Spatial Difference in the Marine Algal Community of Yeongil Bay Inner and Outer Areas on the East Coast of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Marine Algal Community
- fi: frequency of the ith species in the quadrat
2.2. Environmental Factors
2.3. Data Analysis
3. Results
3.1. Marine Environment
3.2. Marine Algal Species and Biomass in Yeongil Bay
3.3. Ecological Index
3.4. Comparison of Marine Algal Community in Outer and Inner Areas
3.4.1. Marine Algal Biomass
3.4.2. Importance Value
3.5. Seasonal Marine Algal Community Structures in the Outer and Inner Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitaker, S.G.; Smith, J.R.; Murray, S.N. Reestablishment of the southern California rocky intertidal brown alga, Silvetia compressa: An experimental investigation of techniques and abiotic and biotic factors that affect restoration success. Restor. Ecol. 2010, 18, 18–26. [Google Scholar] [CrossRef]
- Perkol-Finkel, S.; Ferrario, F.; Nicotera, V.; Airoldi, L. Conservation challenges in urban seascapes: Promoting the growth of threatened species on coastal infrastructures. J. Appl. Ecol. 2012, 49, 1457–1466. [Google Scholar] [CrossRef]
- Satheesh, S.; Wesley, S.G. Diversity and distribution of seaweeds in the Kudankulam coastal waters, south-eastern coast of India. Biodivers. J. 2012, 3, 79–84. [Google Scholar]
- Kim, Y.D.; Ahn, J.K.; Nam, M.M.; Lee, C.; Yoo, H.I.; Yeon, S.Y.; Kim, Y.H.; Kim, J.K.; Choi, J.S. Characteristics of algal succession following rock scraping at Imwon area in the east coast of Korea. J. Ocean Univ. China 2016, 15, 1087–1093. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. An insight to the ecological evaluation index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the european water framework directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef]
- Scherner, F.; Horta, P.A.; Oliveira, E.C.; Simonassi, J.C.; Hall-Spencer, J.M.; Chow, F.; Nunes, J.M.C.; Pereira, S.M.B. Coastal urbanization leads to remarkable seaweed species loss and community shifts along the SW Atlantic. Mar. Pollut. Bull. 2013, 76, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Ocean and Fisheries. A Glossary of Marine and Fisheries; Ministry of Ocean and Fisheries: Sejong, Republic of Korea, 2020. [Google Scholar]
- Raffaelli, D.; Hawkins, S.J. Intertidal Ecology; Son, M.H., Yun, S.G., Eds.; Academy Publishing Company: Seoul, Republic of Korea, 2004. [Google Scholar]
- Dawes, C.J. Marine Botany; Oh, Y.S., Ed.; World Science: Seoul, Republic of Korea, 1998. [Google Scholar]
- Yu, O.H.; Lee, H.G.; Lee, J.H. The intertidal macrobenthic community along an artificial structure. Korean J. Fish. Aquat. Sci. 2006, 39, 132–141. [Google Scholar]
- Mirta, T.; Sophia, E.F.; Ylva, S.O.; Ivan, V.; Paulina, M.; Elizabeti Yuriko, M.O.I.; Monica, A.V.P.; Thaïs, N.C.; Martín, S.J.; Federico, P.O.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Chang. Biol. 2010, 16, 2624–2637. [Google Scholar]
- Allen, L.G. Seasonal abundance, composition and productivity of the littoral fish assemblage in upper Newport Bay. United States Fish. Bull. 1982, 80, 769–790. [Google Scholar]
- Han, K.H.; Hong, J.S.; Kim, Y.S.; Jeon, K.A.; Kim, Y.S.; Hong, B.K.; Hwang, D.S. Species composition and seasonal variations of ichthyoplankton in coastal waters of Yeongil Bay, Korea. Korean J. Ichthyol. 2003, 15, 87–94. [Google Scholar]
- Kim, K.T. A Study on Basic Production and Port Pollution in Youngil Bay; Report Industry-University Cooperation Foundation Research: Seoul, Republic of Korea, 1983; p. 57. [Google Scholar]
- Lee, H.B.; Oh, Y.S. A summer algal vegetation in Youngil Bay, eastern coast of Korea. Algae 1986, 1, 225–240. [Google Scholar]
- Lee, J.W.; Lee, H.B. A floristic study on marine benthic algae of Yongil Bay and adjacent areas, eastern coast of Korea. Algae 1988, 3, 165–182. [Google Scholar]
- Nam, K.W.; Kim, Y.S.; Kim, Y.H.; Sohn, C.H. Benthic marine algae in the east coast of Korea: Flora, distribution and community structure. Korean J. Fish. Aquat. Sci. 1996, 29, 727–743. [Google Scholar]
- Lee, S.Y.; Lee, J.W.; Lee, H.B. Marine benthic algal flora of Yongil Bay and its adjacent areas, the eastern coast of Korea. Algae 1997, 12, 303–311. [Google Scholar]
- Lee, I.K.; Kim, Y.H. Biodiversity and distribution of marine benthic organisms and uses of algal resources in the coastal zone of Korea and Japan I. benthic marine algae in the east coast of Korea. Algae 1999, 14, 91–110. [Google Scholar]
- Park, G.J.; Choi, C.G. A study on the community structure of intertidal benthic marine algae in Youngil Bay, eastern coast of Korea. Korean J. Fish. Aquat. Sci. 2009, 42, 664–673. [Google Scholar]
- Kwak, H.S. General oceanographic factors in Yeongil Bay of Korea, late October 1973. Ocean Sci. J. 1976, 11, 89–95. [Google Scholar]
- Shim, J.M.; Kwon, K.Y.; Jeong, H.D.; Choi, Y.K.; Kim, S.W. Spatial and temporal variability of phytoplankton in relation to environmental factors in Youngil Bay. J. Environ. Sci. Int. 2013, 22, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.D.; Kim, J.I.; Ryu, C.R. Study on current and water quality characteristics in Yongil Bay. J. Ocean Eng. Technol. 2001, 15, 28–37. [Google Scholar]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. Mathematical Ecology; Wiley Company: New York, NY, USA, 1977; pp. 2055–2057. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; Univ-Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688–692. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Meybeck, M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- Pohang City. 2020 White Paper of Environment; Pohang City: Pohang, Republic of Korea, 2020. [Google Scholar]
- Ministry of Land, Transport and Maritime Affairs. Hyeongsan River Basic Plan (Supplementary) Report; Busan Regional Construction and Management Administration: Busan, Republic of Korea, 2011. [Google Scholar]
- Dayton, P.K. Experimental evaluation of ecological dominance in a rocky intertidal algal community. Ecol. Monogr. 1975, 45, 137–159. [Google Scholar] [CrossRef]
- Rivers, F.S.; Peckol, P. Summer decline of Ulva lactuca (chlorophyta) in a eutrophic bayment: Interactive effects of temperature and nitrogen availability? J. Phycol. 1995, 31, 223–228. [Google Scholar] [CrossRef]
- Kang, J.W. Marine Algae. In Illustrated Encyclopedia of Fauna & Flora of Korea; Ministry of Education: Seoul, Republic of Korea, 1968; Volume 8. [Google Scholar]
- Song, Y.C.; Kim, S.R.; Park, S.J.; Kang, G.M.; Oh, S.S. A Study on the causes of Ulva pertusa kjellman large breeding in Bangdu Bay of Jeju Island. Rep. JIHE 2016, 27, 59–69. [Google Scholar]
- Krause-Jensen, D.; Middelboe, A.; Christensen Eelgrass, P.B. Zostera marina, growth along depth gradients: Upper boundaries of the variation as a powerful predictive tool. Oikos 2000, 91, 233–244. [Google Scholar] [CrossRef]
- Human, L.R.D.; Adams, J.B.; Allanson, B.R. Insights into the cause of an Ulva lactuca Linnaeus bloom in the Knysna Estuary. S. Afr. J. Bot. 2016, 107, 55–62. [Google Scholar] [CrossRef]
- Villares, R.; Puente, X.; Carballeira, A. Ulva and Enteromorpha as indicators of heavy metal pollution. Hydrobiologia 2001, 462, 221–232. [Google Scholar] [CrossRef]
- Farias, D.R.; Hurd, C.L.; Eriksen, R.S.; Macleod, C.K. Ulva australis as a tool for monitoring zinc in the Derwent Estuary and implications for environmental assessment. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Bonanno, G.; Veneziano, V.; Piccione, V. The alga Ulva lactuca (ulvaceae, chlorophyta) as a bioindicator of trace element contamination along the coast of Sicily, Italy. Sci. Total Environ. 2019, 699, 134329. [Google Scholar] [CrossRef]
- Mourad, F.A.; El-Azim, H.A. Use of green alga Ulva lactuca (L.) as an indicator to heavy metal pollution at intertidal waters in Suez Gulf, Aqaba Gulf and Suez Canal, Egypt. Egypt J. Aquat. Biol. Fish. 2019, 23, 437–449. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Fisheries Science. Information of Oceanographic Conditions in the East Sea; NIFS East Sea Fisheries Research Institute: Gangneung, Republic of Korea, 2017; Volume 219, pp. 1–10. [Google Scholar]
- Wilkinson, M. Survival strategies of attached algae in estuaries. In Feeding and Survival Strategies of Estuarine Organisms; Jones, N.V., Wolff, W.J., Eds.; Plenum Publishing Company: New York, NY, USA, 1981; pp. 29–38. [Google Scholar]
- Josselyn, M. Do nutrients or physical factors control macroalgal growth in temperate estuaries? Estuaries 1985, 8, 304. [Google Scholar]
- Carballo, J.L.; Olabarria, C.; Osuna, T.G. Analysis of four macroalgal assemblages along the Pacific Mexican coast during and after the 1997–98 El Niño. Ecosystems 2002, 5, 749–760. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, H.G. Epiphytic algae growing on Sargassum thunbergii in southern and western coasts of Korea. J. Ecol. Environ. 2004, 27, 173–177. [Google Scholar]
- Yoshida, G.; Yoshikawa, K.; Terawaki, T. Growth and maturation of two populations of Sargassum horneri (fucales, phaeophyta) in Hiroshima Bay, the Seto Inland Sea. Fish. Sci. 2001, 67, 1023–1029. [Google Scholar] [CrossRef]
- McCourt, R.M. Seasonal patterns of abundance, distributions, and phenology in relation to growth strategies of three Sargassum species. J. Exp. Mar. Biol. Ecol. 1984, 74, 141–156. [Google Scholar] [CrossRef]
- Mathieson, A.C.; Burns, R.L. Ecological studies of economic red algae. V. growth and reproduction of natural and harvested populations of Chondrus crispus stackhouse in New Hampshire. J. Exp. Mar. Biol. Ecol. 1975, 17, 137–156. [Google Scholar] [CrossRef]
- Prathep, A.; Lewmanomont, K.; Buapet, P. Effects of wave exposure on population and reproductive phenology of an algal turf, Gelidium pusillum (Gelidales, Rhodophyta), Songkhla, Thailand. Aquat. Bot. 2009, 90, 179–183. [Google Scholar] [CrossRef]
- Seapy, R.R.; Littler, M.M. The distribution, abundance, community structure, and primary productivity of macroorganisms from two central California rocky intertidal habitats. Pac. Sci. 1978, 71, 87–96. [Google Scholar]
- Dayton, P.K. Experimental studies of algal canopy interactions in a sea otter-dominated kelp community at Amchitka Island, Alaska. Fish. Bull. 1975, 73, 230–237. [Google Scholar]
- Daly, M.A.; Mathieson, A.C. The effects of sand movement on intertidal seaweeds and selected invertebrates at Bound Rock, New Hampshire, USA. Mar. Biol. 1977, 43, 45–55. [Google Scholar] [CrossRef]
- Mitsch, W.J. Ecological engineering: The seven-year itch. Ecol. Eng. 1998, 10, 119–138. [Google Scholar] [CrossRef]
- William, J.M.; Jorgensen, S.E. Ecological Engineering and Ecosystem Restoration; Kang, D.S., Kim, D.M., Sung, K.J., Ahn, C.W., Lee, S.M., Eds.; Hanteemedia: Seoul, Republic of Korea, 2003. [Google Scholar]



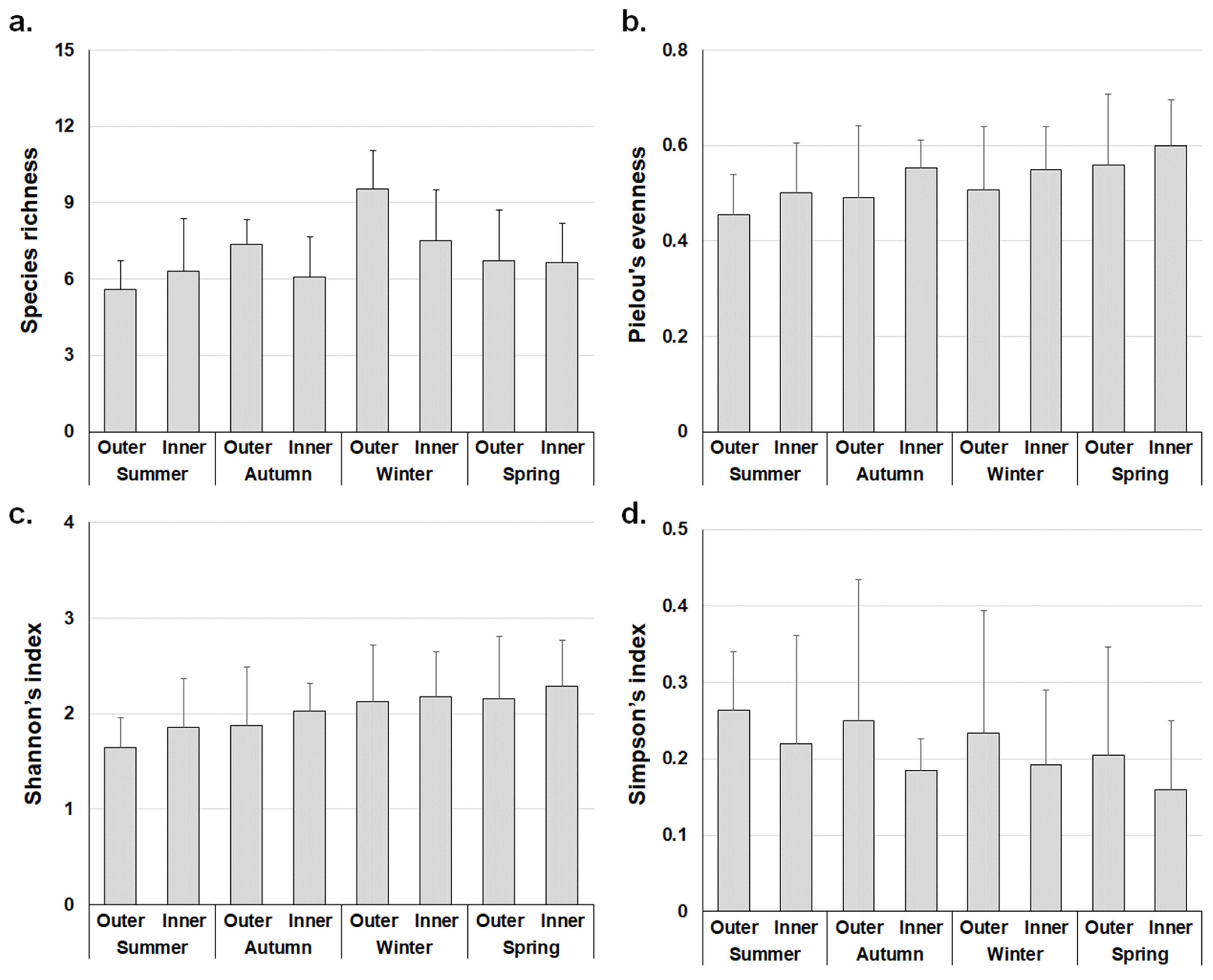
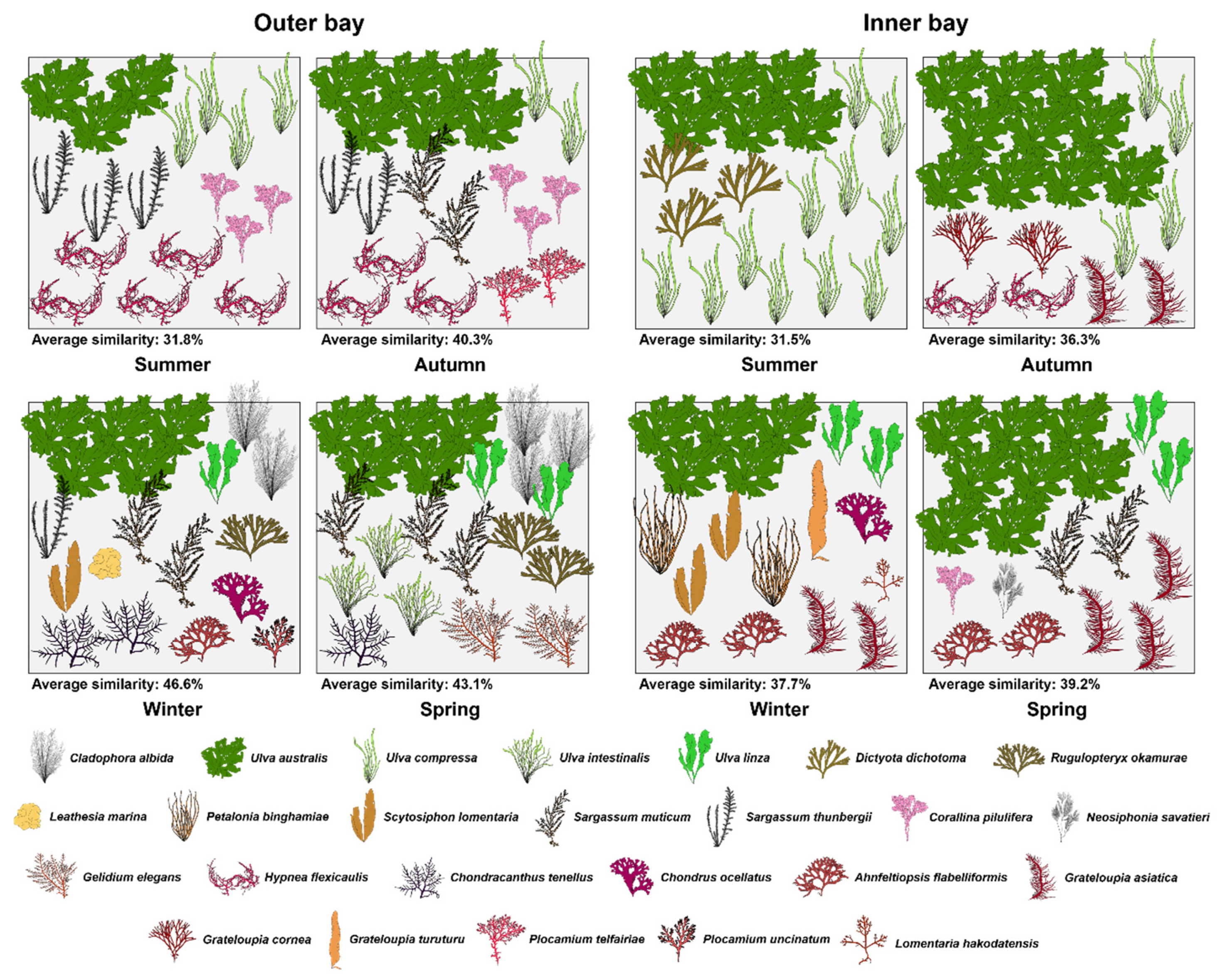
| Type | Aug. | Nov. | Feb. | May | Mean | |
|---|---|---|---|---|---|---|
| Water temperature (°C) | Outer | 25.1 ± 0.3 | 18.3 ± 0.8 | 10.0 ± 0.8 | 18.5 ± 2.0 | 18.0 ± 0.7 |
| Inner | 26.0 ± 0.9 | 19.4 ± 1.3 | 9.7 ± 0.8 | 18.9 ± 1.0 | 18.5 ± 0.4 | |
| pH | Outer | 8.2 ± 0.2 | 8.4 ± 0.3 | 8.4 ± 0.4 | 8.5 ± 0.2 | 8.4 ± 0.2 |
| Inner | 8.3 ± 0.1 | 8.6 ± 0.2 | 8.7 ± 0.2 | 8.5 ± 0.1 | 8.5 ± 0.2 | |
| Salinity (psu) | Outer | 32.3 ± 1.8 | 35.3 ± 0.4 | 36.8 ± 0.2 | 36.7 ± 0.3 | 35.3 ± 0.5 |
| Inner | 29.3 ± 1.7 | 32.4 ± 1.8 | 36.3 ± 0.9 | 35.8 ± 1.7 | 33.4 ± 1.2 | |
| Species | Average Abundance | Contribution (%) | ||
|---|---|---|---|---|
| Outer | Inner | Outer | Inner | |
| Ulva australis | 6.13 | 8.54 | 23.8 | 11.2 |
| Sargassum muticum | 7.29 | 4.79 | 3.1 | 11.4 |
| Ulva compressa | 2.16 | 3.89 | 7.4 | 3.8 |
| Corallina pilulifera | 4.72 | 4.52 | 4.1 | 7.2 |
| Hypnea flexicaulis | 3.30 | 2.69 | 3.4 | 6.8 |
| Sargassum thunbergii | 5.77 | 6.21 | 4.7 | 6.6 |
| Ahnfeltiopsis flabelliformis | 2.91 | 2.92 | 4.2 | 3.4 |
| Grateloupia cornea | 2.86 | 2.74 | 3.7 | 2.2 |
| Ulva linza | 2.30 | 2.47 | 3.3 | 3.3 |
| Average similarity: Outer area (30.9%), Inner area (31.0%) | ||||
| Average Dissimilarity: 69.1% | |
|---|---|
| Species | Contribution (%) |
| Ulva australis | 8.6 |
| Sargassum thunbergii | 6.8 |
| Ulva compressa | 5.8 |
| Corallina pilulifera | 4.8 |
| Sargassum muticum | 4.2 |
| Grateloupia asiatica | 3.8 |
| Cladophora albida | 3.6 |
| Hypnea flexicaulis | 3.6 |
| Ulva intestinalis | 3.2 |
| Ulva linza | 2.7 |
| Grateloupia cornea | 2.6 |
| Chaetomorpha moniligera | 2.4 |
| Species | Contribution (%) | |
|---|---|---|
| Outer Area | Inner Area | |
| Genus Ulva | ||
| Ulva australis | 23.2 | 36.3 |
| Ulva compressa | 9.6 | 11.1 |
| Ulva intestinalis | 3.8 | |
| Ulva linza | 3.1 | 2.8 |
| Cumulative Contribution (%) | 39.7 | 50.2 |
| Genus Sargassum | ||
| Sargassum muticum | 7.5 | 2.4 |
| Sargassum thunbergii | 4.3 | 3.8 |
| Sargassum horneri | 1.6 | 0.8 |
| Cumulative Contribution (%) | 13.4 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; Jung, S.W.; Han, S.Y.; Choi, C.G. Spatial Difference in the Marine Algal Community of Yeongil Bay Inner and Outer Areas on the East Coast of Korea. J. Mar. Sci. Eng. 2023, 11, 1486. https://doi.org/10.3390/jmse11081486
Park SJ, Jung SW, Han SY, Choi CG. Spatial Difference in the Marine Algal Community of Yeongil Bay Inner and Outer Areas on the East Coast of Korea. Journal of Marine Science and Engineering. 2023; 11(8):1486. https://doi.org/10.3390/jmse11081486
Chicago/Turabian StylePark, Se Jeong, Seung Wook Jung, Seung Yeop Han, and Chang Geun Choi. 2023. "Spatial Difference in the Marine Algal Community of Yeongil Bay Inner and Outer Areas on the East Coast of Korea" Journal of Marine Science and Engineering 11, no. 8: 1486. https://doi.org/10.3390/jmse11081486
APA StylePark, S. J., Jung, S. W., Han, S. Y., & Choi, C. G. (2023). Spatial Difference in the Marine Algal Community of Yeongil Bay Inner and Outer Areas on the East Coast of Korea. Journal of Marine Science and Engineering, 11(8), 1486. https://doi.org/10.3390/jmse11081486







