Impacts of the Establishment of Biofoulants on Greek Aquaculture: Farmers’ Expert Knowledge
Abstract
1. Introduction
1.1. The Problem
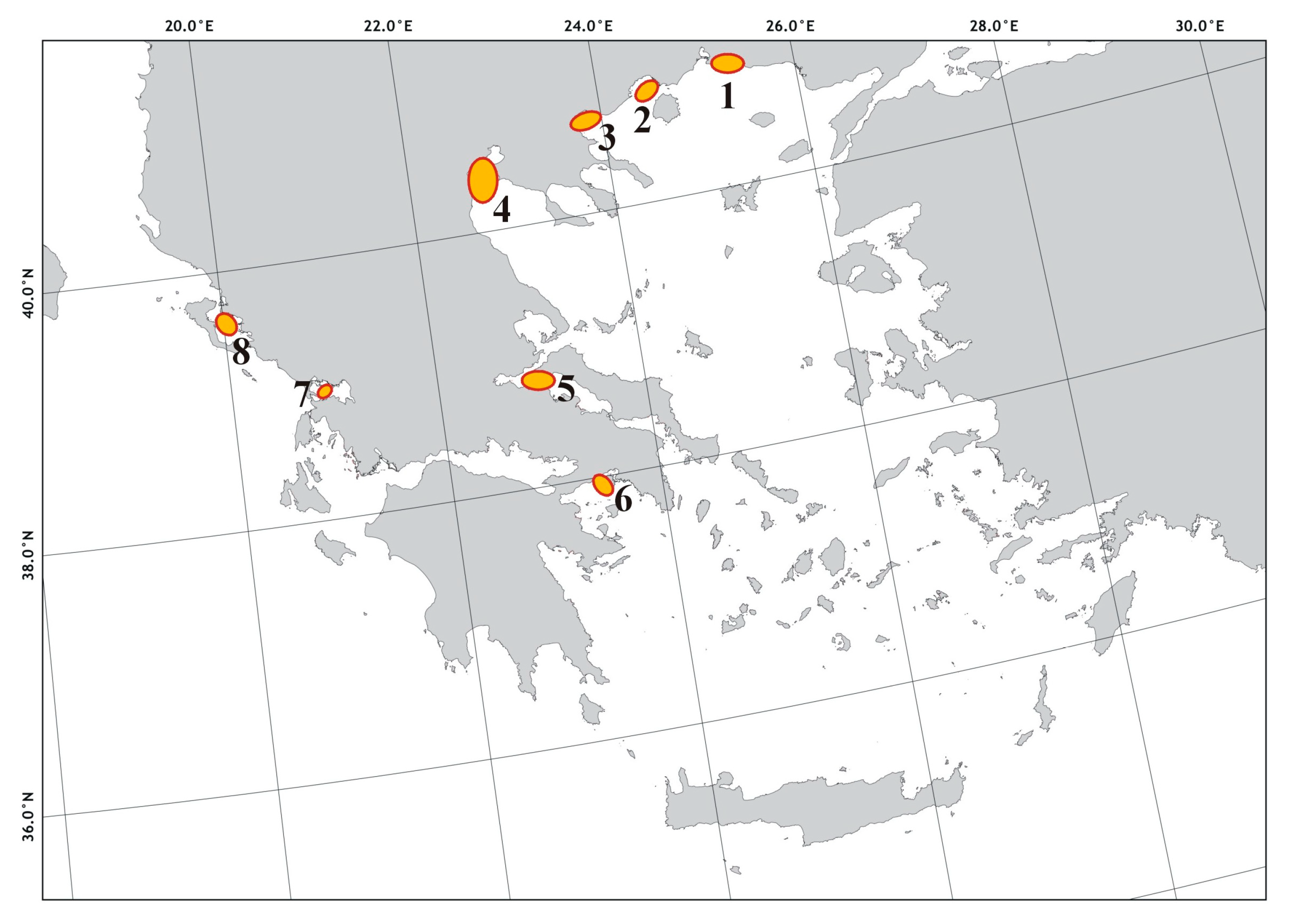
1.2. Focus Area
1.3. Aim of the Study
2. Materials and Methods
2.1. Survey Design
2.2. Interview Survey
2.3. Data Analysis
3. Results
3.1. Typology of the Shellfish Farming Units
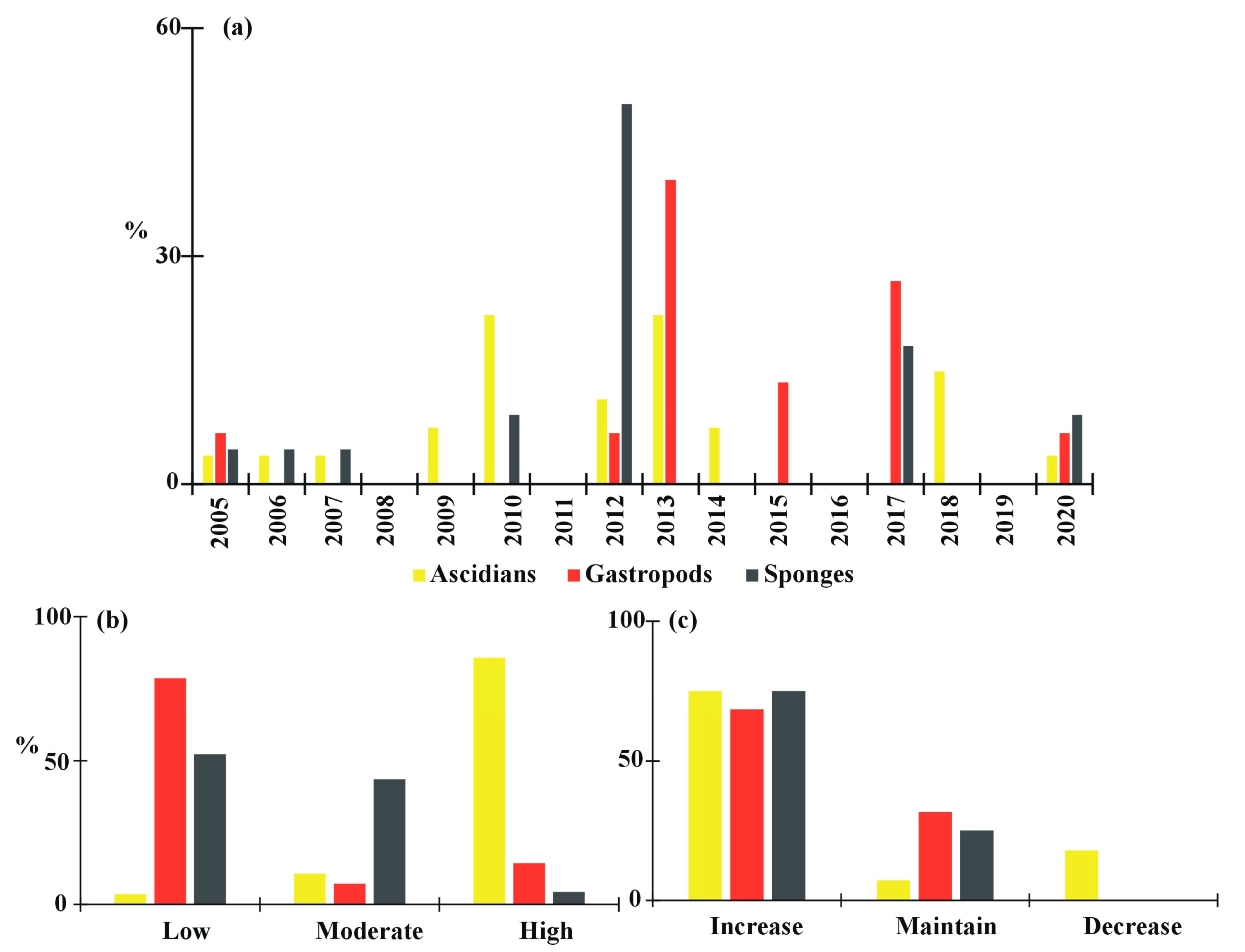
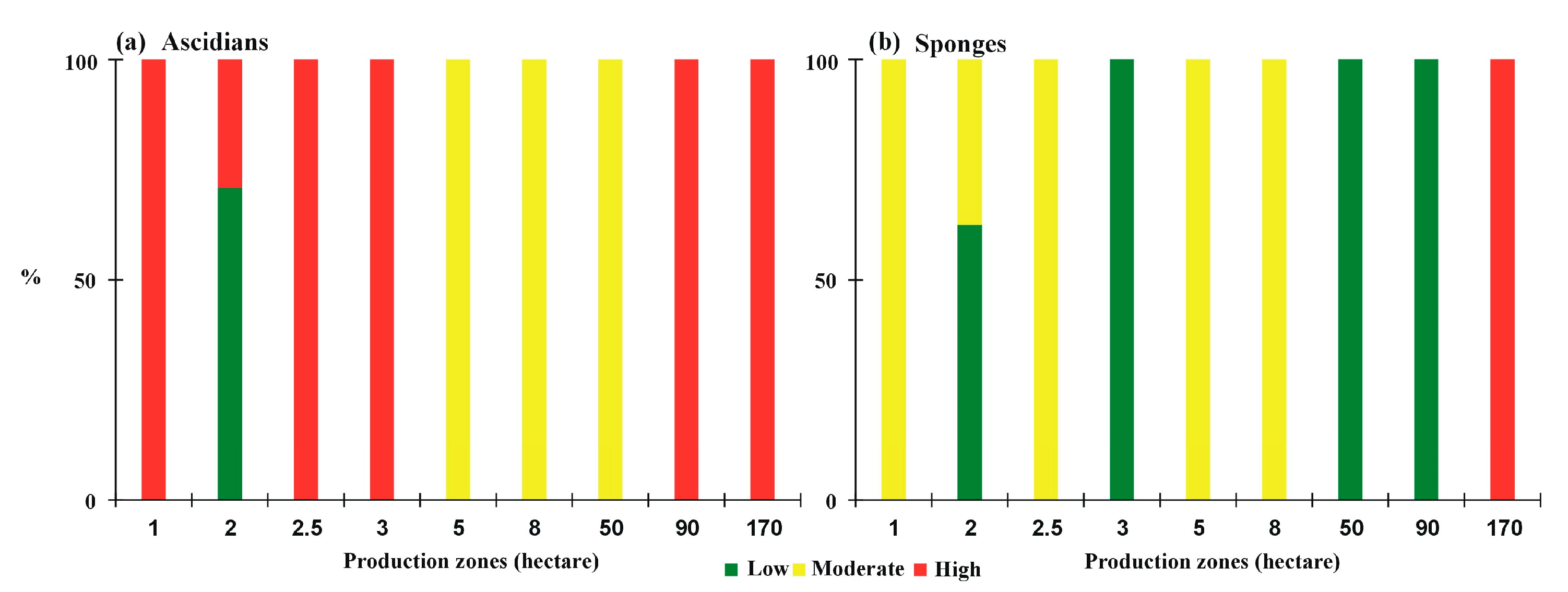
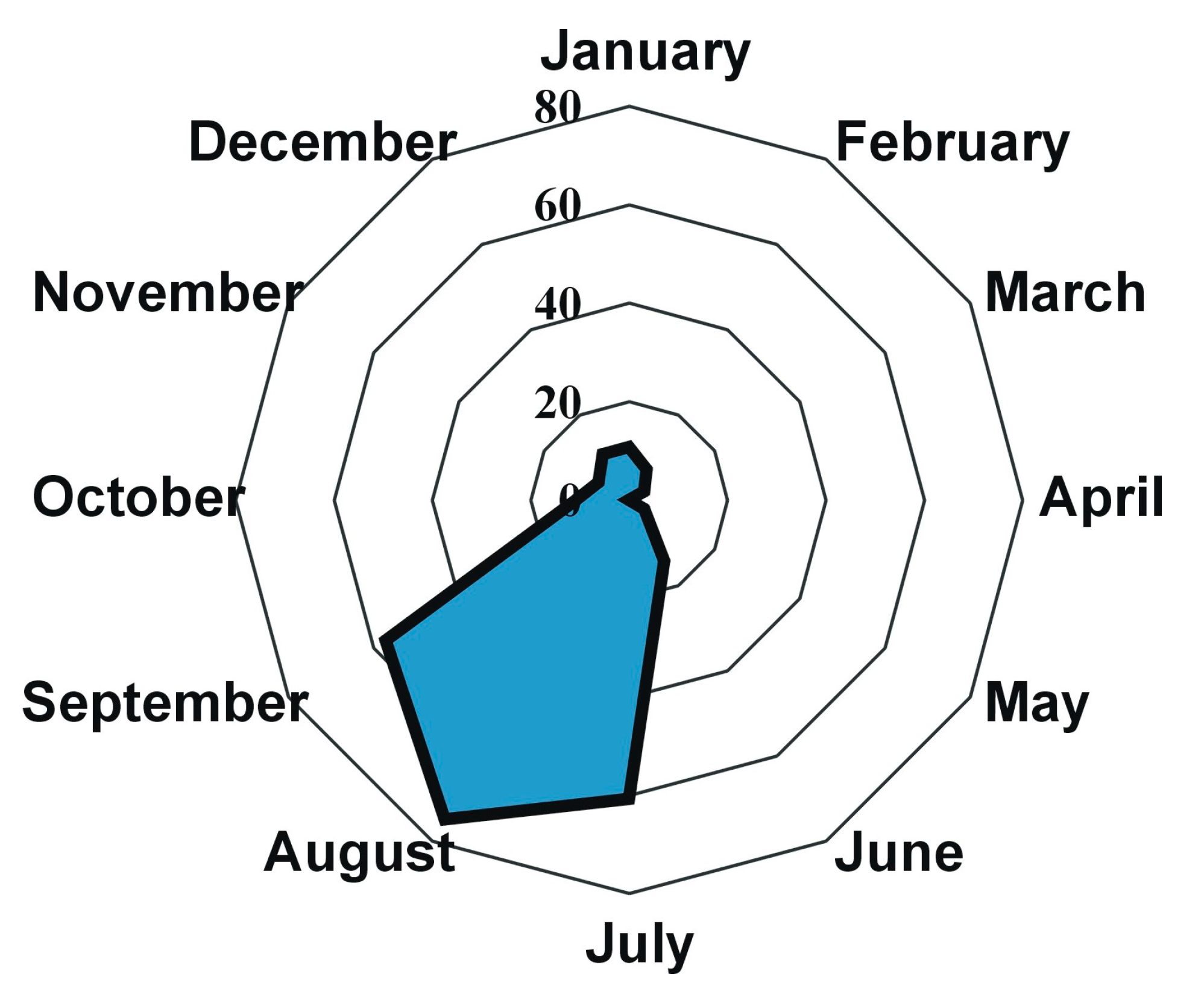
3.2. Problems in Farming
3.3. Final Product and Cost to Address the Problem
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tidwell, J.H. Characterization and categories of aquaculture production systems. In Aquaculture Production Systems; Tidwell, J.H., Ed.; Wiley-Blackwell: Ames, IO, USA, 2012. [Google Scholar]
- Available online: https://download.e-bookshelf.de/download/0000/4076/98/L-G-0000407698-0002356040.pdf (accessed on 1 April 2023).
- Naylor, R.L.; Williams, S.L.L.; Strong, D.R. Aquaculture—A gateway for exotic species. Science 2001, 294, 1655–1656. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Mastitsky, S.E.; Padilla, D.K.; Burlakova, L.E.; Hajduk, M.M. Differences in growth and survivorship of zebra and quagga mussels: Size matters. Hydrobiologia 2011, 668, 183–194. [Google Scholar] [CrossRef]
- Lacoste, E.; Gaertner-Mazouni, N. Biofouling impact on production and ecosystem functioning: A review for bivalve aquaculture. Rev. Aquac. 2015, 7, 187–196. [Google Scholar] [CrossRef]
- Aldred, N.; Clare, A.S. Mini-review: Impact and dynamics of surface fouling by solitary and compound ascidians. Biofouling 2014, 30, 259–270. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; Denys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G. Invasive sea squirts: A growing global problem. J. Exp. Mar. Biol. Ecol. 2007, 342, 3–4. [Google Scholar] [CrossRef]
- Simkanin, C.; Davidson, I.C.; Dower, J.F.; Jamieson, G.; Therriault, T.W. Anthropogenic structures and the infiltration of natural benthos by invasive ascidians. Mar. Ecol. 2012, 33, 499–511. [Google Scholar] [CrossRef]
- Airoldi, L.; Turon, X.; Perkol-Finkel, S.; Rius, M. Corridors for aliens but not for natives: Effects of marine urban sprawl at a regional scale. Divers. Distrib. 2015, 21, 755–768. [Google Scholar] [CrossRef]
- López-Legentil, S.; Turon, X.; Erwin, P.M. Feeding cessation alters host morphology and bacterial communities in the ascidian Pseudodistoma crucigaster. Feeding cessation alters host morphology and bacterial communities in the ascidian Pseudodistoma crucigaster. Front. Zool. 2016, 13, 2. [Google Scholar] [CrossRef]
- Carver, C.E.; Chisholm, A.; Mallet, A.L. Strategies to mitigate the impact of Ciona intestinalis (L) biofouling on shellfish production. J. Shell. Res. 2003, 22, 621–631. Available online: https://www.vliz.be/imisdocs/publications/315992.pdf (accessed on 1 April 2023).
- Blum, J.C.; Chang, A.L.; Liljesthröm, M.; Schenk, M.E.; Steinberg, M.K.; Ruiz, G.M. The non-native solitary ascidian Ciona intestinalis (L) depresses species richness. J. Exp. Mar. Biol. Ecol. 2007, 342, 5–14. [Google Scholar] [CrossRef]
- Lutz-Collins, V.; Ramsay, A.; Quijón, P.A.; Davidson, J. Invasive tunicates fouling mussel lines: Evidence of their impact on native tunicates and other epifaunal invertebrates. Aquat. Invasions 2009, 4, 213–220. [Google Scholar] [CrossRef]
- Ordóñez, V.; Pascual, M.; Rius, M.; Turon, X. Mixed but not admixed: A spatial analysis of genetic variation of an invasive ascidian on natural and artificial substrates. Mar. Biol. 2013, 160, 1645–1660. [Google Scholar] [CrossRef]
- Ordóñez, V.; Rius, M.; McQuaid, C.D.; Pineda, M.C.; Pascual, M.; Turon, X. Early biotic interactions among introduced and native species reveal cryptic predation and shifts in larval behaviour. Mar. Ecol. Prog. Ser. 2013, 488, 65–79. [Google Scholar] [CrossRef]
- Tagliapietra, D.; Keppel, E.; Sigovini, M.; Gretchen, L. First record of the colonial ascidian Didemnum vexillum Kott, 2002 in the Mediterranean: Lagoon of Venice (Italy). BioInvasions Rec. 2012, 1, 247–254. [Google Scholar] [CrossRef]
- Casso, M.; Navarro, M.; Ordóñez, V.; Fernández-Tejedor, M.; Pascual, M.; Turonet, X. Seasonal patterns of settlement and growth of introduced and native ascidians in bivalve cultures in the Ebro Delta (NE Iberian Peninsula). Reg. Stud. Mar. Sci. 2016, 23, 12–22. [Google Scholar] [CrossRef]
- Davis, M.H.; Davis, M.E. Styela clava (Tunicata, Ascidiacea)—A new threat to the Mediterranean shellfish industry? Aquat. Invasions 2009, 4, 283–289. [Google Scholar] [CrossRef]
- Çinar, M.E. The alien ascidian Styela clava now invading the Sea of Marmara (Tunicata: Ascidiacea). ZooKeys 2016, 563, 1–10. [Google Scholar] [CrossRef]
- Ordóñez, V.; Pascual, M.; Fernández-Tejedor, M.; Turon, X. When invasion biology meets taxonomy: Clavelina oblonga (Ascidiacea) is an old invader in the Mediterranean Sea. Biol. Invasions 2016, 18, 1203–1215. [Google Scholar] [CrossRef]
- Papadopoulos, D.K.; Lattos, A.; Giantsis, I.A.; Theodorou, J.A.; Michaelidis, B.; Feidantsis, K. The impact of ascidian biofouling on the farmed Mediterranean mussel Mytilus galloprovincialis physiology and welfare, revealed by stress biomarkers. Biofouling 2023, in press. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Leech, B.S.; Perdikaris, C.; Hellio, C.; Katselis, G. Performance of the cultured Mediterranean mussel Mytilus galloprovincialis Lamark, 1819 after summer post-harvest re-immersion. Turkish J. Fish. Aquat. Sci. 2019, 19, 221–229. [Google Scholar] [CrossRef]
- Roy, H.E.; Adriaens, T.; Aldridge, D.; Bacher, S.; Bishop, J.; Blackburn, T.M.; Copp, G.H. Invasive Alien Species-Prioritising Prevention Efforts Through Horizon Scanning; ENV.B.2/ETU/2014/0016; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.; Cottier-Cook, E.J. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gómez, F.; Hoffman, R.; Jensen, K.; et al. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Fletcher, L.Μ. Ecology of Biofouling and Impacts on Mussel Aquaculture: A Case Study with Didemnum Vexillum. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2013; p. 234. Available online: https://openaccess.wgtn.ac.nz/articles/thesis/Ecology_of_Biofouling_and_Impacts_on_Mussel_Aquaculture_A_Case_Study_with_Didemnum_Vexillum/17004652/1 (accessed on 1 April 2023).
- Genovesi, P.; Scalera, R.; Brunel, S.; Solarz, W.; Roy, D. Towards an Early Warning and Information System for Invasive Alien Species (IAS) Threatening Biodiversity in Europe; Technical Report; European Environment Agency: Copenhagen, Denmark, 2010; Volume 5, p. 52. ISSN 1725–2237. Available online: https://www.eea.europa.eu/publications/information-system-invasive-alien-species/file (accessed on 1 April 2023).
- Focardi, S.; Corsi, I.; Franchi, E. Safety issues and sustainable development of European aquaculture: New tools for environmentally sound aquaculture. Aquac. Int. 2005, 13, 3–17. [Google Scholar] [CrossRef]
- Shine, C.; Kettunen, M.; Genovesi, P.; Essl, F.; Gollasch, S.; Rabitsch, W.; Scalera, R.; Starfinger, U.; ten Brink, P. Assessment to Support Continued Development of the eu Strategy to Combat Invasive Alien Species. Final Report for the European Commission; Institute for European Environmental Policy (IEEP): Brussels, Belgium, 2010; Available online: https://constantine.typepad.com/files/assessment-to-support-continued-development-of-the-eu-strategy-to-combat-invasive-alien-species.pdf (accessed on 1 April 2023).
- Katsanevakis, S.; Deriu, I.; D’Amico, F.; Nunes, A.L.; Pelaez Sanchez, S.; Crocetta, F.; Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Curto, G.; et al. European Alien Species Information Network (EASIN): Supporting European policies and scientific research. Manag. Biol. Invasions 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Antoniadou, C.; Gerovasileiou, V.; Bailly, N. Ascidiacea (Chordata: Tunicata) of Greece: An updated checklist. Biodivers. Data J. 2016, 4, e9273. [Google Scholar] [CrossRef]
- Koukouras, A.; Voultsiadou-Koukoura, Ε.; Kevrekidis, Τ.; Vafidis, D. Ascidian fauna of the Aegean Sea with a check list of eastern Mediterranean and Black Sea species. Annl. Inst. Océanogr. 1995, 71, 19–34. Available online: https://users.auth.gr/~elvoults/pdf/Ascidiacea%2095.pdf (accessed on 1 April 2023).
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.; Simboura, N.; Tsiamis, K.; Pancucci, A. Deep Cleaning of Alien and Cryptogenic Species Records in the Greek Seas (2018 Update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Morri, C.; Bianchi, C.N.; Cocito, S.; Periano, A.; DeBiase, A.M.; Aliani, S.; Pansini, M.; Boyer, M.; Ferdeghini, F.; Pestarino, M.; et al. Biodiversity of marine sessile epifauna at an Aegean island subject to hydrothermal activity: Milos, eastern Mediterranean Sea. Mar. Biol. 1999, 135, 729–739. [Google Scholar] [CrossRef]
- Antoniadou, C.; Voultsiadou, E.; Chintiroglou, C. Sublittoral megabenthos along cliffs of different profile (Aegean Sea, eastern Mediterranean). Belg. J. Zool. 2006, 136, 69–79. Available online: http://users.auth.gr/elvoults/pdf/Sublitoral%20megabenthos%2006.pdf (accessed on 1 April 2023).
- Antoniadou, C.; Voultsiadou, E.; Rayann, A.; Chintiroglou, C. Sessile biota fouling farmed mussels: Diversity, spatio-temporal patterns, and implications for the basibiont. J. Mar. Biol. Assoc. UK 2013, 93, 1593–1607. [Google Scholar] [CrossRef]
- Sini, M.; Garrabou, J.; Koutsoubas, D. Diversity and structure of coralligenous assemblages dominated by Eunicella cavolini (Koch, 1887) in the Aegean Sea. In Proceedings of the Second Mediterranean Symposium on the Conservation of Coralligenous and Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014; Bouafif, C., Langar, H., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2014. Available online: https://www.vliz.be/imisdocs/publications/345030.pdf (accessed on 1 April 2023).
- Panagiotou, M.; Antoniadou, C.; Krestenitis, Y.; Chintiroglou, C. Stock assessment of the dominant ascidians: Microcosmus savignyi, Styela plicata and Phallusia mammillata, in Thessaloniki Bay (Thermaikos Gulf). Fresenius Environ. Bull. 2007, 16, 1012–1019. [Google Scholar]
- Panagiotou, M.; Antoniadou, C.; Chintiroglou, C. Population dynamics and reproductive status of Microcosmus savignyi Monniot, 1962 (Thermaikos Gulf, Eastern Mediterranean): A preliminary assessment. J. Nat. Hist. 2008, 42, 545–558. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C.; Chintiroglou, C. Population dynamics, allometric relationships and reproductive status of Microcosmus sabatieri (Tunicata: Ascidiacea) in the Aegean Sea. J. Mar. Biol. Assoc. UK 2008, 88, 1043–1051. [Google Scholar] [CrossRef]
- Voultsiadou, E.; Pyrounaki, M.; Chintiroglou, C. The habitat engineering tunicate Microcosmus sabatieri Roule, 1885 and its associated peracarid epifauna. Estuar. Coast. Shelf Sci. 2007, 74, 197–204. [Google Scholar] [CrossRef]
- Voultsiadou, E.; Kyrodimou, M.; Antoniadou, C.; Vafidis, D. Sponge epibionts on ecosystem-engineering ascidians: The case of Microcosmus sabatieri. Estuar. Coast. Shelf Sci. 2010, 86, 598–606. [Google Scholar] [CrossRef]
- Switzer, S.E.; Therriault, T.W.; Dunham, A.; Pearce, C.M. Assessing potential control options for the invasive tunicate Didemnum vexillum in shellfish aquaculture. Aquaculture 2011, 318, 145–153. [Google Scholar] [CrossRef]
- Eurostat (2019) Production from Aquaculture Excluding Hatcheries and Nurseries (from 2008 Onwards). Available online: https://ec.europa.eu/eurostat/databrowser/product/page/fish_aq2a (accessed on 1 April 2023).
- Theodorou, J.A.; Perdikaris, C.; Filippopoulos, N.G. Evolution through innovation in Aquaculture: The case of the Hellenic mariculture industry (Greece). J. Applied Aquac. 2015, 27, 160–181. Available online: http://iraj.gr/IRAJ/Evolution_through_Innovation_in_Aquaculture_SAMPLE.pdf (accessed on 1 April 2023). [CrossRef]
- Theodorou, J.A.; Viaene, J.; Sorgeloos, P.; Tzovenis, I. Production and marketing trends of the cultured Mediterranean mussel Mytilus galloprovincialis L. 1819, in Greece. J. Shell. Res. 2011, 30, 859–874. [Google Scholar] [CrossRef]
- Konstantinou, Z.I.; Krestenitis, Y.N.; Latinopoulos, D.; Pagou, K.; Galinou-Mitsoudi, S.; Savvidis, Y. Aspects of mussel-farming activity in Chalastra, Thermaikos Gulf, Greece: An effort to untie a management Gordian knot. Ecol. Soc. 2012, 17, 1. Available online: https://www.jstor.org/stable/26268989 (accessed on 1 April 2023). [CrossRef]
- Theodorou, J.A.; Moutopoulos, D.K.; Tzovenis, I. Semi-Quantitative Risk Assessment of Mediterranean Mussel (Mytilus galloprovincialis L.) Harvesting Bans due to Harmful Algal Bloom (HAB) Incidents in Greece. Aquac. Econ. Manag. 2020, 24, 273–293. [Google Scholar] [CrossRef]
- Koutsoubas, D.; Galinou-Mitsoudi, S.; Katsanevakis, S.; Leontarakis, P.; Metaxatos, A.; Zenetos, A. Bivalve and Gastropod Molluscs of Commercial Interest for Human Consumption in the Hellenic Seas. SoHelFI, 2007. State of Hellenic Fisheries; Papaconstantinou, C., Zenetos, A., Vassilopoulou, V., Tserpes, G., Eds.; HCMR Publications: Athens, Greece, 2007; p. 466. Available online: https://www.vliz.be/imisdocs/publications/ocrd/288583.pdf (accessed on 1 April 2023).
- Fasoulas, T.A. Spat Dynamic Pattern of Cultured Mussel Mytillys galloprovincialis, Lamarck, 1819 in NW Gulf of Thessaloniki. Master’s Thesis, University of Thessaly, Thessaly, Greece, 2008; p. 123. Available online: https://ir.lib.uth.gr/xmlui/bitstream/handle/11615/1749/P0001749.pdf?sequence=1&isAllowed=y (accessed on 1 April 2023).
- Moutopoulos, D.K.; Minasidis, V.; Ziou, A.; Douligeri, A.S.; Katselis, G.; Theodorou, J.A. Investigating the Acceptance of a New Bivalve Product in the Greek Shellfish Market: The Non-Indigenous Pearl Oyster Pinctada imbricata radiata. J. Mar. Sci. Eng. 2022, 10, 251. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Thurstan, R.H.; Buckley, S.M.; Ortiz, J.C.; Pandolfi, J.M. Setting the Record Straight: Assessing the Reliability of Retrospective Accounts of Change. Conserv. Lett. 2016, 9, 98–105. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Minasidis, V.; Ziou, A.; Douligeri, A.S.; Gkikas, M.; Koutante, E.; Katselis, G.; Anagnopoulos, N.; Bourdaniotis, N.; Moutopoulos, D.K. New Product Value Chain for The Pearl Oyster Pinctada imbricata radiata: A preliminary survey. J. Mar. Sci. Eng. 2023, 11, 95. [Google Scholar] [CrossRef]
- FAO. Aquaculture Newsletter No 31. 2004. Available online: https://www.fao.org/3/y5550e/y5550e00.htm (accessed on 1 April 2023).
- Atalah, J.; Fletcher, L.M.; Davidson, I.C.; South, P.M.; Forrest, B. Artificial habitat and biofouling species distributions in an aquaculture seascape. Aquacult. Environ. Interact. 2020, 12, 495–509. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Tsagarakis, K.; Machias, A. Assessing ecological and fisheries implications of the EU landing obligation in Eastern Mediterranean. J. Sea Res. 2018, 141, 99–111. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Libralato, S.; Morissette, L.; Christensen, V. Global Patterns in Ecological Indicators of Marine Food Webs: A Modelling Approach. PLoS ONE 2014, 9, e95845. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Tzovenis, I.; Sorgeloos, P.; Viaene, J. Risk Perceptions and Risk Management Strategies of the Greek Mussel Farmers. In Proceedings of the Fifteenth Biennial Conference of the International Institute of Fisheries Economics & Trade, Montpellier, France, 13–16 July 2010; Economics of Fish Resources and Aquatic Ecosystems. Balancing Uses, Balancing Costs. Shriver, A.L., Ed.; International Institute of Fisheries Economics & Trade: Corvallis, OR, USA, 2010. ISBN 0-9763432-6-6. [Google Scholar]
- Theodorou, J.A.; Tzovenis, I.; Sorgeloos, P.; Viaene, J. An empirical study of the risk perceptions and risk management strategies of the Mediterranean mussel farmers in Greece. Oceanol. Hydrobiol. Stud. 2018, 50, 455–472. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010; p. 944. Available online: https://scholar.google.com/scholar_lookup?title=Biostatistical+Analysis&author=Zar,+J.H.&publication_year=2010 (accessed on 1 April 2023).
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. Available online: https://link.springer.com/article/10.1007/BF02310555 (accessed on 1 April 2023). [CrossRef]
- Kooij Van Der, A.J.; Meulman, J.J. Murals: Multiple Regression and Optimal Scaling Using Alternative Least Squares; Faulbaun, E., Bandilla, W., Eds.; Sofstat: Stuttgart, Germany, 1997; pp. 99–106. Available online: https://scholar.google.com/scholar_lookup?title=Murals&author=Kooij+Van+Der,+A.J.&author=Meulman,+J.J.&publication_year=1997&pages=99%E2%80%93106 (accessed on 1 April 2023).
- SPSS. SPSS BASE 27.0.1.0. Applications Guide; SPSS Inc.: Chicago, IL, USA, 2020; Available online: https://scholar.google.com/scholar_lookup?title=SPSS+BASE+27.0.1.0.+Applications+Guide&author=SPSS&publication_year=2020 (accessed on 1 April 2023).
- Sievers, M.; Dempster, T.; Keough, M.J.; Fitridge, I. Methods to prevent and treat biofouling in shellfish aquaculture. Aquaculture 2019, 505, 263–270. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef]
- Theodorou, J.; Tzovenis, I.; Adams, C.M.; Sorgeloos, P.; Viaene, J. Risk Factors Affecting the Profitability of the Mediterranean Mussel (Mytilus galloprovincialis Lamarck 1819) Farming in Greece. J. Shell Res. 2014, 33, 695–708. [Google Scholar] [CrossRef]
- Adams, C.M.; Shumway, S.E.; Whitlatch, R.B.; Getchis, T. Biofouling in marine molluscan shellfish aquaculture: A survey assessing the business and economic implications of mitigation. J. World Aquacult. Soc. 2011, 42, 242–252. [Google Scholar] [CrossRef]
- Edwards, P.K.; Leung, B. Re-evaluating eradication of nuisance species: Invasion of the tunicate, Ciona intestinalis. Front. Ecol. Environ. 2009, 7, 326–332. [Google Scholar] [CrossRef]
- Pérez Agúndez, J.A.; Raux, P.; Girard, S.; Mongruel, R. Technological adaptation to harmful algal blooms: Socioeconomic consequences for the shellfish farming sector in Bourgneuf bay (France). Aquac. Econ. Man. 2013, 17, 341–359. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, G.; Villasante, S.; do Carme García-Negro, M. Are red tides affecting economically the commercialization of the Galician (NW Spain) mussel farming? Mar. Pol. 2010, 35, 252–257. [Google Scholar] [CrossRef]
- Forrest, B.M.; Atalah, J. Significant impact from blue mussel Mytilus galloprovincialis biofouling on aquaculture production of green-lipped mussels in New Zealand. Aquacult. Environ. Interact. 2017, 9, 115–126. [Google Scholar] [CrossRef]
- Ordóñez, V.; Pascual, M.; Fernández-Tejedor, M.; Pineda, M.; Tagliapietra, D.; Turon, X. Ongoing expansion of the worldwide invader Didemnum vexillum (Ascidiacea) in the Mediterranean Sea: High plasticity of its biological cycle promotes establishment in warm waters. Biol. Invasions 2015, 17, 2075–2085. [Google Scholar] [CrossRef]
- Watts, A.M.; Hopkins, G.A.; Goldstien, S.J. Chimerism and population dieback alter genetic inference related to invasion pathways and connectivity of biofouling populations on artificial substrata. Ecol. Evol. 2019, 9, 3089–3104. [Google Scholar] [CrossRef]
- Ignatiades, L.l.; Gotsis-Skretas, O. Review on toxic and harmful algae in Greek coastal Waters (E. Mediterranean Sea). Toxins 2010, 2, 1019–1037. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Ioannis Tzovenis, I. A framework for risk analysis of the shellfish aquaculture: The case of the Mediterranean mussel farming in Greece. Aquac. Fish. 2021, 8, 375–384. [Google Scholar] [CrossRef]
- Longo, C.; Pontassuglia, C.; Corriero, G.; Gaino, E. Life-cycle traits of Paraleucilla magna, a calcareous sponge invasive in a coastal Mediterranean Basin. PLoS ONE 2012, 7, e42392. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsotsios, D.; Moutopoulos, D.K.; Lattos, A.; Michaelidis, B.; Theodorou, J.A. Impacts of the Establishment of Biofoulants on Greek Aquaculture: Farmers’ Expert Knowledge. J. Mar. Sci. Eng. 2023, 11, 1077. https://doi.org/10.3390/jmse11051077
Tsotsios D, Moutopoulos DK, Lattos A, Michaelidis B, Theodorou JA. Impacts of the Establishment of Biofoulants on Greek Aquaculture: Farmers’ Expert Knowledge. Journal of Marine Science and Engineering. 2023; 11(5):1077. https://doi.org/10.3390/jmse11051077
Chicago/Turabian StyleTsotsios, Dimitrios, Dimitrios K. Moutopoulos, Athanasios Lattos, Basile Michaelidis, and John A. Theodorou. 2023. "Impacts of the Establishment of Biofoulants on Greek Aquaculture: Farmers’ Expert Knowledge" Journal of Marine Science and Engineering 11, no. 5: 1077. https://doi.org/10.3390/jmse11051077
APA StyleTsotsios, D., Moutopoulos, D. K., Lattos, A., Michaelidis, B., & Theodorou, J. A. (2023). Impacts of the Establishment of Biofoulants on Greek Aquaculture: Farmers’ Expert Knowledge. Journal of Marine Science and Engineering, 11(5), 1077. https://doi.org/10.3390/jmse11051077






