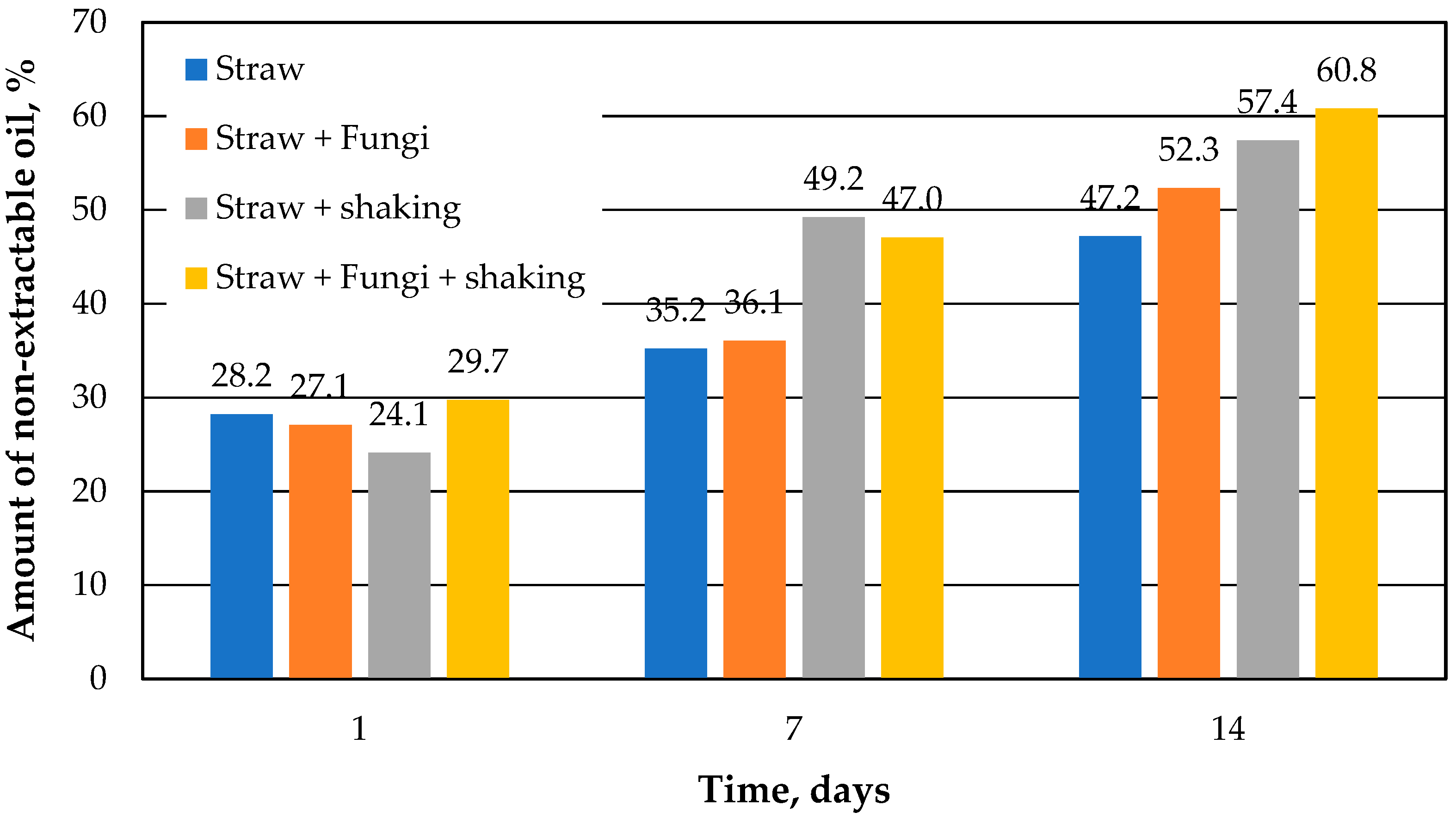1. Introduction
The Baltic Sea is a semi-enclosed water body, and its residence time is very high; therefore, it is sensitive to external influences [
1]. The water is brackish and because of the many islands, wetlands, and lagoons, the Baltic Sea has a specific ecosystem [
1].
At the same time, the Baltic Sea is an inland sea with some of the most intense sea traffic in the world. On average, 300,000 ships enter the Baltic Sea every year [
2]. Two-thirds of the ships in the region are cargo ships, with slightly less than half being oil and oil products tankers [
2]. The annual oil transport [
2,
3] in the sea poses a significant risk for oil spills.
Certain coastal locations on the Baltic Sea, such as ports and the vicinity of industrial facilities that use or refine oil, have suffered from frequent small-scale oil discharges and spills for decades [
4]. The port of Klaipeda in Lithuania, with its oil terminal, is a seaport on the eastern Baltic coast and is considered an important node of the east–west transport corridor linking roads and sea routes in that direction [
5]. Analyses of the port’s sediments show that it is polluted with PAH of pyrogenic origins as well as aliphatic hydrocarbons of technogenic origins [
6,
7,
8].
For surface oil spills, sorbents are used effectively for coastal cleanup and removing small oil slicks [
9,
10]. Sorbents are also unsuitable for use in the open sea due to unfavorable weather conditions [
11]. A sorbent becomes a pollutant itself when remaining on the water surface for too long. Therefore, sorbents must be removed from the water surface at the right time. Then, the best methods for regeneration or recovery of used sorbents (incineration, landfill, biodegradation) must be applied [
10].
A strong focus is, therefore, on exploring the possibilities of natural organic sorbents. These sorbents are sustainable, cost-effective, and biodegradable, and their resources are renewable [
10]. However, as described in [
10], natural sorbents must be hydrophobized to stay on the water after the sorption process of oil in order to not sink and be able to be caught. Another advantage is the equipment of sorbents with an additional function. Multifunctional materials [
12,
13] have an expanded range of properties, efficient manufacturing processes, and a wide range of design options. The use of natural components makes these materials recyclable.
To enhance the effectiveness of sorbents in removing hydrocarbons from the surface, bioremediation techniques can be employed by introducing materials that encourage the microbial biodegradation of oil. These methods involve the addition of microorganisms or nutrients to overcome unfavorable environmental conditions, such as low temperatures, insufficient oil-degrading microorganisms, or a lack of inorganic nutrients in the water. Fungi are among the microorganisms used for this purpose [
14], and their ability to break down hydrocarbons has been well-documented [
15]. However, the challenge is that the immobilization of microorganisms might change the effectiveness of a sorbent in terms of its buoyancy or absorption capacity.
This work describes a hydrophobic straw as a sorbent from the Baltic Sea environment that was extracted using oil-degrading filamentous fungi, as described in European Patent application EP EP3865460 A1 [
16]. The physical properties of the straw without immobilized fungi and its sorption capacity, as well as the evaporation rate of crude oil from the straw’s surface, were previously published in [
10]. Here, we study if the hydrophobized straw as an oil sorbent is affected by the immobilization of filamentous fungi.
2. Materials and Methods
2.1. Chemicals and Materials
For the hydrocarbon extraction process n-hexane and n-heptane (Honeywell/Riedel-de-Haën, Cromasolv for HPLC ≥ 97%), anhydrous sodium sulphate (Sigma-Aldrich (Germany), puriss 99.0–100.5%), aluminum oxide (Sigma-Aldrich, puriss), and acetone (Honeywell, ≥99.5%) were used.
For the gas chromatography analysis, standard mixtures of n-alkanes (Shimadzu) and n-hexane (Honeywell/Riedel-de-Haën, Cromasolv for HPLC ≥ 97%) were used.
Oat straw (Klaipeda, Lithuania) was used as a natural sorbent in this study. It was chopped into 1.0 to 1.5 cm pieces.
Methyltrimethoxysilane (MTMS, C4H12O3Si, 98%; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used for hydrophobization of the straw.
The water was taken from the Baltic Sea next to Karkle (55°48′33″ N, 21°4′38″ E) (Lithuania). The salinity of the water was 5.76 ‰, the pH—8.39.
The crude oil used for the analysis was from SC “ORLEN Lietuva” (Mazeikiai, Lithuania). The density was 867 kg m−3 (T = 20 °C), and the viscosity was 0.0097 Pa s (T = 25 °C).
2.2. Hydrophobization of the Straw
The procedure is identical to the one described in [
10]. For each g of straw, 1 g MTMS was poured into a 50 mL glass dish and positioned at the bottom of a 3 L beaker. The MTMS was chosen partly because it makes chemical vapor deposition into porous materials readily manageable. The glass dish was topped with a plastic fly screen to prevent straw pieces from falling into the MTMS. The straw was placed evenly on the plastic fly screen. The beaker was closed with foil (aluminum or plastic) and put into the oven at 70 °C for 12 h.
2.3. Immobilization on the Hydrophobic Straw with Fungi
The filamentous fungal species with the ability to degrade petroleum hydrocarbon [
16] was used for straw immobilization as follows: the hydrophobized straw was dry-heated to 120 °C for 1.5 h for sterilization. The straw was sterilized and then placed in the sterile breathing Microsac bag (Sac O2 Nevele, Belgium) and mixed gently with a previously prepared 25 mL fungal inoculum in sterile water with Ringer’s solution (NutriSelect
® Basic) and incubated for 72 h at 25 °C. After fungi overgrew the straw, they were frozen, lyophilized (72 h), and kept at room temperature (20–25 °C) until use.
2.4. Determination of the Distribution of Total Petroleum Hydrocarbons in the Water and Straw
The total hydrocarbon content (THC) in the water and straw was analyzed to investigate the distribution of hydrocarbons between the straw and water.
A 1000 mL beaker was half-filled with seawater. A plastic fly screen was fitted inside, as shown in [
10].
Crude oil (1 mL) and the straw (2 g) were placed in the water. After 1, 7, or 14 days, the straw was taken out of the water with the plastic fly screen. The straw was weighed after excess water dripped off for 15 min. All analyses were carried out with and without the straw under the same conditions with three replicates.
The hydrocarbon concentrations in the water and straw were determined according to [
10].
The water was stirred with 50 mL of n-hexane at 500 rpm with a magnetic stirrer for 30 min and then poured into a separatory funnel. The straw was added to a sealed 250 mL DURAN glass bottle along with 100 mL of a solvent mixture of heptane and acetone in a 1:2 ratio. The extraction process was carried out on a shaker at 120 rpm for 60 min and then shaken into another sieve. The plastic fly screen was washed several times with small amounts of n-hexane and added to the straw extraction separatory funnel. After the water phase was separated in each case, the hexane extracts of the seawater phase and the straw were purified separately over a short column of 3 g alumina for the water extracts and 6 g for the straw extracts. A paper filter containing 10 g of sodium sulfate was placed on the column in a glass funnel, and the hexane fraction from the separating funnel was dried in this way. The respective purified extracts were diluted by a factor of 20 with n-hexane for GC analysis and transferred to labeled glass vials with rubber crimp caps.
Hydrocarbon concentration analysis was carried out on a GC-2010 Plus gas chromatograph equipped with a flame ionization detector. Helium (purity > 99.999%) was used as the carrier gas at a flow rate of 30 mL min−1. A Rxi-1MS type 20 m capillary column with an 0.18 mm inner diameter and 0.18 µm thickness cross bond 100% dimethylpolysiloxane polymer film (Restek, Centre County, PA, USA) were used for the analysis. Instrument control and data evaluation were performed with LabSolution software.
Three calibration standard solutions with concentrations of 0.01, 0.05, 0.1, 0.5, and 1.0 mg mL−1 were made of crude oil. A 1.0 mg mL−1 standard solution was made by accurately weighing 100 mg of crude oil and dissolving it in 100 mL of n-hexane. Other standard solutions were made by the dilution method.
The total peak area was measured in the range bounded by the n-decane (C10H22) and n-tetracontane (C40H82) standards. Only compounds with a signal-to-noise ratio higher than 5 were considered. The relative proportions of the constituents in the samples were obtained using the percentage area of chromatographic peaks, as is generally performed.
Total hydrocarbon concentration in the water was calculated as follows [
10]:
Cw is the total hydrocarbon concentration in the seawater, mg L
−1;
Cex is the total hydrocarbon concentration in the seawater extract, mg mL
−1;
Vex is the volume of the seawater extract, and mL;
Vw is the total volume of the seawater sample, mL.
The total hydrocarbon concentration in the straw (sorbent) was calculated as follows [
10]:
Cs is the total hydrocarbon concentration in the straw, g g
−1;
Cex is the total hydrocarbon concentration in the (straw) extract, mg mL
−1;
Vex is the volume of the (straw) extract, mL;
F is the dilution factor; and
Ms is the mass of the straw, mg.
The crude oil mass of the sorbent was calculated according to [
10]:
Wh is the crude oil mass of the straw, mg kg
−1 dry mass;
ρ is the crude oil mass concentration of the extract calculated from the calibration function, mg L
−1;
Vh is the volume of the n-hexane extract, mL;
m is the mass of the sample taken for analysis, g; and
ws is the dry mass content of the straw, wt%.
The percentage of water absorbed by the straw during the experiment (1, 7, and 14 days) was calculated as follows [
10]:
m0 is the initial mass of the dry straw, g;
mmix is the mass of the dry straw with crude oil and seawater, g; and
mH2O is the mass of the seawater, g.
The amount of non-extractable oil
x was calculated from the amount of crude oil, the total amount of extracted oil from the straw, and the seawater:
The sorption measurements for crude oil were carried out in three replicates.
2.5. Fungi Quantification with qPCR
For fungal quantification, sorbents were sampled in three stages under two treatments (with and without agitation): at the initial stage (before the start of the experiment (time 0)), and after 7 and 14 days. The DNeasy PowerSoil Kit (Qiagen, Singapore) was used to extract DNA from the sorbents (up to 200 mg of sterile sorbents). Before extraction, sorbents were cut into smaller pieces under sterile conditions. The extracted DNA was used as a template for PCR reactions. The β-actin gene, characteristic of the fungal genome, was used to quantify fungal cells in the sorbents [
17]. Quantitative real-time PCR was performed using a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) using the SYBR Green PCR Master Mix (Thermo Fisher Scientific). Each 20 μL reaction contained 10 μL SybrGreen, 0.25 μL of a 10 pmol/μL primer solution using the β-actin primers, ACT 512-F (5′ ATG TGC AAG GCC GGT TTC GC 3′) and ACT 783-R (5′ TAC GAG TCC TTC TGG CCC AT 3′) [
18], 8.5 μL H
2O, and 2 μL of the DNA template. The amplification conditions were 95 °C for 10 min and then 40 cycles of 95 °C 15 s, 61 °C 20 s, and 72 °C 15 s. For each sample, the analysis was performed in triplicate. Fluorescence measurements were made at the end of each annealing cycle and at an additional measuring point at 80 °C (for 1 s) to detect the formation of primer dimers during amplification. A melt curve analysis was made by raising the temperature from 65 to 95 °C in 0.5 °C steps for 5 s each. Standard curves based on threshold cycles were generated by re-amplifying the dilution series of PCR products 10 times from fungal genomic DNA, and an aliquot of each dilution in 3 replicates was used for real-time PCR.
3. Results and Discussion
The physical properties of the straw without immobilized fungi and its sorption capacity, as well as the rate of evaporation of the crude oil from the surface of the straw, have already been published in [
10].
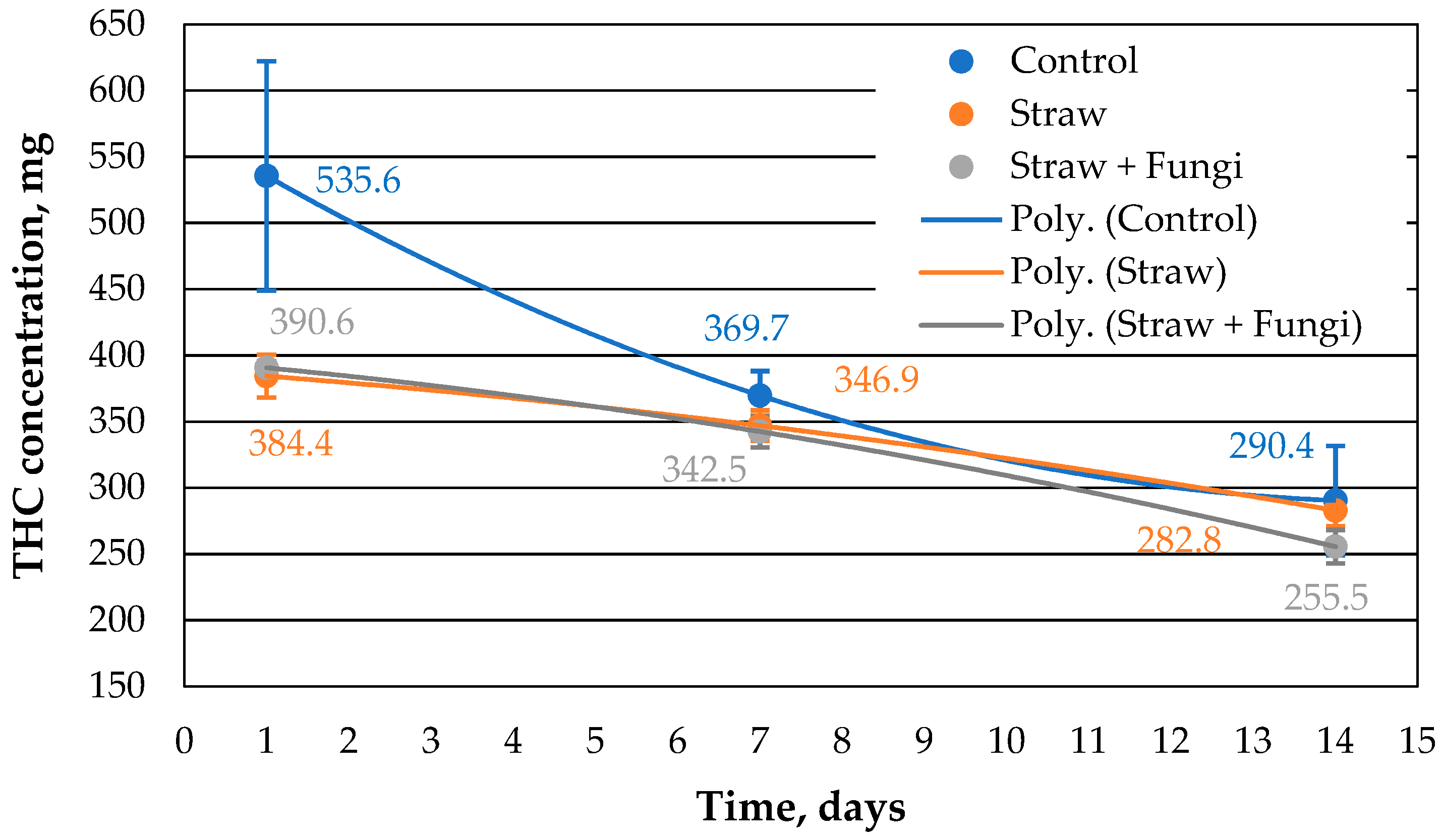
As can be seen in
Figure 1, 2 g of straw with immobilized fungi has a similar sorption capacity as the same amount of straw without immobilized fungi initially and after one week, but it is lower than the control sample consisting of oil on the surface with no sorbent. The sorption capacity of the straw with fungi decreases after 14 days compared to the control sample and the straw without fungi. This could be due to the increased biomass of fungi occupying part of the porosity of the straw or due to straw decomposition affected by fungi, which cannot be decided here.
At the same time,
Figure 1 shows that after 14 days, the amount of extracted oil from the sample with straw without fungi shows the same amount of oil as the control sample, indicating that the straw has completely sorbed the oil at the latest by this point. As already discussed in [
10], the comparative sorption kinetics are probably related to the morphology of the straw.
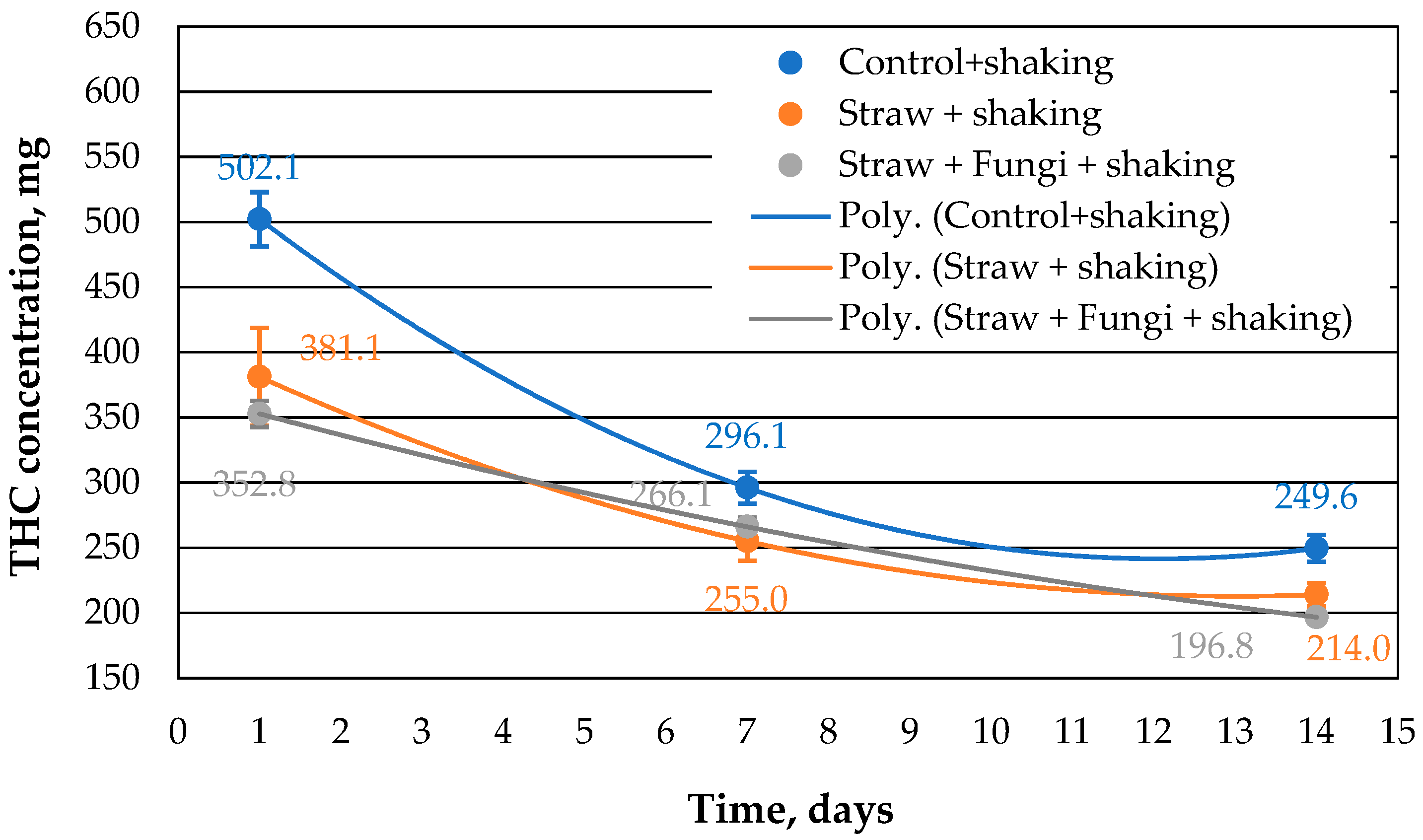
Repeating the same approach with shaking results in a similar trend as in
Figure 1, but with slightly lower values for the extracted THC concentration from the samples (
Figure 2).
Shaking increases the surface area in the beaker track, and of course, more oil can evaporate from the mixture. This is reflected in the systematically lower values for the extractable THC compared to the control sample in
Figure 2. The values of the sorption capacity for straw with and without microbes differ slightly within the error, so that one does not have a clear effect on the effectiveness of the microbes.
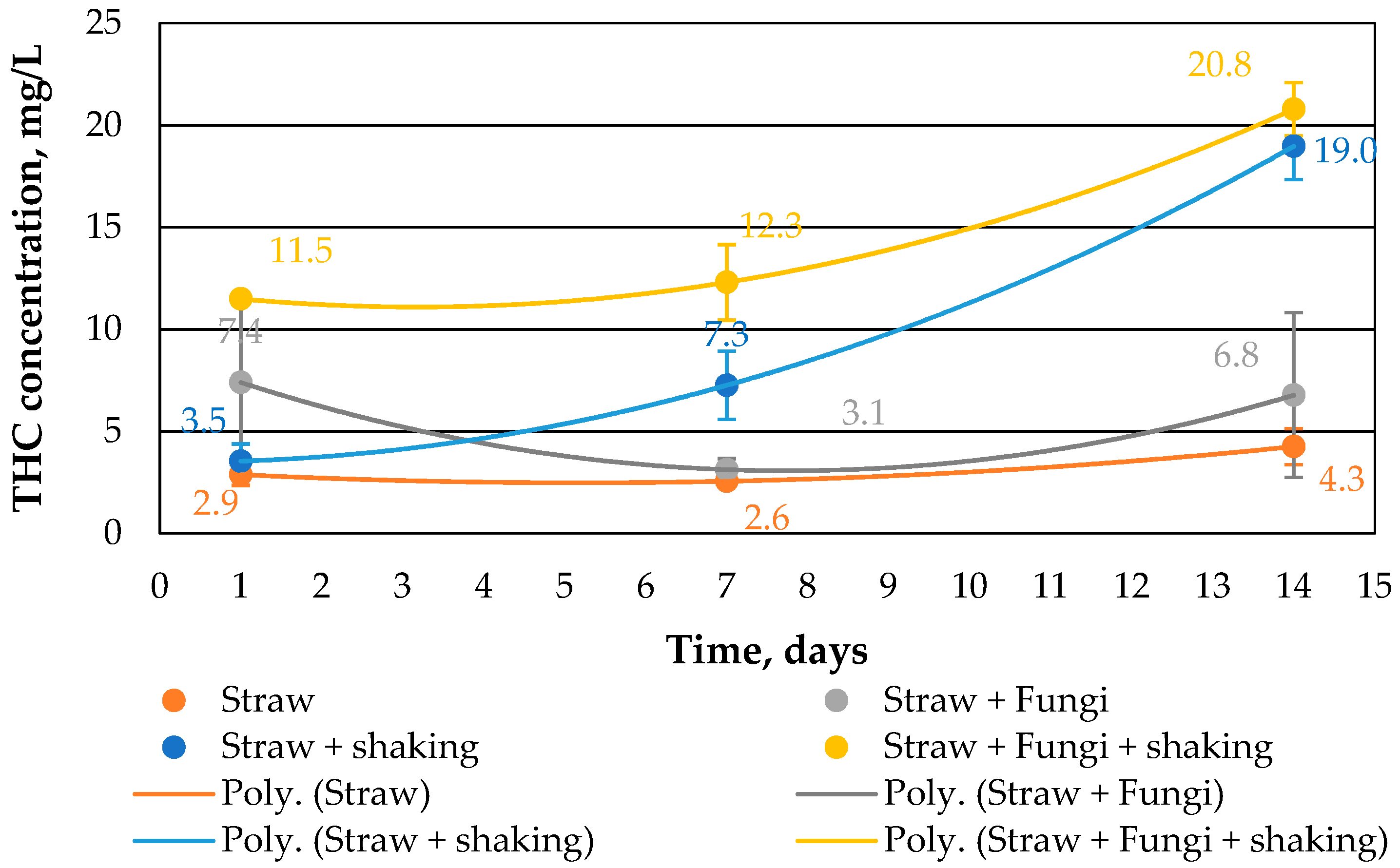
Figure 3 shows the THC concentration in water. Notably, the presence of fungi and simultaneous shaking significantly increases the THC concentration. On the one hand, this could be due to the ability of fungi to form biosurfactants in order to tap the carbon source from the crude oil [
19,
20], and on the other hand, water surface always had access to air through shaking and the straw. As indicated in [
10], the water volume fell partly during the 14-day period. This means that the oil layer on the water was not completely closed, so an exchange of substances in both directions was possible.
However, the shaking of the beakers was not so strong that emulsions of the crude oil could form in water. Accordingly, the water was always clear and never cloudy during this study.
Figure 4 shows a comparison of the percent of the non-extractable oil versus the running time. Non-extractable oil is the difference between the theoretical extractable amount of oil, which is identical to the control sample, and the amount of oil actually determined. It can be seen that the amount of non-extractable oil in the straw increases over the course of 14 days, which has already been shown [
10]. This can probably also be assumed for the straw samples with immobilized microbes. Slow desorption kinetics due to the inner morphology of the straw was already assumed in [
10] to be the cause for the non-extractable amount.
However, it is also noted that after 14 days, the amount of non-extractable oil in the straw samples with microbes is a few percent higher than the respective comparative sample without microbes. Thus, microbes immobilized on the straw increase the sorption capacity of the straw, but only after 14 days.
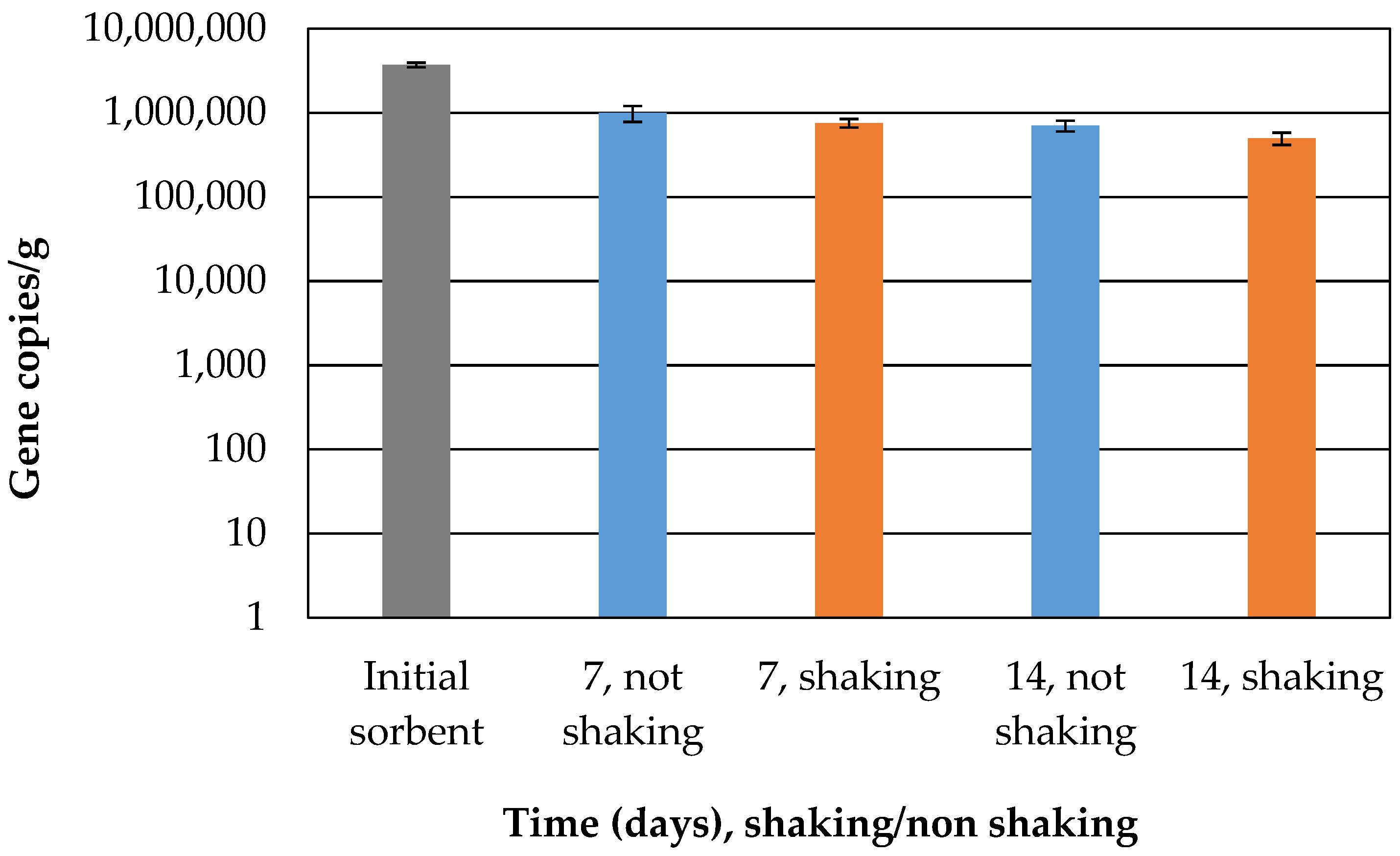
The number of cells of the fungal biomass in the sorbent was determined in order to be able to classify the time delay of the increase in the sorption capacity. The initial number decreased slightly from the initial amount (
Figure 5) but remained stable until the end of the experiment, indicating their potential activity in the presence of adsorbed crude oil. The natural microbial community present in the seawater used for the experiment might have oil-degrading microbes that can cooperate or compete with immobilized fungi. Oil-degrading bacteria could compete with fungi not only for petroleum hydrocarbon but also for other available nutrients [
21].
Despite the decrease in the number of cells, it can also be stated that the fungal community was not extinguished during the experiments.
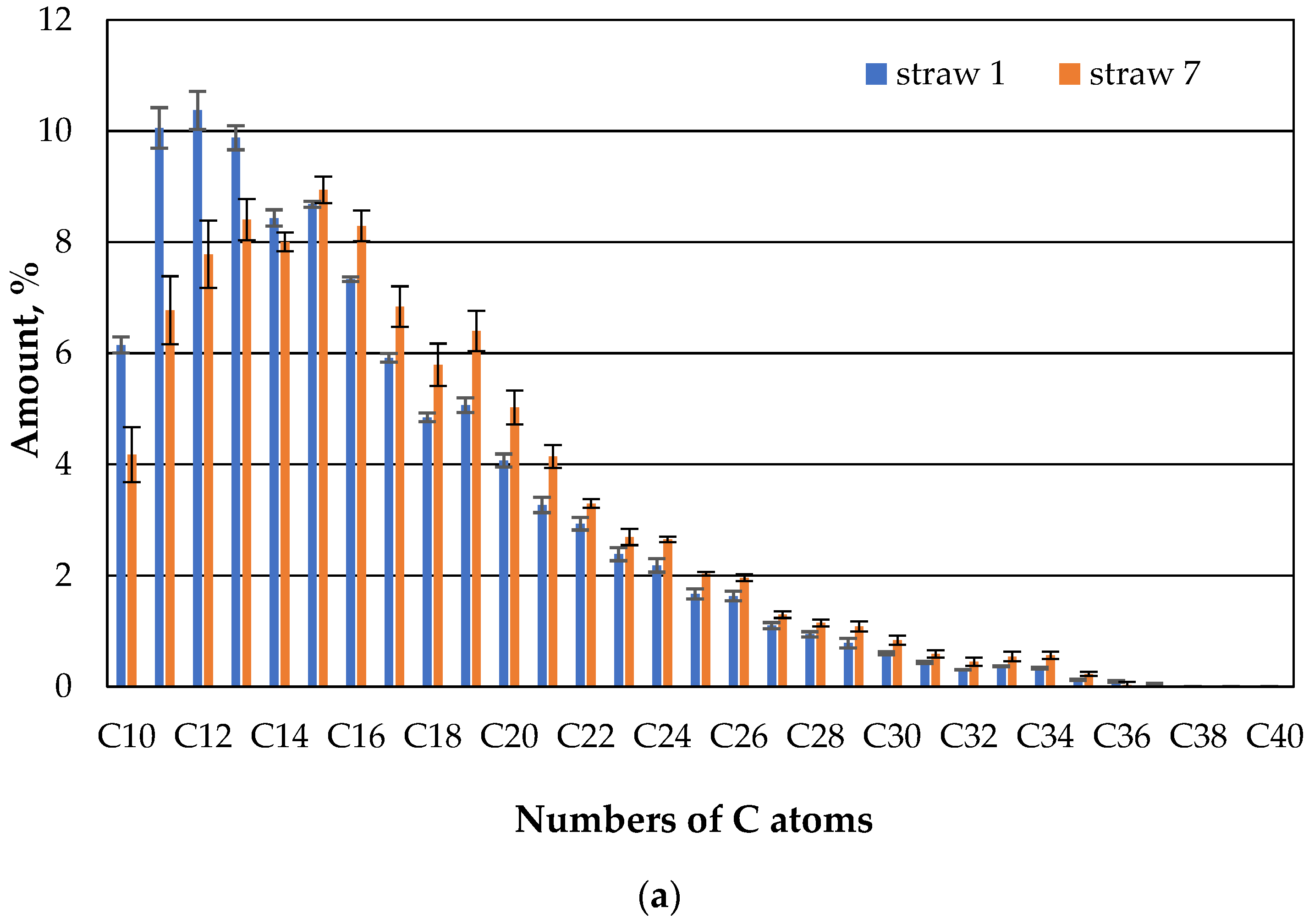
As already described in [
10], hydrophobization of the straw avoids wetting and partial sinking into the water. In this study, it was found that immobilization of the hydrophobized straw with microbes does not affect this behavior. The following figures show the composition of the extracted oil from the beaker which, apart from the water, only contains oil and the hydrophobic straw without fungi (
Figure 6).
As discussed in [
10], it was also found and confirmed here that the light fractions (hydrocarbon with a small number of carbon atoms) in the case of the straw without immobilized microbes already evaporated in the first week, and this composition hardly changed in the second week. It could be discussed here that the light fraction would have sorbed rather than evaporated, but then it should have reappeared after one or two weeks during the extraction due to the small straw pieces. This could not be confirmed here or in previously published research [
10].
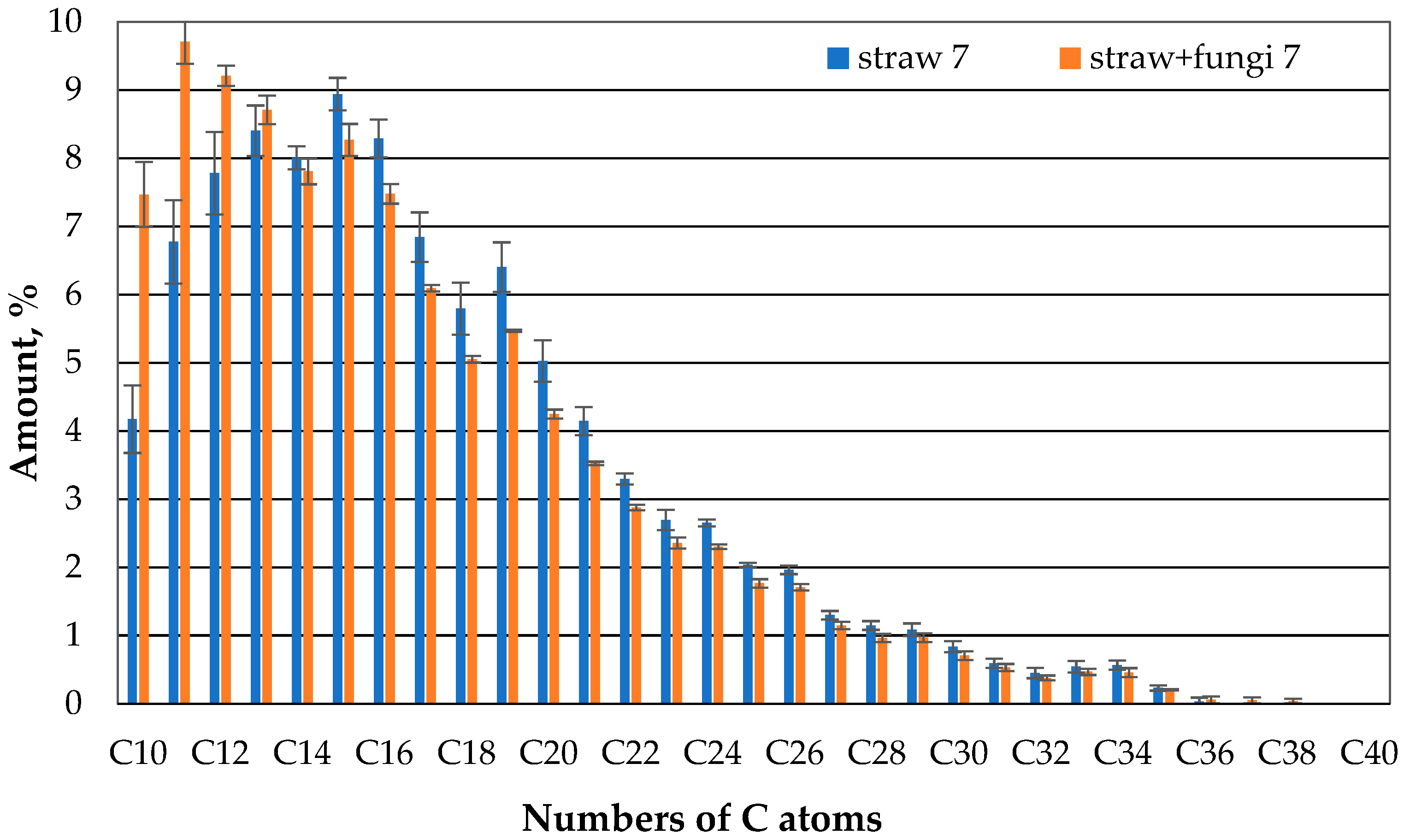
The comparison of the gas chromatographic composition of the oil of the straw with fungi and the straw without microbes after one day shows that clearly less oil evaporates in the case of the straw with microbes than in the case of the straw without microbes compared to the case of compounds with 10 or 11 carbon atoms (
Figure 7). There is no apparent difference between the straw with and without microbes for the higher hydrocarbons.
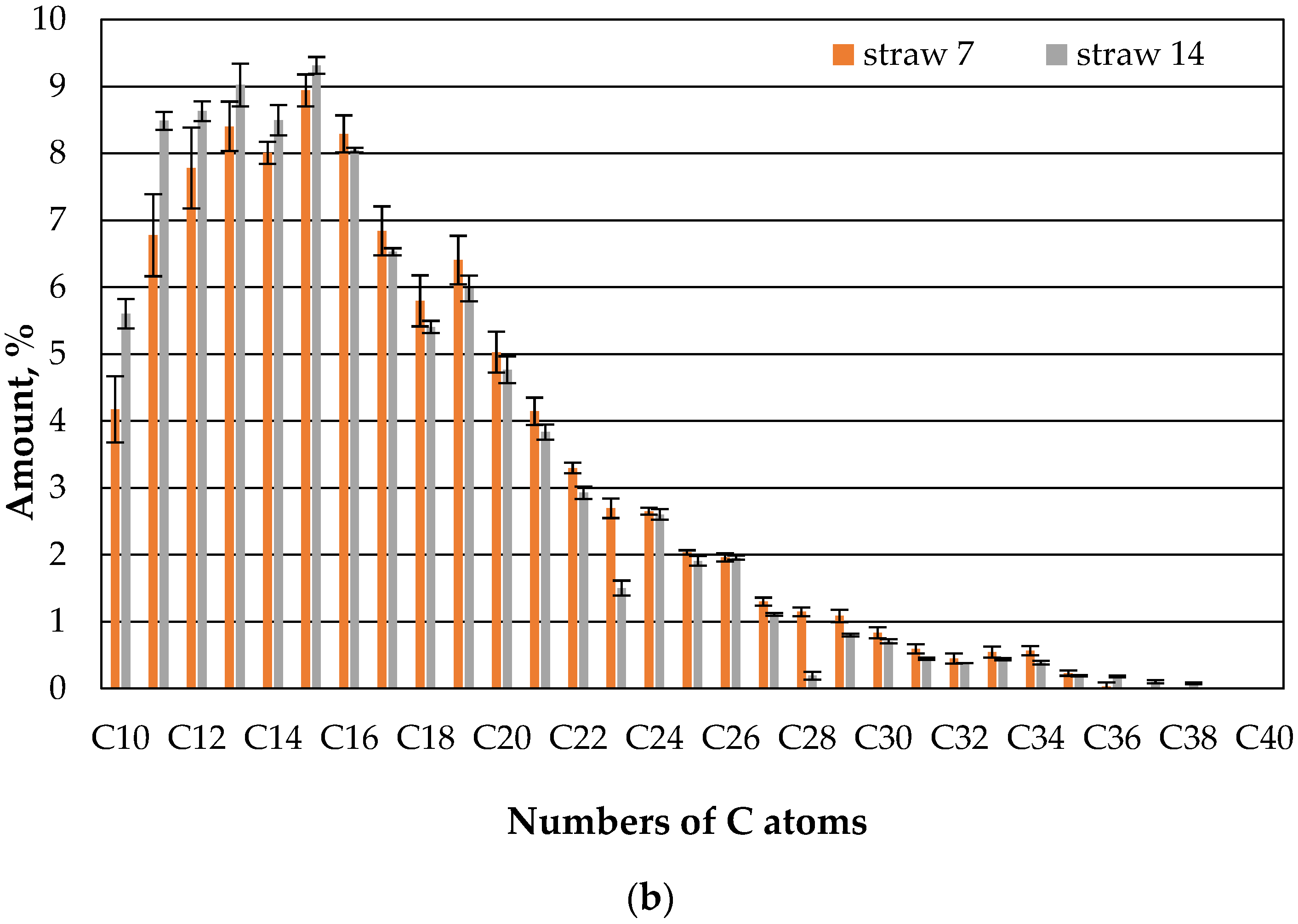
It appears that straw with microbes retains the fractions with low hydrocarbon chains to a greater extent than straw without microbes, even after 7 days (
Figure 8). In the higher KW chains, there is no such clear preference.
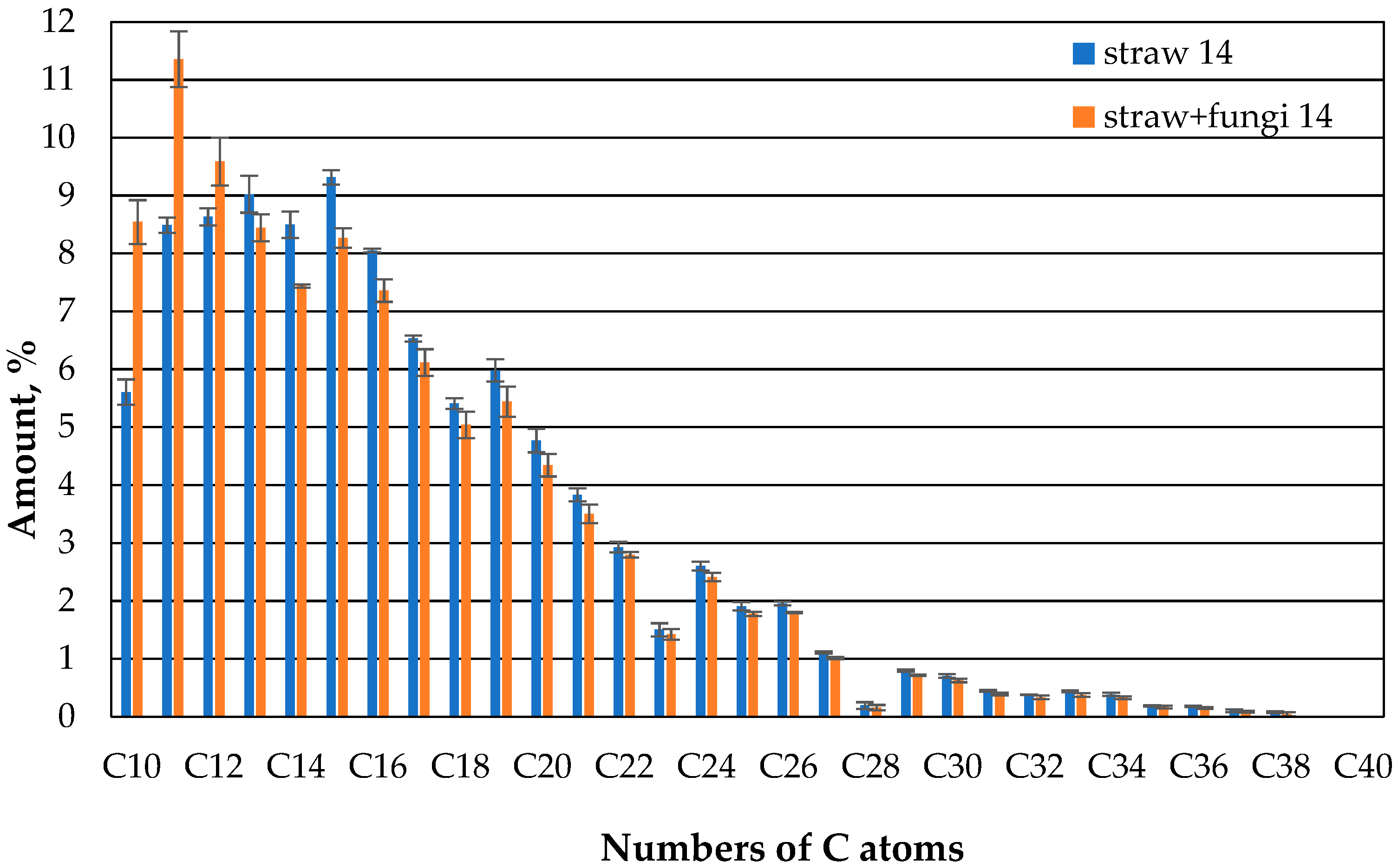
Fungi, therefore, appear to increase the sorption capacity of straw in relation to the hydrocarbons in the oil in the range of 10 to 13 C atoms. At the same time, the sorption capacity for the higher hydrocarbons was the same as that of straw without fungi. This could have to do with the higher diffusion speed of lower hydrocarbons. And it does not change almost in the second week (
Figure 9).
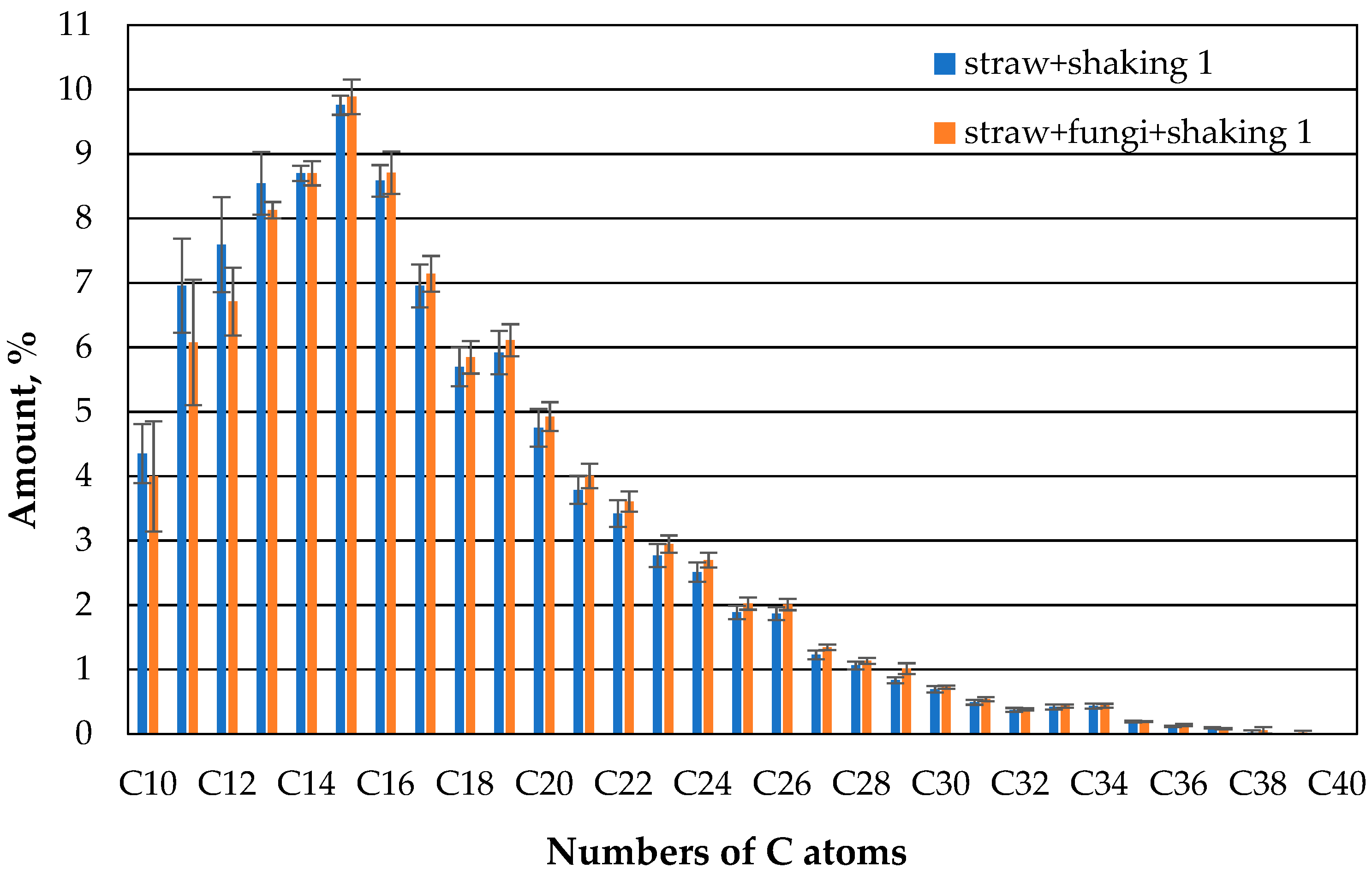
The behavior changes significantly if the beakers are additionally shaken (
Figure 10). On the first day, about the same amount of oil has evaporated from both the microbe-free and the microbe-containing beaker, and the composition of the oil that can still be extracted is about the same.
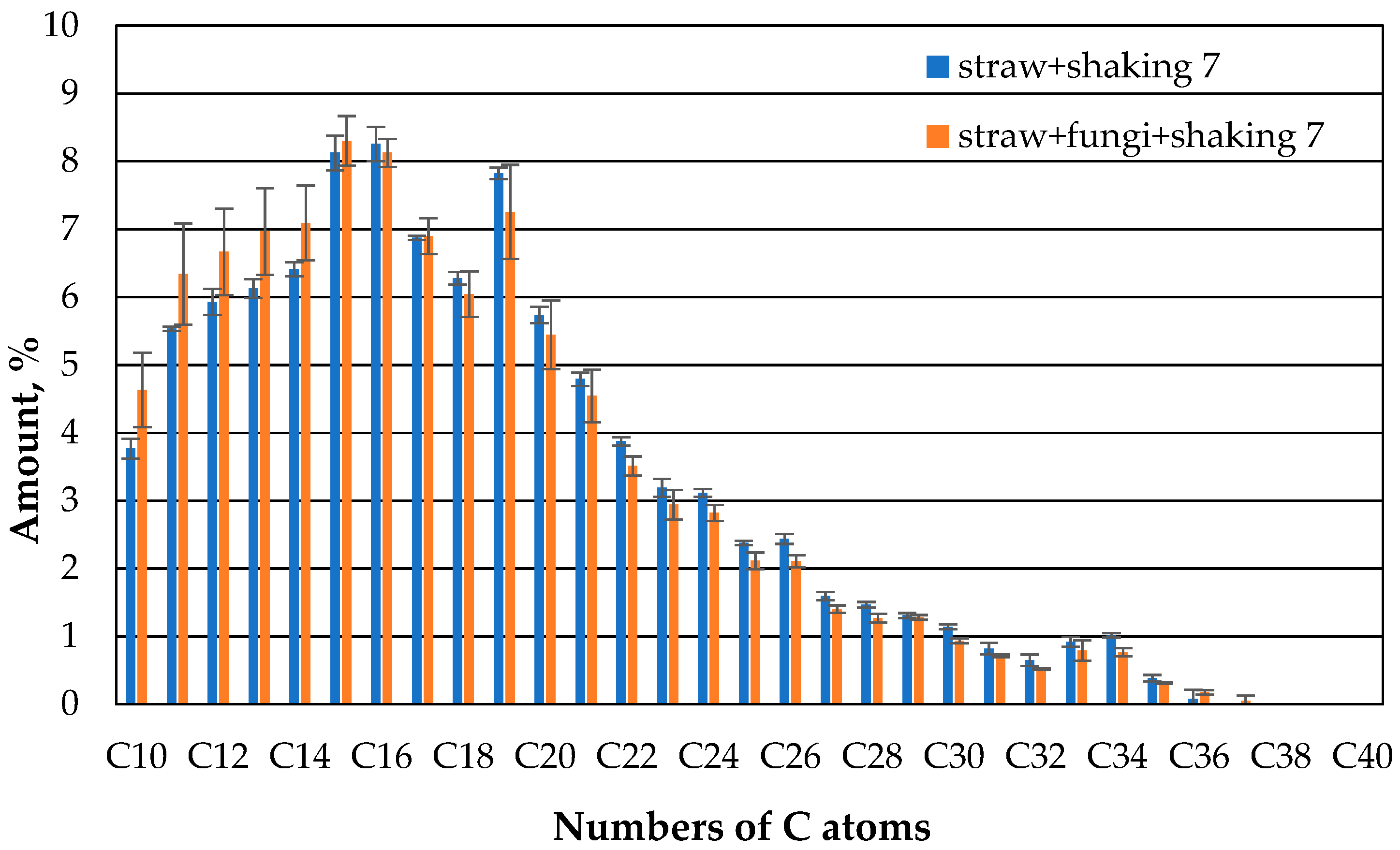
However, after 7 days, a little more oil in the range of the lower hydrocarbon chains up to about 14 carbon atoms can be extracted from the microbe-containing beakers (
Figure 11).
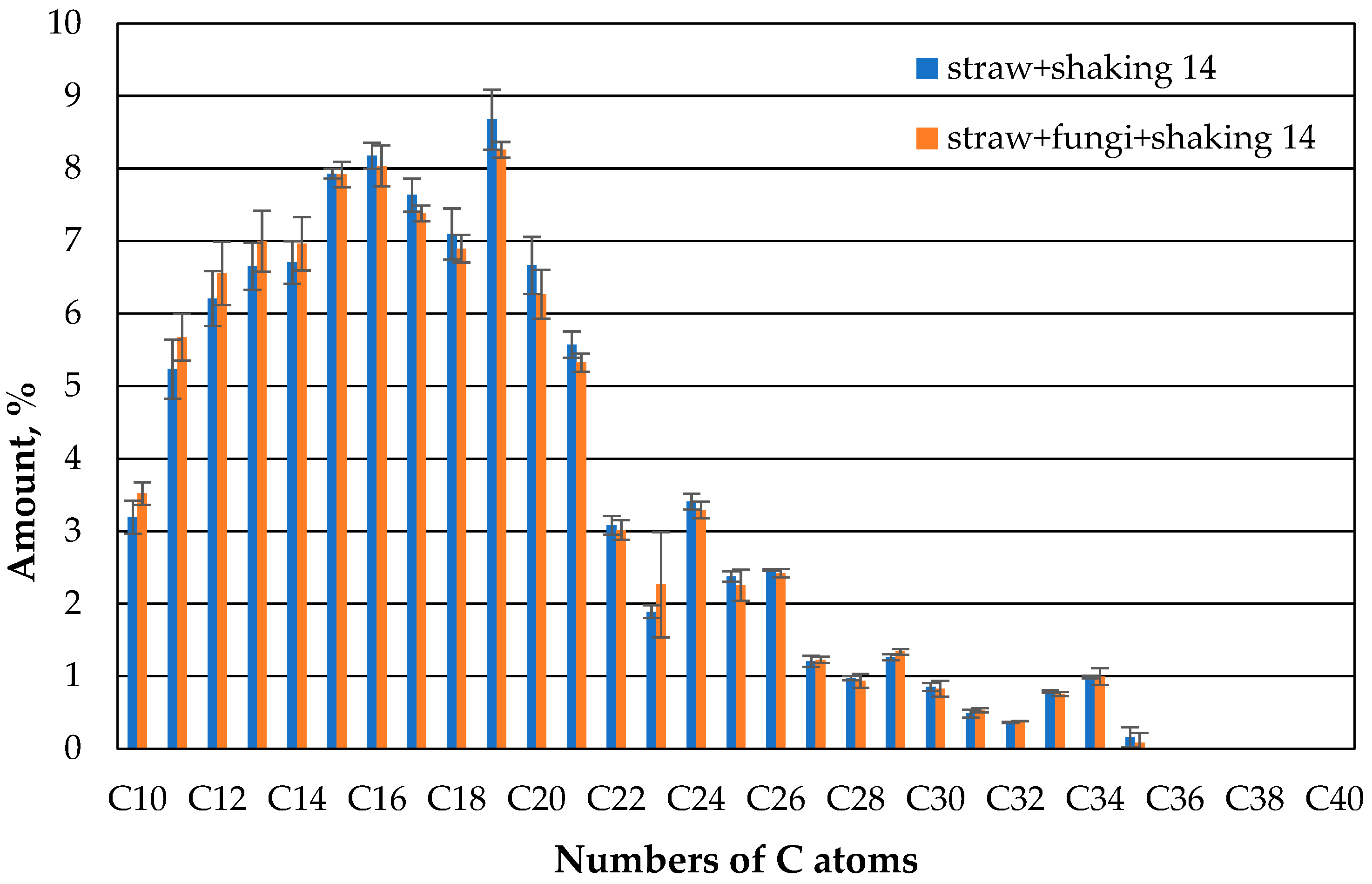
However, this changes again after 14 days (
Figure 12). Here, the KW distribution of the shaken straw fungi has matched the straw without fungi.
As shown in
Figure 12, the fraction of non-extractable oil is slightly higher in shaken straw fungi. The lower proportion of low HCs in the sample with the shaved straw with fungi compared to the unshaken sample could be attributed to the influence of the microbes. As already described in [
10], the straw enables mass transfer, including the transport of oxygen from the environment into the aqueous phase of the beaker. This would support the metabolism of the microbes. In the literature [
22,
23], a reason often cited as preventing microbes from getting into an oil spill is that the microbes lack enough oxygen during the oil spill.
4. Conclusions
The buoyancy of the straw was not affected by the immobilization of filamentous fungi. Sorbed oil still floats on the water during the 14-day test period.
The sorption kinetics of the oil appear to be similar in the straw with and without immobilized biomass and independent of beaker shaking. However, the fraction of non-extractable oil after 14 days is higher in the straw with fungi and shaking compared to without shaking. This could indicate the beginning of oil mining.
It cannot be ruled out that fungi will change and even shrink somewhat during this study. Based on this result, instead of leaving the straw with fungi and oil in the water, it might be beneficial to take it out and let the oil degradation and decomposition of the straw proceed on land. It is advantageous that the straw with fungi and oil is always floating on the water surface during the test.
The shaking and the straw protruding above the oil layer, favored by the sorption of the oil into the straw, allow the microbes to grow unhindered. The growth of the microbes initially immobilized on the straw cannot be confirmed, but a microbial community restructuring could also be questioned.