Abstract
Several inputs of metal contamination can affect port seawater, such as industries and sludges. Despite the urge of developing new techniques to face this problem, most of the studies focus on traditional methods of remediation. Bioremediation by fungi represents an innovative and sustainable tool to efficiently remove metals from seawaters. The study’s aim is to develop a new green technology using fungi (myco-barriers) to maintain a good standard for water quality in port areas. A large commercial port (Port of Genoa) and a small marina (Port of Cavo) in Italy were chosen as pilot sites. Myco-barriers were realised by inoculating sterile straw and sawdust with mycelium of macro- and microfungi. After the incubation, myco-barriers were placed in the ports and sampled after 15 and 30 days to verify metal bioaccumulation. Myco-barriers with macrofungi showed the tendency to bioaccumulate more efficiently after 15 treatment days (Zn 7.0 mg kg−1, Cu 6.5 mg kg−1, Pb 1.2 mg kg−1), while myco-barriers with microfungi showed higher bioaccumulation after 30 days (Ni 0.6 mg kg−1, Pb 0.6 mg kg−1, Cu 5 mg kg−1). Results showed that myco-barriers have metal bioaccumulation potential and can represent a significant alternative to traditional techniques of remediation (chemical–physical).
1. Introduction
Marine water contamination by potentially toxic elements (PTEs) is a recent problem, especially in highly anthropised areas as ports. Different inputs as industrial effluents, terrestrial and freshwater run-off, sea traffic, ballast waters, and accidental spills negatively affect seawaters, both in marinas and industrial/commercial ports, and are potential sources of metal contamination [1,2]. Moreover, in the port basins, sediments can also represent a collector for PTEs [3,4] and a secondary source of PTEs due to their handling during dredging activities or resuspension during vessel manoeuvres. In fact, in these cases, sediment movements can promote elements dissolution and transfer in the water column [5]. Despite the numerous PTEs inputs, the rate of port seawater contamination by PTEs is difficult to evaluate because ports are open systems, and toxic elements tend to be very diluted in the water column. However, some PTEs (e.g., As, Pb, Cd, Fe, Zn, Cu) can be toxic at low concentrations and potentially affect marine biodiversity not only in the ports but also in the neighbouring areas [6,7,8]. Moreover, PTEs, as inorganic elements, cannot be degraded into less harmless compounds and tend to be bioconcentrated and biomagnificated in the food chain, becoming potentially dangerous also for humans [9,10].
Most of the studies focus on remediation of organic pollutants, such as petroleum, sludges, and oil spills, and not on the removal of metals from seawater. These techniques mainly consist of mechanical control of the spreading oil spill (booms), mechanical removal using oil skimmers (devices designed to separate and recover oily liquids emulsified in a water body or floating on the water), sweeping arms or adsorbent materials (sheets, rolls, pillows, absorbent pads), or chemical removal by dispersion of products (surfactants, solvents, and stabilizers) [11,12,13]. Studies dealing with the water decontamination from inorganic contaminants include traditional techniques, such as coagulation, precipitation, electrochemical treatment, membrane filtration and adsorption/ion exchange systems [14]. However, in addition to these techniques, biological methods can be applied for inorganic contaminants removal from marine waters [14,15,16,17]. Detoxification and rehabilitation of contaminated environments (also marine) with the use of microbes has emerged as the safest, easiest, and most effective technology [18]. Unfortunately, to date, international legislation that limits the use of microorganisms in bioremediation in environments does not exist; however, there are national good practises to bioremediation application (e.g., [19]). The absence of a specific law negatively affects the employment of this technology and its knowledge, underlining the need to explore improved training and development of more user-friendly resources.
Bioremediation in sensu strictu involves microorganisms, such as fungi and bacteria [20,21]. Different species of microbes naturally colonise seawaters and can survive in marine environments tolerating high salt concentrations [22]. Several studies showed the bioremediation properties of fungi (mycoremediation) and bacteria for removal of organic and inorganic pollutants [22,23,24]. Fungi, exploiting enzymes, organic acids, and metabolites are able to biodegrade complex organic contaminants, but they can also bioaccumulate and immobilise PTEs [23,24,25]. However, as mentioned above, not a lot of studies show deepened removal techniques of PTEs from port waters by biological methods, and the consequent maintenance of a good standard quality of the seawater over time [26,27,28,29] also reduces the amount of hazardous waste to be disposed of in landfills. Concerning metals, in fact, most studies were carried out on marine sediments bioremediation [29,30,31] because sediments often tend to accumulate high levels of PTEs, despite many metal ions passing in aqueous solution at the sediment–water interface [32].
This study aimed to apply mycoremediation to seawater, developing for the first-time absorbent barriers filled with fungi (myco-barriers), which can be applied for the removal of PTEs from seawater and for the maintenance of good standard quality of port waters. Myco-barriers were developed using different fungal species and tested in both a commercial port (the Port of Genoa, northwestern Italy; Figure 1) and a marina (the Port of Cavo, Elba Island, central Italy; Figure 1) to verify their efficiency in marine environment with a different potential rate of PTEs contamination. All the experimental procedures were carried out within the GEREMIA (“Wastewater management for the improvement of port waters”; http://interreg-maritime.eu/web/geremia accessed on 30 April 2023) and QUALIPORTI (“Water quality by limiting actions and identification of pollutants in ports and organisation of innovative cross-border resources”; http://interreg-maritime.eu/web/qualiporti, accessed on 30 April 2023) projects in the framework of the Interreg Italy-France Maritime 2014–2020 Programme. These projects aimed to find new solutions both to remedy accidental spills and to ensure the optimal environmental quality of the port waters.
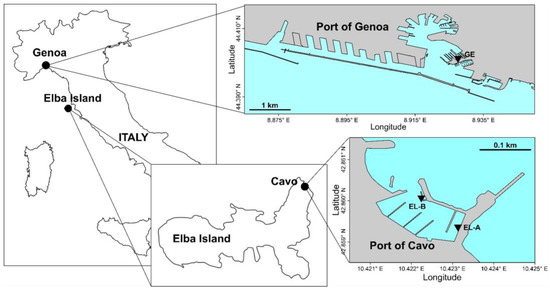
Figure 1.
Study areas (Italy): the Port of Genoa (on top) and the Port of Cavo (on bottom). The inverted triangles represent myco-barrier deployment sites (GE in the Port of Genoa and EL-A and EL-B in the Port of Cavo).
2. Materials and Methods
2.1. Study Areas
The Port of Genoa (Figure 1) is a commercial, industrial, and tourist port at the apex of the Ligurian Sea in the northwestern Mediterranean Sea. The port covers an area of about 7 × 106 m2 and has 47 km of shipping lanes. This port is located within the territory of the city of Genoa (land area of 243.6 km2 and population of about 600,000) and receives run-off water from the roads, freshwater from two relatively big streams (catchment area surfaces of 93 km2 and 140 km2) and numerous small streams, and wastewater from the various port activities. Within the port, there are ferry and cruise terminals, bulk cargo terminals, container terminals, dry docks, numerous marinas, shipyards, an oil depot, and a steel mill [33]. Inside the port basin, water temperature varies between 12–14 °C in February and 14–26 °C in July, while salinity is strongly affected by precipitation and freshwater stream input, varying generally between 36–37 of in spring and autumn and 37–38 of in summer and winter [33].
The Port of Cavo (Figure 1) is located to the northeast of Elba Island (central Italy) and is a small marina overlooked by the village of Cavo (620 inhabitants). Hydrofoils and ferries to the mainland dock at the only pier presents outside the port [34]. The internal landing place of the Port of Cavo is characterised by traffic deriving from small pleasure boats, has a total of about 200 berths, most of which are only occupied during the summer period, and has a petrol station for boats and cars. Water temperature and salinity show values of 13.5–15.2 °C and 38.2–38.3, respectively, inside the Port of Cavo in the first period of the year (from January to April) [35].
2.2. Fungal Selection
Fungi employed for the development of myco-barriers were differently selected in the studied ports.
Concerning the Port of Genoa, both macro- and microfungi were used. Among macrofungi, Ligurian wild strains of Pleurotus ostreatus (PO) (Jacq.) P. Kumm. and Trametes versicolor (TV) (L.) Lloyd were selected, while among microfungi, a strain belonging to the Trichoderma harzianum Rifai group (TH) was selected. The latter was previously isolated from the water of the Port of Genoa within the SEDITERRA project (Interreg Italy-France Maritime 2014–2020 Programme) [36] and cryopreserved in the mycological collection of the University of Genoa ColD—UNIGE JRU MIRRI-IT.
Regarding the Port of Cavo, both macro- and microfungi were employed. Pleurotus ostreatus (PO) was tested together with the Trichoderma harzianum strain native of the Port of Genoa (TH.all), a microfungal autochthonous strain belonging to the T. harzianum Rifai group (TH.aut) and a mix of autochthonous strains belonging to the species Simplicillium lanosoniveum (J.F.H. Beyma) Zare & W. Gams and Trichoderma harzianum Rifai group (MIX). TH.aut was selected to compare its efficiency to the TH.all strain isolated from the Port of Genoa.
2.3. Myco-Barriers Development and Positioning in the Ports
Once fungi were selected, myco-barriers were prepared. First, a specific substrate was studied for the fungal growth. A mixture of vegetable, straw, and sawdust was sterilised (120 °C × 20 min) in apposite autoclavable bags. Then, the mixture in the bags was inoculated with selected fungal strains, 7 days old, grown in pure culture on malt extract agar (MEA, NutriSelect® Plus, Merck Life Science Srl, Milan, Italy) plates (microfungi) and potato dextrose agar (PDA, NutriSelect® Plus, Merck Life Science Srl, Milan, Italy) plates (macrofungi). The bags were incubated in the dark at ±24 °C for the time necessary for the mycelia to grow and completely colonise the substrates (1–2 months). After fungal growth, commercial booms 20 × 20 made only by plastic absorbent polymers (GreanOcean, Amsterdam, The Netherlands) were emptied of their contents and filled with 500 g of the substrates colonised by the fungal mycelia. Together with myco-barriers, commercial barriers and barriers made only by the vegetable substrate without fungi were also prepared and used as control. The different types of barriers were combined in a series consisting of one original barrier (PA), one vegetal barrier (VE), one myco-barrier with microfungi (TV, TH, TH.all, TH.aut, or MIX), and one myco-barrier with macrofungi (PO). Two series were prepared for each study site to permit their recovery at 15 (time 1) and 30 (time 2) days after installation (Figure 2). Finally, the series of myco-barriers were deployed in the Port of Genoa (GE site) and in the Port of Cavo (EL-A and EL-B sites; Figure 1). The GE site was in the shipyards of the Port of Genoa, while the EL-A and EL-B sites were located inside and at the entrance of the Port of Cavo, respectively.
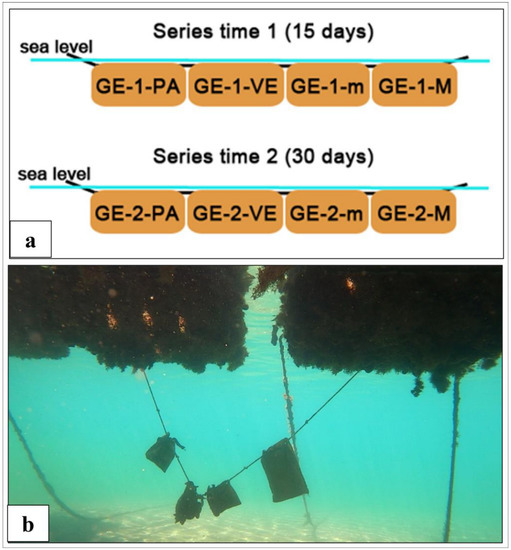
Figure 2.
Barriers’ setup: (a) Scheme of the composition of the barrier series: GE represents the site in the Port of Genoa; 1 and 2 are the two recovering times at 15 and 30 days, respectively; PA is the original barriers; VE is the vegetal barriers; and “m” and “M” are the barriers consisting of micro- and macrofungi, respectively. (The series installed in the Port of Cavo had the same structure with the EL-A and EL-B for the site identification.); (b) image of a barrier series in the Port of Cavo.
2.4. Sampling Program and Analyses of Myco-Barriers Efficiency
After 15 (time 1) and 30 (time 2) days of deployment, the barrier series was recovered. Three sub-samples were taken from each individual barrier and recovered in 50 mL sterile plastic Falcon tubes for chemical analysis and determination of the PTEs concentration. ICP-MS analyses was carried out on the samples by Lifeanalytiscs s.r.l. (Oderzo (TV), Italy)Together with the barriers, 1 L of the port waters was also collected at each site and analysed at the different recovering time (time 0, time 1, and time 2) to estimate the PTEs concentration in the port waters. Elements considered in this study are Al, As, Cd, total Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, and Zn.
2.5. Statistical Analysis
Thanks to the ICP-MS analyses of all the barriers and the waters samples, the bioconcentration factor (BCF) was calculated to better understand the bioaccumulation rate of specific elements by the myco-barriers and the port water quality. BCF was calculated as the ratio of the concentration of the specific element in a specific myco-barrier to the element mean concentration in the seawater during the experiment (it is not calculated for the elements with values below the detection limit in water). Moreover, Fisher’s exact test was calculated with Microsoft Excel to verify the data significance. In particular, the test was carried out to evaluate the behaviour of the myco-barriers made with different fungi towards PTEs.
3. Results
In general, ICP-MS chemical analyses showed how some elements in seawaters are below the detection limit or present as trace elements in both ports (Tables S1 and S2 for the Port of Genoa and Tables S3–S7 for the Port of Cavo). In fact, only As and Zn were constantly found in Genoa seawaters at the three sampling times and ranged between 2 and 4 µg L−1 and between 16.5 and 48 µg L−1, respectively. Similarly, only As and Mn were persistently found in the water of the Port of Cavo, with concentrations from 2 to 13 µg L−1 and from 8 to 76 µg L−1, respectively. Other elements found, but not consistently, were Sb (1.4–5.2 µg L−1) and Fe (11–20 µg L−1) in the Port of Genoa and Al (173–416 µg L−1), Fe (45–59 µg L−1), and Zn (29 µg L−1) in the Port of Cavo.
3.1. Port of Genoa
PTEs concentrations found in seawater, myco-barriers, and control barriers of the Port of Genoa at the three sampling times are shown in Tables S1 and S2. Unfortunately, the myco-barriers inoculated with TV and exposed to 30 d of treatment in port waters were partially destroyed by a sea storm. Data highlighted how the myco-barriers made with an autochthonous microfungal strain belonging to the TH group showed a higher bioaccumulation for Ni, Pb, and Cu after 30 days (0.6, 0.6, 5.0 mg kg−1, respectively), while control barriers (commercial and vegetal) showed PTEs accumulation mainly after 15 days (mainly for Cu) (Figure 3). The bioconcentration factor calculated for Ni after 30 d showed a very high value: 150. On the contrary, myco-barriers inoculated with macrofungi TV and PO show the higher bioaccumulation of PTEs after 15 days for Zn (7.0 mg kg−1), Cu (6.5 mg kg−1), Pb (1.2 mg kg−1) and Pb (0.2 mg kg−1), and Ni (0.8 mg kg−1), while after 30 days the elements’ uptake is unchanged. The control barriers show very low PTEs accumulation only in the TV 15 days series (Figure 3). Concerning the BCF index calculation, TV barriers showed values of bioconcentration up to 700 for Zn, while PO was up to 200 for Ni.
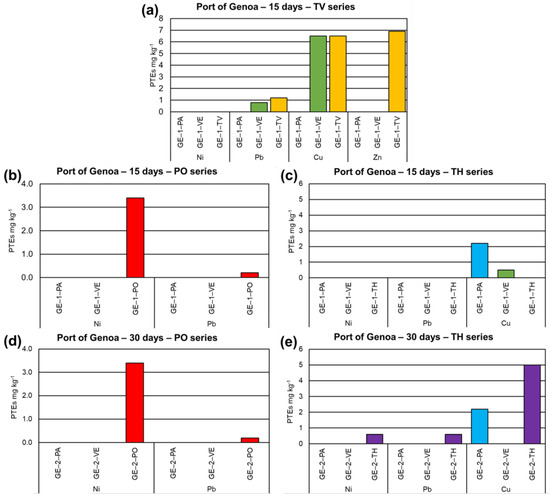
Figure 3.
Results on bioaccumulation of PTEs in myco-barriers, compared to original barriers (PA) and vegetal barriers (VE), from the Port of Genoa (GE): (a) T. versicolor barriers (TV) after 15 days (GE−1−TV); (b) P. ostreatus barriers (PO) after 15 days (GE−1−PO); (c) T. harzianum (TH) after 15 days (GE−1−TH); (d) PO after 30 days (GE−2−PO); and (e) TH after 30 days (GE−2−TH).
Moreover, Fisher’s exact test, calculated for the PTEs accumulated and not accumulated by TH and PO myco-barriers, showed a p-value of 0.4003, and it is not significant at p < 0.05.
3.2. Port of Cavo
The PTEs found in seawater, myco-barriers, and control barriers of the Port of Cavo at the three sampling times are shown in Tables S3–S7. Data showed no significant differences between the two sites (A and B) selected in the Port of Cavo for the treatment. Myco-barriers constituted by TH.all bioaccumulated Al, Cu, and Fe during the treatment reaching maximum values after 30 days (28.5, 2.9, 72.2 mg kg−1, respectively, in site A), while vegetal barriers VE accumulated Al and Fe only in the first period of treatment (8.0 and 20.0 mg kg−1, respectively) (Figure 4). TH.all showed a high bioconcentration rate of these metals: up to 142 for Al and over 2000 for Fe. Concerning site B, T.H.all confirmed the capability to bioaccumulate Al (61 mg kg−1) and Fe (253.4 mg kg−1) with corresponding BCFs values of 610 and over 8000, respectively. Regarding autochthonous inocula in site A, unlike the allochthonous strain, TH.aut showed the capability to better uptake Al, Fe, Cu, and Zn after 15 d of treatment (11.4, 7.6, 0.3, and 0.7 mg kg−1, respectively) with corresponding BCFs values of 57 (Al), 253.3 (Fe), and 35 (Zn). On the contrary, myco-barriers inoculated with the mix inoculum (MIX) highlighted the best bioaccumulation capability after 30 d of treatment (55.7 mg kg−1 Fe, 2.2 mg kg−1 Zn; Figure 5) with a BCF of over 1000 for Fe and 110 for Zn. Concerning site B, data showed a lower value of bioaccumulation and bioconcentration of PTEs by both TH.aut and MIX inoculum in both the sites of treatment (Figure 5). Concerning the macrofungus PO, myco-barriers bioaccumulate Al (2.5 mg kg−1 after 15 d in the site A) and Fe (up to 93.23 mg kg−1 after 30 d in the site A) (Figure 4), and their BCFs were 12.5 for Al and over 3000 for Fe, respectively.
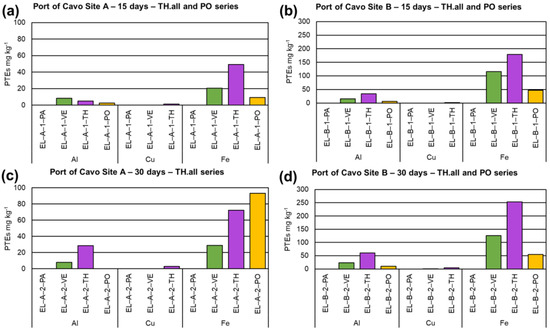
Figure 4.
Results on bioaccumulation of PTEs in myco-barriers, compared to original barriers (PA) and vegetal barriers (VE), from the Port of Cavo (EL): (a) T. harzianum (TH.all) and P. ostreatus (PO) in site A after 15 days (EL−A−1−TH and EL−A−1−PO); (b) TH.all and PO in site B after 15 days (EL−B−1−TH and EL−B−1−PO); (c) TH.all and PO in site A after 30 days (EL−A−2−TH and EL−A−2−PO); and (d) TH.all and PO in site B after 30 days (EL−B−2−TH and EL−B−2−PO).
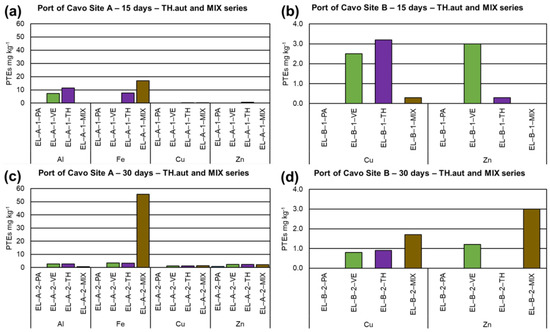
Figure 5.
Results on bioaccumulation of PTEs in myco-barriers, compared to original barriers (PA) and vegetal barriers (VE), from the Port of Cavo (EL): (a) T. harzianum Rifai group (TH.aut) and Simplicillium with Trichoderma genera (MIX) in site A after 15 days (EL−A−1−TH and EL−A−1−MIX); (b) TH.aut and MIX in site B after 15 days (EL−B−1−TH and EL−B−1−MIX); (c) TH.aut and MIX in site A after 30 days (EL−A−2−TH and EL−A−2−MIX); and (d) TH.aut and MIX in site B after 30 days (EL−B−2−TH and EL−B−2−MIX).
Moreover, Fisher’s exact test showed, in both sites and for both treatments with autochthonous and allochthonous fungi, an insignificant p-value (p > 0.05).
4. Discussion
This study aimed to investigate the possibility to employ fungi for the decontamination of port seawaters by PTEs exploiting floating absorbent myco-barriers. In fact, fungi are known to also bioaccumulate PTEs in seawaters [29]. Some macrofungal strains of T. versicolor and P. ostreatus are known to grow well in an aquatic environment, tolerate saline waters, and biodegrade pollutants from contaminated waters [37,38]. Therefore, we decided to select the mycelia of two wild Ligurian strains of macrofungi (P. ostratus and T. versicolor, conserved in the ColD), testing their potentiality together with autochthonous and allochthonous microfungi, to verify possible differences in terms of efficiency and efficacy of the treatment. Trichoderma harzianum group strains also are well-known marine-derived fungi able to survive in marine environments thanks to the development of salt tolerance mechanisms [39,40]. Moreover, other studies showed how T. harzianum strains are employable in mycoremediation protocol of organic pollutants and toxic metals [39,41]. Simplicillium lanosoniveum is recently and successfully tested for the treatment of wastewaters [42], and it is characterised by a wide ecological range: it was isolated in marine environment too, and it is studied to produce various bioactive compounds [43].
Results showed how PTEs contamination in ports is difficult to assess. Many elements in fact are diluted in seawaters and often below detection limits. This situation is typical of the port marine environment, which is affected by various environmental and anthropic forcings, such as the circulation of water masses forced by strong winds or the action of nautical propellers, and the introduction of concentrated chemical elements by individual and temporary activities that can either dilute or concentrate, respectively, the chemicals in the water masses in a short period of time.
Despite values often below detection limits, elements can be bioconcentrated by marine organisms and represent a threat for the environment. In fact, despite the high dilution of some elements in port seawaters, myco-barriers showed the capability to bioconcentrate PTEs in a different rate and way. In particular, the most bioaccumulated elements were Fe, Cu, Zn, Al, Ni, and Pb. These elements can be very toxic for the marine environments and can threaten marine biodiversity [44]. The majority of these elements are essential nutrients in little concentrations, and fungi are characterised by a specific biological system for their bioaccumulation (e.g., membrane proteins, metallothioneins, chelators, siderophores), but others (such as Pb and As) are indifferent elements and their mistaken bioaccumulation into the cells, due to phenomena of replacement with essential ions, is toxic [25]. For these elements, generally, some fungi develop exclusion mechanisms to protect themselves, but other fungi, exploitable in mycoremediation processes, chelate and actively transport these elements into the cells, stocking them into vacuoles and reducing their toxicity [25]. This is particularly evident in the Port of Genoa where the BCF index for Zn and Ni (essential trace metals) reached high values. On the contrary, in both the study sites, some elements are totally excluded, such as As, which is constantly present in seawaters, and Sb, Cd, and Hg, which are under the detection limit. Moreover, these phenomena explain how selected fungi showed a different behaviour towards elements: microfungi gradually bioconcentrated PTEs during the 30 days of the test, while macrofungi tend to bioaccumulate PTEs within 15 days and then the metals concentration was constant. The life cycle, growth, and metabolism of a microfungus are faster than a macrofungus (which is more susceptible to environmental factors) [45,46], favouring the continuous and increasing accumulation of PTEs during the exposition time. Macrofungi, in fact, despite their higher complexity of metabolites and enzymatic products, are characterised by a slower growth rate [47] and a less capability to bioaccumulate elements, which remains constant after 15 days. Data highlighted and confirmed that these features make macrofungi easily employable in the biodegradaton of organic contaminants than in the bioaccumulation of inorganic elements [48].
Moreover, tests carried out in the Port of Cavo highlighted how the allochthonous strain of T. harzianum (isolated from the Port of Genoa) exhibited a higher bioaccumulation rate of PTEs than the local strains. The literature shows that native fungal strains (in a compromised site) are generally the most suitable for the bioremediation of the site itself, due to their adaptation to local environmental conditions [32,49]. However, our results showed that the allochthonous species performed better than the local ones. This may happen because the Port of Cavo is characterised by a low contamination by PTEs. In fact, Cavo is a marina where metals are very diluted in water, while metals are more abundant in the Port of Genoa (one of the main commercial ports in Italy); thus, the fungal strains isolated from seawaters of Genoa are better adapted to tolerate and bioaccumulate PTEs respect to the ones from Cavo.
Fisher’s exact test results confirmed this different behaviour among macrofungi, autochthonous microfungi, and allochthonous microfungi. The tests, in fact, were not significant (p > 0.05) in all cases, underlining that the differences in the PTEs bioaccumulation is not casual but due to the physiological properties of the selected strains.
Overall, the present study assessed local and tolerant fungal strains as an alternative tool to the traditional techniques for the remediation of PTEs and the maintenance of a good standard quality of port seawaters. Fungi adaptability to limiting environmental conditions and PTEs contamination, enhance the production of biological compounds useful in the bioremediation processes, allowing their exploitation in various biotechnological applications. Moreover, the employment of new green technologies, such as myco-barriers in the marine environment, is desirable not only for protecting marine habitats but also for reducing plastic debris due to the use of commercial barriers made of plastic polymers.
In this first application, no consideration was given to the costs of production, management, and disposal of the myco-barriers, as this was a first small-scale test with a reduced number of myco-barriers carried out to verify their efficiency and potential.
5. Conclusions
This study developed and applied a new biotechnological tool for the removal of PTEs from port waters and for the maintenance of a good standard quality. Results showed that myco-barriers can represent a useful alternative to traditional techniques of remediation (chemical–physical), reducing the negative impact on the environment and its biodiversity. Both macro- and microfungi, in fact, showed not only the capability to remove PTEs, but also that this property is affected by fungal species-specific physiological characteristics.
Future studies will be necessary to develop a better understanding of the bioaccumulation mechanisms of fungi and to select the most suitable and efficient ones, even with low levels of metal contamination. In addition, fungi isolation from other pilot sites (other ports in the Mediterranean Sea but also in other seas/oceans) will make it possible to expand the knowledge acquired so far on the marine fungal community and to identify cosmopolitan fungal strains that can be applied at multiple sites and thus make the procedure as standardised and robust as possible. Investigation of the physical–chemical characteristics of water masses in the pilot sites (such as temperature, salinity, dissolved oxygen, concentration of organic matter, pH, etc.) during the myco-barrier deployment will be important in the future development of the study because they can influence to a greater or lesser extent the dynamics of bioaccumulation by fungi of the PTEs. Finally, future applications and investigations will have to involve the industrial phases of production of the barriers and those of disposal after their use at sea (which was not performed in this first experimental application), in order to identify and resolve any management criticalities and evaluate the entire process from production to disposal from an economic point of view.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse11061117/s1, Table S1: Results of the ICP-MS analyses of the PTEs at initial time in Genoa waters; Table S2: PTEs concentration after 15 and 30 days in Genoa waters; Table S3: Results of the ICP-MS analyses of the PTEs concentration at the initial time in Cavo waters; Tables S4 and S5: PTEs concentration after 15 and 30 days in Cavo site A waters; Tables S6 and S7: PTEs concentration after 15 and 30 days in Cavo site B waters.
Author Contributions
Conceptualization, G.C., L.C., M.Z. and M.C.; methodology, G.C., L.C., M.Z. and M.C.; validation, M.Z. and M.C.; formal analysis, S.D.P. and E.R.; investigation, G.C., S.D.P. and E.R.; data curation, G.C. and L.C.; writing—original draft preparation, G.C. and L.C.; writing—review and editing, M.Z. and M.C.; resources, M.C. and M.Z.; supervision, M.Z. and M.C.; project administration, M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was funded by the GEREMIA (CUP D41I18000600005) and QUALIPORTI (CUP J96F17000040007) projects in the framework of the European Interreg Italy-France Maritime 2014–2020 Programme and partially by the RETURN project (CUP D33C22001290002) in the framework of the Italian PNRR (EU-funded National Recovery and Resilience Plan).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article Supplementary Materials and on request from the corresponding author, M.C.
Acknowledgments
The authors would like to thank the Servizi Ecologici Porto di Genova for hosting the barriers at their quay during the experiment, and the Circolo Nautico Cavo (Elba Island) for its cooperation during the project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Spagnoli, F.; De Marco, R.; Dinelli, E.; Frapiccini, E.; Frontolani, F.; Giordano, P. Sources and metal pollution of sediments from a coastal area of the Central Western Adriatic Sea (Southern Marche Region, Italy). Appl. Sci. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Tornero, V.; Hank, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Chen, Q.; Bao, B.; Li, Y.; Liu, M.; Zhu, B.; Mu, J.; Chen, Z. Effects of marine oil pollution on microbial diversity in coastal waters and stimulating indigenous microorganism bioremediation with nutrients. Reg. Stud. Mar. Sci. 2020, 39, 101395. [Google Scholar] [CrossRef]
- Merhaby, D.; Ouddane, B.; Net, S.; Halwani, J. Assessment of trace metals contamination in surficial sediments along Lebanese Coastal Zone. Mar. Pollut. Bull. 2018, 133, 881–890. [Google Scholar] [CrossRef]
- Monte, C.N.; Rodrigues, A.P.C.; de-Freitas, A.R.; Freire, A.S.; Santelli, R.E.; Braz, B.F.; Machado, W. Dredging impact on trace metal behavior in a polluted estuary: A discussion about sampling design. Braz. J. Oceanogr. 2019, 67, e19227. [Google Scholar] [CrossRef]
- Shree, B.V.; Nishikant, G. Examining the heavy metal contents of an estuarine ecosystem: Case study from Maharashtra, India. J. Coast. Conserv. 2019, 23, 977–984. [Google Scholar]
- Wang, X.; Zhao, L.; Xu, H.; Zhang, X. Spatial and seasonal characteristics of dissolved heavy metals in the surface seawater of the Yellow River Estuary, China. Mar. Pollut. Bull. 2018, 137, 465–473. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Szynkowska, M.I.; Pawlaczyk, A.; Mackiewicz, E. Bioaccumulation and biomagnification of trace elements in the environment. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Dave, D.; Ghaly, A.E. Remediation technologies for marine oil spills: A critical review and comparative analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar] [CrossRef]
- Malhas, R.; Al-Ibrahim, Y.; Al-Meraj, A.; Abdullah, H.; Alshatti, A. Application of magnetic separation for oil spill remediation and recovery in Kuwait sea water. Desal. Water Treat. 2021, 209, 114–120. [Google Scholar] [CrossRef]
- Tewari, S.; Sirvaiya, A. Oil spill remediation and tis regulation. Int. J. Res. Sci. Engin. 2015, 1, 1–7. [Google Scholar]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Product. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Dewi, E.R.S.; Nuravivah, R. Potential of microalgae Chlorella vulgaris as bioremediation agents of heavy metal Pb (Lead) On Culture Media. In E3S Web of Conferences 31; EDP Sciences: Les Ulis, France, 2018; p. 05010. [Google Scholar] [CrossRef]
- Ferrante, M.; Vassallo, M.; Mazzola, A.; Brundo, M.V.; Pecoraro, R.; Grasso, A.; Copat, C. In vivo exposure of the marine sponge Chondrilla nucula Schmidt, 1862 to cadmium (Cd), copper (Cu) and lead (Pb) and its potential use for bioremediation purposes. Chemosphere 2018, 193, 1049–1057. [Google Scholar] [CrossRef]
- Seidel, H.; Löser, C.; Zehnsdorf, A.; Hoffmann, P.; Schmerold, R. Bioremediation process for sediments contaminated by heavy metals: Feasibility study on a pilot scale. Environ. Sci. Technol. 2004, 38, 1582–1588. [Google Scholar] [CrossRef]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Paul, D. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Consorzio Italbiotec. Available online: www.italbiotec.it (accessed on 30 April 2023).
- Gunyar, O.A.; Uztan, A.H. Environmental mycobiotechnology in special reference to fungal bioremediation. In Nanotechnology Applications in Health and Environmental Sciences; Saglam, N., Korkusuz, F., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2021; pp. 361–383. [Google Scholar]
- Kumar, V.; Shahi, S.K.; Singh, S. Bioremediation: An eco-sustainable approach for restoration of contaminated sites. In Microbial Bioprospecting for Sustainable Development; Singh, J., Sharma, D., Kumar, G., Sharma, N., Eds.; Springer: Singapore, 2018; pp. 115–136. [Google Scholar]
- Fulke, A.B.; Kotian, A.; Giripunje, M.D. Marine microbial response to heavy metals: Mechanism, implications and future prospect. Bull. Environ. Contam. Toxicol. 2020, 105, 182–197. [Google Scholar] [CrossRef]
- Ceci, A.; Pinzari, F.; Russo, F.; Persiani, A.M.; Gadd, G.M. Roles of saprotrophic fungi in biodegradation or transformation of organic and inorganic pollutants in co-contaminated sites. Appl. Microbiol. Biotechnol. 2019, 103, 53–68. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Areco, M.M.; Salomone, V.N.; dos Santos Afonso, M. Ulva lactuca: A bioindicator for anthropogenic contamination and its environmental remediation capacity. Mar. Environ. Res. 2021, 171, 105468. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshanee, M.; Das, S. Bioremediation potential of biofilm forming multi-metal resistant marine bacterium Pseudomonas chengduensis PPSS-4 isolated from contaminated site of Paradip Port, Odisha. J. Earth Syst. Sci. 2021, 130, 1–17. [Google Scholar] [CrossRef]
- Singh, J.K.; Yadav, A.K.; Gupta, S.; Verma, R. Heavy metal pollution in coastal Environment and its remediation using mangroves. An eco-sustainable approach. In Wetlands Conservation: Current Challenges and Future Strategies; Sharma, S., Singh, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 201–228. [Google Scholar]
- Cecchi, G.; Cutroneo, L.; Di Piazza, S.; Besio, G.; Capello, M.; Zotti, M. Port Sediments: Problem or Resource? A Review Concerning the Treatment and Decontamination of Port Sediments by Fungi and Bacteria. Microorganisms 2021, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, A.; Beolchini, F.; Corinaldesi, C.; Amato, A.; Becci, A.; Rastelli, E.; Hekeu, M.; Regoli, F.; Astarita, E.; Greco, S.; et al. Assessing the efficiency and eco-sustainability of bioremediation strategies for the reclamation of highly contaminated marine sediments. Mar. Environ. Res. 2020, 162, 105101. [Google Scholar] [CrossRef]
- Negrin, V.L.; Gironés, L.; Serra, A.V. Eco-friendly strategies of remediation in the marine system: Bioremediation and phytoremediation. In Coastal and Deep Ocean Pollution; Arias, A.H., Botté, S.E., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 184–214. [Google Scholar]
- Chen, Y.; Liu, Q.; Xu, M.; Wang, Z. Inter-annual variability of heavy metals pollution in surface sediments of Jiangsu coastal region, China: Case study of the Dafeng Port. Mar. Pollut. Bull. 2020, 150, 110720. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, L.; Carbone, C.; Consani, S.; Vagge, G.; Canepa, G.; Capello, M. Environmental complexity of a port: Evidence from circulation of the water masses, and composition and contamination of bottom. Mar. Pollut. Bull. 2017, 119, 184–194. [Google Scholar] [CrossRef]
- Autorità di Sistema Portuale del Mar Tirreno Settentrionale. Available online: https://www.portialtotirreno.it/i-porti/pontile-di-cavo/ (accessed on 30 April 2023).
- QUALIPORTI Project. Rapport Résultats et Comparaisons Entre les Zones Enquêtes. Available online: https://interreg-maritime.eu/documents/780767/0/QUALIPORTI_T2.2.2_Synthe%CC%80se+transfrontalie%CC%80re+du+monitorage+de+la+qualite%CC%81+des+eaux.pdf/a2594384-ae2a-9fc9-c6b0-480ec48a3a1b?t=1664287497081 (accessed on 30 April 2023).
- Cecchi, G.; Cutroneo, L.; Di Piazza, S.; Capello, M.; Zotti, M. Culturable fungi from dredged and marine sediments from six ports studied in the framework of the SEDITERRA Project. J. Soils Sed. 2021, 21, 1563–1573. [Google Scholar] [CrossRef]
- Lalitha, N.; Patil, P.K.; Rajesh, R.; Muralidhar, M. Usage of Pleurotus ostreatus for Degradation of Oxytetracycline in Varying Water Salinities in Brackishwater Aquaculture System. J. Coast Res. 2019, 86, 138–141. [Google Scholar] [CrossRef]
- Mori, T.; Sudo, S.; Kawagishi, H.; Hirai, H. Biodegradation of diuron in artificially contaminated water and seawater by wood colonized with the white-rot fungus Trametes versicolor. J. Wood Sci. 2018, 64, 690–696. [Google Scholar] [CrossRef]
- Ayad, F.; Matallah-Boutiba, A.; Rouane–Hacene, O.; Bouderbala, M.; Boutiba, Z. Tolerance of Trichoderma sp. to heavy metals and its antifungal activity in Algerian marine environment. J. Pure Appl. Microbiol. 2018, 12, 855–870. [Google Scholar] [CrossRef]
- De Padua, J.C.; dela Cruz, T.E.E. Isolation and characterization of nickel-tolerant Trichoderma strains from marine and terrestrial environments. J. Fungi 2021, 7, 591. [Google Scholar] [CrossRef]
- Hussain, D.F.; Mutlag, N.H. Assessment the ability of Trichoderma harzianum Fungi in Bioremediation of some of Heavy Metals in Waste Water. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 790, p. 012087. [Google Scholar]
- Liu, X.; Xing, X.; Dong, Q.; Liu, W.; Li, W. Efficient removal of nitrogen/phosphorous by mix-cultivation of Haematococcus pluvialis and Simplicillium lanosoniveum in wastewater supplemented with NaHCO3. Biochem. Eng. J. 2022, 182, 108433. [Google Scholar] [CrossRef]
- Wei, D.P.; Wanasinghe, D.N.; Hyde, K.D.; Mortimer, P.E.; Xu, J.; Xiao, Y.P.; Bhunjun, C.S.; To-Anun, C. The genus Simplicillium. MycoKeys 2019, 60, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, C.; Gaulier, C.; Bratkic, A.; Galceran, J.; Puy, J.; Baeyens, W. Labile trace metal concentration measurements in marine environments: From coastal to open ocean areas. Trends Analyt. Chem. 2019, 116, 92–101. [Google Scholar] [CrossRef]
- Gams, W.; Van der Aa, H.A.; Van der Plaats-Niterink, A.J.; Samson, R.A.; Stalpers, J.A. CBS Course of Mycology; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 1975; p. 104. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007; p. 672. [Google Scholar]
- Hawksworth, D.L.; Sridhar, K.R.; Deshmukh, S.K. The macrofungal resource. In Advances in Macrofungi: Diversity, Ecology and Biotechnology; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–9. [Google Scholar]
- Haider, K.; Trojanowski, J. A comparison of the degradation of 14C-labeled DHP and corn stalk lignins by micro-and macrofungi and bacteria. In Lignin Biodegradation: Microbiology, Chemistry, and Potential Applications; CRC Press: Boca Raton, FL, USA, 2018; pp. 111–134. [Google Scholar]
- Văcar, C.L.; Covaci, E.; Chakraborty, S.; Li, B.; Weindorf, D.C.; Frențiu, T.; Podar, D. Heavy metal-resistant filamentous fungi as potential mercury bioremediators. J. Fungi 2021, 7, 386. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).