Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta
Abstract
1. Introduction
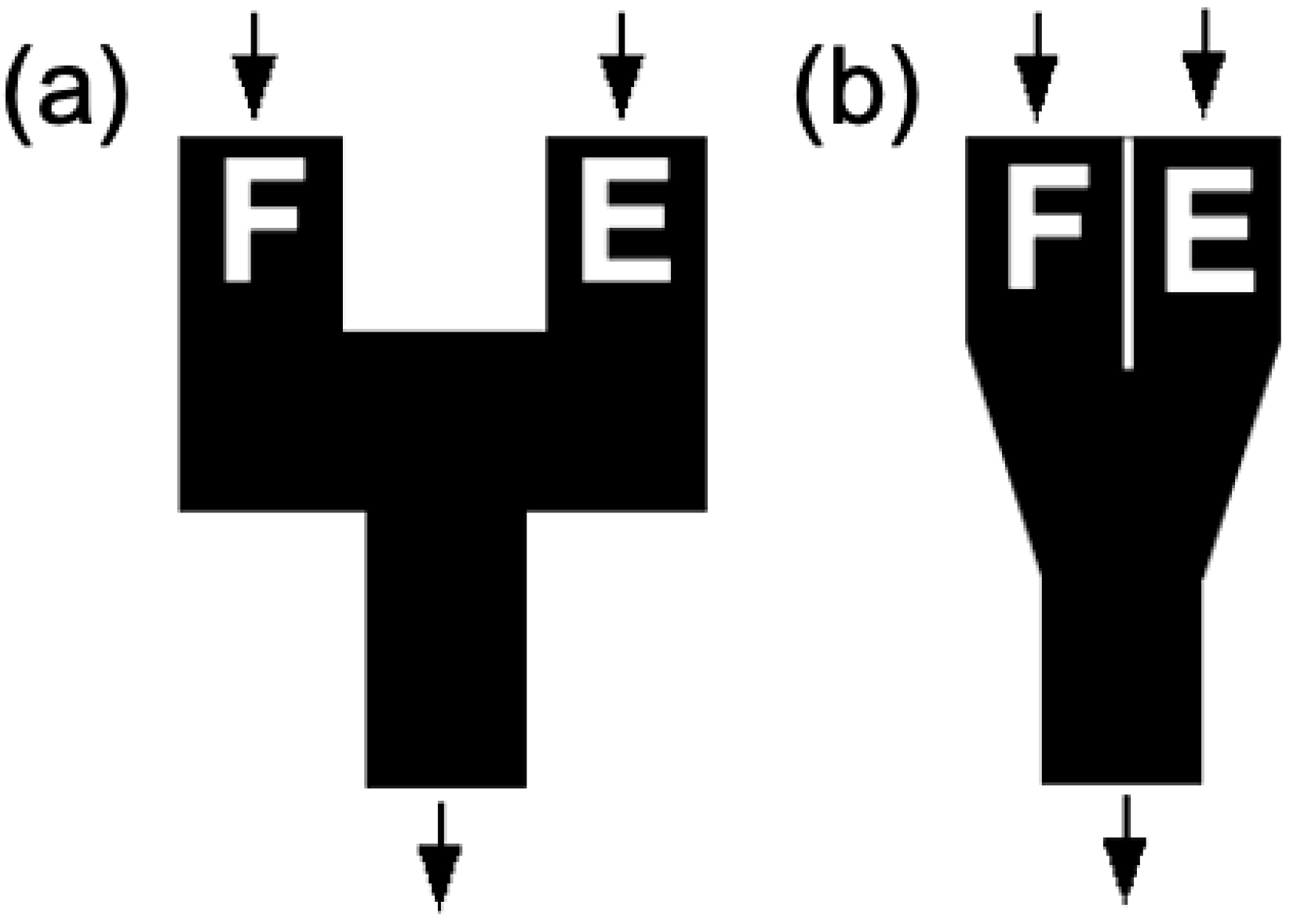
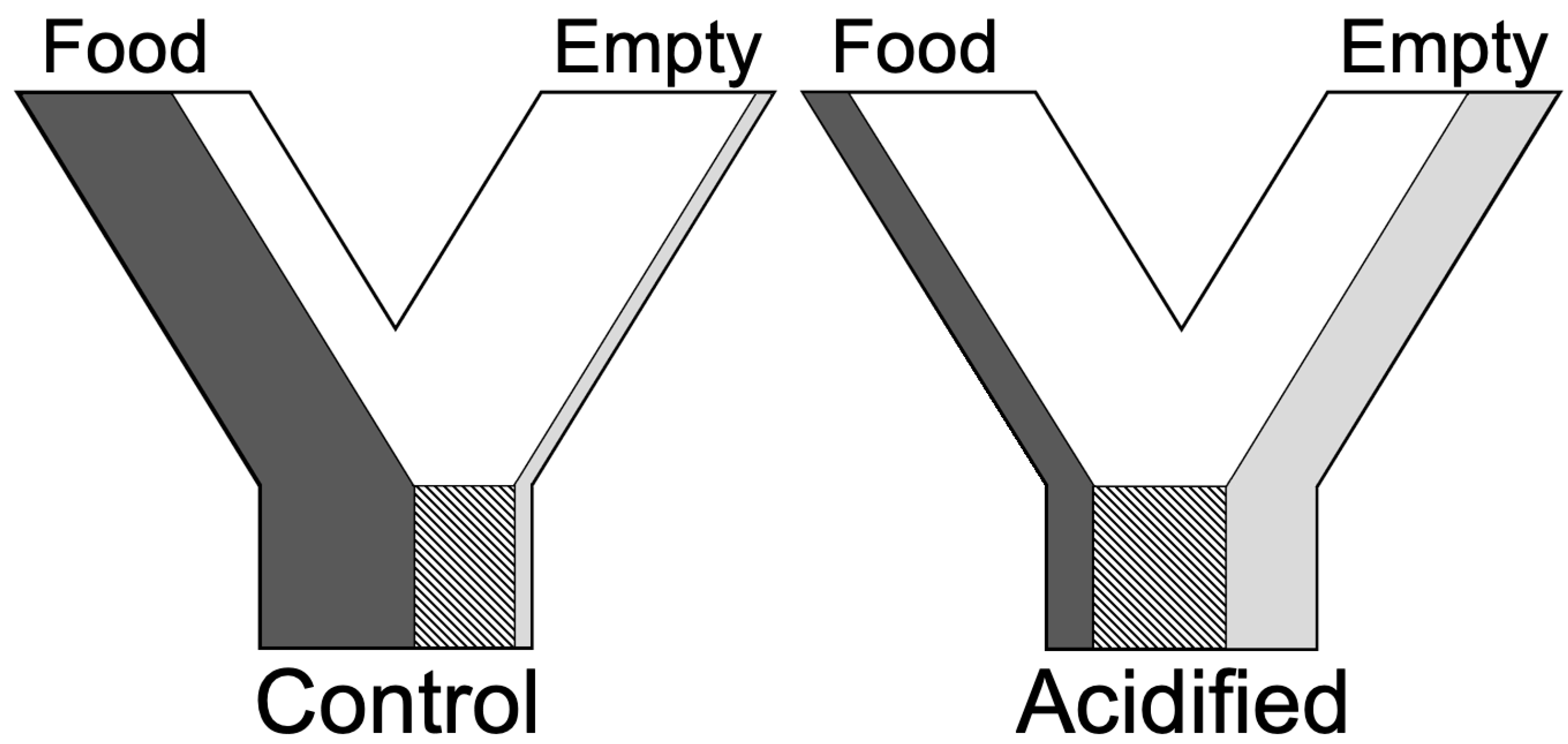
2. Materials and Methods
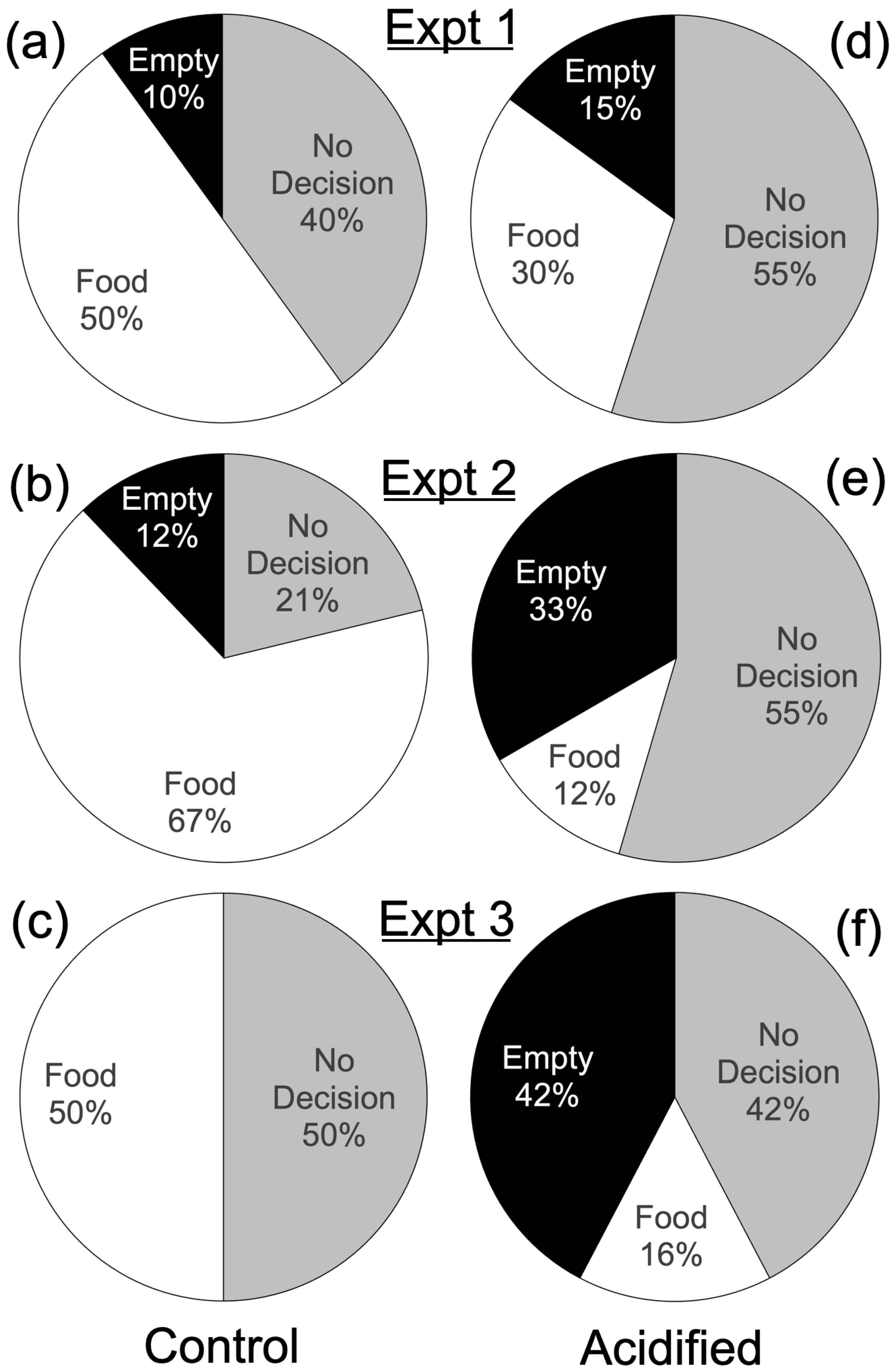
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Expt. | Length of Expt. | Treatment | Empty | No Decision | Food | Fisher’s Exact Test Results | |
|---|---|---|---|---|---|---|---|
| n | p | ||||||
| 1 | 20 min | Acid | 3 | 11 | 6 | 40 | 0.55 |
| Control | 2 | 8 | 10 | ||||
| 2 | 10 min | Acid | 11 | 18 | 4 | 66 | <0.001 |
| Control | 4 | 7 | 22 | ||||
| 3 | 5 min | Acid | 11 | 11 | 4 | 42 | 0.002 |
| Control | 0 | 8 | 8 | ||||
| All decisions under 5 min | Acid | 11 | n/a | 4 | 47 | <0.001 | |
| Control | 5 | 27 | |||||
References
- Kwiatkowski, L.; Torres, O.; Bopp, L.; Aumont, O.; Chamberlain, M.; Christian, J.R.; Dunne, J.P.; Gehlen, M.; Ilyina, T.; John, J.G.; et al. Twenty-First Century Ocean Warming, Acidification, Deoxygenation, and Upper-Ocean Nutrient and Primary Production Decline from CMIP6 Model Projections. Biogeosciences 2020, 17, 3439–3470. [Google Scholar] [CrossRef]
- Terhaar, J.; Kwiatkowski, L.; Bopp, L. Emergent Constraint on Arctic Ocean Acidification in the Twenty-First Century. Nature 2020, 582, 379–383. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core writing team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151.
- Kaniewska, P.; Campbell, P.R.; Kline, D.I.; Rodriguez-Lanetty, M.; Miller, D.J.; Dove, S.; Hoegh-Guldberg, O. Major Cellular and Physiological Impacts of Ocean Acidification on a Reef Building Coral. PLoS ONE 2012, 7, e34659. [Google Scholar] [CrossRef] [PubMed]
- Doo, S.S.; Kealoha, A.; Andersson, A.; Cohen, A.L.; Hicks, T.L.; Johnson, Z.I.; Long, M.H.; McElhany, P.; Mollica, N.; Shamberger, K.E.F.; et al. The Challenges of Detecting and Attributing Ocean Acidification Impacts on Marine Ecosystems. ICES J. Mar. Sci. 2020, 77, 2411–2422. [Google Scholar] [CrossRef]
- Feely, R.A.; Alin, S.R.; Newton, J.; Sabine, C.L.; Warner, M.; Devol, A.; Krembs, C.; Maloy, C. The Combined Effects of Ocean Acidification, Mixing, and Respiration on PH and Carbonate Saturation in an Urbanized Estuary. Estuar. Coast. Shelf Sci. 2010, 88, 442–449. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-Frequency Dynamics of Ocean PH: A Multi-Ecosystem Comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef]
- Cai, W.-J.; Feely, R.A.; Testa, J.M.; Li, M.; Evans, W.; Alin, S.R.; Xu, Y.-Y.; Pelletier, G.; Ahmed, A.; Greeley, D.J.; et al. Natural and Anthropogenic Drivers of Acidification in Large Estuaries. Annu. Rev. Mar. Sci. 2021, 13, 23–55. [Google Scholar] [CrossRef]
- Jian, S.; Zhang, J.; Zhang, H.-H.; Yang, G.-P. Effects of Ocean Acidification and Short-Term Light/Temperature Stress on Biogenic Dimethylated Sulfur Compounds Cycling in the Changjiang River Estuary. Environ. Chem. 2019, 16, 197. [Google Scholar] [CrossRef]
- Scanes, E.; Scanes, P.R.; Ross, P.M. Climate Change Rapidly Warms and Acidifies Australian Estuaries. Nat. Commun. 2020, 11, 1803. [Google Scholar] [CrossRef]
- Moyle, P.B.; Bennett, W.A.; Fleenor, W.E.; Lund, J.R. Habitat Variability and Complexity in the Upper San Francisco Estuary. San Fr. Estuary Watershed Sci. 2010, 8. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Russell, B.D.; Connell, S.D.; Ng, J.C.Y.; Lo, M.M.Y. Acid Dulls the Senses: Impaired Locomotion and Foraging Performance in a Marine Mollusc. Anim. Behav. 2015, 106, 223–229. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Comeau, S.; Kornder, N.A.; Perry, C.T.; van Hooidonk, R.; DeCarlo, T.M.; Pratchett, M.S.; Anderson, K.D.; Browne, N.; Carpenter, R.; et al. Global Declines in Coral Reef Calcium Carbonate Production under Ocean Acidification and Warming. Proc. Natl. Acad. Sci. USA 2021, 118, e2015265118. [Google Scholar] [CrossRef]
- Hyun, B.; Kim, J.-M.; Jang, P.-G.; Jang, M.-C.; Choi, K.-H.; Lee, K.; Yang, E.J.; Noh, J.H.; Shin, K. The Effects of Ocean Acidification and Warming on Growth of a Natural Community of Coastal Phytoplankton. J. Mar. Sci. Eng. 2020, 8, 821. [Google Scholar] [CrossRef]
- Lord, J.; Harper, E.; Barry, J. Ocean Acidification May Alter Predator-Prey Relationships and Weaken Nonlethal Interactions between Gastropods and Crabs. Mar. Ecol. Prog. Ser. 2019, 616, 83–94. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium Carbonate Formation and Dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic Ocean Acidification over the Twenty-First Century and Its Impact on Calcifying Organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Pistevos, J.C.A.; Nagelkerken, I.; Rossi, T.; Olmos, M.; Connell, S.D. Ocean Acidification and Global Warming Impair Shark Hunting Behaviour and Growth. Sci. Rep. 2015, 5, 16293. [Google Scholar] [CrossRef]
- Rosa, R.; Baptista, M.; Lopes, V.M.; Pegado, M.R.; Ricardo Paula, J.; Trübenbach, K.; Leal, M.C.; Calado, R.; Repolho, T. Early-Life Exposure to Climate Change Impairs Tropical Shark Survival. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141738. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Furnas, M.; Lønborg, C. Coral Reefs on the Edge? Carbon Chemistry on Inshore Reefs of the Great Barrier Reef. PLoS ONE 2014, 9, e109092. [Google Scholar] [CrossRef]
- Zhan, Y.; Cui, D.; Xing, D.; Zhang, J.; Zhang, W.; Li, Y.; Li, C.; Chang, Y. CO2-Driven Ocean Acidification Repressed the Growth of Adult Sea Urchin Strongylocentrotus intermedius by Impairing Intestine Function. Mar. Pollut. Bull. 2020, 153, 110944. [Google Scholar] [CrossRef]
- Froehlich, K.R.; Lord, J.P. Can Ocean Acidification Interfere with the Ability of Mud Snails (Tritia obsoleta) to Sense Predators? J. Exp. Mar. Biol. Ecol. 2020, 526, 151355. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Martin, R.A.; Fallows, C. Effects of Environmental Conditions on Predator–Prey Interactions between White Sharks (Carcharodon carcharias) and Cape Fur Seals (Arctocephalus pusillus pusillus) at Seal Island, South Africa. Environ. Biol. Fishes 2006, 76, 341–350. [Google Scholar] [CrossRef]
- Wisenden, B.D. Olfactory Assessment of Predation Risk in the Aquatic Environment. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1205–1208. [Google Scholar] [CrossRef]
- Jiahuan, R.; Wenhao, S.; Xiaofan, G.; Wei, S.; Shanjie, Z.; Maolong, H.; Haifeng, W.; Guangxu, L. Ocean Acidification Impairs Foraging Behavior by Interfering with Olfactory Neural Signal Transduction in Black Sea Bream, Acanthopagrus schlegelii. Front. Physiol. 2018, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.-A.; Lefevre, S.; McCormick, M.I.; Domenici, P.; Nilsson, G.E.; Munday, P.L. Marine Mollusc Predator-Escape Behaviour Altered by near-Future Carbon Dioxide Levels. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132377. [Google Scholar] [CrossRef] [PubMed]
- Manríquez, P.; Jara, M.; Mardones, M.; Torres, R.; Navarro, J.; Lardies, M.; Vargas, C.; Duarte, C.; Lagos, N. Ocean Acidification Affects Predator Avoidance Behaviour but Not Prey Detection in the Early Ontogeny of a Keystone Species. Mar. Ecol. Prog. Ser. 2014, 502, 157–167. [Google Scholar] [CrossRef]
- Queirós, A.M.; Fernandes, J.A.; Faulwetter, S.; Nunes, J.; Rastrick, S.P.S.; Mieszkowska, N.; Artioli, Y.; Yool, A.; Calosi, P.; Arvanitidis, C.; et al. Scaling up Experimental Ocean Acidification and Warming Research: From Individuals to the Ecosystem. Glob. Chang. Biol. 2015, 21, 130–143. [Google Scholar] [CrossRef]
- Clements, J.C.; Hunt, H.L. Marine Animal Behaviour in a High CO2 Ocean. Mar. Ecol. Prog. Ser. 2015, 536, 259–279. [Google Scholar] [CrossRef]
- Chatzinikolaou, E.; Grigoriou, P.; Martini, E.; Sterioti, A. Impact of Ocean Acidification and Warming on the Feeding Behaviour of Two Gastropod Species. Mediterr. Mar. Sci. 2019, 20, 669. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Levinton, J.S.; Matthew Hoch, J. Foraging by the Mud Snail, Ilyanassa obsoleta (Say), Modulates Spatial Variation in Benthic Community Structure. J. Exp. Mar. Biol. Ecol. 2003, 292, 139–157. [Google Scholar] [CrossRef]
- Atema, J.; Burd, G.D. A Field Study of Chemotactic Responses of the Marine Mud Snail, Nassarius obsoletus. J. Chem. Ecol. 1975, 1, 243–251. [Google Scholar] [CrossRef]
- Coffin, M.R.S.; Drolet, D.; Hamilton, D.J.; Barbeau, M.A. Effect of Immersion at Low Tide on Distribution and Movement of the Mud Snail, Ilyanassa obsoleta (Say), in the Upper Bay of Fundy, Eastern Canada. J. Exp. Mar. Biol. Ecol. 2008, 364, 110–115. [Google Scholar] [CrossRef]
- Trott, T.J.; Dimock, R.V. Intraspecific Trail Following by the Mud Snail Ilyanassa obsoleta. Mar. Behav. Physiol. 1978, 5, 91–101. [Google Scholar] [CrossRef]
- Bibby, R.; Cleall-Harding, P.; Rundle, S.; Widdicombe, S.; Spicer, J. Ocean Acidification Disrupts Induced Defences in the Intertidal Gastropod Littorina littorea. Biol. Lett. 2007, 3, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Jellison, B.M.; Ninokawa, A.T.; Hill, T.M.; Sanford, E.; Gaylord, B. Ocean Acidification Alters the Response of Intertidal Snails to a Key Sea Star Predator. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160890. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols; Guest, P.C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 105–111. ISBN 978-1-4939-8994-2. [Google Scholar]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean Acidification Impairs Olfactory Discrimination and Homing Ability of a Marine Fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Nilsson, G.E.; Dixson, D.L.; Domenici, P.; McCormick, M.I.; Sørensen, C.; Watson, S.-A.; Munday, P.L. Near-Future Carbon Dioxide Levels Alter Fish Behaviour by Interfering with Neurotransmitter Function. Nat. Clim. Chang. 2012, 2, 201–204. [Google Scholar] [CrossRef]
- Briffa, M.; de la Haye, K.; Munday, P.L. High CO2 and Marine Animal Behaviour: Potential Mechanisms and Ecological Consequences. Mar. Pollut. Bull. 2012, 64, 1519–1528. [Google Scholar] [CrossRef]
- Dodd, L.F.; Grabowski, J.H.; Piehler, M.; Westfield, I.; Ries, J.B. Ocean Acidification Impairs Crab Foraging Behaviour. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150333. [Google Scholar] [CrossRef]
- de la Haye, K.L.; Spicer, J.I.; Widdicombe, S.; Briffa, M. Reduced Sea Water PH Disrupts Resource Assessment and Decision Making in the Hermit Crab Pagurus bernhardus. Anim. Behav. 2011, 82, 495–501. [Google Scholar] [CrossRef]
- Booth, J.A.T.; McPhee-Shaw, E.E.; Chua, P.; Kingsley, E.; Denny, M.; Phillips, R.; Bograd, S.J.; Zeidberg, L.D.; Gilly, W.F. Natural Intrusions of Hypoxic, Low PH Water into Nearshore Marine Environments on the California Coast. Cont. Shelf Res. 2012, 45, 108–115. [Google Scholar] [CrossRef]
- Wallace, R.B.; Baumann, H.; Grear, J.S.; Aller, R.C.; Gobler, C.J. Coastal Ocean Acidification: The Other Eutrophication Problem. Estuar. Coast. Shelf Sci. 2014, 148, 1–13. [Google Scholar] [CrossRef]
- Feller, R.J. Dietary Immunoassay of Ilyanassa obsoleta, the Eastern Mud Snail. Biol. Bull. 1984, 166, 96–102. [Google Scholar] [CrossRef]
- Kimbro, D.L.; Grabowski, J.H.; Hughes, A.R.; Piehler, M.F.; White, J.W. Nonconsumptive Effects of a Predator Weaken Then Rebound over Time. Ecology 2017, 98, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, R.; Norin, T.; Watson, S.-A.; Pistevos, J.C.A.; Beldade, R.; Hacquart, S.; Gattuso, J.-P.; Rodolfo-Metalpa, R.; Vidal-Dupiol, J.; Killen, S.S.; et al. Near-Future Ocean Warming and Acidification Alter Foraging Behaviour, Locomotion, and Metabolic Rate in a Keystone Marine Mollusc. Sci. Rep. 2020, 10, 5461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manz, M.; Lord, J.; Morales, M. Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta. J. Mar. Sci. Eng. 2023, 11, 623. https://doi.org/10.3390/jmse11030623
Manz M, Lord J, Morales M. Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta. Journal of Marine Science and Engineering. 2023; 11(3):623. https://doi.org/10.3390/jmse11030623
Chicago/Turabian StyleManz, Maria, Joshua Lord, and Melissa Morales. 2023. "Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta" Journal of Marine Science and Engineering 11, no. 3: 623. https://doi.org/10.3390/jmse11030623
APA StyleManz, M., Lord, J., & Morales, M. (2023). Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta. Journal of Marine Science and Engineering, 11(3), 623. https://doi.org/10.3390/jmse11030623






