRef]
- Lombarte, A.; Aguirre, H. Quantitative differences of the chemoreceptor systems in the barbels of two species of Mullidae, Mullus surmuletus and Mullus barbatus. Mar. Ecol. Prog. Ser. 1997, 150, 57–64. [Google Scholar] [CrossRef]
- Bauchot, M.L.; Hureau, J.C. Sparidae. In Check-List of the Fishes of the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; SEI: Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 790–812. [Google Scholar]
- Pauly, D. Anecdotes and the Shifting Baseline Syndrome of Fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Sogard, S.M. Size-selective mortality in the juvenile stage of teleost fishes: A review. Bull. Mar. Sci. 1997, 60, 1129–1157. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: London, UK, 1992; 249p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).