No Observed Effects of Subsea Renewable Energy Infrastructure on Benthic Environments
Abstract
1. Introduction
2. Materials and Methods
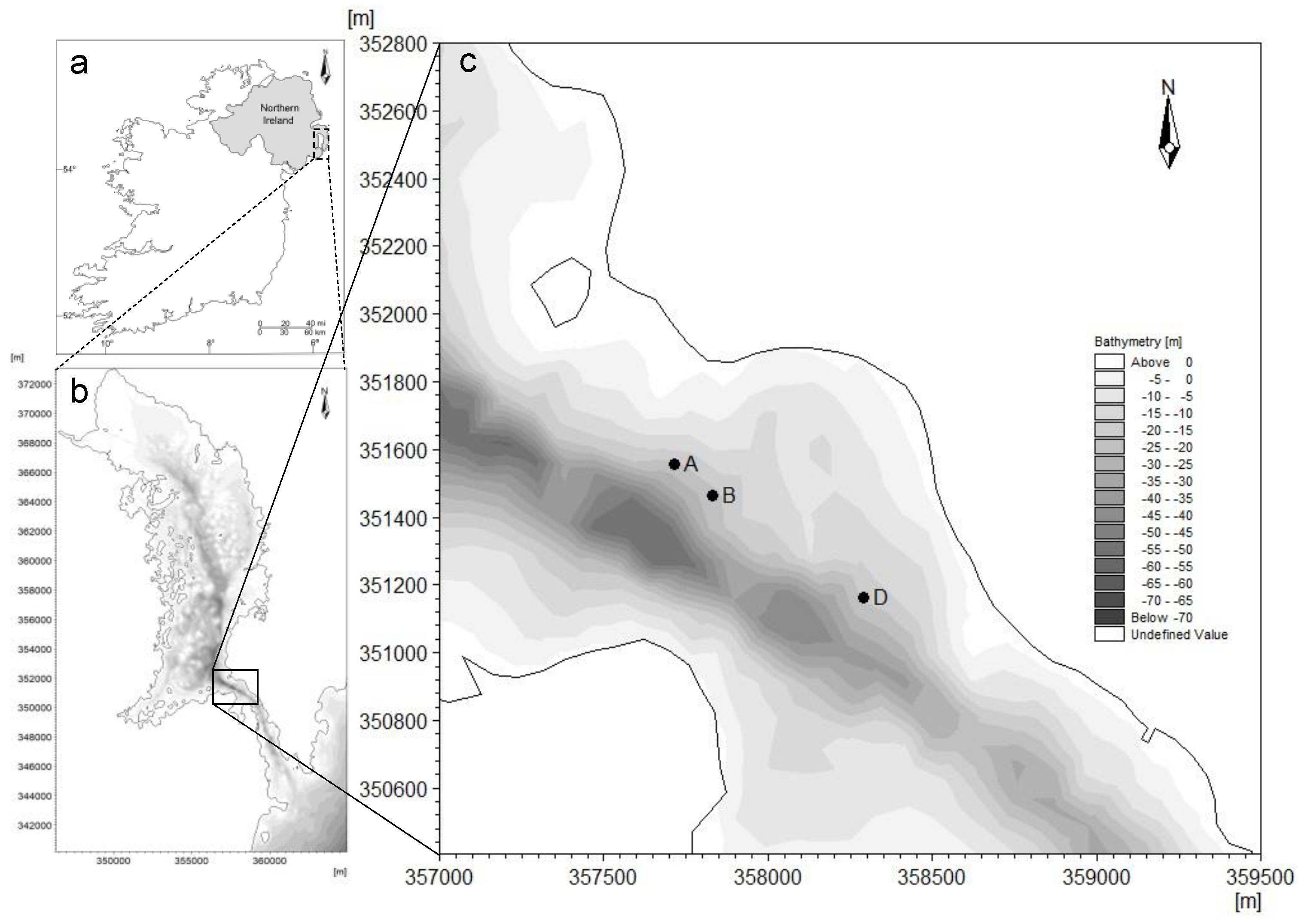
2.1. Site Selection
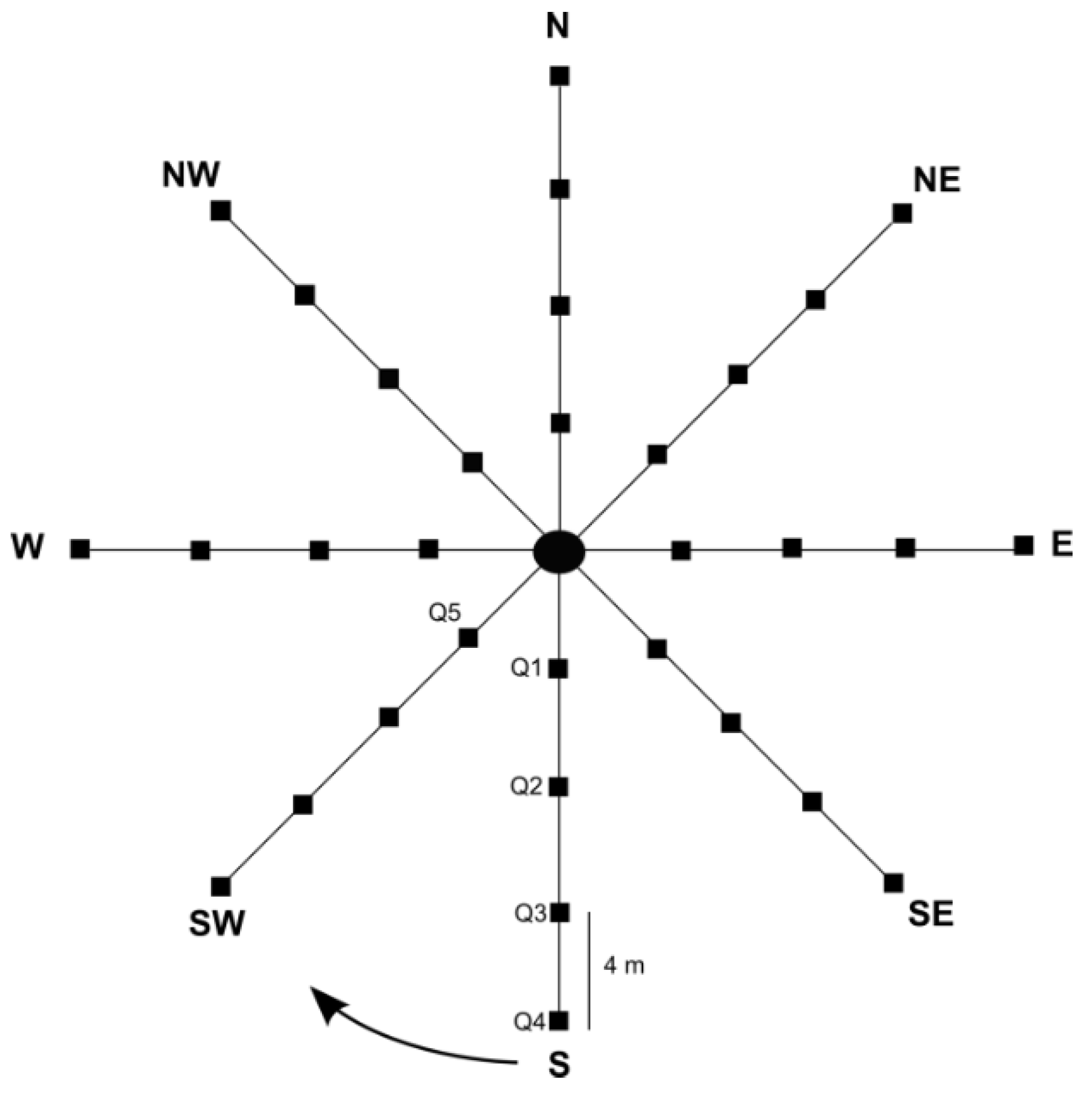
2.2. Survey Techniques
2.3. Data Analysis
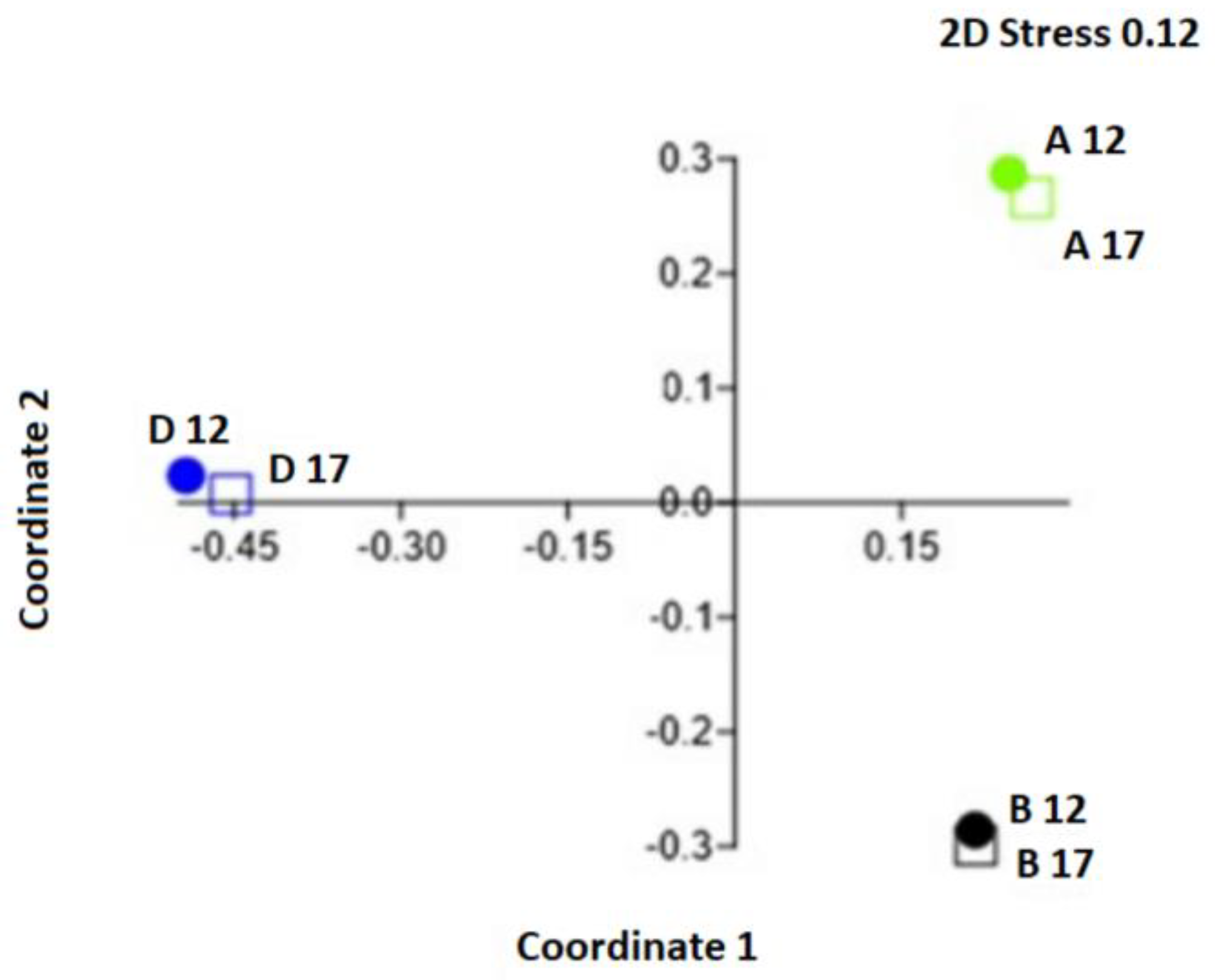
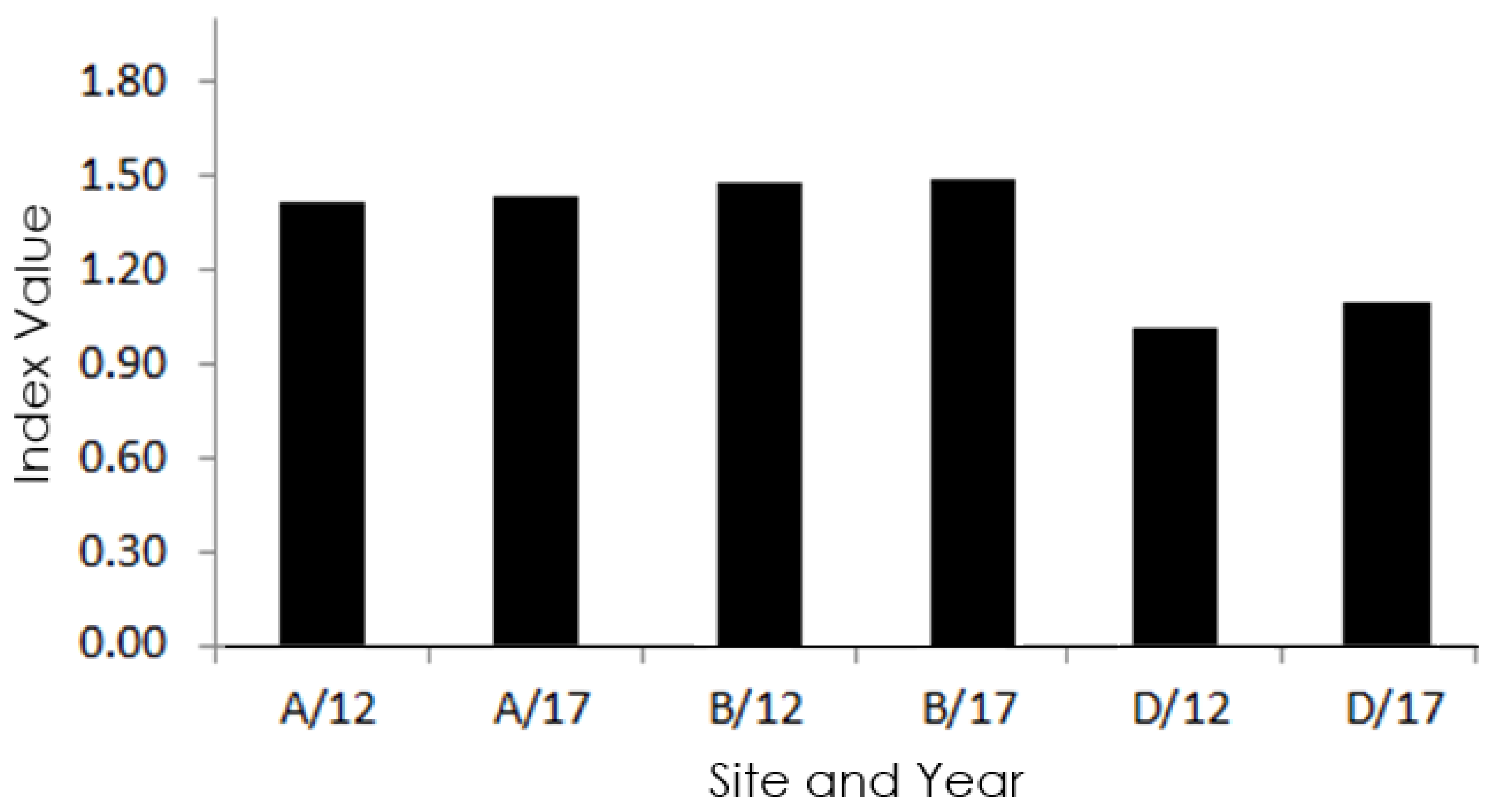
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. 2018: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; 616p. [Google Scholar] [CrossRef]
- Rosenberg, R. Eutrophication—The future marine coastal nuisance? Mar. Pollut. Bull. 1985, 16, 227–231. [Google Scholar] [CrossRef]
- Williams, R.; Wright, A.; Ashe, E.; Blight, L.; Bruintjes, R.; Canessa, R.; Clark, C.; Cullis-Suzuki, S.; Dakin, D.; Erbe, C.; et al. Impacts of anthropogenic noise on marine life: Publication patterns, new discoveries, and future directions in research and management. Ocean Coast. Manag. 2015, 115, 17–24. [Google Scholar] [CrossRef]
- Pine, M.K.; Schmitt, P.; Culloch, R.M.; Lieber, L.; Kregting, L.T. Providing ecological context to anthropogenic subsea noise: Assessing listening space reductions of marine mammals from tidal energy devices. Renew. Sustain. Energy Rev. 2018, 103, 49–57. [Google Scholar] [CrossRef]
- Gill, A.B. Offshore renewable energy: Ecological implications of generating electricity in the coastal zone. J. Appl. Ecol. 2005, 42, 605–615. [Google Scholar] [CrossRef]
- Segura, E.; Morales, R.; Somolinos, J. A strategic analysis of tidal current energy conversion systems in the European Union. Appl. Energy 2018, 212, 527–551. [Google Scholar] [CrossRef]
- Kregting, L.; Elsaesser, B.; Kennedy, R.; Smyth, D.; O’carroll, J.; Savidge, G. Do Changes in Current Flow as a Result of Arrays of Tidal Turbines Have an Effect on Benthic Communities? PLoS ONE 2016, 11, e0161279. [Google Scholar] [CrossRef]
- Connell, S.; Glasby, T. Do urban structures influence local abundance and diversity of subtidal epibiota? A case study from Sydney Harbour, Australia. Mar. Environ. Res. 1999, 47, 373–387. [Google Scholar] [CrossRef]
- Causon, P.D.; Gill, A.B. Linking ecosystem services with epibenthic biodiversity change following installation of offshore wind farms. Environ. Sci. Policy 2018, 89, 340–347. [Google Scholar] [CrossRef]
- Raoux, A.; Robin, I.; Pezy, J.-P.; Bennis, A.-C.; Dauvin, J.-C. Multi-Disciplinary and Multi-Scale Assessment of Marine Renewable Energy Structure in a Tidal System. J. Energy Power Technol. 2021, 3, 16. [Google Scholar] [CrossRef]
- Langhamer, O. Artificial Reef Effect in relation to Offshore Renewable Energy Conversion: State of the Art. Sci. World J. 2012, 2012, 386713. [Google Scholar] [CrossRef]
- Koschier, D.; Bender, J.; Solenthaler, B.; Teschner, M. Smoothed particle hydrodynamics techniques for the physics based simulation of fluids and solids. arXiv 2020, arXiv:2009.06944. [Google Scholar] [CrossRef]
- Ashley, M.; Mangi, S.; Rodwell, L. The potential of offshore windfarms to act as marine protected areas—A systematic review of current evidence. Mar. Policy 2014, 45, 301–309. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Zerebecki, R.A. Ocean warming increases threat of invasive species in a marine fouling community. Ecology 2010, 91, 2198–2204. [Google Scholar] [CrossRef]
- Bulleri, F.; Airoldi, L. Artificial marine structures facilitate the spread of a non-indigenous green alga, Codium fragile ssp. tomentosoides, in the north Adriatic Sea. J. Appl. Ecol. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Glasby, T.M.; Connell, S.; Holloway, M.G.; Hewitt, C. Nonindigenous biota on artificial structures: Could habitat creation facilitate biological invasions? Mar. Biol. 2006, 151, 887–895. [Google Scholar] [CrossRef]
- Zambrano, C. Lessons learned from subsea tidal kite quarter scale ocean trials. In WTE16—Second Workshop on Wave and Tidal Energy; Valdivia, Chile, 2016. [Google Scholar]
- Guy, C.; Smyth, D.; Roberts, D. The importance of population density and inter-individual distance in conserving the European oyster Ostrea edulis. J. Mar. Biol. Assoc. UK 2018, 99, 587–593. [Google Scholar] [CrossRef]
- Smyth, D.; Mahon, A.M.; Roberts, D.; Kregting, L. Settlement of Ostrea edulis is determined by the availability of hard substrata rather than by its nature: Implications for stock recovery and restoration of the European oyster. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 662–671. [Google Scholar] [CrossRef]
- Smyth, D. The Conservation of Habitats and Species Regulations 2010 Legislation; Government UK: London, UK, 2010; pp. 35–39. [Google Scholar]
- Pikesley, S.K.; Solandt, J.-L.; Trundle, C.; Witt, M.J. Benefits beyond ‘features’: Cooperative monitoring highlights MPA value for enhanced seabed integrity. Mar. Policy 2021, 134, 104801. [Google Scholar] [CrossRef]
- Keenan, R.J.; Kimmins, J.P. The ecological effects of clear-cutting. Environ. Rev. 1993, 1, 121–144. [Google Scholar] [CrossRef]
- Hemery, L.G. Changes in Benthic and Pelagic Habitats Caused by Marine Renewable Energy Devices. In 2020 State of the Science Report; Pacific Northwest National Lab.: Richland, WA, USA, 2020. [Google Scholar] [CrossRef]
- Carleton, J.H.; Done, T.J. Quantitative video sampling of coral reef benthos: Large-scale application. Coral Reefs 1995, 14, 35–46. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Frederiksen, M.; Mavor, R.; Wanless, S. Seabirds as environmental indicators: The advantages of combining data sets. Mar. Ecol. Prog. Ser. 2007, 352, 205–211. [Google Scholar] [CrossRef]
- Bento, N.L.; Ferraz, G.A.E.S.; Barata, R.A.P.; Santana, L.S.; Barbosa, B.D.S.; Conti, L.; Becciolini, V.; Rossi, G. Overlap influence in images obtained by an unmanned aerial vehicle on a digital terrain model of altimetric precision. Eur. J. Remote Sens. 2022, 55, 263–276. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological Statistics, version 1; Palaeontologia Electronica: online, 2009; p. 92. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities. An Approach to Statistical Analysis and Interpretation; Primer-E Ltd.: Plymouth, UK, 2001; Volume 2, pp. 1–68. [Google Scholar]
- O’carroll, J.; Kennedy, R.; Savidge, G. Identifying relevant scales of variability for monitoring epifaunal reef communities at a tidal energy extraction site. Ecol. Indic. 2017, 73, 388–397. [Google Scholar] [CrossRef]
- Bender, A.; Langhamer, O.; Sundberg, J. Colonisation of wave power foundations by mobile mega- and macrofauna—A 12 year study. Mar. Environ. Res. 2020, 161, 105053. [Google Scholar] [CrossRef] [PubMed]
- Taormina, B.; Percheron, A.; Marzloff, M.P.; Caisey, X.; Quillien, N.; Lejart, M.; Desroy, N.; Dugornay, O.; Tancray, A.; Carlier, A. Succession in epibenthic communities on artificial reefs associated with marine renewable energy facilities within a tide-swept environment. ICES J. Mar. Sci. 2020, 77, 2656–2668. [Google Scholar] [CrossRef]
- Shields, M.A.; Woolf, D.K.; Grist, E.P.; Kerr, S.A.; Jackson, A.; Harris, R.E.; Bell, M.C.; Beharie, R.; Want, A.; Osalusi, E.; et al. Marine renewable energy: The ecological implications of altering the hydrodynamics of the marine environment. Ocean Coast. Manag. 2011, 54, 2–9. [Google Scholar] [CrossRef]
- Miller, R.G.; Hutchison, Z.L.; Macleod, A.K.; Burrows, M.T.; Cook, E.J.; Last, K.S.; Wilson, B. Marine renewable energy development: Assessing the Benthic Footprint at multiple scales. Front. Ecol. Environ. 2013, 11, 433–440. [Google Scholar] [CrossRef]
- Newman, S.P.; Meesters, E.H.; Dryden, C.S.; Williams, S.M.; Sanchez, C.; Mumby, P.J.; Polunin, N.V.C. Reef flattening effects on total richness and species responses in the Caribbean. J. Anim. Ecol. 2015, 84, 1678–1689. [Google Scholar] [CrossRef]
- Bernstein, B.B. Decision framework for platform decommissioning in California. Integr. Environ. Assess. Manag. 2015, 11, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Leijon, M.; Boström, C.; Danielsson, O.; Gustafsson, S.; Haikonen, K.; Langhamer, O.; Strömstedt, E.; Stålberg, M.; Sundberg, J.; Svensson, O.; et al. Wave Energy from the North Sea: Experiences from the Lysekil Research Site. Surv. Geophys. 2008, 29, 221–240. [Google Scholar] [CrossRef]
| % Cover | Density | SACFOR Scale |
|---|---|---|
| >80% | >10,000/m2 | S = Superabundant |
| 40–79% | 1000–9999/m2 | A = Abundant |
| 20–39% | 100–999/m2 | C = Common |
| 10–19% | 10–99/m2 | F = Frequent |
| 5–9% | 1–9/m2 | O = Occasional |
| 1–5% | 1–9/10 m2 | R = Rare |
| Site A 2012 | Site A 2017 | Site B 2012 | Site B 2017 | Site D 2012 | Site D 2017 | |
|---|---|---|---|---|---|---|
| Phylum | ||||||
| ANNELIDA | ||||||
| Spirobis spirobis | R | R | R | R | - | - |
| Spirobranchus lamarcki | - | R | - | R | - | R |
| Spirobranchus triqueter | R | O | A | A | R | R |
| ARTHROPODA | ||||||
| Balanus crenatus | - | F | F | A | - | - |
| Cancer pagurus | R | - | R | - | - | O |
| Liocarcinus puber | - | R | - | - | - | - |
| BRYOZOA | ||||||
| Alcyonidium diaphanum | R | R | O | R | - | - |
| Alcyonium digitatum | C | A | R | O | F | F |
| Flustra foliacea | - | C | F | F | - | - |
| CHORDATA | ||||||
| Ctenolabrus rupestris | - | R | R | - | - | - |
| Dendrodoa grossularia | - | - | - | R | - | - |
| Pholis gunnellus | - | R | - | - | - | R |
| CNIDARIA | ||||||
| Actinothoe sphyrodeta | - | R | - | - | - | - |
| Corynactis viridis | R | R | - | - | - | - |
| Sagartia elegans | R | C | F | R | R | R |
| Tubularia indivisa | R | O | R | R | R | O |
| Urticina felina | R | O | - | - | - | R |
| ECHINODERMATA | ||||||
| Asterias rubens | O | - | O | R | - | - |
| Echinus esculentus | - | R | R | R | R | O |
| Henricia oculata | - | - | R | R | - | - |
| Marthasterias glacialis | - | R | - | R | - | - |
| Solaster popposus | - | R | R | O | R | F |
| MOLLUSCA | ||||||
| Calliostoma zizyphinum | - | R | - | - | - | - |
| Gibbula cineraria | - | - | - | - | - | R |
| Nucella lapillus | - | R | - | - | - | - |
| OCHROPHYTA | ||||||
| Laminaria hyperborea | - | R | - | - | - | - |
| PORIFERA | ||||||
| Cliona celata | - | - | R | R | - | - |
| Esperiopsis fucorum | R | R | R | - | - | - |
| Halichondria panicea | O | F | O | O | O | O |
| Myxilla incrustans | R | R | - | - | - | - |
| Myxilla fimbriata | R | R | R | - | - | - |
| Pachymatisma johnstonia | R | R | - | R | - | - |
| RHODOPHYTA | ||||||
| Lithothamnion sp. | O | O | A | A | O | R |
| Taxon | Agility | Av. Dissim. | Contrib. % | Cumul. % |
|---|---|---|---|---|
| Macroalgae | Sessile | 28.59 | 30.5 | 28.74 |
| Bryozoa | Sessile | 24.8 | 26.58 | 24.93 |
| Porifera | Sessile | 15.56 | 17.37 | 15.64 |
| Annelida | Vagile | 5.81 | 6.94 | 5.84 |
| Cnidaria | Sessile/Mobile | 4.89 | 5.84 | 4.91 |
| Arthropoda | Mobile | 6.55 | 5.43 | 6.57 |
| Hydrozoa | Sessile | 4.58 | 4.98 | 4.6 |
| Echinoid | Vagile | 3.89 | 2.16 | 3.9 |
| Fish | Mobile | 1.02 | 0.1 | 1.02 |
| Mollusca | Vagile | 1.4 | 0.05 | 1.4 |
| Ascidian | Vagile | 2.49 | 0.04 | 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyth, D.; Kregting, L. No Observed Effects of Subsea Renewable Energy Infrastructure on Benthic Environments. J. Mar. Sci. Eng. 2023, 11, 1061. https://doi.org/10.3390/jmse11051061
Smyth D, Kregting L. No Observed Effects of Subsea Renewable Energy Infrastructure on Benthic Environments. Journal of Marine Science and Engineering. 2023; 11(5):1061. https://doi.org/10.3390/jmse11051061
Chicago/Turabian StyleSmyth, David, and Louise Kregting. 2023. "No Observed Effects of Subsea Renewable Energy Infrastructure on Benthic Environments" Journal of Marine Science and Engineering 11, no. 5: 1061. https://doi.org/10.3390/jmse11051061
APA StyleSmyth, D., & Kregting, L. (2023). No Observed Effects of Subsea Renewable Energy Infrastructure on Benthic Environments. Journal of Marine Science and Engineering, 11(5), 1061. https://doi.org/10.3390/jmse11051061







