Abstract
The study of acoustic signals in aquatic animals contributes to developing new monitoring systems based on passive acoustics and improves our knowledge of their behaviors and ecology. Here, the sounds produced by the invasive species crayfish Cherax destructor and their possible role in intraspecific interactions are analyzed. Synchronized acoustic and video monitoring systems were used in a tank to record acoustic signals and associated behavioral events (tail flips, number of encounters, number of fights) and states (velocity and distance moved, angular velocity, duration of fighting and proximity). The crayfish were monitored in seven layouts combining males (M) and females (F) (F, M, FF, MM, MF, MMF, FFM). Both males and females produced two types of acoustic signals (high- and low-frequency sounds). Grouped animals produced fewer low-frequency sounds than single animals. In a grouped layout, more sounds were recorded when animals were in proximity (distance between two specimen less than 6 cm). In a single layout, sounds were not associated with a specific event or behavioral state. The number of signals emitted in the FF group and single M group were significantly higher than those in other layouts. Our study indicates that low-frequency sounds are produced non-accidentally and provide a baseline for future tests on intraspecific acoustic communication on this species. This study could help implement low-cost passive acoustic monitoring able to identify this species and the possible negative effect of its dispersion in a non-native environment.
1. Introduction
Today, passive acoustic monitoring (PAM) has become a widely used tool in various studies on behavior, conservation, invasive species identification and the anthropogenic impact of noise [1,2,3,4]. For potentially invasive species that emit sound, PAM allows for rapid identification, large-scale monitoring and reduced response times for management. Although visual signals are particularly used in aquatic environments, deep and murky waters reduce visibility very quickly and can negatively affect visual communication [5,6]. However, acoustic communication has advantages related to specific species, regardless of light conditions or transmission capacity for long distances. In fact, the production of acoustic signals allows for very efficient communication, especially for species that inhabit extreme or turbid habitats, making other communication systems inefficient [7].
Many species of aquatic vertebrates and invertebrates use acoustic signals in various contexts, such as mating [8,9,10,11], agonistic activities [12,13], group cohesion and coordination [14,15], socializing [16] and spatial orientation [17]. The importance of the acoustic environment has been revealed by several authors who evaluated the effects of noise pollution at biochemical and behavioral levels [18,19,20,21,22,23,24,25]. Many decapod crustaceans have been shown to be very adept at acoustic detection and communication [11,26,27]. Some species possess external structures are specialized in the production of sounds that generate a screech [28,29,30,31], snap [32,33] and carapace vibrations [34]. Although many crustaceans produce sounds, little is known of their hearing skills. A sound wave has two physical components, the pressure variations of the medium and the vibrations of water particles. Aquatic crustaceans are sensitive to the latter component. In crustaceans, sensory hairs on the body surface, internal chordal organs and statocysts can be stimulated by water vibrations [35]. Some authors [36] found that the external cuticular hairs of the Homarus americanus are probably responsible for sound detection and in Cherax destructor the sensory hairs are sensitive to water vibration frequencies [37]. The association between some specific behaviors and acoustic emission was described in some species of decapods. For example, in Palinuridae, tail flip movements were described to be related to the production of sounds in anti-predatory contexts, such as during encounters with octopus and after being caught [30,38,39]. In other species, i.e., Ovalipes catharus, Neohelice granulate and Ocypode platytarsus, the signals’ emission is related to courtship, post-mating guarding behavior and competition [11,31,40,41]. Although many studies have been conducted on marine decapod species, little is known about freshwater species. As of today, the acoustic production of Procambarus clarkii, Euastacus armatus and Faxonius limosus [42] are the only ones to have been studied. P. clarkii generates sound through the movement of the scaphognathite [43] and tends to emit signals during intraspecific interactions [39]. E. armatus produces audible sounds through abdominal stridulatory organs [44].
One of the most common and well-known freshwater decapods is the crayfish Cherax destructor, commonly referred to as a ‘yabby’. This species is able to tolerate variable physical and chemical conditions, allowing it to inhabit a wide range of aquatic habitats, including temporary and permanent systems [45,46]. Invasive species are more tolerant to water pollution, to the detriment of native species. Biodiversity loss is one of the consequences of pollution in freshwater systems. Biodiversity is often used as an indicator of water quality, but such indicators may be unreliable due to highly tolerant invasive species [47]. Invasive species can change their behavior depending on whether they are in their natural environment or in a new area; the invasive crayfish Pacifastacus leniusculus is more aggressive than the same species in their natural environment [48]. The species also adapts easily to artificial environments such as irrigation canals and reservoirs [49]. The yabby feeds on small arthropods or plants, burrowing and living alone in underwater burrows, which it defends against intruders [45,50]. Due to its ability to tolerate suboptimal water quality conditions, this species is considered an ideal candidate for freshwater aquaculture. Crayfish with aquaculture potential have been the targets of translocations. C. destructor is a crayfish native to South-Eastern Australia that has been introduced into aquaculture in many parts of Europe, raising concerns that it may become an invasive species and pose a threat to biodiversity [51,52]. C. destructor has colonized many areas of Australia and Europe, particularly Spain; natural colonies were reported in central Italy in 2009 [49,53,54], but information on the species in the wild is still scarce [55]. In light of this, it is important to study C. destructor’s behavior in order to implement effective monitoring actions. However, despite its wide distribution, few studies have been reported on this species and they are mostly focused on dominance behaviors [56,57,58]. Some authors [59] showed that C. destructor prefers to share resources rather than fight, displaying placid behavior in both inter- and intraspecific interactions. Sex and body mass are important factors in determining dominance between C. destructor and the other Cherax species, showing as larger animals tending to dominate during agonistic interactions [60]. As of today, no studies are available about the ability to produce sounds in this species. The aim of this work was to ascertain whether males and females of Cherax destructor emit acoustic signals and to understand whether these signals are associated with particular behavioral states or events.
2. Materials and Methods
2.1. Collection and Housing of Animals
The experiment was conducted at the Department of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF) of the University of Palermo from March to June 2021. The animals were supplied by the yabby aquaculture facility located in Fiumefreddo di Sicilia (Eastern Sicily).
For this study, 46 adult freshwater crayfish Cherax destructor (23 males and 23 females) aged 12 months were used. To determine the sex of each animal, the two female and male genital openings were checked in the ventral part; the female openings are located at the base of the third pair of legs and the male openings at the base of the fifth pair of legs. Carapace, claw and cephalothorax lengths were 10.35 ± 1.26 cm, 5.8 ± 0.99 cm, 5.34 ± 0.84 cm (mean ± SD), respectively. The animals were singularly housed in holding tanks (dimensions 35.6 × 23.4 × 22.8 cm) with continuous aeration O2 > 5.0 mg/L, constant temperature of 21° ± 1 °C and under a controlled photoperiod of 12/12 h of light and dark. They were fed daily with commercial diet (5% of body weight, Malta Cleyton, Mexico).
2.2. Experimental Setup
The study was divided into two different experimental settings: single experiments and group experiments (see Table 1). The single experiments consisted of two experimental layouts: 1 male (M) or 1 female (F). The group experiments consisted of five experimental layouts: (1) 1 female plus 1 female (FF); (2) 1 male plus 1 male (MM); (3) 1 male plus 1 female (MF); (4) 2 males plus 1 female (MMF); (5) 2 females plus 1 male (FFM). The setup and experimental conditions of the study are shown in Table 1. The experiments were conducted from 8 a.m. to 4 p.m. using two rectangular tanks of the same dimensions (85 × 50 × 45 cm, water height 26 cm): one used for control trials (no animals in the tank) and one used for test trials. The crayfish were not fed for 1 day before the start of the experimental phase. For the experiments, crayfish were randomly selected from the holding tanks and placed in the test tank (Figure 1). No shelters were present in the tanks. After an acclimatization period of 15 min, the behavior and acoustic signals emitted were filmed and recorded for 45 min. Animals in each setting (Table 1) were monitored for a total of 18 h and 45 min. Each crayfish was used only for one trial.

Table 1.
Layout of the experimental design and number of acoustic and video monitoring analyzed. M = male; F = female.
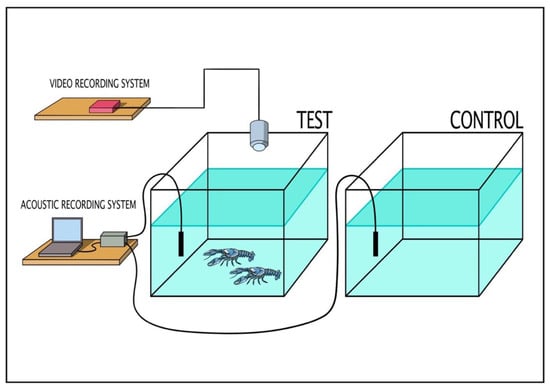
Figure 1.
Schematic representation of experimental tanks equipped with hydrophone and video camera.
2.3. Acoustic-Video Monitoring System and Analysis
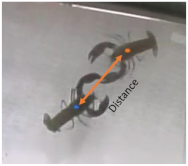
Behavior was recorded with a video camera placed on top of only the test tank. The video acquisition system consisted of an analogue video camera (ScubaLight, Mantova, Italy) placed in the top of the test tank (Figure 1). The camera was linked to a digital video recorder INCH H.264 LCD DVR (NTSC/PAL video system with H.264 video compression format, motion detection area 16 × 12 grid, hard disk storage accommodating 1 SATA HDD). Video files were saved in AVI format in the recorder and later exported via USB. The acoustic system was composed of two calibrated hydrophones simultaneously monitoring both the experimental and control tanks in order to avoid attributing external environmental noise or internal acquisition system noise to the species under investigation (Figure 1) [7]. The two calibrated hydrophones (model 8104, Bruel & Kjaer, Naerum, Denmark) had a sensitivity of −205.6 ± 4.0 dB re 1 V μPa in the 0.1 Hz–80 kHz frequency band (+4 dB and −12 dB in the frequency range of 0.1 Hz to 120 kHz). The hydrophones were connected to two synchronized channels of an analogue/digital acquisition board (USGH416HB Avisoft Bioacoustics, Berlin, Germany, set with a preamplification of 40 dB), managed by a dedicated software (Avisoft Recorder). The signals of both channels were acquired at 300 k samples s-1 at 16-bit resolution. The acoustic and video systems clocks were synchronized at every test. Moreover, to avoid any delay in time among the two clocks, at the start and the end of every test we produced an impulsive sound using two bars under the camera. The videos were analyzed using the software EthoVision XT 9.0 (Noldus Information Technology, Netherlands) in a semi-automated mode. The EthoVision system is able to distinguish and follow the subjects from the background based on their grayscale/brightness. To do this, an experimental arena needs to be set. The dimensions of the experimental arena were calibrated by taking the actual dimensions of the pool as a reference, using two calibration axes, one vertical and one horizontal.
Eleven behaviors (4 events and 7 states) were considered for this study:
- Behavioral events: acoustic signals, tail flips, encounters, fights;
- Behavioral states: fights’ duration, velocity of movement, distance moved, angular velocity, proximity.
The description of all behaviors is reported in Table 2.

Table 2.
Description of the events and behavioral states of Cherax destructor measured during the different trials. See also videos in the Supplementary Materials.
Velocity of movement, distance moved, angular velocity, proximity, walking and resting were measured by EthoVision with a temporal resolution of 2 s. Velocity of movement, distance moved and angular velocity were then summarized/averaged using a resolution of 120 s. Tail flips, encounters, fights and their duration were manually measured for each trial by an expert operator viewing the videos and noted the latter. To identify and quantify the sounds emitted by the yabby, all Waveform Audio File Format (WAV) were visualized using the spectrograms (1024-sample Hanning window, sample rate 300 k) and oscillogram (SASLab Pro 5.3.2-16 software, Avisoft Bioacoustcs, Germany) of both synchronized channels recorded from the test and the control tank. The signals were categorized in two groups based on their first peak of frequency (Buscaino et al., in preparation), assessed by visual inspection of spectrogram (frequency with maximum energy) (see Table 2). The acoustic signals were considered as behavioral events and the number of signals emitted every 120 s was summarized and divided by the number of specimens of each replicate. To couple sound, behavioral events and status, the behavior which was recorded in the same time interval as the detection of a sound characterized by a high signal-to-noise ratio was verified. Among the states, it was also verified whether the specimens were walking or resting during sound emission and if the specimens were in proximity or not. The proximity was considered 1 if the distance among the animals was <6 cm. The proximity was 0 if the distance was >6 cm. This measurement was calculated based on the length of the carapace and claws so that animals were in physical contact when the distance between their central points was less of 6 cm. For the proximity, we considered all the trials in each group (FF, MM, MF, FFM, MMF).
2.4. Statistical Analysis
Using the chi-square test, it can be seen that the distribution of the data did not show a normal distribution. Acoustic emissions, behavioral events and states, the differences between the two sexes (males vs. females), between test settings (singles vs. group) and the seven different layouts (M, F, FF, MM; MF; MMF; FFM) were investigated using the Mann–Whitney U-test (for two parameters comparison) and Kruskal–Wallis test (for multi-parameter comparisons). In the latter case, the post hoc multiple comparison test was applied. Moreover, differences in sound emission considering proximity condition were evaluated using the Mann–Whitney U-test. The results were considered statistically significant at p < 0.05. All statistical analyses were performed with STATISTICA 8.0 (StatSoft Europe, Hamburg, Germany).
3. Results
3.1. Acoustic Signals
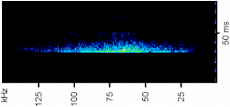
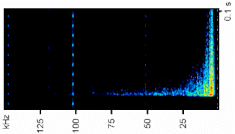
A total of 3929 sounds were recorded during the tests and they were distinguished in two classes, high- and low-frequency sounds (Figure 2). Specifically, we recorded 653 high-frequency sounds and 3276 low-frequency sounds (Table 3). The yabby of both sexes produces both impulsive signals (Figure 3). Grouped animals produced fewer low-frequency sounds than single animals (grouped animals 64.7 ± 36; single animals 109.6 ± 10 mean ± SE; Mann–Whitney U test: Z = 2.9, p < 0.001). In contrast, high-frequency sounds were mostly emitted by grouped animals rather than single animals (Mann–Whitney U test: Z = −5.4, p < 0.0001). Single males emitted significantly more low-frequency sounds than single females (Mann–Whitney U test: Z = −5.06, p < 0.001; Figure 3, Table 3). No significant differences were found in higher-frequency sounds between single males and females (Figure 3, Table 3). Comparing the low-frequency sounds between the different layouts, the results showed that the presence of two males with a female determined an increase of sounds emission compared to MF layout (Kruskal–Wallis multiple comparison test: df = 6, N = 997, Z = 4.9, p < 0.0001). In the FF groups, the specimens emitted a higher number of signals than all the other layouts (Kruskal–Wallis multiple comparison test p < 0.05) (Table S1). Considering the high-frequency sounds, the number of signals emitted in FFM group were significantly higher than the ones emitted in single layouts and FF group (Kruskal–Wallis multiple comparison test p < 0.05) (see Figure 3, Table S1).
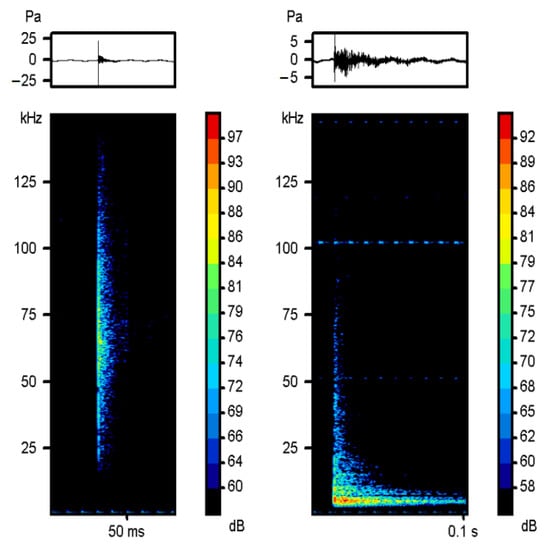
Figure 2.
Spectrograms of a high sound and low sound from a male. Spectrograms show frequency (kHz) vs. time (s), with intensity in dB re 1 μPa on color scale, 1024-sample Hanning window. The oscillogram is at the top. WAV files of these sounds are available in Supplementary Materials.

Table 3.
Total number of sounds and mean number (± standard error) per specimen and per replica recorded for test layout typology.
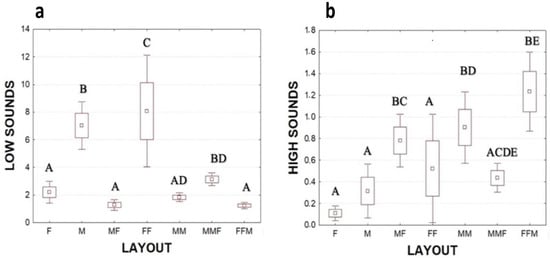
Figure 3.
Number of (a) low sounds and (b) high sounds produced in different layouts per specimen in 120 s. Showing mean ± 25th to 75th percentiles; error bars: mean ± 1.96*SE; the different letters indicate significant differences (p < 0.05) among the experimental layouts.
3.2. Behavioral Events and States
Considering all states and event variables, only angular velocity showed significantly higher values in males than in female animals (Mann–Whitney U test: Z = −2.2 p < 0.002) and in grouped animals compared to single animals (Mann–Whitney U test: Z = −6.5, p < 0.0001) (Figure 4). Comparing the behavioral states and events between the different layouts, the results of multiple comparisons are reported in Figure 4. In particular, the group FFM showed lower values in the distance moved (Kruskal–Wallis multiple comparison test p < 0.05,) and higher duration of fights compared to other layouts (Kruskal–Wallis multiple comparison test p < 0.05) (Figure 4, Table S1). The group FF showed higher values in velocity of movement and distance moved compared to single males (Kruskal–Wallis multiple comparison test p < 0.05; Table S1).
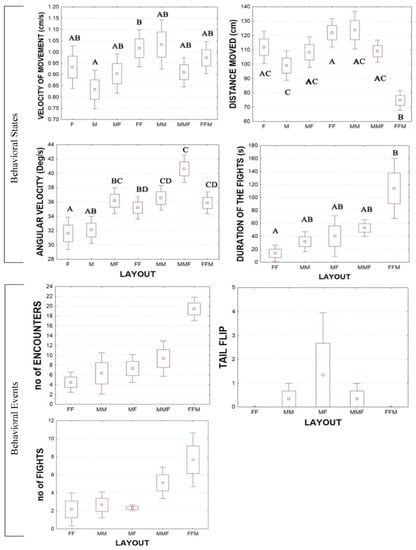
Figure 4.
Behavioral states and events in different layouts showing mean ± 25th to 75th percentiles (box) and error bars: mean ± 1.96*SE. The different letters indicate significant differences (p < 0.05) among the experimental layouts.
3.3. Acoustic and Behavior Association
The analysis of synchronized acoustic and video data did not show any particular behavioral event/state associated with sound emission. Individuals of both sexes in single layout emitted signals both while walking and in a resting state, and both while moving their claws and not. Animals in the groups emitted significantly more low-frequency sounds (Figure 5, Mann–Whitney U test p < 0.05 Z = −4.2) and high-frequency sounds (Figure 5, Mann–Whitney U test p < 0.05 Z = −3.8) when the distance between the individuals was less than 6 cm (proximity = 1). This result is stronger if we consider that 75% of our observations had proximity equal to 0.
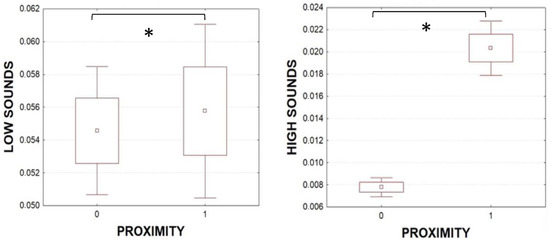
Figure 5.
Mean number of low (left) and high (right) sounds per specimen, (averaged on 2 s) in the groups, considering the proximity (proximity = 1 if distance ≤ 6 cm; proximity = 0 if distance is > 6 cm). Boxplot: mean ± 25th to 75th percentiles (box) and error bars: mean ± 1.96*SE. Mann–Whitney U test (* p < 0.05).
4. Discussion
This study shows that Cherax destructor produces acoustic signals of two different types, high- and low-peak frequency impulses. C. destructor has a highly developed sense of sight, but in the wild often lives in murky waters where the visibility is reduced very quickly and this can adversely affect visual communication [5], necessitating the use of non-visual techniques [50], such as chemical [62] or, likely, acoustic signals. When the crayfish are burrowed into mud or on land, they resort to chemical communication [63], but this may not be fully effective. As a consequence, visual and chemical communication might be complemented with the use of acoustic signals [64]. Moreover, C. destructor emitting more low-frequency sounds compared to high-frequency sounds could make the transmission of any information in the aquatic environment more effective, especially considering that sounds at lower frequency can travel for a longer distance than the higher-frequency sounds [65]. However, in decapod crustaceans little is known about their sensitivity to sound [26], and sound-based communication in C. destructor needs still be investigated with further studies.
We found that the males in the single M experiments emitted more low sounds than both the females from the single F experiments and in groups, with the exception of FF. Even if a possible communication role of sounds in this species has yet to be proven, in males they could serve in maintaining contact with other, not nearby conspecifics. Cherax destructor prefers to stay close to a familiar animal by using chemical signals or visual identification [66,67]. This hypothesis was previously proposed in lobsters [30], where their stridulations could be seen not only as an anti-predator strategy, but also as signals to warn conspecifics of the danger of predation or to potentially recruit other conspecifics to help them. In females, low-frequency signals were emitted more when they were in pairs, in the FF group. In this layout, they showed also the lowest values of encounters, fights, duration of fights and no tail flips. Mostly studies have been focused on the agonistic activities of males in crustaceans but females also tend to fight. It has been demonstrated that in crab species, males tend to fight to determine male dominance in the group, while females fight for food and shelter [68]. In C. destructor, the different behaviors of males and females was investigated by [69], with research finding that they showed different behaviors during social interactions: females mainly relied on body signals (unreliable signals) to resolve disputes, as opposed to males of the same species, who preferred to opt for fighting. The emission of acoustic signals in C. destructor could be used by females as an indirect way of establishing dominance and avoiding injuring interaction. It has been seen how, thanks to indirect signals exchanged between crayfish conspecifics, dominant or subordinate animals can avoid injury by reducing the number of competitive interactions or the intensity of conflicts [62]. There are no external changes that indicate receptivity in Cherax females [70], but the presence of the eggs in the weeks following the experiments indicates that they could be receptive and suggests the role of the high number of sounds produced.
High-frequency sounds did not tend to be emitted differently depending on sex, but were emitted more if the animals were in groups and in close proximity. These signals were fewer compared to the low-frequency sounds and could be emitted accidently; however, further studies could help to explain their possible role in the ecology of this species.
Considering the behavioral events in different layouts, no significant differences were found. The duration of fights was longer in the FFM than FF groups. Moreover, in the FFM groups, the number of encounters and fights was higher compared to in other layouts (although there is no statistical significance, a difference could emerge by increasing replicas). In the behavioral states, the angular velocity was higher in the grouped animals than singles. Among crayfish, the interactions of a third crayfish have implications for hierarchies and social behavior [71]. In this arrangement, the animals also emitted more high-frequency sounds. In our data, the agonistic approaches were stronger if two females and a male were present; no tail flips were performed during the fights, probably because no clear hierarchy was formed [72].
The groups MMF did not show any particular differences compared to the other layouts; no increase of the fights number or the duration of the fights, or in sound emissions. These results suggest that these males did not compete for females. In contrast, in other decapod species, previous research demonstrated an increase of agonistic interactions when two males were in the presence of a female. For example, when the crabs Ovalipes trimaculatus and Neohelice granulata were put into groups consisting of two males and one female, an increase in motility parameters was observed depending on the receptivity state of female [10,11].
The comparison among the low-sounds emission events and the synchronized behavior events and states did not reveal a specific movement/mechanism of sound emission. C. destructor sound emission probably involves structures not monitored by the camera, such as the mouth, missiles, organs imputed for this purpose or other mechanisms. The low sounds are emitted both when the animals are moving and when they are resting, suggesting that the production of sounds is not, or to a reduced extent, determined by the organs imputed to movement. In the freshwater decapod P. clarkii, sound is generated by the movement of the scaphognathite up and down within the chamber formed by the efferent gill channel [43]. The freshwater Euastacus armatus also produces audible sounds through abdominal stridulatory organs [44]. In our study, the video camera on top of the tank and the resolution of the videos was not high enough to detect a specific movement suggesting that the sound emission should be internal or localized in the ventral part of the body. Moreover, the sounds were emitted both when the animals were moving and when they were resting, suggesting that the production of sounds was not determined by the organs imputed to movement, or if it was, this was to a reduced extent. In other decapod crustaceans such as lobsters and P. clarkii [30,39], tail flips are associated with the production of sounds. However, in this work, no correlation was found between tail flip events and acoustic emission.
5. Conclusions
C. destructor emits acoustic signals (about 2 sound per minutes, see Table 3). Considering animals modify their behavior in the presence of a conspecific or in response to its signal, which can be chemical, postural, or acoustic, we could hypothesize that this species uses these cues to communicate with conspecifics, to court, to try to keep the group cohesive and to avoid fights. Specific studies should be carried out to test if sounds can be used to communicate. These results, within a further study on acoustic characterization of C. destructor sounds in the laboratory and natural environment, could help to implement low-cost passive acoustic monitoring able to identify this species and the possible negative effect of its dispersion in a non-native environment [73].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse11061147/s1, Table S1: K–W multiple comparisons z’ values; Video S1: Velocity layout F; Video S2: Fight layout FF; Video S3: tail flip layout MF; Audio S1: High sound; Audio S2: Low sound.
Author Contributions
Conceptualization, C.D.V., G.B. and M.V.; methodology, C.D.V., G.B., M.V., M.M. and M.C.; software, C.D.V. and G.B.; validation, G.B., M.C., M.V., V.A. and M.A.; formal analysis, C.D.V., G.B. and M.C.; investigation, C.D.V., M.M., G.B. and M.C.; resources, M.A. and V.A.; data curation, C.D.V. and G.B.; writing—original draft preparation, C.D.V., G.B., M.V., M.C. and M.M.; writing—review and editing, all authors; visualization, C.D.V., G.B. and M.C.; supervision, G.B., M.V., V.A. and M.A.; project administration, G.B. and M.V.; funding acquisition, M.A. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study performed on a freshwater invertebrate other than cephalopods (shrimp) which do not require ethical review and approval according to EU directive 2010/63/EU (22 September 2010).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We express our thanks to Giampaolo Badalamenti and Pietro Chirco for their collaboration in the management of the animals used in the experiments. Additionally, POFEAMP Project (02/INA/17, PO-FEAMP 2014–2020—CUP: B76C18000910006) of the Sicily Region, and A. Pulizzi (Dipartimento Pesca Mediterranea—Regione Siciliana, Italy) are acknowledged for the support provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buscaino, G.; Ceraulo, M.; Pieretti, N.; Corrias, V.; Farina, A.; Filiciotto, F.; Maccarrone, V.; Grammauta, R.; Caruso, F.; Alonge, G.; et al. Temporal patterns in the soundscape of the shallow waters of a Mediterranean marine protected area. Sci. Rep. 2016, 6, 34230. [Google Scholar] [CrossRef]
- Caruso, F.; Alonge, G.; Bellia, G.; De Domenico, E.; Grammauta, R.; Larosa, G.; Mazzola, S.; Riccobene, G.; Pavan, G.; Papale, E.; et al. Long-Term Monitoring of Dolphin Biosonar Activity in Deep Pelagic Waters of the Mediterranean Sea. Sci. Rep. 2017, 7, 4321. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.M.; Humphrey, S.R. Passive acoustic monitoring shows no effect of anthropogenic noise on acoustic communication in the invasive round goby (Neogobius melanostomus). Freshw. Biol. 2018, 65, 66–74. [Google Scholar] [CrossRef]
- Gregorietti, M.; Papale, E.; Ceraulo, M.; de Vita, C.; Pace, D.S.; Tranchida, G.; Mazzola, S.; Buscaino, G. Acoustic Presence of Dolphins through Whistles Detection in Mediterranean Shallow Waters. J. Mar. Sci. Eng. 2021, 9, 78. [Google Scholar] [CrossRef]
- Abrahams, M.V.; Kattenfeld, M.G. The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behav. Ecol. Sociobiol. 1997, 40, 169–174. [Google Scholar] [CrossRef]
- Frommen, J.G. Aggressive communication in aquatic environments. Funct. Ecol. 2019, 34, 364–380. [Google Scholar] [CrossRef]
- Buscaino, G.; Ceraulo, M.; Canale, D.; Papale, E.; Marrone, F. First evidence of underwater sounds emitted by the living fossils Lepidurus lubbocki and Triops cancriformis (Branchiopoda: Notostraca). Aquat. Biol. 2021, 30, 101–112. [Google Scholar] [CrossRef]
- Salmon, M.; Atsaides, S.P. Visual and Acoustical Signalling during Courtship by Fiddler Crabs (GenusUca). Am. Zool. 1968, 8, 623–639. [Google Scholar] [CrossRef]
- Amorim, M.C.P.; Vasconcelos, R.O. Variability in the mating calls of the Lusitanian toadfish Halobatrachus didactylus: Cues for potential individual recognition. J. Fish Biol. 2008, 73, 1267–1283. [Google Scholar] [CrossRef]
- Buscaino, G.; Gavio, A.; Galvan, D.; Filiciotto, F.; Maccarrone, V.; de Vincenzi, G.; Mazzola, S.; Orensanz, J. Acoustic signals and behaviour of Ovalipes trimaculatus in the context of reproduction. Aquat. Biol. 2015, 24, 61–73. [Google Scholar] [CrossRef]
- Filiciotto, F.; Moyano, M.P.S.; Hidalgo, F.; de Vincenzi, G.; Bazterrica, M.C.; Ceraulo, M.; Corrias, V.; Quinci, E.M.; Lorusso, M.; Mazzola, S.; et al. Underwater acoustic communication during the mating behaviour of the semi-terrestrial crab Neohelice granulata. Sci. Nat. 2019, 106, 35. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.M.; Duarte, I.G.; Fonseca, P.J.; Turner, G.F.; Amorim, M.C. Courtship and agonistic sounds by the cichlid fish Pseudotropheus zebra. J. Acoust. Soc. Am. 2008, 124, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Ceraulo, M.; Moyano, M.P.S.; Bazterrica, M.C.; Hidalgo, F.J.; Snitman, S.; Papale, E.; Buscaino, G.; Gavio, M.A. Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters. J. Mar. Sci. Eng. 2022, 10, 1370. [Google Scholar] [CrossRef]
- Herzing, D. Synchronous and Rhythmic Vocalizations and Correlated Underwater Behavior of Free-ranging Atlantic Spotted Dolphins (Stenella frontalis) and Bottlenose Dolphins (Tursiops truncatus) in the Bahamas. Anim. Behav. Cogn. 2015, 2, 14–29. [Google Scholar] [CrossRef]
- Van Oosterom, L.; Montgomery, J.C.; Jeffs, A.G.; Radford, C.A. Evidence for contact calls in fish: Conspecific vocalisations and ambient soundscape influence group cohesion in a nocturnal species. Sci. Rep. 2016, 6, 19098. [Google Scholar] [CrossRef]
- Papale, E.; Fanizza, C.; Buscaino, G.; Ceraulo, M.; Cipriano, G.; Crugliano, R.; Grammauta, R.; Gregorietti, M.; Renò, V.; Ricci, P.; et al. The Social Role of Vocal Complexity in Striped Dolphins. Front. Mar. Sci. 2020, 7, 584301. [Google Scholar] [CrossRef]
- Jeffs, A.; Tolimieri, N.; Montgomery, J.C. Crabs on cue for the coast: The use of underwater sound for orientation by pelagic crab stages. Mar. Freshw. Res. 2003, 54, 841–845. [Google Scholar] [CrossRef]
- Celi, M.; Filiciotto, F.; Parrinello, D.; Buscaino, G.; Damiano, A.; Cuttitta, A.; D’Angelo, S.; Mazzola, S.; Vazzana, M. Physiological and agonistic behavioural response of Procambarus clarkii to an acoustic stimulus. J. Exp. Biol. 2012, 216, 709–718. [Google Scholar] [CrossRef]
- Celi, M.; Filiciotto, F.; Vazzana, M.; Arizza, V.; Maccarrone, V.; Ceraulo, M.; Mazzola, S.; Buscaino, G. Shipping noise affecting immune responses of European spiny lobster (Palinuruselephas). Can. J. Zool. 2015, 93, 113–121. [Google Scholar] [CrossRef]
- Filiciotto, F.; Vazzana, M.; Celi, M.; Maccarrone, V.; Ceraulo, M.; Buffa, G.; Di Stefano, V.; Mazzola, S.; Buscaino, G. Behavioural and biochemical stress responses of Palinurus elephas after exposure to boat noise pollution in tank. Mar. Pollut. Bull. 2014, 84, 104–114. [Google Scholar] [CrossRef]
- Filiciotto, F.; Vazzana, M.; Celi, M.; Maccarrone, V.; Ceraulo, M.; Buffa, G.; Arizza, V.; de Vincenzi, G.; Grammauta, R.; Mazzola, S.; et al. Underwater noise from boats: Measurement of its influence on the behaviour and biochemistry of the common prawn (Palaemon serratus, Pennant 1777). J. Exp. Mar. Biol. Ecol. 2016, 478, 24–33. [Google Scholar] [CrossRef]
- Vazzana, M.; Ceraulo, M.; Mauro, M.; Papale, E.; Dioguardi, M.; Mazzola, S.; Arizza, V.; Chiaramonte, M.; Buscaino, G. Effects of acoustic stimulation on biochemical parameters in the digestive gland of Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819). J. Acoust. Soc. Am. 2020, 147, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Mauro, M.; Ceraulo, M.; Dioguardi, M.; Papale, E.; Mazzola, S.; Arizza, V.; Beltrame, F.; Inguglia, L.; Buscaino, G. Underwater high frequency noise: Biological responses in sea urchin Arbacia lixula (Linnaeus, 1758). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110650. [Google Scholar] [CrossRef]
- Mauro, M.; Pérez-Arjona, I.; Perez, E.J.B.; Ceraulo, M.; Bou-Cabo, M.; Benson, T.; Espinosa, V.; Beltrame, F.; Mazzola, S.; Vazzana, M.; et al. The effect of low frequency noise on the behaviour of juvenile Sparus aurata. J. Acoust. Soc. Am. 2020, 147, 3795–3807. [Google Scholar] [CrossRef]
- Solé, M.; Kaifu, K.; Mooney, T.A.; Nedelec, S.L.; Olivier, F.; Radford, A.N.; Vazzana, M.; Wale, M.A.; Semmens, J.M.; Simpson, S.D.; et al. Marine invertebrates and noise. Front. Mar. Sci. 2023, 10, 1129057. [Google Scholar] [CrossRef]
- Popper, A.N.; Salmon, M.; Horch, K.W. Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A 2001, 187, 83–89. [Google Scholar] [CrossRef]
- Nakamachi, T.; Ishida, H.; Hirohashi, N. Sound Production in the Aquatic Isopod Cymodoce japonica (Crustacea: Peracarida). Biol. Bull. 2015, 229, 167–172. [Google Scholar] [CrossRef]
- Guinot-Dumortier, D.; Dumortier, B. La Stridulation Chez Les Crabes. Crustaceana 1960, 1, 117–155. [Google Scholar] [CrossRef]
- Boon, P.; Yeo, D.; Todd, P. Sound production and reception in mangrove crabs Perisesarma spp. (Brachyura: Sesarmidae). Aquat. Biol. 2009, 5, 107–116. [Google Scholar] [CrossRef]
- Buscaino, G.; Filiciotto, F.; Gristina, M.; Bellante, A.; Buffa, G.; Di Stefano, V.; Maccarrone, V.; Tranchida, G.; Mazzola, S. Acoustic behaviour of the European spiny lobster Palinurus elephas. Mar. Ecol. Prog. Ser. 2011, 441, 177–184. [Google Scholar] [CrossRef]
- Moyano, M.P.S.; Ceraulo, M.; Mazzola, S.; Buscaino, G.; Gavio, M.A. Sound production mechanism in the semiterrestrial crab Neohelice granulata (Brachyura, Varunidae). J. Acoust. Soc. Am. 2019, 146, 3466–3474. [Google Scholar] [CrossRef] [PubMed]
- Au, W.W.L.; Banks, K. The acoustics of the snapping shrimp Synalpheus parneomeris in Kaneohe Bay. J. Acoust. Soc. Am. 1998, 103, 41–47. [Google Scholar] [CrossRef]
- Versluis, M.; Schmitz, B.; von der Heydt, A.; Lohse, D. How Snapping Shrimp Snap: Through Cavitating Bubbles. Science 2000, 289, 2114–2117. [Google Scholar] [CrossRef]
- Henninger, H.P.; Watson, W.H. Mechanisms underlying the production of carapace vibrations and associated waterborne sounds in the American lobster, Homarus americanus. J. Exp. Biol. 2005, 208, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, T.; Tautz, J. The Sensitivity of Crayfish Mechanoreceptors to Hydrodynamic and Acoustic Stimuli. In Frontiers in Crustacean Neurobiology; Wiese, K., Krenz, W.-D., Tautz, J., Reichert, H., Mulloney, B., Eds.; Birkhäuser: Basel, Switzerland, 1990; pp. 114–120. [Google Scholar] [CrossRef]
- Jézéquel, Y.; Jones, I.T.; Bonnel, J.; Chauvaud, L.; Atema, J.; Mooney, T.A. Sound detection by the American lobster (Homarus americanus). J. Exp. Biol. 2021, 224, jeb240747. [Google Scholar] [CrossRef]
- Tautz, J.; Sandeman, D.C. The Detection of Waterborne Vibration by Sensory Hairs on the Chelae of the Crayfish. J. Exp. Biol. 1980, 88, 351–356. [Google Scholar] [CrossRef]
- Bouwma, P.E.; Herrnkind, W.F. Sound production in Caribbean spiny lobster Panulirus argus and its role in escape during predatory attack by Octopus briareus. N. Z. J. Mar. Freshw. Res. 2009, 43, 3–13. [Google Scholar] [CrossRef]
- Buscaino, G.; Filiciotto, F.; Buffa, G.; Di Stefano, V.; Maccarrone, V.; Buscaino, C.; Mazzola, S.; Alonge, G.; D’angelo, S. The underwater acoustic activities of the red swamp crayfish Procambarus clarkii. J. Acoust. Soc. Am. 2012, 132, 1792–1798. [Google Scholar] [CrossRef]
- Clayton, D. Singing and dancing in the ghost crab Ocypode platytarsus (Crustacea, Decapoda, Ocypodidae). J. Nat. Hist. 2008, 42, 141–155. [Google Scholar] [CrossRef]
- Flood, A.S.; Goeritz, M.L.; Radford, C.A. Sound production and associated behaviours in the New Zealand paddle crab Ovalipes catharus. Mar. Biol. 2019, 166, 162. [Google Scholar] [CrossRef]
- Breithaupt, T. Sound Perception in Aquatic Crustaceans. In The Crustacean Nervous System; Wiese, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 548–558. [Google Scholar] [CrossRef]
- Favaro, L.; Tirelli, T.; Gamba, M.; Pessani, D. Sound production in the red swamp crayfish Procambarus clarkii (Decapoda: Cambaridae). Zoöl. Anz. A J. Comp. Zool. 2011, 250, 143–150. [Google Scholar] [CrossRef]
- Sandeman, D.C.; Wilkens, L.A. Sound Production by Abdominal Stridulation in the Australian Murray River Crayfish, Euastacus Armatus. J. Exp. Biol. 1982, 99, 469–472. [Google Scholar] [CrossRef]
- Reynolds, K.M. Aspects of the Biology of the Freshwater Crayfish Cherax Destructor in Farm Dams in Far-Western NSW; University of New South Wales: Sydney, NSW, Australia, 1980. [Google Scholar] [CrossRef]
- Austin, C.; Knott, B. Systematics of the Freshwater Crayfish Genus Cherax Erichson (Decapoda: Parastacidae) in South-Western Australia: Electrophoretic, Morphological and Habitat Variation. Aust. J. Zool. 1996, 44, 223–258. [Google Scholar] [CrossRef]
- MacNeil, C.; Boets, P.; Platvoet, D. ‘Killer Shrimps’, Dangerous Experiments and Misguided Introductions: How Freshwater Shrimp (Crustacea: Amphipoda) Invasions Threaten Biological Water Quality Monitoring in the British Isles. Freshw. Rev. 2012, 5, 21–35. [Google Scholar] [CrossRef]
- Pintor, L.M.; Sih, A.; Bauer, M.L. Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 2008, 117, 1629–1636. [Google Scholar] [CrossRef]
- Beatty, S.; Morgan, D.; Gill, H. Role of Life History Strategy in the Colonisation of Western Australian Aquatic Systems by the Introduced Crayfish Cherax destructor Clark, 1936. Hydrobiologia 2005, 549, 219–237. [Google Scholar] [CrossRef]
- Basil, J.; Sandeman, D. Crayfish (Cherax destructor) use Tactile Cues to Detect and Learn Topographical Changes in Their Environment. Ethology 2000, 106, 247–259. [Google Scholar] [CrossRef]
- McNeely, J. Invasive species: A costly catastrophe for native biodiversity. Land Use Water Resour. Res. 2001, 2, 1–10. [Google Scholar]
- Lynas, J.; Lindhjem, P.; Storey, A.; Knott, B. Is the Yabby, Cherax Destructor (Parastacidae) in Western Australia an Ecological Threat? Freshwater Crayfish; International Association of Astacology: Hinterthal, Austria, 2004. [Google Scholar]
- Holdich, D.; Reynolds, J.; Souty-Grosset, C.; Sibley, P. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 11. [Google Scholar] [CrossRef]
- Coughran, J.; McCormack, R.; Daly, G. Translocation of the Yabby Cherax destructor into eastern drainages of New South Wales, Australia. Aust. Zool. 2009, 35, 100–103. [Google Scholar] [CrossRef]
- Vecchioni, L.; Marrone, F.; Chirco, P.; Arizza, V.; Tricarico, E.; Arculeo, M. An update of the known distribution and status of Cherax spp. in Italy (Crustacea, Parastacidae). BioInvasions Rec. 2022, 11, 1045–1055. [Google Scholar] [CrossRef]
- Baird, H.P.; Patullo, B.W.; Macmillan, D.L. Reducing aggression between freshwater crayfish (Cherax destructor Clark: Decapoda, Parastacidae) by increasing habitat complexity. Aquac. Res. 2006, 37, 1419–1428. [Google Scholar] [CrossRef]
- Patullo, B.W.; Baird, H.P.; Macmillan, D.L. Altered aggression in different sized groups of crayfish supports a dynamic social behaviour model. Appl. Anim. Behav. Sci. 2009, 120, 231–237. [Google Scholar] [CrossRef]
- Mauro, M.; Arizza, V.; Arculeo, M.; Attanzio, A.; Pinto, P.; Chirco, P.; Badalamenti, G.; Tesoriere, L.; Vazzana, M. Haemolymphatic Parameters in Two Aquaculture Crustacean Species Cherax destructor (Clark, 1836) and Cherax quadricarinatus (Von Martens, 1868). Animals 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Balcombe, S.; Capon, S.; Cockayne, B. Do opposites attack? Resource interactions between an alien and native crayfish from the Lake Eyre Basin. Mar. Freshw. Res. 2022, 73, 873–883. [Google Scholar] [CrossRef]
- Lynas, J.; Storey, A.W.; Knott, B. Aggressive interactions between three species of freshwater crayfish of the genusCherax(Decapoda: Parastacidae). Mar. Freshw. Behav. Physiol. 2007, 40, 105–116. [Google Scholar] [CrossRef]
- Bergman, D.A.; Moore, P.A. Field Observations of Intraspecific Agonistic Behavior of Two Crayfish Species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 2003, 205, 26–35. [Google Scholar] [CrossRef]
- Moore, P.A. The Smell of Success and Failure: The Role of Intrinsic and Extrinsic Chemical Signals on the Social Behavior of Crayfish. Integr. Comp. Biol. 2005, 45, 650–657. [Google Scholar] [CrossRef]
- Bergman, D.A.; Moore, P.A. The Role of Chemical Signals in the Social Behavior of Crayfish. Chem. Senses 2005, 30, i305–i306. [Google Scholar] [CrossRef]
- Bradbury, J.W.; Vehrencamp, S.L. Economic models of animal communication. Anim. Behav. 2000, 59, 259–268. [Google Scholar] [CrossRef]
- Medwinn, H.; Clay, C.S. Fundamentals of Acoustical Oceanography; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Crook, R.; Patullo, B.W.; Macmillan, D.L. Multimodal individual recognition in the crayfish cherax destructor. Mar. Freshw. Behav. Physiol. 2004, 37, 271–285. [Google Scholar] [CrossRef]
- Van der Velden, J.; Zheng, Y.; Patullo, B.W.; Macmillan, D.L. Crayfish Recognize the Faces of Fight Opponents. PLoS ONE 2008, 3, e1695. [Google Scholar] [CrossRef] [PubMed]
- Dalosto, M.M.; Ayres-Peres, L.; Araujo, P.B.; Santos, S.; Palaoro, A.V. Pay attention to the ladies: Female aggressive behavior and weapon allometry provide clues for sexual selection in freshwater anomurans (Decapoda: Aeglidae). Behav. Ecol. Sociobiol. 2019, 73, 127. [Google Scholar] [CrossRef]
- Walter, G.M.; van Uitregt, V.O.; Wilson, R.S. Social control of unreliable signals of strength in male but not female crayfish, Cherax destructor. J. Exp. Biol. 2011, 214, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Barki, A.; Karplus, I. Mating Behavior and a Behavioral Assay for Female Receptivity in the Red-claw Crayfish Cherax Quadricarinatus. J. Crustac. Biol. 1999, 19, 493–497. [Google Scholar] [CrossRef]
- Zulandt, T.; Zulandt-Schneider, R.A.; Moore, P.A. Observing agonistic interactions alters subsequent fighting dynamics in the crayfish, Orconectes rusticus. Anim. Behav. 2008, 75, 13–20. [Google Scholar] [CrossRef]
- Graham, M.E.; Herberholz, J. Stability of dominance relationships in crayfish depends on social context. Anim. Behav. 2009, 77, 195–199. [Google Scholar] [CrossRef]
- Gottesman, B.L.; Francomano, D.; Zhao, Z.; Bellisario, K.; Ghadiri, M.; Broadhead, T.; Gasc, A.; Pijanowski, B.C. Acoustic monitoring reveals diversity and surprising dynamics in tropical freshwater soundscapes. Freshw. Biol. 2018, 65, 117–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).