The African Striped Grunt, Parapristipoma octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Laboratory Analysis
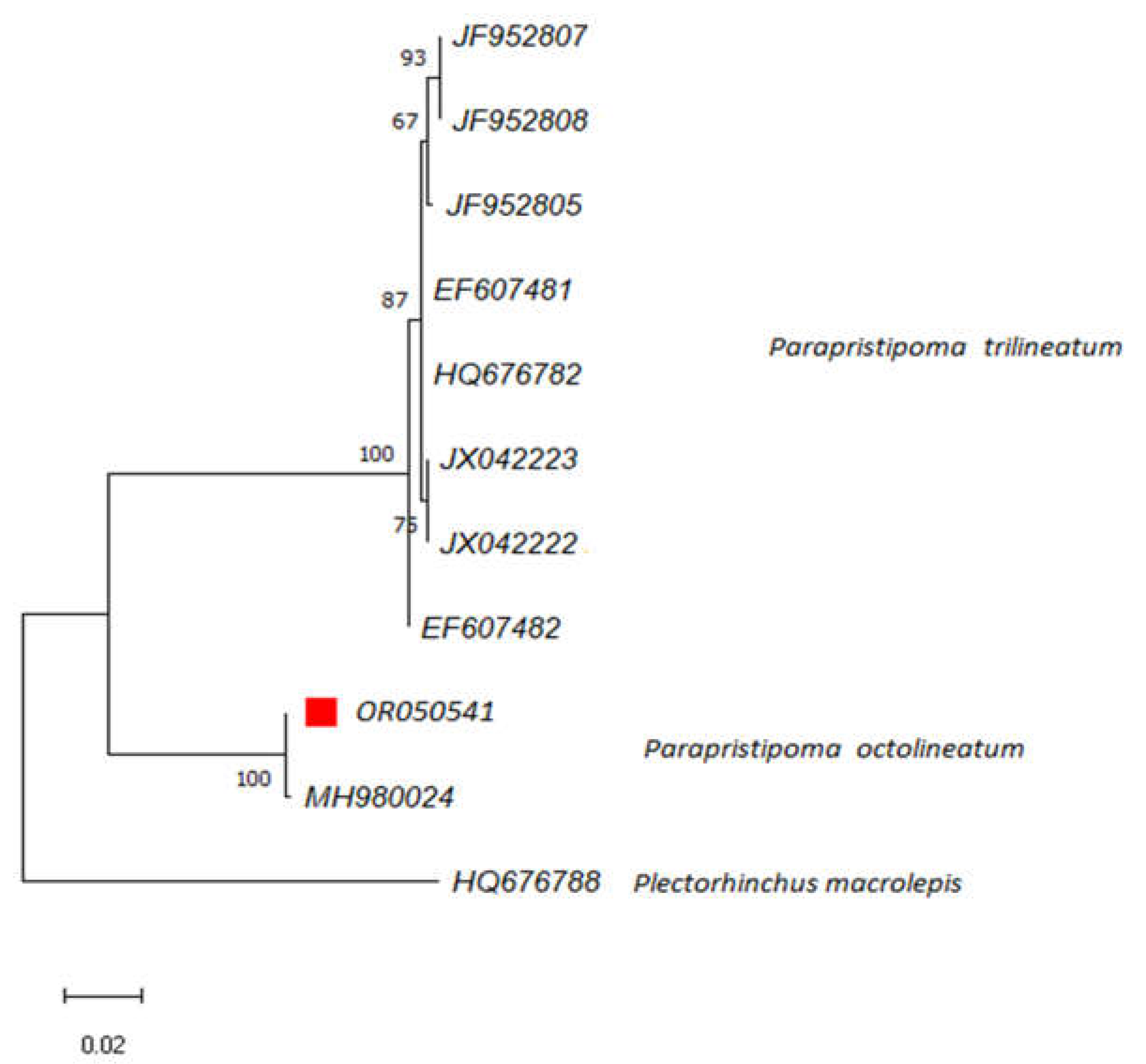
2.3. Molecular Identification via DNA Barcoding
2.4. Preparation of a Rapid Identification Key
3. Results
3.1. Biological, Morphological and Genetic Characterization of the Analyzed Specimen
3.2. Identification Key for Rapid Identification of Haemulidae Species Recorded in the Mediterranean Sea
- -
- Body overall uniform dark (grey to dark purple-blackish) or light (silvery-whitish), or uniformly scattered with irregular bright yellow-orange spots and irregular narrow stripes of the same color………………………………………..……………...…...1
- -
- Body with highly visible and regular longitudinal bars (white or yellow-brown) extending on the sides from the head/anterior part of the body to the caudal peduncle.........................................................................................................................................2
- -
- Body uniformly scattered with narrow and irregular small bright yellow-orange spots and stripes…………………………………...………….Orthopristis chrysoptera
- -
- Body color overall light/dark brown on dorsal area and whitish/light brown ventrally with 3–4 highly visible longitudinal white stripes on dorsal sides…………………………………………………….…Parapristipoma octolineatum
- -
- Body color overall whitish; black spot at upper angle of opercle; pectoral, pelvic and anal fins yellowish or orange ……………………......…..……..Pomadasys incisus
3.3. Remarks on Identification Key
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Mannion, A.M.; Sarris, D. Mediterranean island biodiversity and climate change: The last 10,000 years and the future. Biodivers. Conserv. 2016, 25, 2597–2627. [Google Scholar] [CrossRef]
- Giannakopoulos, C.; Bindi, M.; Moriondo, M.; LeSager, P.; Tin, T. Climate Change Impacts in the Mediterranean Resulting from a 2 °C Global Temperature Rise; WWF: Gland, Switzerland, 2005. [Google Scholar]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: A recent changes. Mar. Biodivers. Rec. 2020, 13, 10. [Google Scholar] [CrossRef]
- Azzurro, E. The advance of thermophilic fishes in the Mediterranean Sea: Overview and methodological questions. CIESM Workshop Monogr. 2008, 35, 39–45. [Google Scholar]
- Tiralongo, F.; Crocetta, F.; Riginella, E.; Lillo, A.O.; Tondo, E.; Macali, A.; Mancini, E.; Russo, F.; Coco, S.; Paolillo, G.; et al. Snapshot of rare, exotic and overlooked fish species in the Italian seas: A citizen science survey. J. Sea Res. 2020, 164, 101930. [Google Scholar] [CrossRef]
- Nota, A.; Ignoto, S.; Bertolino, S.; Tiralongo, F. First record of Caranx crysos (Mitchill, 1815) in the Ligurian Sea (northwestern Mediterranean Sea) suggests northward expansion of the species. Ann. Ser. Hist. Nat. 2023, 33, 55–60. [Google Scholar]
- Coco, S.; Roncarati, A.; Tiralongo, F.; Felici, A. Meridionalization as a possible resource for fisheries: The case study of Caranx rhonchus Geoffroy Saint-Hilaire, 1817, in southern Italian waters. J. Mar. Sci. Eng. 2022, 10, 274. [Google Scholar] [CrossRef]
- Kletou, D.; Hall-Spencer, J.M.; Kleitou, P. A lionfish (Pterois miles) invasion has began in the Mediterranean Sea. Mar. Biodivers. Rec. 2016, 9, 46. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. United Kingd. 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Boero, F.; Féral, J.P.; Azzurro, E.; Cardin, V.; Riedel, B.; Despalatović, M.; Munda, I.; Moschella, P.; Zaouali, J.; Fonda Umani, S.; et al. Climate warming and related changes in Mediterranean marine biota. CIESM Workshop Monogr. 2008, 35, 5–21. [Google Scholar]
- Pyšek, P.; Richardson, D. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World; John Willey & Sons. Inc.: New York, NY, USA, 1994; p. 600. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. (Eds.) Catalog of Fishes: Genera, Species, References.; California Academy of Sciences: San Francisco, CA, USA, 2023. [Google Scholar]
- Tiralongo, F.; Isgrò, C.; Tibullo, D. Orthopristis chrysoptera (Actinopterygii: Perciformes: Haemulidae): A new alien fish for the Mediterranean Sea. Acta Ichthyol. Piscat. 2020, 50, 539–542. [Google Scholar] [CrossRef]
- Akyol, O.; Ünal, V. First record of a Lessepsian migrant, Pomadasys stridens (Actinopterygii: Perciformes: Haemulidae), from the Aegean Sea, Turkey. Acta Ichthyol. Piscat. 2016, 46, 53–55. [Google Scholar] [CrossRef][Green Version]
- Doumpas, N.; Tanduo, V.; Crocetta, F.; Giovos, I.; Langeneck, J.; Tiralongo, F.; Kleitou, P. The bastard grunt Pomadasys incisus (Bowdich, 1825) (Teleostei: Haemulidae) in Cyprus (eastern Mediterranean Sea)—A late arrival or just a neglected species? Biodivers. Data J. 2020, 8, e58646. [Google Scholar] [CrossRef] [PubMed]
- Torchio, M. Minacce per l’ittiofauna Mediterranea: Le forme esotiche. Atti Della Soc. Ital. Delle Sci. Naturali. 1969, 109, 91–96. [Google Scholar]
- Stern, N.; Badreddine, A.; Bitar, G.; Crocetta, F.; Deidun, A.; Dragičević, B.; Dulčić, J.; Durgham, H.; Galil, B.S.; Galiya, M.Y.; et al. New Mediterranean Biodiversity Records. Mediterr. Mar. Sci. 2019, 20, 230–247. [Google Scholar]
- Bilecenoglu, M.; Alfaya, J.; Azzurro, E.; Baldacconi, R.; Boyaci, Y.; Circosta, V.; Compagno, L.; Coppola, F.; Deidun, A.; Durgham, H.; et al. New Mediterranean Marine Biodiversity Records. Mediterr. Mar. Sci. 2013, 14, 463–480. [Google Scholar] [CrossRef]
- Ben-Tuvia, A.; McKay, R. Haemulidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume II, pp. 858–864. [Google Scholar]
- Lipej, L.; Spoto, M.; Dulcîc, J. Plectorhinchus mediterraneus from off north east Italy and Slovenia—the first record of fish of the family Haemulidae from the Adriatic Sea. J. Fish Biol. 1996, 48, 805–806. [Google Scholar] [CrossRef]
- Hattour, A.; Bradai, M.N. First record of the Rubber-lip grunt Plectorhinchus mediterraneus (Guichenot 1850) (Osteichthyes: Haemulidae) in Tunisian waters (Central Mediterranean). Bull. Inst. Natn. Scien. Tech. Mer. Salammbô 2011, 38, 159–163. [Google Scholar]
- Corsini-Foka, M.; Sarlis, N. A strange occurrence of Plectorhinchus gaterinus (Actinopterygii: Perciformes: Haemulidae) in the Thracian Sea (Eastern Mediterranean). Acta Ichthyol. Piscat. 2016, 46, 37–41. [Google Scholar] [CrossRef][Green Version]
- Dieuzeide, R.; Novella, M.; Roland, J. Catalogue des poissons des côtes algériennes. In Bulletin Station d’Aquaculture et de Pêche de Castiglione; University of California: Algeria, 1955; Volume III, 3844p. [Google Scholar]
- Djabali, F.; Brahmi, B.; Mammasse, M. Poissons des Côtes Algériennes; ISMAL: Calicut, India, 1993; 215p. [Google Scholar]
- Wirtz, P.; Fricke, R.; Biscoito, M.J. The coastal fishes of Madeira Island—New records and an annotated checklist. Zootaxa 2008, 1715, 1–26. [Google Scholar] [CrossRef]
- Casamajor, M.N. First record of Parapristipoma octolineatum (Haemulidae) on the French Atlantic coast. Cybium 2016, 40, 263–264. [Google Scholar]
- Báez, J.C.; Rodríguez-Cabello, C.; Bañón, R.; Brito, A.; Falcón, J.M.; Maño, T.; Baro, J.; Macías, D.; Meléndez, M.J.; Camiñas, J.A.; et al. Updating the national checklist of marine fishes in Spanish waters: An approach to priority hotspots and lessons for conservation. Mediterr. Mar. Sci. 2019, 20, 260–270. [Google Scholar] [CrossRef]
- Bañón, R.; Tejerina, R.; Morales, X.; Alonso-Fernández, A.; Barros-García, D.; De Carlos, A. Unusual occurrence of fishes along the Northeast Atlantic: New biological and distributional data. Mediterr. Mar. Sci. 2019, 20, 189–196. [Google Scholar] [CrossRef]
- Tsagarakis, K.; Darmanin, S.A.; AL Mabruk, S.A.; Auriemma, R.; Azzurro, E.; Badouvas, N.; Bakiu, R.; Bariche, M.; Battaglia, P.; Betti, F.; et al. New records of rare species in the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 627–652. [Google Scholar] [CrossRef]
- Bauchot, M.-L. Poisson osseaux. In Fiches FAO d’identification des espèces pour les besoins de la pêche (Révision 1). Méditerranée et mer Noire; Zone de pêche 37. Vertébrés., 1987, Fischer, W., Bauchot, M.-L., Schneider, M., Eds.; FAO: Rome, Italy, 1987; Volume 2, p. 1118. [Google Scholar]
- Schneider, W. FAO Species Identification Sheets for Fishery Purposes. In Field Guide to Commercial Marine Resources of the Gulf of Guinea; FAO: Rome, Italy, 1990; p. 268. [Google Scholar]
- Louisy, P. Guida All’identificazione dei pesci d’Europa e del Mediterraneo; Il Castello: Milano, Italy, 2020; p. 512. [Google Scholar]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Pappalardo, A.M.; Federico, C.; Sabella, G.; Saccone, S.; Ferrito, V. A COI non-synonymous mutation as diagnostic tool for intraspecific discrimination in European anchovy Engraulis encrasicolus (Linnaeus). PLoS ONE 2015, 10, e0143297. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bodilis, P.; Crocetta, F.; Langeneck, J.; Francour, P. The spread of an Atlantic fish species, Pomadasys incisus (Bowdich, 1825) (Osteichthyes: Haemulidae), within the Mediterranean Sea with new additional records from the French Mediterranean coast. Ital. J. Zool. 2013, 80, 273–278. [Google Scholar] [CrossRef][Green Version]
- Froese, R.; Pauly, D. (Eds.) FishBase. Available online: http://www.fishbase.org (accessed on 20 July 2023).
- Lasram, F.B.R.; Mouillot, D. Increasing southern invasion enhances congruence between endemic and exotic Mediterranean fish fauna. Biol. Invasions 2009, 11, 697–711. [Google Scholar] [CrossRef]
- Evans, J.; Tonna, R.; Schembri, P.J. Portent or accident? Two new records of thermophilic fish from the central Mediterranean. BioInvasions Rec. 2015, 4, 299–304. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Lipej, L. A modified key for rapid determination of Blennioidea (Pisces: Perciformes) in the Adriatic Sea. Acta Adriat. 2010, 51, 55–65. [Google Scholar]
- Tiralongo, F.; La Mesa, G.; Paladini De Mendoza, F.; Massari, F.; Azzurro, E. Underwater photo contests to complement coastal fish inventories: Results from two Mediterranean Marine Protected Areas. Mediterr. Mar. Sci. 2021, 22, 436–445. [Google Scholar]
- Azzurro, E. Unusual occurrences of fish in the Mediterranean Sea: An insight into early detection. In Fish Invasions of the Mediterranean Sea: Change and Renewal; Golani, D., Appelbaum-Golani, B., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2010; pp. 99–126. [Google Scholar]
- Guichenot, A. Historie naturelle des reptiles et des poisons. In Exploration Scientifique de l’Algérie Pendant les Années 1840, 1841, 1842; Paris Imprimerie Royale: Paris, Frances, 1850. [Google Scholar]
- Pajuelo, J.; Lorenzo, J.; Gregoire, M. Age and growth of the bastard grunt (Pomadasys incisus: Haemulidae) inhabiting the Canarian archipelago, Northwest Africa. Fish. Bull. 2003, 101, 851–859. [Google Scholar]
- Pajuelo, J.; Lorenzo, J.; Gregoire, M.; Domínguez-Seoane, R. Life history of the Pomadasys incisus (Osteichthyes: Haemulidae) of the Canarian Archipelago. Sci. Mar. 2003, 67, 241–248. [Google Scholar] [CrossRef][Green Version]
- Fehri-Bedoui, R.; Gharbi, H. Sex-ratio, reproduction and feeding habits of Pomadasys incisus (Haemulidae) in the Gulf of Tunis (Tunisia). Acta Adriatica 2008, 49, 5–19. [Google Scholar]
- Bilecenoğlu, M.; Kaya, M.; Eryiğit, A. New data on the occurrence of two alien fishes Pisodonophis semicinctus and Pomadasys stridens from Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2009, 10, 151–155. [Google Scholar] [CrossRef][Green Version]
- Akyol, O.; Çoker, T. On the presence of the Lessepsian Pomadasys stridens (Haemulidae) in the Aegean Sea (Marmaris Bay, Turkey). Turk. J. Marit. Mar. Sci. 2018, 4, 163–166. [Google Scholar]
- Avşar, D.; Mavruk, S.; Yeldan, H.; Manaşirli, M. Population dynamics of an emergent invasive fish, striped piggy, Pomadasys stridens (Actinopterygii, Perciformes, Haemulidae) in the Gulf of Iskenderun, north-eastern Mediterranean. Acta Ichthyol. Et Piscat. 2021, 51, 13–21. [Google Scholar] [CrossRef]
- Dooley, J.K.; Van Tassell, J.; Brito, A. An annotated checklist of the shorefishes of the Canary Islands. Am. Mus. Nat. Hist. 1985, 2824, 1–49. [Google Scholar]
- Tiralongo, F.; Lillo, A.O.; Tibullo, D.; Tondo, E.; Martire, C.L.; D’agnese, R.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring uncommon and non-indigenous fishes in Italian waters: One year of results for the AlienFish project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Tiralongo, F.; Villani, G.; Arciprete, R.; Mancini, E. Filling the gap on Italian records of an invasive species: First records of the Blue Crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), in Latium and Campania (Tyrrhenian Sea). Acta Adriatica 2021, 61, 99–104. [Google Scholar] [CrossRef]
- Peyton, J.; Martinou, A.F.; Pescott, O.L.; Demetriou, M.; Adriaens, T.; Arianoutsou, M.; Bazos, I.; Bean, C.W.; Booy, O.; Botham, M.; et al. Horizon scanning for invasive alien species with the potential to threaten biodiversity and human health on a Mediterranean island. Biol. Invasions 2019, 21, 2107–2125. [Google Scholar] [CrossRef]

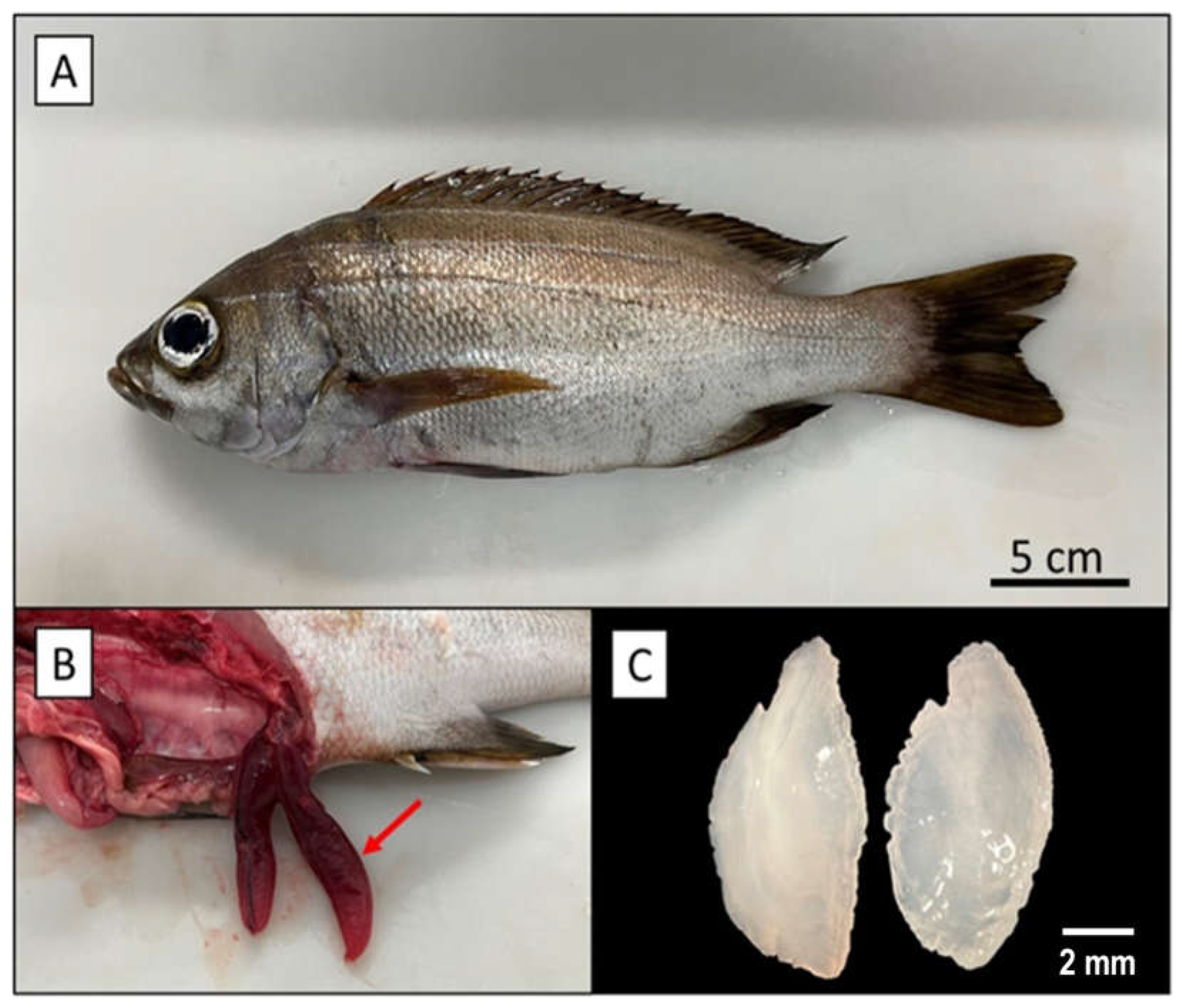
 ).
).
 ).
).
| Morphometric and Meristic Data | |
|---|---|
| cm | |
| Total length (TL) | 35.0 |
| Standard length (SL) | 29.5 |
| Fork length (FL) | 33.5 |
| Head length (HL) | 9.2 |
| Pre-anal length (PAL) | 18.0 |
| g | |
| Weight | 542 |
| Dorsal fin | XIII + 14 |
| Anal fin | III + 7 |
| Pectoral fin | 12 |
| Pelvic fin | I + 5 |
| Date | Country | Location | Coordinates | Depth | Size | References |
|---|---|---|---|---|---|---|
| 4 October 2008 | Spain | Off Salobreña | 36.7230 N 3.5808 W | 1 m | n.a. | Tiralongo and Azzurro; In: Tsagarakis et al., 2021 [31] |
| 21 October 2020 | Algeria | Off Béni Saf | 35.3014 N 1.4250 W | 15 m | 24.8 cm | Al Mabruk et al.; In: Tsagarakis et al., 2021 [31] |
| 5 December 2022 | Spain | Off Teulada-Moraira | 38.6701 N 0.1327 W | 20 m | 35.0 cm | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiralongo, F.; Pappalardo, A.M.; Ignoto, S.; Lombardo, B.M.; Ferrito, V.; Campos Sosa, A.; Spinelli, A. The African Striped Grunt, Parapristipoma octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean. J. Mar. Sci. Eng. 2023, 11, 1688. https://doi.org/10.3390/jmse11091688
Tiralongo F, Pappalardo AM, Ignoto S, Lombardo BM, Ferrito V, Campos Sosa A, Spinelli A. The African Striped Grunt, Parapristipoma octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean. Journal of Marine Science and Engineering. 2023; 11(9):1688. https://doi.org/10.3390/jmse11091688
Chicago/Turabian StyleTiralongo, Francesco, Anna Maria Pappalardo, Sara Ignoto, Bianca Maria Lombardo, Venera Ferrito, Aitor Campos Sosa, and Andrea Spinelli. 2023. "The African Striped Grunt, Parapristipoma octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean" Journal of Marine Science and Engineering 11, no. 9: 1688. https://doi.org/10.3390/jmse11091688
APA StyleTiralongo, F., Pappalardo, A. M., Ignoto, S., Lombardo, B. M., Ferrito, V., Campos Sosa, A., & Spinelli, A. (2023). The African Striped Grunt, Parapristipoma octolineatum (Valenciennes, 1833), in the Mediterranean Sea: The Third Record with Biological and Ecological Notes, and Identification Key for Haemulidae Recorded in the Mediterranean. Journal of Marine Science and Engineering, 11(9), 1688. https://doi.org/10.3390/jmse11091688









