Abstract
Microplastics (MPs) have been given considerable attention due to their risk to aquatic organisms in marine environments. In this study, MPs’ abundance and their potential correlation with environmental factors were investigated from 26 sites in Daya Bay, South China Sea. The results showed that the abundance of MPs was 1.8–13.87 items/L in surface water and 190–823 items/kg (dry weight) in sediment. The most abundant shape of MPs in both water and sediment was fiber, the most abundant particle size was 0.5–1 mm and the most abundant color was transparent. In addition, the most common polymer type of MPs was polyethylene terephthalate (PET), followed by rayon (RY), polypropylene (PP), cellulose (CL) and polyethylene (PE). The abundance of MPs in sediment was significantly correlated with sediment organic N and C (p < 0.05), while that in surface water had no significant correlation with the environmental factors except dissolved oxygen (p > 0.05). A factor analysis showed that MPs in sediment might share similar sources with organic N and C, which were mainly from the autochthonous sedimentation of marine organisms, and MPs might sediment jointly with organic matter. In summary, this study reflects on MP pollution and the potential correlation with environmental factors, providing essential data for governmental agencies to formulate microplastic pollution control policies.
1. Introduction
Plastic is an organic synthetic polymer compound that has been widely utilized in the world due to its low cost, light weight and corrosion resistance [1]. The excessive use of plastic and its poor management and incorrect disposal may result in the accumulation of plastic fragments in the marine environment. The used plastic that is discharged into the environment as waste may degrade and weather into pieces or particles less than 5 mm in diameter or smaller, generally referred as microplastics (MPs) [2,3]. In recent years, various MPs have been discovered in seawater and sediment, mainly derived from coastal industries [4], the fishing industry [5] and the fragmentation of large plastic [6]. MPs in coastal areas originate from both the land and sea. It is estimated that about 80% of marine plastic particles come from the land, with 1.15 million to 2.41 million tons of plastic waste currently entering the ocean from rivers each year [7]. In recent decades, MPs have been increasingly detected in marine areas around the world, such as the Yellow Sea [8], the Adriatic Sea from the Croatian marine protected area [9], the western Pacific [10] and even Antarctica [11]. These studies indicate that MPs are present in water, sediment and beach substrates along the coastline, offshore and in the deep sea [12,13]. Numerous essential functions of marine ecosystems, including food supply and climate regulation, are being gradually lost as enormous anthropogenic plastic waste is discharged into the oceans [14].
Due to their relatively small size, MPs may easily be accidentally ingested by aquatic organisms and transferred into the marine food chain, presenting serious threats to the health of marine animals [15]. Numerous marine organisms have been reported to accidentally ingest MPs, such as zooplankton [16], shrimp [17], fish [18], sea turtles [19] and whales [20]. The ingestion of MPs and their further bioaccumulation can cause a significant threat to marine wildlife. Directly, MPs cause toxicity to organisms through ingestion, inhalation and dermal contact, resulting in oxidative stress, inflammation and cellular damage in organisms [21]. Additionally, due to the large surface area and hydrophobicity, MPs can serve as carriers of pathogens such as Escherichia coli and parasites, threatening organisms [22], as well as various hydrophobic/hydrophilic organic pollutants and heavy metals [3,23,24]. It has been reported that hydrophobic/hydrophilic organic pollutants can be effectively adsorbed on the surface of MPs and release toxicity with migration to organisms [25,26,27]. It has also been found that the presence of MPs enhances the toxicity of cadmium to zebrafish (Danio rerio) and that combined exposure leads to oxidative damage and inflammation in zebrafish tissues. In conclusion, as carriers of toxic pollutants and pathogens, MPs will cause serious health problems for humans once they enter the human body through the food chain. Therefore, it cannot be denied that MP pollution has increasingly become a crucial issue that must be addressed.
To reduce MPs’ pollution in the environment, it is necessary to understand the processes by which they are formed. The occurrence of MPs is extremely complex, which makes them particularly difficult to study. Plastics are predominantly degraded by slow weathering processes in the aquatic environment, which include thermal degradation, photo-oxidative degradation and wave-driven fragmentation. The authors of [28,29] discovered that the weathering rate of MPs in the aquatic environment is slower than in the terrestrial environment due to the lower temperature. Additionally, high salinity affected the microbial composition of the water and sediment, reducing the chances of the microbial degradation of plastics [30]. Ariza-Tarazona et al. (2020) demonstrated that low pH incorporates H+ into the system, which facilitates plastic degradation [31]. Consequently, the monitoring of environmental factors in coastal areas is highly beneficial and critical for the study and management of MPs. Current information on some MPs in the marine environment is well established [32,33], but the correlation of MP contamination and environmental factors is still poorly documented [34]. Daya Bay, a large semi-enclosed gulf in the northern part of the South China Sea, is located to the left of the Pearl River in Guangdong Province and southwest of the Dapeng Peninsula in Shenzhen, which is surrounded by one of the most densely populated and industrialized areas in China [35]. With the construction of the Daya Bay Nuclear Power Station in 1994, the Ling’ao Nuclear Power Station in 2002 and the continuous development of the petrochemical industry and auto electronics industry in Daya Bay, the marine ecology of the bay in the area will suffer great environmental pressure [36]. The investigation of pollution in the Daya Bay area has mainly focused on organic pollutants, heavy metals and water quality, but the investigation of MP pollution remains unexplored [37,38,39] Therefore, this study aimed to explore the potential correlation of environmental factors and the abundance, spatial distribution and compositional characteristics of MPs in surface water and sediment in Daya Bay. It provides a basis for monitoring MPs in the Daya Bay waters and calls for the reduction in plastic use and the protection of local water resources.
2. Materials and Methods
2.1. Study Site
Daya Bay (22°5′–22°9′ N, 114°5′–114° E) in Huizhou City, Guangdong Province, is an essential bay in the South China Sea. The total area is about 650 km2 and its coastline reaches 52 km [40]. Daya Bay is a semi-enclosed bay with tidal currents and a relatively slow water cycle in the South China Sea. There are no major rivers flowing into it except the small Danao River. It is one of the most valuable economic development zones and aquaculture areas in China.
2.2. Sampling
The sample site information of this study is shown in Figure 1. The sampling period was from 22 to 24 July 2020, with a total of 26 sampling sites (sites S1–S22 were evenly distributed in the Daya Bay area and D1–D4 were located in the Danao estuary and coastal area of Daya Bay). MP samples were collected from surface water (MPwater) and sediment (MPsediment). Temperature (Temp), salinity, pondus hydrogenii (pH), dissolved oxygen (DO) and depth were measured in situ using a YSI 6600 multi-probe sensor (Yellow Springs Instrument Co., OH, 45387-1107, USA). Chlorophyll a (Chl a) was extracted with 90% acetone in the dark for 24 h at 4 °C and analyzed using a Turner Design 10 fluorometer [31,41]. For each subsample, 20 L of surface water was collected with a pump. The surface water was filtered through a 48 μm standard steel sieve and the substance on the sieve was transferred into 250 mL glass bottles after being rinsed with distilled water to collect the microplastics [42,43]. The sediment samples for MP extraction and nutrient analysis were collected with a pre-cleaned Peterson mud picker (PBS-411) at the same location as the water samples; approximately 7–8 L of sediment samples was grabbed by the PBS-411 and placed onto the deck of the boat for us to collect samples. The sediment samples were transferred to pre-cleaned glass vials and stored in a refrigerator at −20 °C until transfer to the laboratory for the next analysis [44].
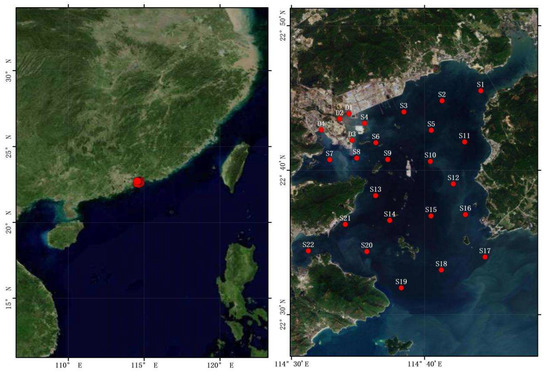
Figure 1.
Sampling sites of Daya Bay. S1–S22 are sites in the bay area and D1–D4 are the sites in coastal area of the bay. Red point indicate the sampling sites.
2.3. Extraction of MPs
All surface water samples were combined with 30% hydrogen peroxide and kept in the dark for 24 h to ensure complete digestion of all organic substances then filtered through a 0.45 μm micro-porous filter membrane (Aqueous system) under a vacuum pump [45]. The filter membrane was placed in a covered glass Petri dish and air dried at room temperature for the next step in the analysis. The density method was used to separate MPs from sediment samples [46]. Firstly, the collected sediment samples were dried in an oven at 60 °C to constant weight. Then, they were gently ground and mixed thoroughly. Each sediment subsample of about 30 g was added to a glass beaker containing a 200 mL saturated solution of sodium chloride (NaCl). The mixture sediment was stirred uniformly and left to stand for 24 h until the MPs floated to the surface. An amount of 90 mL of 30% hydrogen peroxide digest was then added to the floating material to degrade the natural organic matter, similar to the water sample treatment. It is necessary to emphasize that MPs, such as PET material, may float by adhering to attached materials on shells and other objects.
2.4. Identification of MPs
The characteristics (e.g., abundance, shape, size and color) of the suspicious MPs on the filter were observed and photographed by a stereomicroscope (Optec SZ680). The shape, color and size of all suspicious MPs were measured digitally on the filter using the Leica Application Suite System (Version 4.3.0) [47]. The length of all MPs was based on the length of the longest axis. The observed MPs were divided into four categories according to their shape: fiber, pellet, fragment and film. A film is a thin piece of plastic debris, a pellet is a spherical or cylindrical piece and a fiber is a thin and lengthy item; other irregular shapes were designated as fragments. When an item could not be defined as fiber or film, it was classified as a fragment [43]. The MPs were divided into six categories according to their color: white (both white and transparent), red, yellow, green, blue and black. They were also divided into six categories according to their size: <0.5 mm, 0.5–1 mm, 1–2 mm, 2–3 mm, 3–4 mm and 4–5 mm.
No organic or cellular structures were visible to the naked eye. The consistent thickness and color of filaments along their full length, clear particles, uniform color, and transparent white MPs were observed under high-magnification microscopy [16,47]. It was inaccurate to make assumptions by simple microscopic observation and subjectivity alone. The suspicious MPs collected in the samples were identified by micro-Raman spectroscopy (Thermo Fisher Scientific Waltham, MA, USA DXR2, 532 nm laser, Raman shift 50–3500 cm−1). In total, 5177 and 920 MPs were observed in 26 surface water samples and sediment samples, respectively. Then, 110 suspicious MPs were randomly taken and tested in the spectra of 500–3500 cm−1. The absorbances of the infrared spectra of the samples were detected in this range and compared in a searchable library. The result with the highest confidence level was identified as the material of the sample [48,49]. A spectral match of higher than 80% is considered to be the chemical composition of the particle as an MP, otherwise it is not [43].
2.5. Sediment Total Organic Carbon and Nitrogen Analyses
Total organic carbon (C) and nitrogen (N) in sediments were analyzed through a Flash EA 1112 Series Elemental Analyzer (EA-IRMS, all Thermo Finnigan, Bremen, Germany). Prior to carbon analysis, 1 g of each subsample was acidified by 10% HCl for 3 h to remove inorganic carbon. The elemental composition of C and N was defined as the percentage of mass fraction of dw (dry weight) [50]. The atomic ratio of C to N (C/N) was also calculated.
2.6. Quality Control
To prevent contamination, contact with plastic materials was avoided during sampling and experiments. All the sampling tools were cleaned three times with distilled water to avoid site cross-contamination. During sample separation and analysis, the laboratories involved in this study were prohibited from using any plastic equipment and synthetic clothing. To ensure that the laboratory air was contaminant free, a blank Petri dish was placed on the lab bench during the experiment. The control Petri dishes were finally confirmed to be free of MP contamination.
2.7. Data Analysis
A statistical analysis of MP abundance, size, shape, color and composition was performed using Microsoft Office software (Excel 2021), and graphics were produced using prism and ArcGIS 10.3.1. Pearson correlation and factor analysis were analyzed through SPSS 25.0 (IBM, Amenk, NY, USA). A statistical significance level of p = 0.05 was employed.
3. Results
3.1. Environmental Factors and Abundance of Microplastics
The environmental factors Temp, salinity, pH, DO and Chl a of the water column are shown in Figure 2A–E. The distribution of temperature and salinity showed a quite opposite pattern. The water temperature of the inner bay (D1, D2, D3, D4, S1, S2, S3, S4, S6, S7, S8 and S9) ranged from 30.3 to 33.9 °C, which was significantly higher than the other sites (p < 0.05). The salinity of the sampling sites along the northern coast was relatively lower than that of the outer bay (S5, S12~S21). The water of D1, D2, D3 and D4 was slightly alkaline with a pH beyond 8.10. The pH at the other sampling sites was relatively similar, except for S7 where the pH was the lowest.
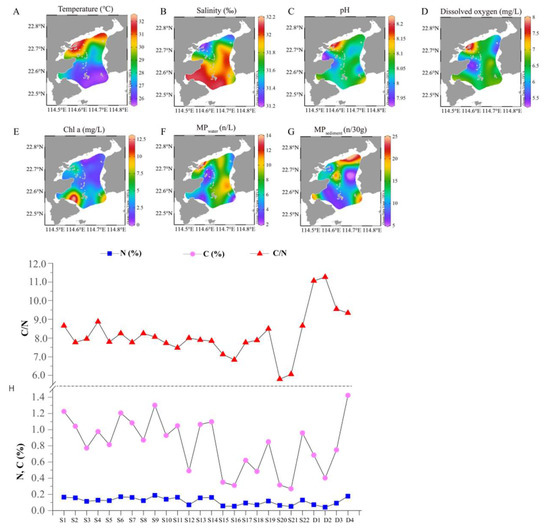
Figure 2.
Distribution of the environmental factors and microplastic abundance in Daya Bay. (A–G) show the distributions of water temperature, salinity, pH. and Chl a and the abundance of microplastics in surface water and sediments, respectively. (H) shows the contents of N (%), C (%) and C/N at different sampling sites.
The abundance of MPs (Figure 2F) in surface water ranged from 1.8 to 13.87 items/L. Generally, MPs in the northwest and east coast had a relatively high abundance. The northwest coast is near Aotou Town, which has a relatively large population, a long history of cage aquaculture and a hurry port and is also near the Danao estuary, while the east bay is close to the famous tourist bay of Shunliao. Dapeng Bay has a relatively low MP abundance. The abundance of MPs in sediment ranged from 5.7 to 24.7 items/30 g dw (relevant to 190~823 items/kg dw) (Figure 2G). It has a quite a different spatial distribution pattern than that of the MP abundance in surface water. The highest abundance occurred at the northeast coast near Xiachong Town (S1, S2), followed by site S9 near an island where a crude oil terminal is located, then followed by site S17, which is at the east corner of the outer bay.
The contents of total organic carbon (C) and nitrogen (N) and the carbon to nitrogen ratio (C/N) of the sediment in the coastal areas in Daya Bay are shown in Figure 2H. The content of C in different sites varied similarly to N content. The content of C at S12, S15, S16, S20, S21 and D2 was relatively low with less than 0.5%, while that of S1, S6, S9 and D4 was higher than 1.2%. The content of N at all sample sites ranged from 0.04% to 0.17%. The C/N ratio of the sediment varied from 5.8 to 11.3. Comparatively, S20 and S21 had the lowest values, while D1–D5 had the highest values.
3.2. Shape, Sizes and Color of MPs
According to the images of the MPs under the microscope (Figure 3), four shapes, fibers, fragments, films and pellets, were found in Daya Bay. The fibers were the most common shape, with the proportion ranging from 93.0% to 100.0% in surface water, and the proportion ranged from 50.9% to 100% in sediment (Figure 4B).
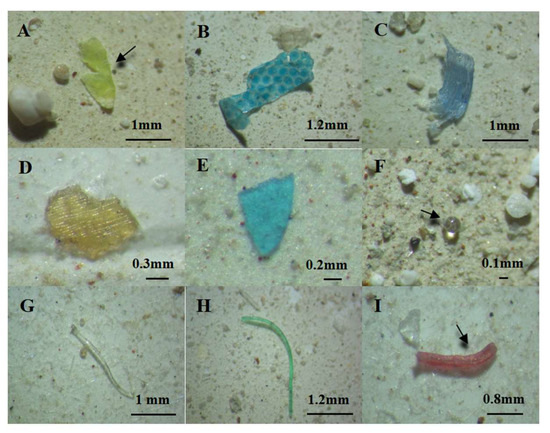
Figure 3.
Shape of microplastics in surface water and sediment in Daya Bay: (A–C) film, (D,E) fragments, (F) pellets, (G–I) fibers. (A–D) Captured at 2× magnification; (D–I) captured at 3× magnification.
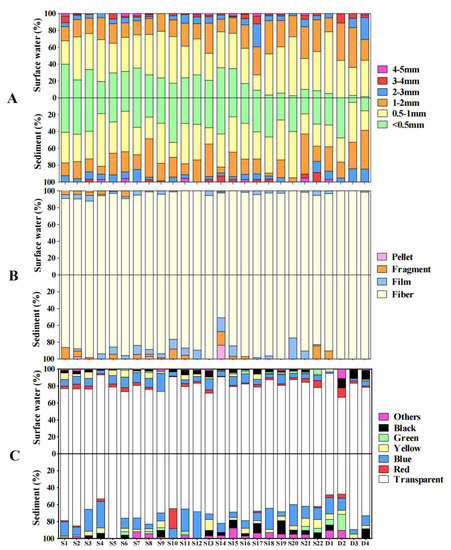
Figure 4.
The proportion based on size (A), shape (B) and color (C) of microplastics in surface water and sediment in Daya Bay.
The size and color of the MPs in the surface water and sediment at all sampling sites were also identified (Figure 4A). In the surface water, a size of 0.5–1 mm accounted for 18.17–70.00% of the total MPs, with an average of 44.73%, followed by sizes of 1–2 mm and <0.5 mm with averages of 24.09% and 22.52%, respectively Similarly, MPs sized 0.5–1 mm dominated in the sediment, and the proportion ranged from 17.65 to 55.00% with an average percentage of 36.17%. The size of <0.5 mm was the second most predominant with an average of 33.12%, while the size of 1–2 mm was the third most predominant size with an average of 21.57%. The observed MP colors were transparent (both white and transparent), red, yellow, green, blue and black (Figure 4C). The color of both the water and sediment samples were predominantly transparent, accounting for 82.27% and 71.49% of the total MPs, which was significantly higher than the blue ones with 7.88% and 13.82%, respectively (p < 0.05).
3.3. Types of Microplastics
The Raman spectra of MPs show the degree of conformity to the spectra of the standards (Figure 5A). The degree of conformity of all identified MPs to the standard Raman spectra was higher than 80%, which ensured the accuracy of the identified MPs. According to the results, 89 particles were identified as MPs. Ten types of microplastics were identified, including polyethylene terephthalate (PET), rayon (RY), polypropylene (PP), cellulose (CL) and polyethylene (PE), as well as a small proportion of other components. The predominant polymer types in surface water were PET (28%) and RY (15%) and those in sediment were PP (20%) and PE (17.78%) (Figure 5B).
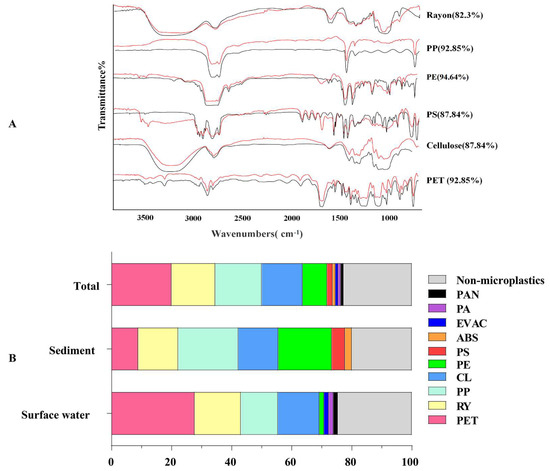
Figure 5.
Raman spectroscopy (A) and the composition (B) of the selected microplastics. Red curves are the selected spectra of microplastic samples and black curves are the spectra of standard polymers in spectral libraries. The types and similarity of the microplastic spectra compared to the standard spectra are shown on the right. PET, polyethylene terephthalate; RY, rayon; PP, polypropylene; CL, cellulose; PE, polyethylene; PS, polystyrene; ABS, acrylonitrile butadiene styrene; EVAC, ethylene-vinyl acetate resin; PA, polyamide; PAN, polyacrylonitrile.
3.4. Pearson Correlation and Factor Analysis for Microplastics and Environmental Factors
The Pearson correlation showed that Temp, salinity, pH, DO and Chl a were significantly correlated with each other (p < 0.05) (Figure 6A). The abundance of MPs in surface water neither correlated significantly with that in sediments nor correlated with other environmental factors, except that it positively correlated with DO (p < 0.05) (Figure 6A). The abundance of MPs in sediment was positively correlated with the contents of sediment organic N and C (p < 0.05).
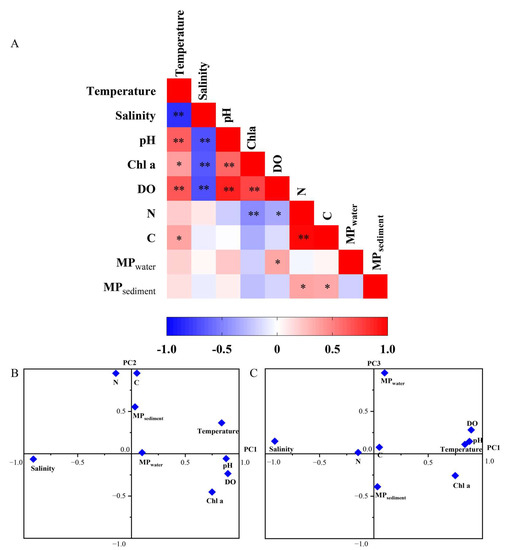
Figure 6.
Pearson correlation (A) and factor analysis (B,C) between the abundance of MPs and the environmental factors. Red indicates the positive correlation and blue indicated the negative correlation; * and ** indicate the significant correlation at levels p < 0.05 and p < 0.01, respectively.
Based on a factor analysis, three components (PC1, PC2 and PC3) accounting for 83.2% of variance were extracted (Figure 6B,C). PC1 had high positive loadings (>0.83) on the variables DO, pH, Chl a and Temp and negative loading (−0.91) on salinity, accounting for 41.2% of the total variance. All the above variables are relevant to water eutrophication, which may suggest that factor PC1 represents the water eutrophication level. PC2 had high loadings for the variables total organic N and C and MPs in sediment, accounting for 27.8% of the total variance. PC3 showed high loading for MPwater alone, accounting for 16.0% of the total variance.
4. Discussions
4.1. Characteristics of Microplastics of Daya Bay
Numerous studies have investigated MP pollution in seawater. In this study, the abundance of MPs in surface water ranged from 1.8 to 13.87 items/L in Daya Bay. A similar abundance of MPs has also been found in the Pearl River estuary (7.8–10.95 items/L) [43]. Nevertheless, a comparatively lower abundance of MPs was found in the Bohai Sea (0.4–5.2 items/L) [51], and a relatively higher abundance of MPs was detected in Chabahar Bay (86–362 items/L) [52]. The abundance of MPs in sediment ranged from 5.7 to 24.7 items/30 g dw (relevant to 190~823 items/kg dw). The abundance of MPs observed in the present study was much higher than that found in the south of the Caspian Sea, reaching 0–48 items/kg dw [53], and in the Bay of Bengal of India, reaching 0.33–317.67 items/kg, but it was comparatively lower than that found in lake shore sediment of the Montenegrin Coast of the Adriatic Sea basin (150–2500 items/kg dw) [54] and in coastal sediment of La Palma in Spain (2682 ± 827 items/kg dw) [55]. Overall, the pollution status of the MPs in the Daya Bay was in the middle level. The data we collected were from a sampling survey during the summer, because summer is the time when human activities are most frequent in the coastal areas of Daya Bay. Conducting the survey at this time of the year helps us to understand the severity of the situation of microplastic pollution in Daya Bay. In this way, we can more accurately evaluate the connection between microplastic pollution and local anthropogenic activities and environmental factors. However, the importance of managing and cleaning plastic waste still deserves attention.
In the current study, the sizes of 0.5–1 mm and <0.5 mm accounted for the highest proportion of MPs. Generally, the abundance of MPs decreased successively along with size enlargement, which is consistent with the size distribution of MPs in other research areas [56]. Larger MP materials eventually break up into smaller MPs in the marine environment through mechanical action, photo-oxidation and biodegradation [57,58], which results in a high abundance of smaller-sized MPs. In addition, previous studies found that biofouling increased the density of MPs and accelerated their sedimentation. As a result, there is accelerated accumulation of smaller MPs, and thus a considerable amount of small-sized MPs were detected in the water and sediment [59,60].
Fibers were the most predominant shape of MPs observed in this study in both surface water and sediment. This has also been observed in the Xijin Wetland Park, Nanning [61], the Pearl River estuary [43,62], Poyang Lake [63] and the Atlantic Ocean south of the Algarve Coast (Portugal) [64]. To a great extent, the fibers may be due to the decomposition of fishing instruments and sewage containing clothing fibers [65]. As a potential source of MPs, fiber could be stripped from plastics by fishing activities, atmospheric deposition and domestic sewage containing laundry fibers [66]. There are a great number of fishing-related activities in Daya Bay every year, such as boat fishing and cage culture, which may be a predominant factor in increasing the fiber content in the water environment [67].
As for the color of MPs, both water and sediment samples were predominantly transparent, accounting for 82.27% and 71.49% of the total MPs in this study. The fact that transparency accounts for such a high proportion can be explained by domestic sewage and fishing activities. It is a fact that a large number of transparent plastic products are commonly used, such as fishing line, nylon nets and plastic bags. Their intensive use inevitably generates large amounts of transparent MPs [68]. Furthermore, polychromatic MPs are faded in water by light, heat, water and biological weathering, resulting in transparent MPs [69,70].
The most common polymers in Daya Bay were PET, RY, PP, CL and PE, which are widely utilized in fishing activities and the packaging industry [71,72], suggesting that human activities are probably significant sources of these MPs [73]. In Daya Bay, the well-developed local textile industry may be a unique source of MPs, and the processing and production of various garments is inseparable from PET and RY [74,75]. PET can be processed into polyester fiber, which is excellent in wrinkle resistance and conformability. Therefore, it is generally used to make curtains, conveyor belts, ropes, electrical insulation materials, etc. [35]. As for RY, it is an artificially manufactured fiber, usually used in clothing, upholstery, industrial surgical instruments, feminine hygiene products, tires, etc. [76]. Once the waste from the use of these household products enters the seawater system through domestic sewage or fishing activities, it will decompose and produce large amounts of fibrous MPs [77]. PP and PE are the most frequent MP components in sediment [78,79] and had a relatively high proportion of the total sample in this study. It was previously noted that biofouling increased the density of MPs and accelerated their settling, which explains the predominance of PP and PE in the sediment of Daya Bay instead of in the surface water [80].
4.2. Potential Correlation between Environmental Factors and Microplastics
The development of MP pollution in coastal areas primarily depends on the local climate and water environment, with its abundance mainly attributed to anthropogenic sources and population density [81]. Human living conditions vary from one site to another, such as daily effluents, industrial wastewater, ships or fishing, all of which contribute different types of plastics or MPs [52,82]. Therefore, it is hard to track the sources of coastal MPs. A factor analysis is a possible approach to obtain potential associations among different environmental indicators. In the present experiment, the factor analysis showed that neither MPs in surface water nor in sediments correlated with PC1, which indicates the eutrophication level of the bay. However, the content of total organic N and C and the abundance of MPs in sediments were related to PC2. It was indicated that sediment MPs shared similar sources with total organic N and C in the sediments, which was also suggested by a significant Pearson correlation between the contents of total organic N and C and sediment MP abundance (p < 0.05). The C/N ratio is frequently used to identify the proportions of sedimentary organic matter originating from marine autogenic and terrigenous inputs [83]. Generally, the C/N ratio of sediment organic matter from terrestrial sources is equal to or above 20, while that from marine origins typically varies between 5 and 8 [84]. In the present study, the C/N ratio of sediment organic matter ranged from 6.8 to 11.2 with the highest value in the estuary area, indicating higher terrigenous inputs in the estuary area. It also indicated that the source of organic C and N was mainly from the autochthonous sedimentation of marine organisms. MPs in sediment deposited jointly with the organic sediment N and C, as biofouling increased the sedimentation of MPs. The abundance of MPs in surface water has no significant correlation with that in sediment and it was highly related to PC3, explaining 0.95 of the loading. MPs in surface water change frequently and complexly due to water flow [8,85], as does their abundance.
5. Conclusions
This study provided the first report of MPs in surface water and sediment in Daya Bay, South China Sea. The abundance of MPs ranged from 1.8 to 13.87 items/L in surface water and 190 to 823 items/kg dw in sediment. The predominant size of MPs is 0.5–1 mm. The predominant shape of the observed MPs was fiber, and the majority of MPs were transparent in color. On average, PET was the most common polymer type. The Pearson correlation and factor analysis showed that MPs in sediment might share similar sources with organic N and C and might sediment jointly with organic matter. In addition, the distribution of surface MPs was not correlated with the water trophic level. All in all, these results indicate the MP contamination in Daya Bay and provide essential data for governmental agencies to formulate microplastic pollution control policies. Our study was the first to jointly recognize the distribution characteristics of MPs and environmental factors. It provides a new perspective for future research about MP pollution.
Author Contributions
P.L.: conceptualization, research performance, methodology, formal analysis, data curation, writing—original draft; H.L.: visualization, investigation, validation, writing—original draft; H.T.: conceptualization, methodology, formal analysis, writing—review, funding acquisition; A.Z.: formal analysis, writing—review; Y.D., W.Z., Z.Z. and D.S.: conceptualization, research performance; Z.K.: sampling, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (32171548) and Guangzhou Science and Technology Project: 2023B03J1359.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Xiaofeng Chen, Zhaolin Lv and Qinqi Guo for providing help in sampling and figures and Muting Yan and Han Gong for providing the microscope.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pol-lution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Mao, R.; Song, J.; Yan, P.; Ouyang, Z.; Wu, R.; Liu, S.; Guo, X. Horizontal and vertical distribution of microplastics in the Wul-iangsuhai Lake sediment, northern China. Sci. Total Environ. 2021, 754, 142426. [Google Scholar] [CrossRef]
- Lechner, A.; Ramler, D. The discharge of certain amounts of industrial microplastic from a production plant into the River Danube is permitted by the Austrian legislation. Environ. Pollut. 2015, 200, 159–160. [Google Scholar] [CrossRef]
- de Carvalho, A.R.; Imbert, A.; Parker, B.; Euphrasie, A.; Bouletreau, S.; Britton, J.R.; Cucherousset, J. Microplastic in angling baits as a cryptic source of contamination in European freshwaters. Sci. Rep. 2021, 11, 11255. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Peng, G.; Xu, P.; Li, D. Coastal ocean dynamics reduce the export of microplastics to the open ocean. Sci. Total Environ. 2020, 713, 136634. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Wang, X.; Yang, F.; Chen, M.; Wang, J. Characterization of microplastics in the surface seawater of the South Yellow Sea as affected by season. Sci. Total Environ. 2020, 724, 138375. [Google Scholar] [CrossRef]
- Blaskovic, A.; Fastelli, P.; Cizmek, H.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the Croatian marine protected area of the natural park of Telascica bay (Adriatic Sea). Mar. Pollut. Bull. 2017, 114, 583–586. [Google Scholar] [CrossRef]
- Chen, M.; Du, M.; Jin, A.; Chen, S.; Dasgupta, S.; Li, J.; Xu, H.; Ta, K.; Peng, X. Forty-year pollution history of microplastics in the largest marginal sea of the western Pacific. Geochem. Perspect. Let. 2020, 13, 42–47. [Google Scholar] [CrossRef]
- Munari, C.; Infantini, V.; Scoponi, M.; Rastelli, E.; Corinaldesi, C.; Mistri, M. Microplastics in the sediments of Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 2017, 122, 161–165. [Google Scholar] [CrossRef]
- Sagawa, N.; Kawaai, K.; Hinata, H. Abundance and and size of microplastics in a coastal sea: Comparison among bottom sediment, beach sediment, and surface water. Mar. Pollut. Bull. 2018, 133, 532–542. [Google Scholar] [CrossRef]
- Manbohi, A.; Mehdinia, A.; Rahnama, R.; Dehbandi, R. Microplastic pollution in inshore and offshore surface waters of the southern Caspian Sea. Chemosphere 2021, 281, 130896. [Google Scholar] [CrossRef]
- Borja, A.; Andersen, J.H.; Arvanitidis, C.D.; Basset, A.; Buhl-Mortensen, L.; Carvalho, S.; Dafforn, K.A.; Devlin, M.J.; Esco-bar-Briones, E.G.; Grenz, C.; et al. Past and Future Grand Challenges in Marine Ecosystem Ecology. Front. Mar. Sci. 2020, 7, 362. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frere, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.M.; et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Change Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L.; Baini, M.; Giannetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Hossain, M.R.; Jiang, M.; Wei, Q.; Leff, L.G. Microplastic surface properties affect bacterial colonization in freshwater. J. Basic Microbiol. 2019, 59, 54–61. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Naqash, N.; Prakash, S.; Kapoor, D.; Singh, R. Interaction of freshwater microplastics with biota and heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1813–1824. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of micro-plastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Rubin, A.E.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Rivera De la Rosa, J.; Barbieri, V.; Siligardi, C.; Cedil-lo-González, E.I. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Antao Barboza, L.G.; Garcia Gimenez, B.C. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Lei, Z.; Fuad, M.T.I.; Wang, Q.; Sun, S.; Fang, J.K.-H.; Liu, X. Distribution and potential sources of microplastics in sediments in remote lakes of Tibet, China. Sci. Total Environ. 2022, 806, 150526. [Google Scholar] [CrossRef]
- Du, J.Z.; Song, H.Q.; Mu, D.H.; Li, D.J.; Yan, S.P.; Gu, Y.J. Sorption/desorption of radioruthenium on the surface sediments of Daya Bay, China. J. Radioanal. Nucl. Chem. 2007, 273, 119–122. [Google Scholar] [CrossRef]
- Tang, H.; Ke, Z.; Yan, M.; Wang, W.; Nie, H.; Li, B.; Zhang, J.; Xu, X.; Wang, J. Concentrations, Distribution, and Ecological Risk Assessment of Heavy Metals in Daya Bay, China. Water 2018, 10, 780. [Google Scholar] [CrossRef]
- Wu, M.-L.; Wang, Y.-S.; Dong, J.-D.; Sun, C.-C.; Wang, Y.-T.; Sun, F.-L.; Cheng, H. Investigation of Spatial and Temporal Trends in Water Quality in Daya Bay, South China Sea. Int. J. Environ. Res. Public Health 2011, 8, 2352–2365. [Google Scholar] [CrossRef]
- Zhou, J.L.; Maskaoui, K. Distribution of polycyclic aromatic hydrocarbons in water and surface sediments from Daya Bay, China. Environ. Pollut. 2003, 121, 269–281. [Google Scholar] [CrossRef]
- Wu, M.-L.; Wang, Y.-S.; Sun, C.-C.; Wang, H.; Dong, J.-D.; Yin, J.-P.; Han, S.-H. Identification of coastal water quality by statistical analysis methods in Daya Bay, South China Sea. Mar. Pollut. Bull. 2010, 60, 852–860. [Google Scholar] [CrossRef]
- Xiang, C.; Ke, Z.; Li, K.; Liu, J.; Zhou, L.; Lian, X.; Ta, Y. Effects of terrestrial inputs and seawater intrusion on zooplankton community structure in Daya Bay, South China Sea. Mar. Pollut. Bull. 2021, 167, 112331. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Mao, R.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X.; Cai, Z.; Li, M.; Liu, Z.; Gong, H.; Yan, M. Characteristics of microplastic pollution and analysis of colonized-microbiota in a freshwater aquaculture system. Environ. Pollut. 2022, 306, 119385. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Xu, K.; Huang, K.; Wang, J. Microplastics Environmental Effect and Risk Assessment on the Aquaculture Systems from South China. Int. J. Environ. Res. Public Health 2021, 18, 1869. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Ek, C.; Gerdes, Z.; Garbaras, A.; Adolfsson-Erici, M.; Gorokhova, E. Growth Retardation and Altered Isotope Composition As Delayed Effects of PCB Exposure in Daphni a magna. Environ. Sci. Technol. 2016, 50, 8296–8304. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242, 1557–1565. [Google Scholar] [CrossRef]
- Hosseini, R.; Sayadi, M.H.; Aazami, J.; Savabieasfehani, M. Accumulation and distribution of microplastics in the sediment and coastal water samples of Chabahar Bay in the Oman Sea, Iran. Mar. Pollut. Bull. 2020, 160, 111682. [Google Scholar] [CrossRef]
- Nematollahi, M.J.; Moore, F.; Keshavarzi, B.; Vogt, R.D.; Saravi, H.N.; Busquets, R. Microplastic particles in sediments and waters, south of Caspian Sea: Frequency, distribution, characteristics, and chemical composition. Ecotoxicol. Environ. Saf. 2020, 206, 111137. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, N.; Joksimovic, D.; Pekovic, M.; Perosevic-Bajceta, A.; Bajt, O. Microplastics in Surface Sediments along the Monte-negrin Coast, Adriatic Sea: Types, Occurrence, and Distribution. J. Mar. Sci. Eng. 2021, 9, 841. [Google Scholar] [CrossRef]
- Villanova-Solano, C.; Diaz-Pena, F.J.; Hernandez-Sanchez, C.; Gonzalez-Salamo, J.; Gonzalez-Pleiter, M.; Vega-Moreno, D.; Fernandez-Pinas, F.; Fraile-Nuez, E.; Machin, F.; Hernandez-Borges, J. Microplastic pollution in sublittoral coastal sediments of a North Atlantic island: The case of La Palma (Canary Islands, Spain). Chemosphere 2022, 288, 132530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, L.; Li, D.; Wu, S.; Kong, L.; Wang, J.; Shi, H. The microplastic pollution in beaches that served as historical nesting grounds for green turtles on Hainan Island, China. Mar. Pollut. Bull. 2021, 173, 113069. [Google Scholar] [CrossRef] [PubMed]
- Arhant, M.; Le Gall, M.; Le Gac, P.Y.; Davies, P. Impact of hydrolytic degradation on mechanical properties of PET—Towards an understanding of microplastics formation. Polym. Degrad. Stab. 2019, 161, 175–182. [Google Scholar] [CrossRef]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Degli Innocenti, F. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 225. [Google Scholar] [CrossRef]
- Kooi, M.; van Nes, E.H.; Scheffer, M.; Koelmans, A.A. Ups and Downs in the Ocean: Effects of Biofouling on Vertical Transport of Microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, K.; Li, Y.; Zhang, Y.; Yan, L.; Xu, K.; Huang, S.; Junaid, M.; Wang, J. Microplastics abundance, distribution, and composition in freshwater and sediments from the largest Xijin Wetland Park, Nanning, South China. Gondwana Res. 2022, 108, 13–21. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, Z.; Liu, P.; Zhao, X.; Wu, R.; Zhang, C.; Lin, C.; Li, Y.; Guo, X. Distribution and characteristics of microplastics in the basin of Chishui River in Renhuai, China. Sci. Total Environ. 2021, 773, 145591. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Lechthaler, S.; Schwarzbauer, J.; Reicherter, K.; Stauch, G.; Schuttrumpf, H. Regional study of microplastics in surface waters and deep sea sediments south of the Algarve Coast. Reg. Stud. Mar. Sci. 2020, 40, 101488. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Vazquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Betti, F.; Rindi, F.; Tregrosso, A.; Bo, M. Fate of lost fishing gears: Experimental evidence of bio-fouling colonization patterns from the northwestern Mediterranean Sea. Environ. Pollut. 2021, 268, 115746. [Google Scholar] [CrossRef]
- Micheluz, A.; Angelin, E.M.; Lopes, J.A.; Melo, M.J.; Pamplona, M. Discoloration of Historical Plastic Objects: New Insight into the Degradation of beta-Naphthol Pigment Lakes. Polymers 2021, 13, 2278. [Google Scholar] [CrossRef]
- Marti, E.; Martin, C.; Galli, M.; Echevarria, F.; Duarte, C.M.; Cozar, A. The Colors of the Ocean Plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated Microplastic Pollution Derived from Fishery Ac-tivities and “Hidden” in Deep Sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(ethylene terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Chouchene, K.; Prata, J.C.; Da Costa, J.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Microplastics on Barra beach sediments in Aveiro, Portugal. Mar. Pollut. Bull. 2021, 167, 112264. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, L.; Huang, W.; Liu, J.; Dou, S. Assessment of plastic pollution in the Bohai Sea: Abundance, distribution, morpho-logical characteristics and chemical components. Environ. Pollut. 2021, 278, 116874. [Google Scholar] [CrossRef]
- Chen, D.; Ke, Z.; Tan, Y. Distribution of C/N/P stoichiometry in suspended particulate matter and surface sediment in a bay under serious anthropogenic influence: Daya Bay, China. Environ. Sci. Pollut. Res. 2021, 28, 29177–29187. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Tan, Y.; Huang, L.; Zhao, C.; Jiang, X. Spatial distributions of δ13C, δ15N and C/N ratios in suspended particulate organic matter of a bay under serious anthropogenic influences: Daya Bay, China. Mar. Pollut. Bull. 2017, 114, 183–191. [Google Scholar] [CrossRef]
- Zhdanov, I.; Lokhov, A.; Belesov, A.; Kozhevnikov, A.; Pakhomova, S.; Berezina, A.; Frolova, N.; Kotova, E.; Leshchev, A.; Wang, X.; et al. Assessment of seasonal variability of input of microplastics from the Northern Dvina River to the Arctic Ocean. Mar. Pollut. Bull. 2022, 175, 113370. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).