Disentangling Population Level Differences in Juvenile Migration Phenology for Three Species of Salmon on the Yukon River
Abstract
1. Introduction
2. Materials and Methods
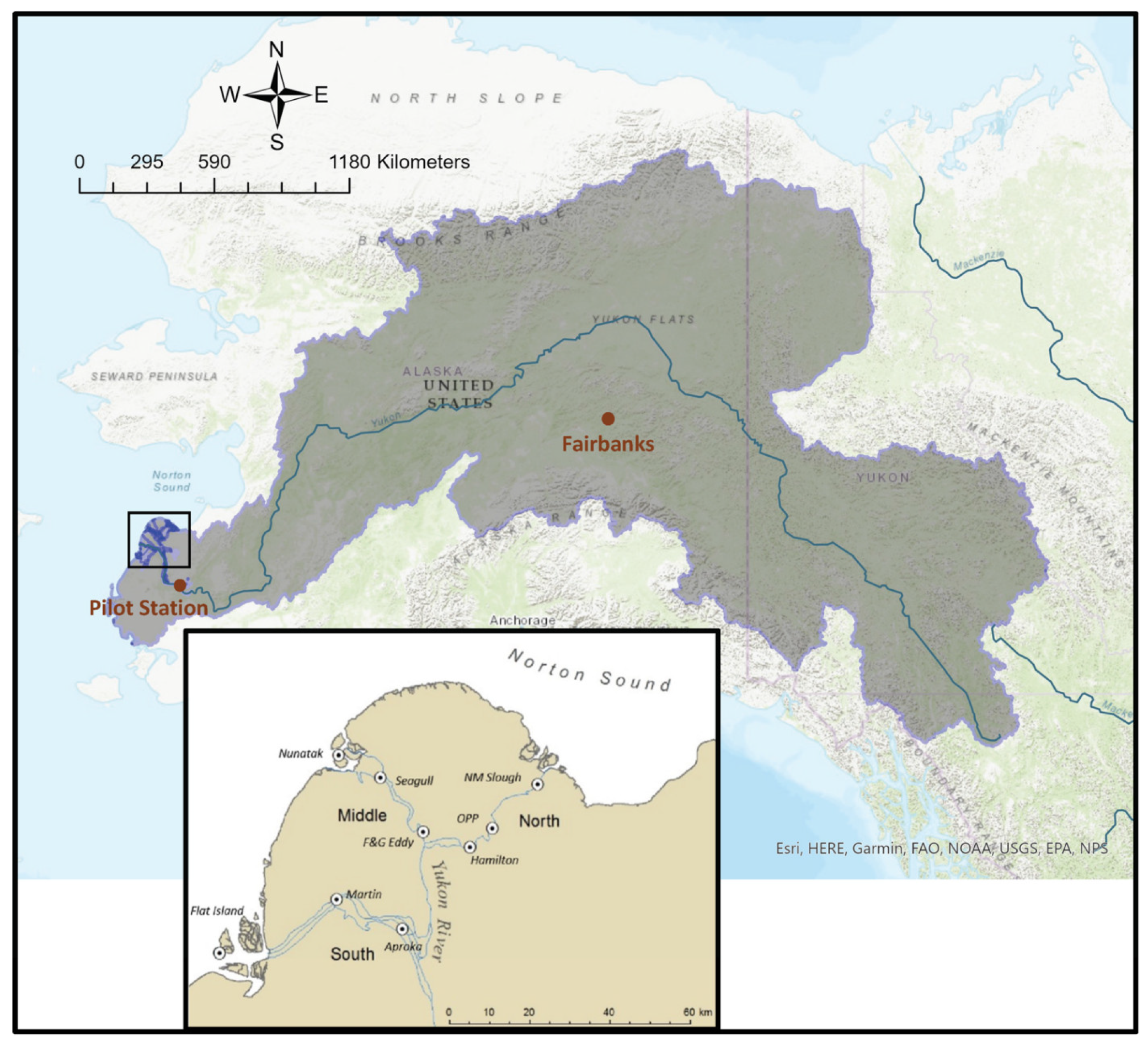
2.1. Study Area
2.2. Biological Data
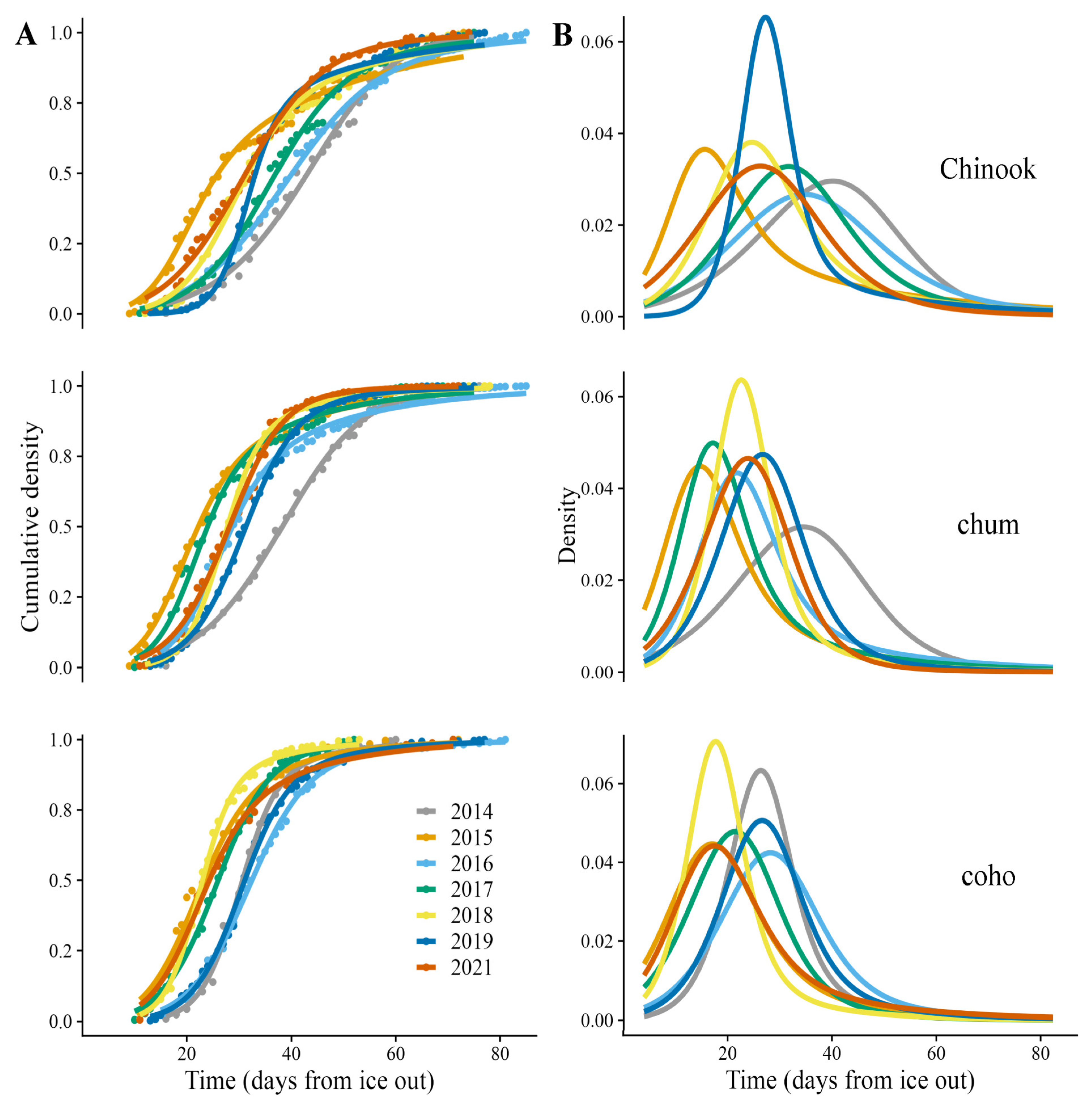
2.3. Phenology Modeling
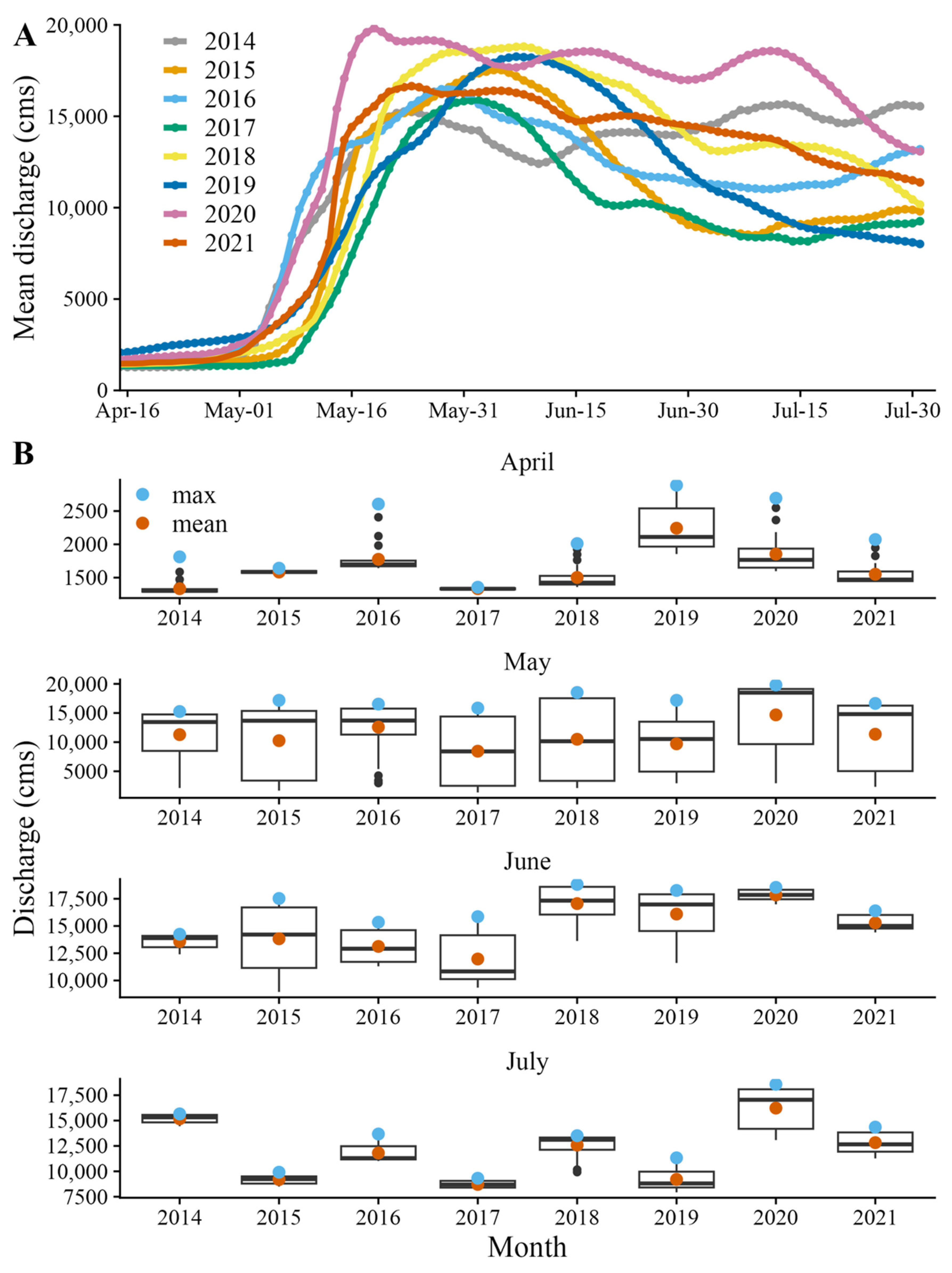
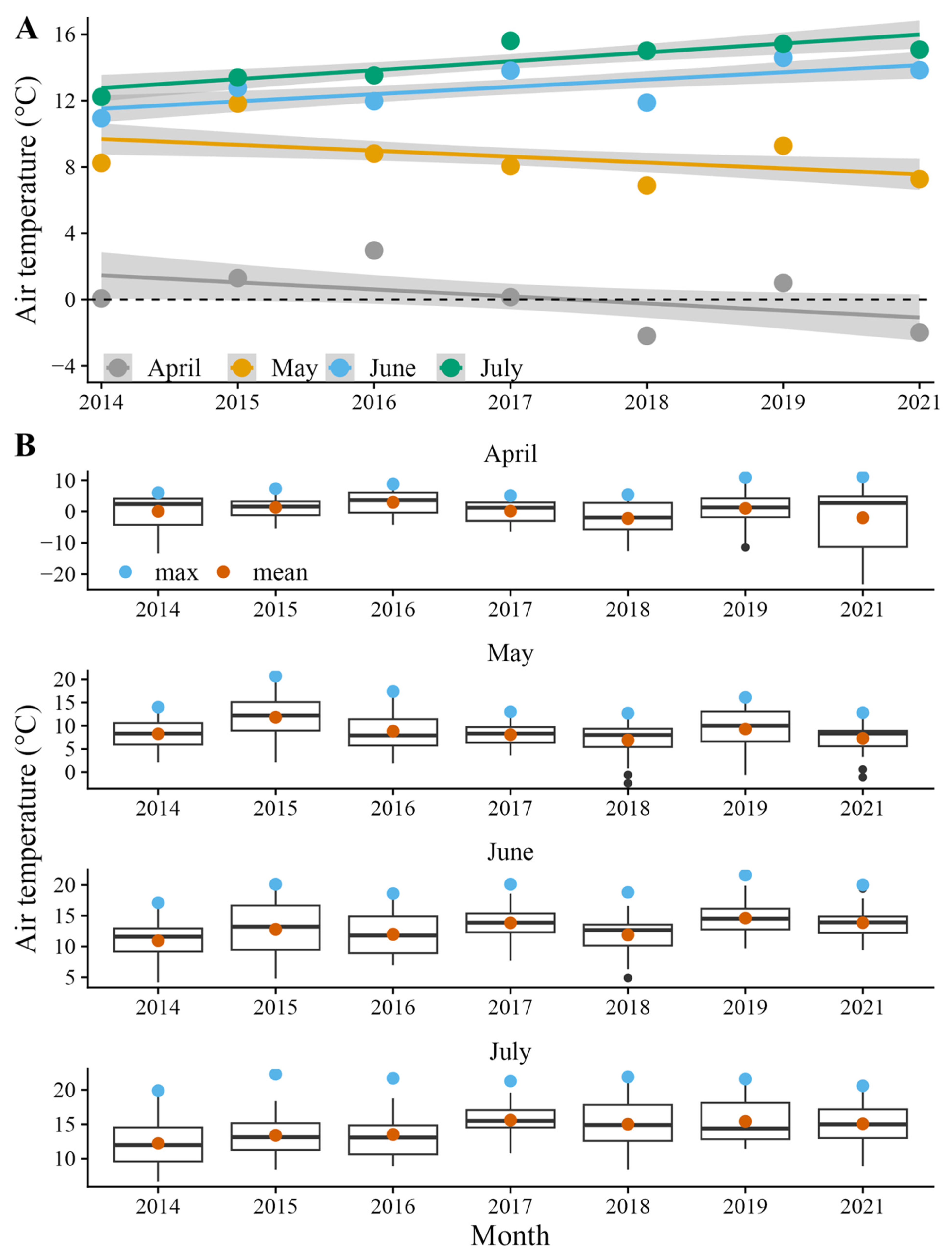
2.4. Environmental Data and Modeling
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, J.M.; Templin, W.D.; Farley, E.V.; Seeb, J.E. Stock-Structured Distribution of Western Alaska and Yukon Juvenile Chinook Salmon (Oncorhynchus tshawytscha) from United States Basis Surveys, 2002–2007. North Pac. Anadromous Fish Comm. Bull. 2009, 5, 51–59. [Google Scholar]
- Howard, K.; Murphy, J.M.; Wilson, L.; Moss, J.H.; Farley, E.V. Size Selective Mortality of Chinook Salmon in Relation to Body Energy after the First Summer in Nearshore Marine Habitats. North Pac. Anadromous Fish Comm. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Murphy, J.M.; Howard, K.; Eisner, L.; Andrews, A.G.; Templin, W.D.; Guthrie, C.; Cox, M.K.; Farely, E.V. Linking Abundance, Distribution, and Size of Juvenile Yukon River Chinook Salmon to Survival in the Northern Bering Sea. North Pac. Anadromous Fish Comm. 2013, 9, 25–30. [Google Scholar]
- Simmons, O.M.; Gregory, S.D.; Gillingham, P.K.; Riley, W.D.; Scott, L.J.; Britton, J.R. Biological and Environmental Influences on the Migration Phenology of Atlantic Salmon Salmo Salar Smolts in a Chalk Stream in Southern England. Freshwat. Biol. 2021, 66, 1581–1594. [Google Scholar] [CrossRef]
- McCormick, S.D.; Hansen, L.P.; Quinn, T.P.; Saunders, R.L. Movement, Migration, and Smolting of Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1998, 55, 77–92. [Google Scholar] [CrossRef]
- Scheuerell, M.D.; Zabel, R.W.; Sandford, B.P. Relating Juvenile Migration Timing and Survival to Adulthood in Two Species of Threatened Pacific Salmon (Oncorhynchus spp.). J. Appl. Ecol. 2009, 46, 983–990. [Google Scholar] [CrossRef]
- Harvey, A.C.; Glover, K.A.; Wennevik, V.; Skaala, O. Atlantic Salmon and Sea Trout Display Synchronised Smolt Migration Relative to Linked Environmental Cues. Sci. Rep. 2020, 10, 3529. [Google Scholar] [CrossRef]
- Erkinaro, J.; Julkunen, M.; Niemelä, E. Migration of Juvenile Atlantic Salmon Salmo Salar in Small Tributaries of the Subarctic River Teno, Northern Finland. Aquaculture 1998, 168, 105–119. [Google Scholar] [CrossRef]
- Satterthwaite, W.H.; Carlson, S.M.; Allen-Moran, S.D.; Vincenzi, S.; Bograd, S.J.; Wells, B.K. Match-Mismatch Dynamics and the Relationship between Ocean-Entry Timing and Relative Ocean Recoveries of Central Valley Fall Run Chinook Salmon. Mar. Ecol. Prog. Ser. 2014, 511, 237–248. [Google Scholar] [CrossRef]
- Carlsen, K.T.; Berg, O.K.; Finstad, B.; Heggberget, T.G. Diel Periodicity and Environmental Influence on the Smolt Migration of Arctic Charr, Salvelinus Alpinus, Atlantic Salmon, Salmo Salar, and Brown Trout, Salmo Trutta, in Northern Norway. Environ. Biol. Fishes 2004, 70, 403–413. [Google Scholar] [CrossRef]
- Berg, O.K.; Jonsson, B. Migratory Patterns of Anadromous Atlantic Salmon, Brown Trout, and Arctic Charr from the Vardes River in Northern Norway. In Proceedings of the Salmonid Migration and Distribution Symposium, Trondheim, Norway, 23–25 June 1989; University of Washington, School of Fisheries: Seattle, WA, USA, 1989; pp. 106–115. [Google Scholar]
- Bjerck, H.B.; Urke, H.A.; Haugen, T.O.; Alfredsen, J.A.; Ulvund, J.B.; Kristensen, T. Synchrony and Multimodality in the Timing of Atlantic Salmon Smolt Migration in Two Norwegian Fjords. Sci. Rep. 2021, 11, 6504. [Google Scholar] [CrossRef] [PubMed]
- Munsch, S.H.; Greene, C.M.; Johnson, R.C.; Satterthwaite, W.H.; Imaki, H.; Brandes, P.L. Warm, Dry Winters Truncate Timing and Size Distribution of Seaward-Migrating Salmon across a Large, Regulated Watershed. Ecol. Appl. 2019, 29, e01880. [Google Scholar] [CrossRef] [PubMed]
- Sykes, G.; Johnson, C.; Shrimpton, J.M. Temperature and Flow Effects on Migration Timing of Chinook Salmon Smolts. Trans. Am. Fish. Soc. 2009, 138, 1252–1265. [Google Scholar] [CrossRef]
- Otero, J.; L’Abée-Lund, J.H.; Castro-Santos, T.; Leonardsson, K.; Storvik, G.O.; Jonsson, B.; Dempson, B.; Russell, I.C.; Jensen, A.J.; Baglinière, J.-L.; et al. Basin-Scale Phenology and Effects of Climate Variability on Global Timing of Initial Seaward Migration of Atlantic Salmon (Salmo salar). Glob. Change Biol. 2014, 20, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Riley, W.D.; Eagle, M.O.; Ives, S.J. The Onset of Downstream Movement of Juvenile Atlantic Salmon, Salmo salar L., in a Chalk Stream. Fish. Manag. Ecol. 2002, 9, 87–94. [Google Scholar] [CrossRef]
- Kovach, R.P.; Joyce, J.E.; Echave, J.D.; Lindberg, M.S.; Tallmon, D.A. Earlier Migration Timing, Decreasing Phenotypic Variation, and Biocomplexity in Multiple Salmonid Species. PLoS ONE 2013, 8, e53807. [Google Scholar] [CrossRef]
- Giorgi, A.E.; Hillman, T.W.; Stevenson, J.R.; Hays, S.G.; Peven, C.M. Factors That Influence the Downstream Migration Rates of Juvenile Salmon and Steelhead through the Hydroelectric System in the Mid-Columbia River Basin. N. Am. J. Fish. Manag. 1997, 17, 268–282. [Google Scholar] [CrossRef]
- Flitcroft, R.L.; Lewis, S.L.; Arismendi, I.; LovellFord, R.; Santelmann, M.V.; Safeeq, M.; Grant, G. Linking Hydroclimate to Fish Phenology and Habitat Use with Ichthyographs. PLoS ONE 2016, 11, e0168831. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C. Global Climate Change Impacts in the United States; National Science and Technology Council, Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate Change and Freshwater Ecosystems: Impacts across Multiple Levels of Organization. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Brabets, T.P.; Wang, B.; Meade, R.H. Environmental and Hydrologic Overview of the Yukon River Basin, Alaska and Canada; U.S. Geological Survey: Anchorage, AK, USA, 2000. [Google Scholar]
- Pan, C.G.; Kirchner, P.B.; Kimball, J.S.; Du, J.; Rawlins, M.A. Snow Phenology and Hydrologic Timing in the Yukon River Basin, AK, USA. Remote Sens. 2021, 13, 2284. [Google Scholar] [CrossRef]
- Yang, D.; Marsh, P.; Ge, S. Calculation and Analysis of Yukon River Heat Flux. In Proceedings of the Cold and Mountain Region Hydrological Systems Under Climate Change: Towards Improved Projections, Gothenburg, Sweden, 22–26 July 2013; International Association of Hydrological Sciences: Wallingford, UK, 2013. [Google Scholar]
- Jensen, D.; Mahoney, A.; Resler, L. The Annual Cycle of Landfast Ice in the Eastern Bering Sea. Cold Reg. Sci. Technol. 2020, 174, 103059. [Google Scholar] [CrossRef]
- Martin, D.J.; Whitmus, C.J.; Hachmeister, L.E. Distribution and Seasonal Abundance of Juvenile Salmon and Other Fishes in the Yukon River Delta. In Outer Continental Shelf Environmental Assessment Program, Final Reports of Principal Investigators; U.S. Department of Commerce, NOAA: Washington, DC, USA, 1987; Volume 63, pp. 123–279. [Google Scholar]
- USGS. U.S. Geological Survey National Water Information System: Usgs 15565447 Yukon R at Pilot Station Ak. 2022. Available online: https://nwis.waterdata.usgs.gov/nwis/inventory/?site_no=15565447 (accessed on 20 November 2022).
- Semmens, K.A.; Ramage, J.M. Recent Changes in Spring Snowmelt Timing in the Yukon River Basin Detected by Passive Microwave Satellite Data. Cryosphere 2013, 7, 905–916. [Google Scholar] [CrossRef]
- Walvoord, M.A.; Striegl, R.G. Increased Groundwater to Stream Discharge from Permafrost Thawing in the Yukon River Basin: Potential Impacts on Lateral Export of Carbon and Nitrogen. Geophys. Res. Lett. 2007, 34, L12402. [Google Scholar] [CrossRef]
- Brabets, T.P.; Walvoord, M.A. Trends in Streamflow in the Yukon River Basin from 1944 to 2005 and the Influence of the Pacific Decadal Oscillation. J. Hydrol. 2009, 371, 108–119. [Google Scholar] [CrossRef]
- Elshamy, M.; Loukili, Y.; Pomeroy, J.W.; Pietroniro, A.; Richard, D.; Princz, D. Physically Based Cold Regions River Flood Prediction in Data-Sparse Regions: The Yukon River Basin Flow Forecasting System. J. Flood Risk Manag. 2022, e12835. [Google Scholar] [CrossRef]
- Pan, C.G.; Kirchner, P.B.; Kimball, J.S.; Du, J. A Long-Term Passive Microwave Snowoff Record for the Alaska Region 1988–2016. Remote Sens. 2020, 12, 153. [Google Scholar] [CrossRef]
- DFO, Division of Fisheries and Oceans Canada. Yukon River Chinook, Fall Chum and Coho Salmon Integrated Fisheries Management Plan; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- Jallen, D.M.; Gleason, C.M.; Borba, B.M.; West, F.M.; Decker, K.S.; Ransbury, S.R. Yukon River Salmon Stock Status and Salmon Fisheries, 2022: A Report to the Alaska Board of Fisheries, January 2023; Alaska Department of Fish and Game: Anchorage, AK, USA, 2022. [Google Scholar]
- ADF&G, Alaska Department of Fish & Game. Yukon River Chinook Salmon Mixed Stock Analysis, Genetic Baseline. Available online: https://www.adfg.alaska.gov/index.cfm?adfg=fishinggeneconservationlab.yukonchinook_baseline (accessed on 1 January 2023).
- Decovich, N.A.; Drobny, P. Genetic Stock Identification of Fall Chum Salmon in Subsistence Harvest from the Tanana Area, Yukon River, 2014; Report to the Yukon River Panel: Project No. URE-05014N; Alaska Department of Fish and Game: Anchorage, AK, USA, 2015. [Google Scholar]
- Bradford, M.J.; Grout, J.A.; Moodie, S. Ecology of Juvenile Chinook Salmon in a Small Non-Natal Stream of the Yukon River Drainage and the Role of Ice Conditions on Their Distribution and Survival. Can. J. Zool. 2001, 79, 2043–2054. [Google Scholar] [CrossRef]
- Daum, D.W.; Flannery, B.G. Canadian-Origin Chinook Salmon Rearing in Nonnatal U.S. Tributary Streams of the Yukon River, Alaska. Trans. Am. Fish. Soc. 2011, 140, 207–220. [Google Scholar] [CrossRef]
- Anderson, T.L.; Earl, J.E.; Hocking, D.J.; Osbourn, M.S.; Rittenhouse, T.A.G.; Johnson, J.R. Demographic Effects of Phenological Variation in Natural Populations of Two Pond-Breeding Salamanders. Oecologia 2021, 196, 1073–1083. [Google Scholar] [CrossRef]
- Gienapp, P.; Hemerik, L.; Visser, M.E. A New Statistical Tool to Predict Phenology under Climate Change Scenarios. Glob. Change Biol. 2005, 11, 600–606. [Google Scholar] [CrossRef]
- Steer, N.C.; Ramsay, P.M.; Franco, M.; Price, S. Nlstimedist: An R Package for the Biologically Meaningful Quantification of Unimodal Phenology Distributions. Methods Ecol. Evol. 2019, 10, 1934–1940. [Google Scholar] [CrossRef]
- Inouye, B.D.; Ehrlén, J.; Underwood, N. Phenology as a Process Rather than an Event: From Individual Reaction Norms to Community Metrics. Ecol. Monogr. 2019, 89, e01352. [Google Scholar] [CrossRef]
- Franco, M. The Time Distribution of Environmental Phenomena—Illustrated with the London Marathon. PeerJ Prepr. 2018, 6, e27175v1. [Google Scholar]
- Austen, E.J.; Jackson, D.A.; Weis, A.E. Describing Flowering Schedule Shape through Multivariate Ordination. Int. J. Plant Sci. 2014, 175, 70–79. [Google Scholar] [CrossRef]
- Encinas-Viso, F.; Revilla, T.A.; Etienne, R.S. Phenology Drives Mutualistic Network Structure and Diversity. Ecol. Lett. 2012, 15, 198–208. [Google Scholar] [CrossRef]
- Linden, A. Adaptive and Nonadaptive Changes in Phenological Synchrony. Proc. Natl. Acad. Sci. USA 2018, 115, 5057–5059. [Google Scholar] [CrossRef]
- Malo, J.E. Modelling Unimodal Flowering Phenology with Exponential Sine Equations. Funct. Ecol. 2002, 16, 413–418. [Google Scholar] [CrossRef]
- Belitz, M.W.; Larsen, E.A.; Ries, L.; Guralnick, R.P.; Ellison, A. The Accuracy of Phenology Estimators for Use with Sparsely Sampled Presence-Only Observations. Methods Ecol. Evol. 2020, 11, 1273–1285. [Google Scholar] [CrossRef]
- McDevitt-Galles, T.; Moss, W.E.; Calhoun, D.M.; Johnson, P.T.J. Phenological Synchrony Shapes Pathology in Host-Parasite Systems. Proceedings. Biol. Sci. R. Soc. 2020, 287, 20192597. [Google Scholar] [CrossRef]
- National Weather Service Breakup Database. Available online: https://www.weather.gov/aprfc/breakupDB?site=471 (accessed on 12 January 2022).
- Miller, K.; Howard, K.; Murphy, J.M.; Neff, A.D. Spatial Distribution, Nutritional Status and Community Composition of Juvenile Chinook Salmon and Other Fishes in the Yukon River Estuary; NOAA Technical Memorandum NMFS AFSC-2985; Alaska Fisheries Science Center (U.S.): Seattle, WA, USA, 2016. [Google Scholar]
- Miller, K.; Shaftel, R.; Bogan, D. Diets and Prey Items of Juvenile Chinook (Oncorhynchus Tshawytscha) and Coho (O. Kisutch) Salmon on the Yukon Delta; NOAA Technical Memorandum NMFS-AFSC-410’; National Oceanic and Atmospheric Administration, U.S. Department of Commerce: Washington, DC, USA, 2020; p. 54.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 1 November 2022).
- Eastwood, N.; Franco, M.; Ramsay, P.; Steer, N. Nlstimedist: Non-Linear Model Fitting of Time Distribution of Biological Phenomena. R Package Version 2.0.0. 2020. Available online: https://rdrr.io/cran/nlstimedist/ (accessed on 1 November 2022).
- Yang, D.; Marsh, P.; Ge, S. Heat Flux Calculations for Mackenzie and Yukon Rivers. Polar Sci. 2014, 8, 232–241. [Google Scholar] [CrossRef]
- Zedonis, P.A.; Newcomb, T.J. An Evaluation of Flow and Water Temperatures During the Spring for Protection of Salmon and Steelhead Smolts in the Trinty River, California; United States Fish and Wildlife Service, Coastal California Fish and Wildlife Office: Arcata, CA, USA, 1997. [Google Scholar]
- Yang, D.; Peterson, A. River Water Temperature in Relation to Local Air Temperature in the Mackenzie and Yukon Basins. Arctic 2017, 70, 47–58. [Google Scholar] [CrossRef]
- USDA, N.R.C.S. National Water and Climate Center; Little Chena Ridge (947). 2022. Available online: https://wcc.sc.egov.usda.gov/nwcc/site?sitenum=947 (accessed on 15 November 2022).
- Benjamin, D.J.; Berger, J.O.; Johannesson, M.; Nosek, B.A.; Wagenmakers, E.J.; Berk, R.; Bollen, K.A.; Brembs, B.; Brown, L.; Camerer, C.; et al. Redefine Statistical Significance. Nat. Hum. Behav. 2018, 2, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, G.; Sardanelli, F. Statistical Significance: P Value, 0.05 Threshold, and Applications to Radiomics—Reasons for a Conservative Approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G. The Reign of the “P-Value” Is Over: What Alternative Analyses Could We Employ to Fill the Power Vacuum? Biol. Lett. 2019, 15, 20190174. [Google Scholar] [CrossRef] [PubMed]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2022. [Google Scholar]
- AMAP. Snow, Water, Ice and Permafrost in the Arctic (Swipa) 2017; AMAP: Oslo, Norway, 2017; p. 269. [Google Scholar]
- Fullerton, A.H.; Burke, B.J.; Lawler, J.J.; Torgersen, C.E.; Ebersole, J.L.; Leibowitz, S.G. Simulated Juvenile Salmon Growth and Phenology Respond to Altered Thermal Regimes and Stream Network Shape. Ecosphere 2017, 8, e02052. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.; Garcia, S.; Murphy, J.M.; Dann, T.H. Juvenile Chinook Abundance Index and Survey Feasibility Assessment in the Northern Bering Sea, 2014–2016; Alaska Department of Fish & Game: Juneau, AK, USA, 2019. [Google Scholar]
- Murphy, M.L.; Heifetz, J.; Thedinga, J.F.; Johnson, S.W.; Koski, K.V. Habitat Utilization by Juvenile Pacific Salmon (Onchorynchus) in the Glacial Taku River, Southeast Alaska. Can. J. Fish. Aquat. Sci. 1989, 46, 1677–1685. [Google Scholar] [CrossRef]
- Giannico, G.R.; Hinch, S.G. The Effect of Wood and Temperature on Juvenile Coho Salmon Winter Movement, Growth, Density and Survival in Side-Channels. River Res. Appl. 2003, 19, 219–231. [Google Scholar] [CrossRef]
- Miller, B.A.; Sadro, S. Residence Time and Seasonal Movements of Juvenile Coho Salmon in the Ecotone and Lower Estuary of Winchester Creek, South Slough, Oregon. Trans. Am. Fish. Soc. 2003, 132, 546–559. [Google Scholar] [CrossRef]
- Weybright, A.D.; Giannico, G.R. Juvenile Coho Salmon Movement, Growth and Survival in a Coastal Basin of Southern Oregon. Ecol. Freshwat. Fish 2018, 27, 170–183. [Google Scholar] [CrossRef]
- Poletto, J.B.; Cocherell, D.E.; Baird, S.E.; Nguyen, T.X.; Cabrera-Stagno, V.; Farrell, A.P.; Fangue, N.A. Unusual Aerobic Performance at High Temperatures in Juvenile Chinook Salmon, Oncorhynchus tshawytscha. Conserv. Physiol. 2017, 5, cow067. [Google Scholar] [CrossRef]
- Lo, V.K.; Martin, B.T.; Danner, E.M.; Cocherell, D.E.; Cech, J.; Joseph, J.; Fangue, N.A. The Effect of Temperature on Specific Dynamic Action of Juvenile Fall-Run Chinook Salmon, Oncorhynchus tshawytscha. Conserv. Physiol. 2022, 10, coac067. [Google Scholar] [CrossRef] [PubMed]
- Konzela, C.M.; Whittle, J.A.; Marvin, C.T.; Myurphy, J.M.; Howard, K.G.; Borba, B.M.; Farely, E.V.; Templin, W.D.; Guyon, J. Genetic Analysis Identifies Consisten Proportions of Season Life History Types in Yukon River Juvenile and Adult Chum Salmon. North Pac. Anadromous Fish Comm. 2016, 6, 439–450. [Google Scholar] [CrossRef]
- Baughman, C.A.; Conaway, J.S. Comparison of Historical Water Temperature Measurements with Landsat Analysis Ready Data Provisional Surface Temperature Estimates for the Yukon River in Alaska. Remote Sens. 2021, 13, 2394. [Google Scholar] [CrossRef]

| Variable Name | Meaning |
|---|---|
| Response variables | |
| c | A measure of the temporal concentration of the phenology distribution. Increasing values of c reduce the spread of the pdf. |
| t skew | A measure of the migration time lag. Higher values of t shift the cdf and pdf to the right. A measure of distributional asymmetry in the pdf. |
| Explanatory variables—Discharge | |
| Range of discharge | The difference between the maximum and minimum values of discharge for each month in the migration period (May, June, and July) and for April, which is at the beginning or prior to the onset of peak discharge in spring. |
| Average discharge Maximum discharge | The average discharge for each month in the migration period (May, June, and July) and for April. The maximum discharge for each month in the migration period (May, June, and July) and for April. |
| Explanatory variables—Air Temperature | |
| Maximum temperature | The maximum air temperature for each month in the migration period (May, June, and July) and for April as a proxy for water temperature. |
| Average temperature | Average monthly temperatures for the migration period (May, June, and July) and for April as proxies for water temperatures. |
| Chinook Salmon | Chum Salmon | Coho Salmon | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Skew | c | t | Skew | c | t | Skew | c | t |
| Discharge | |||||||||
| Range—Apr | 0.19 | 0.22 | 0.23 | −0.21 | −0.24 | 0.46 | 0.41 | 0.10 | 0.43 |
| (0.68) | (0.63) | (0.62) | (0.66) | (0.61) | (0.30) | (0.33) | (0.67) | (0.34) | |
| Range—May | 0.61 | 0.37 | −0.80 | 0.56 | 0.76 | −0.57 | 0.11 | 0.27 | −0.66 |
| (0.15) | (0.42) | (0.03 *) | (0.19) | (0.05 *) | (0.18) | (0.82) | (0.56) | (0.11) | |
| Range—Jun | 0.76 | 0.68 | −0.76 | 0.67 | 0.62 | −0.75 | −0.44 | −0.51 | −0.06 |
| (0.05 *) | (0.09) | (0.05 *) | (0.10) | (0.14) | (0.05 *) | (0.32) | (0.24) | (0.90) | |
| Range—Jul | 0.52 | 0.36 | −0.28 | 0.04 | 0.22 | 0.08 | 0.43 | 0.38 | −0.15 |
| (0.24) | (0.42) | (0.55) | (0.93) | (0.64) | (0.86) | (.33) | (0.40) | (0.74) | |
| Max—Apr | 0.38 | 0.43 | 0.03 | −0.10 | −0.16 | 0.27 | 0.29 | −0.03 | 0.43 |
| (0.400) | (0.34) | (0.96) | (0.82) | (0.72) | (0.56) | (0.53) | (0.96) | (0.34) | |
| Max—May | 0.74 | 0.52 | −0.75 | 0.49 | 0.65 | −0.39 | 0.30 | 0.29 | −0.44 |
| (0.05 *) | (0.23) | (0.05 *) | (0.27) | (0.11) | (0.39) | (0.51) | (0.52) | (0.32) | |
| Max—June | 0.89 | 0.77 | −0.83 | 0.40 | 0.59 | −0.40 | 0.21 | 0.20 | −0.43 |
| (0.01 *) | (0.04 *) | (0.02 *) | (0.38) | (0.17) | (0.38) | (0.64) | (0.66) | (0.33) | |
| Max—July | −0.61 | −0.50 | 0.62 | −0.59 | −0.57 | 0.77 | 0.69 | 0.65 | 0.06 |
| (0.08) | (0.25) | (0.14) | (0.17) | (0.19) | (0.04 *) | (0.08) | (0.11) | (0.89) | |
| Average—Apr | 0.67 | 0.76 | −0.30 | 0.03 | −0.03 | 0.00 | 0.10 | −0.25 | 0.33 |
| (0.150) | (0.05 *) | (0.52) | (0.96) | (0.96) | (0.99) | (0.83) | (0.99) | (0.46) | |
| Average—May | −0.47 | −0.45 | 0.33 | −0.26 | −0.36 | 0.33 | 0.44 | 0.16 | 0.12 |
| (0.10) | (0.25) | (0.46) | (0.58) | (0.42) | (0.47) | (0.33) | (0.73) | (0.70) | |
| Average—Jun | 0.49 | 0.51 | −0.39 | −0.15 | 0.10 | 0.23 | 0.75 | 0.69 | −0.38 |
| (0.29) | (0.32) | (0.39) | (0.75) | (0.87) | (0.62) | (0.05 *) | (0.09) | (0.40) | |
| Average—Jul | −0.73 | −0.60 | 0.64 | −0.20 | −0.55 | 0.73 | 0.67 | 0.71 | −0.01 |
| (0.06) | (0.15) | (0.12) | (0.20) | (0.23) | (0.07) | (0.10) | (0.07) | (0.98) | |
| Air Temperature | |||||||||
| Max—Apr | −0.09 | 0.18 | −0.02 | −0.56 | −0.62 | 0.38 | 0.49 | −0.01 | 0.0–4 |
| (0.84) | (0.70) | (0.96) | (0.19) | (0.14) | (0.40) | (0.26) | (0.99) | (0.93) | |
| Max—May | 0.52 | 0.62 | −0.45 | −0.16 | 0.17 | −0.45 | −0.32 | −0.69 | 0.34 |
| (0.24) | (0.14) | (0.32) | (0.37) | (0.72) | (0.31) | (0.48) | (0.90) | (0.45) | |
| Max—Jun | 0.77 | 0.75 | −0.66 | −0.71 | 0.44 | −0.56 | −0,24 | −0.35 | −0.28 |
| (0.04 *) | (0.05 *) | (0.10) | (0.50) | (0.32) | (0.19) | (0.60) | (0.43) | (0.55) | |
| Max—Jul | 0.80 | (0.10) | −0.82 | −0.84 | 0.81 | −0.83 | −0.17 | −0.08 | −0.13 |
| (0.03 *) | (0.83) | (0.02 *) | (0.02 *) | (0.03 *) | (0.02 *) | (0.72) | (0.87) | (0.78) | |
| Avg—Apr | 0.46 | 0.58 | −0.45 | −0.17 | 0.10 | −0.44 | −0.29 | −0.71 | 0.30 |
| (0.30) | (0.17) | (0.31) | (0.47) | (0.84) | (0.33) | (0.53) | (0.07) | (0.51) | |
| Average—May | 0.46 | 0.51 | −0.50 | −0.25 | 0.30 | −0.57 | −0.37 | −0.66 | 0.24 |
| (0.30) | (0.24) | (0.26) | (0.22) | (0.51) | (0.18) | (0.41) | (0.10) | (0.61 | |
| Average—Jun | 0.43 | 0.52 | −0.38 | −0.47 | −0.07 | −0.12 | −0.12 | −0.18 | −0.29 |
| (0.34) | (0.23) | (0.40) | (0.57) | (0.88) | (0.78) | (0.79) | (0.71) | (0.52) | |
| Average—Jul | 0.66 | 0.38 | −0.24 | −0.40 | 0.54 | −0.33 | −0.44 | −0.17 | 0.06 |
| (0.11) | (0.40) | (0.60) | (0.41) | (0.21) | (0.48) | (0.32) | (0.72) | (0.89) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, K.B.; Weiss, C.M. Disentangling Population Level Differences in Juvenile Migration Phenology for Three Species of Salmon on the Yukon River. J. Mar. Sci. Eng. 2023, 11, 589. https://doi.org/10.3390/jmse11030589
Miller KB, Weiss CM. Disentangling Population Level Differences in Juvenile Migration Phenology for Three Species of Salmon on the Yukon River. Journal of Marine Science and Engineering. 2023; 11(3):589. https://doi.org/10.3390/jmse11030589
Chicago/Turabian StyleMiller, Katharine B., and Courtney M. Weiss. 2023. "Disentangling Population Level Differences in Juvenile Migration Phenology for Three Species of Salmon on the Yukon River" Journal of Marine Science and Engineering 11, no. 3: 589. https://doi.org/10.3390/jmse11030589
APA StyleMiller, K. B., & Weiss, C. M. (2023). Disentangling Population Level Differences in Juvenile Migration Phenology for Three Species of Salmon on the Yukon River. Journal of Marine Science and Engineering, 11(3), 589. https://doi.org/10.3390/jmse11030589






