Molluscs from Tidal Channels of the Gulf of Gabès (Tunisia): Quantitative Data and Comparison with Other Lagoons and Coastal Waters of the Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
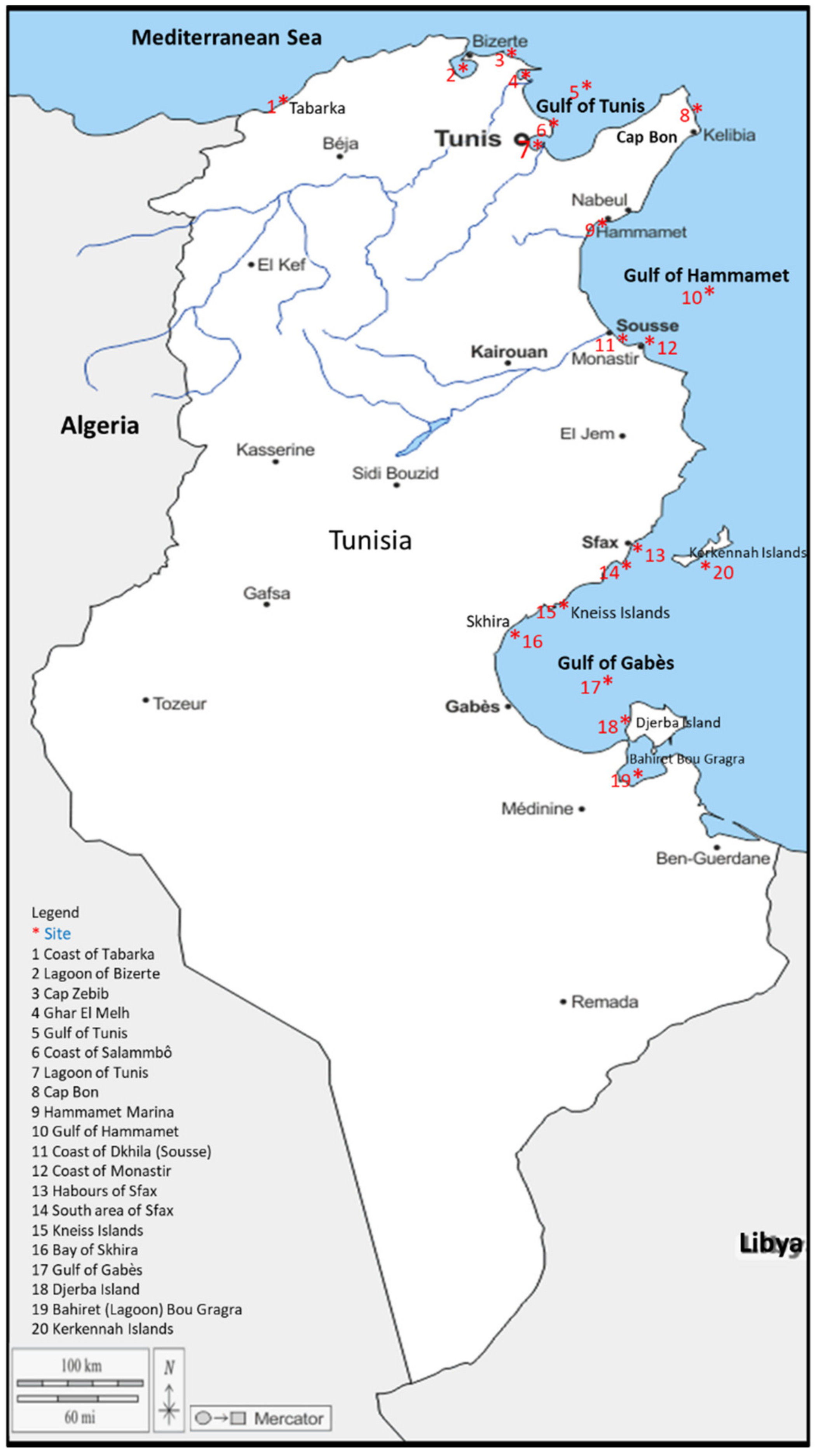
2.1. Study Area
2.2. Sampling and Laboratory Procedures
2.3. Statistical Analysis of Biological Parameters
2.4. Environmental Parameters
3. Results
3.1. General Characteristics of the Molluscan Fauna
3.2. Spatial Patterns
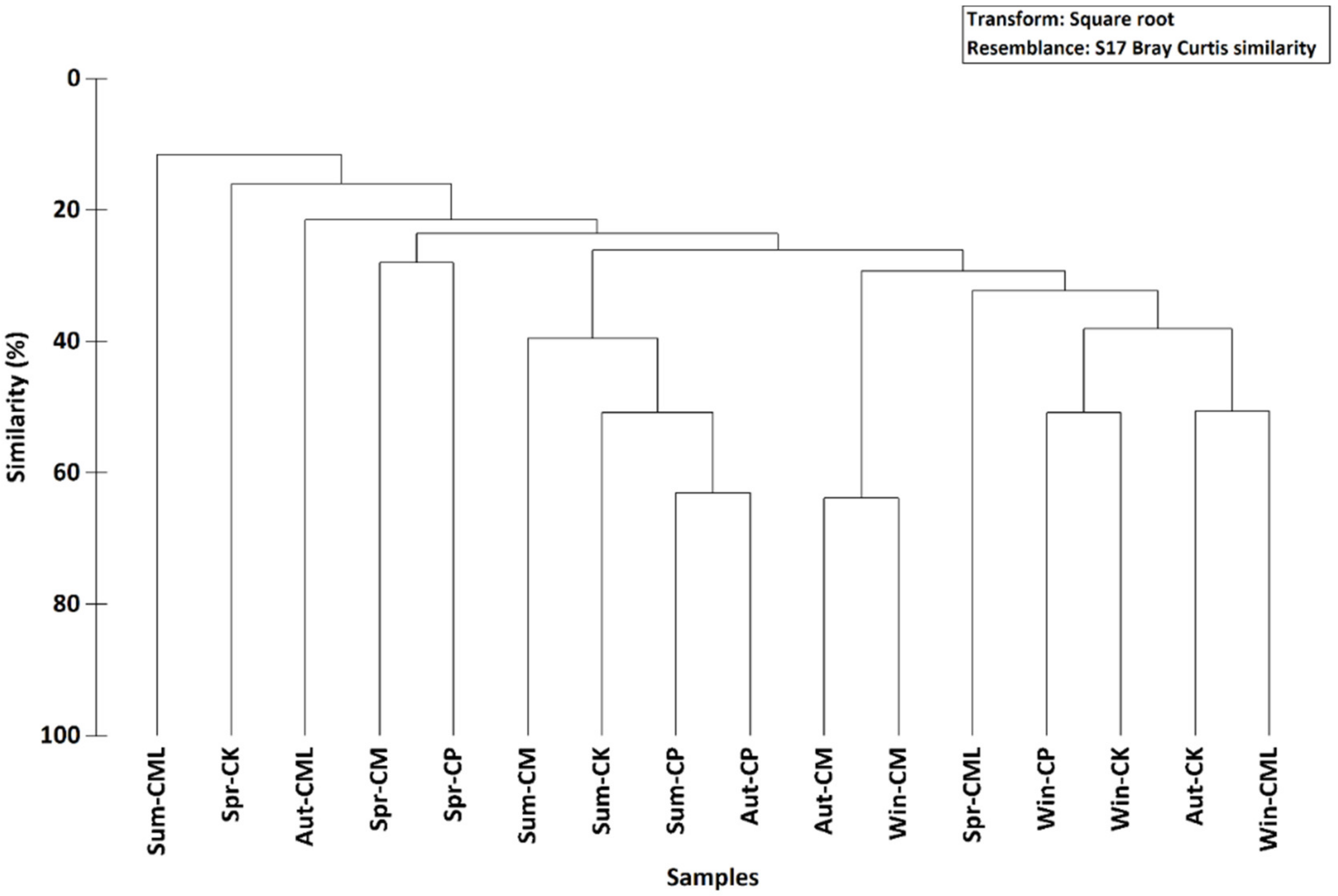
3.3. Seasonal Patterns
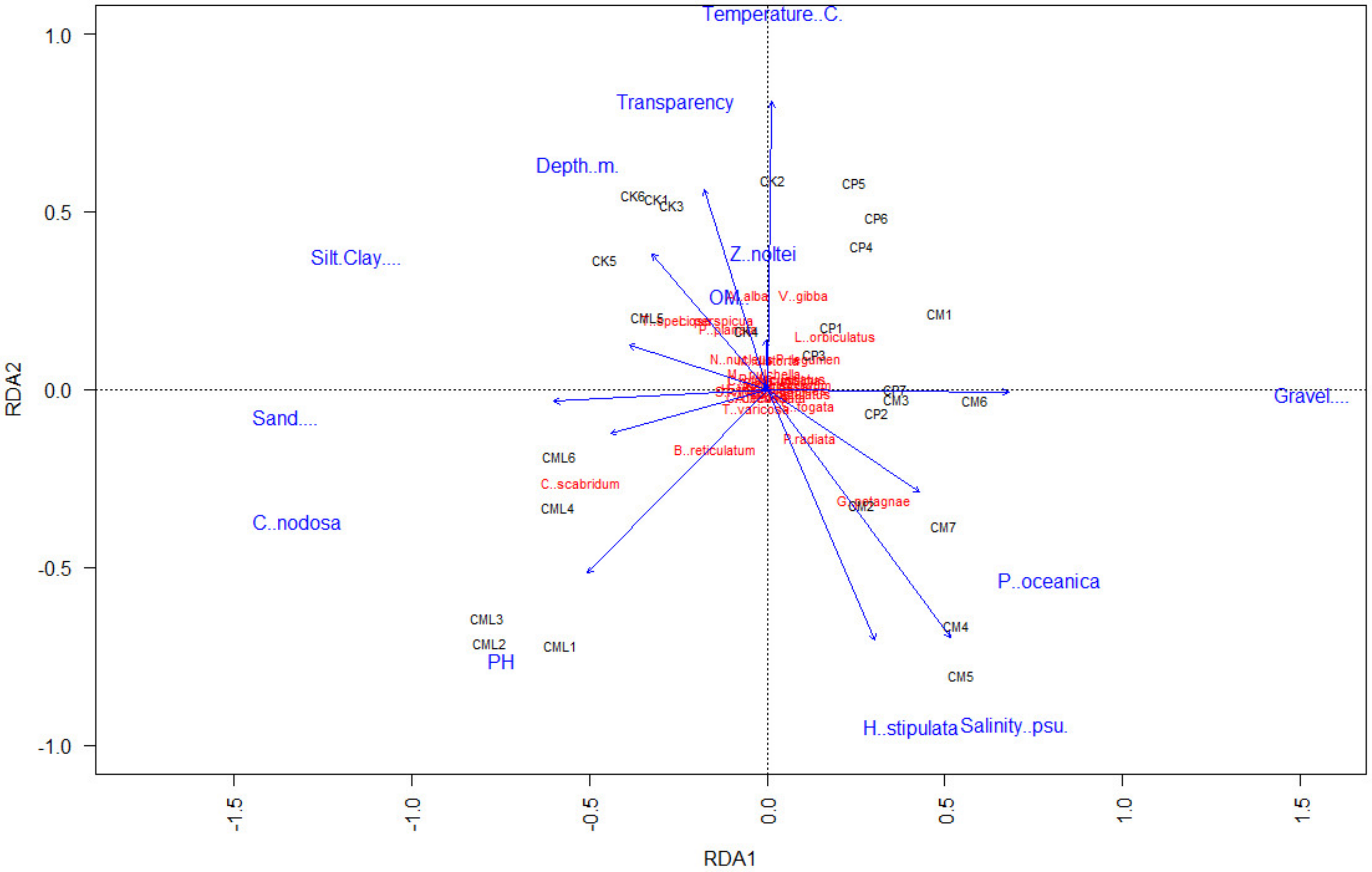
3.4. Role of Environmental Factors
4. Discussion
4.1. Main Characteristics of the Malacofauna of the Tidal Channel of the Gulf of Gabès
4.2. Comparison with Other Mediterranean Malacofauna Assemblages
5. Conclusions
- (1)
- A decrease in species richness from the shallower to the deeper zones of the channels.
- (2)
- Seasonal changes in species richness and abundance, with higher values in autumn and winter than during the other two seasons. The seasons of autumn and winter appear favourable for the accumulation of algae and detritus in the channels after the period of macro-algae growth and reproduction.
- (3)
- Depth, sediment type and presence of the marine phanerogams are the main factors explaining the structuration of the malacofauna of the tidal channel of the Gulf of Gabès, forming four distinct assemblages. Fine sand and gravel suspension bivalve species account for the structure of the mollusc assemblages associated with each channel.
- (4)
- The Maltine channel shows higher abundances than the three other channels, which could be linked to the more extensive development of seagrasses and macroalgae at this site [47,48,49,50,51,52] Moreover, a spatial pattern can be recognized in terms of species richness and abundance: the Maltine channel has the richest fauna, while the Mimoun channel has the poorest, with the Ben Khlaf and Kneiss channels showing intermediate values.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaouti, A.; Bayed, A. Diversité taxonomique et structure de la macrofaune benthique des substrats meubles de la lagune de Smir. In Ecosystèmes Côtiers Sensibles de la Méditerranée: Cas du Littoral de Smir; Bayed, A., Scapini, F., Eds.; Université Mohammed V-Agdal: Rabat, Morocco, 2005; Volume 4, pp. 33–42. [Google Scholar]
- Draredja, B.; Melouah Beldi, H.; Benmarce, S. Diversité de la macrofaune benthique de la lagune Mellah (Parc national d’El-Kala, Algérie nord-est). Bull. Soc. Zool. Fr. 2012, 137, 73–86. [Google Scholar]
- Belgacem, W.; Langar, H.; Pergent, G.; Ben Hassinea, O.K. Associated mollusk communities of a Posidonia oceanica meadow in Cap Zebib (off North East Tunisia). Aquat. Bot. 2013, 104, 170–175. [Google Scholar] [CrossRef]
- Sabelli, B.; Taviani, M. The Making of the Mediterranean Molluscan Biodiversity. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Berlin, Germany, 2014; pp. 285–306. [Google Scholar]
- Elgharsalli, R.; Rabaoui, L.; Aloui-Bejaoui, N. Community structure of a molluscan assemblage in an anthropized environment, Hammamet Marina, North-Eastern Tunisia. Turk. J. Fish Aqua. Sci. 2015, 15, 751–760. [Google Scholar]
- Khedhri, I.; Atoui, A.; Ibrahim, M.; Afli, A.; Aleya, L. Assessment of surface sediment dynamics and response of benthic macrofauna assemblages in Boughrara Lagoon (SW Mediterranean Sea). Ecol. Ind. 2016, 70, 77–88. [Google Scholar] [CrossRef]
- Khedhri, I.; Afli, A.; Aleya, L. Structuring factors of the spatio-temporal variability of macrozoobenthos assemblages in a southern Mediterranean lagoon: How useful for bioindication is a multi-biotic indices approach? Mar. Poll. Bull. 2017, 114, 515–527. [Google Scholar] [CrossRef]
- Zaabar, W.; Zakhama-Sraieb, R.; Charfi-Cheikhrouha, F.; Sghaıer Achouri, M. Composition of a molluscan assemblage associated with macrophytes in Menzel Jemil Bizerte lagoon, SW Mediterranean Sea). Afr. J. Ecol. 2017, 7, 537–547. [Google Scholar] [CrossRef]
- Gofas, S.; Zenetos, A. Exotic molluscs in the Mediterranean Basin: Current status and perspectives. Oceanogr. Mar. Biol. Ann. Rev. 2003, 41, 237–277. [Google Scholar]
- Zenetos, A.; Gofas, S.; Russo, G.; Templado, J. CIESM Atlas of Exotic Species in the Mediterranean; CIESM Publishers: Rabat, Monaco, 2004; Volume 3, 376p. [Google Scholar]
- Antit, M.; Gofas, S.; Salas, C.; Azzouna, A. One hundred years after Pinctada: An update on alien Mollusca in Tunisia. Medit. Mar. Sci. 2011, 12, 53–73. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Sabelli, B.; Taviani, M. Réflexions sur les endémiques (Mollusques Marins) du Golfe de Gabès (Tunisie). In Proceedings of the Commission International Pour Exploration Scientifique de la Mer Mediterranee, Cagliari, Monaco, 13–14 October 1980; pp. 149–150. [Google Scholar]
- Ayari, R.; Afli, A. Bionomie benthique du petit Golfe de Tunis. Bull. Inst. Natn. Sci. Tech. Mer Salammbô 2003, 30, 79–90. [Google Scholar]
- Aloui-Bejaoui, N.; Afli, A. Functional diversity of the macro-invertebrate community in the port area of Kerkennah Islands (Tunisia). Medit. Mar. Sci. 2012, 13, 93–102. [Google Scholar] [CrossRef]
- Antit, M.; Daoulatli, A.; Urra, J.; Rueda, J.L.; Gofas, S.; Salas, C. Seasonality and trophic diversity in molluscan assemblages from the Bay of Tunis (southern Mediterranean Sea). Medit. Mar. Sci. 2016, 17, 692–707. [Google Scholar] [CrossRef]
- Mosbahi, N.; Dauvin, J.C.; Neifar, L. Molluscs associated with intertidal Zostera noltei Hornemann beds in southern Tunisia (Central Mediterranean): Seasonal dynamics and environmental drivers. Vie Milieu 2018, 68, 221–235. [Google Scholar]
- Chambost, L. Essai sur la région littorale dans les environs de Salammbô. Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1928, 8, 29. [Google Scholar]
- Seurat, L.G. Observations nouvelles sur les faciès et les associations animales de l’étage intercotidal de la petite Syrte (Golfe de Gabès). Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1929, 12, 59. [Google Scholar]
- Seurat, L.G. Observations sur les limites, les faciès et les associations animales de l’étage intercotidal de la petite Syrte (Golfe de Gabès). Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1929, 3, 72. [Google Scholar]
- Seurat, L.G. Formations littorales et estuaires de la Syrte mineure (Golfe de Gabès). Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1934, 32, 65. [Google Scholar]
- Molinier, R.; Picard, J. Eléments de bionomie marine sur les côtes de Tunisie. Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1954, 48, 47. [Google Scholar]
- Pérès, J.M.; Picard, J. Recherches sur les peuplements benthiques du seuil siculo-tunisien. Résultats scientifiques des campagnes de la Calypso. Ann. Inst. Océanogr. Paris 1956, 32, 233–264. [Google Scholar]
- Gaillande, D. Note sur les peuplements de la zone centrale du Golfe de Gabès (Campagne Calypso 1965). Téthys 1970, 2, 131–138. [Google Scholar]
- Ktari-Chakroun, F.; Azzouz, A. Les fonds chalutables de la région Sud Est de la Tunisie (Golfe de Gabes). Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1971, 2, 5–48. [Google Scholar]
- Azouz, A. Les fonds chalutables de la région nord de la Tunisie. Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1973, 2, 473–564. [Google Scholar]
- Zaouali, J. Les peuplements malacologiques de la mer de Bou Grara. Bull. Off. Nat. Pêche Tunisie 1978, 2, 199–209. [Google Scholar]
- Rabaoui, L.; El Zrelli, R.; Ben Mansour, M.; Balti, R.; Mansour, L.; Tlig-Zouari, S.; Guerfel, M. On the relationship between the diversity and structure of benthic macroinvertebrate communities and sediment enrichment with heavy metals in Gabes Gulf, Tunisia. J. Mar. Biol. Ass. UK 2015, 95, 233–245. [Google Scholar] [CrossRef]
- Mosbahi, N.; Boudaya, L.; Dauvin, J.C.; Neifar, N. Spatial Distribution and Abundance of Intertidal Benthic Macrofauna in the Kneiss Islands (Gulf of Gabès, Tunisia). Cah. Biol. Mar. 2015, 56, 319–328. [Google Scholar]
- Mosbahi, N.; Pezy, J.P.; Dauvin, J.C.; Neifar, L. Short-term impact of bait digging on intertidal macrofauna of tidal mudflats around the Kneiss Islands (Gulf of Gabès, Tunisia). Aquat. Living Resour. 2015, 28, 111–118. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.P.; Dauvin, J.C.; Neifar, N. Spatial and temporal structures of the macrozoobenthos from the intertidal zone of the Kneiss Islands (Central Mediterranean Sea). Open J. Mar. Sci. 2016, 6, 223–237. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.P.; Dauvin, J.C.; Neifar, L. Immediate effect of clam harvesting on intertidal benthic communities in the mudflat zones of Kneiss Islands (central Mediterranean Sea). J. Aqua. Res. Dev. 2016, 7, 454. [Google Scholar] [CrossRef]
- Mosbahi, N.; Blanchet, H.; Lavesque, N.; De Montaudouin, X.; Dauvin, J.C.; Neifar, L. Main ecological features of benthic macrofauna between Mediterranean and Atlantic intertidal sea grass bed—A case study. J. Mar. Biol. Oceanogr. 2017, 6, 2. [Google Scholar] [CrossRef]
- Mosbahi, N.; Moncef Serbaji, M.; Pezy, J.P.; Neifar, L.; Dauvin, J.C. Response of benthic macrofauna to multiple anthropogenic pressures in the shallow coastal zone south of Sfax (Tunisia, Central Mediterranean Sea). Environ. Pollut. 2019, 253, 474–487. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.P.; Neifar, N.; Dauvin, J.C. Ecological status assessment and non-indigenous species in industrial and fishing harbours of the Gulf of Gabès (central Mediterranean Sea). Environ. Sci. Poll. Res. 2021, 28, 65278–65299. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.P.; Dauvin, J.C.; Neifar, L. COVID-19 Pandemic Lockdown: An excellent opportunity to study the effects of trawling disturbance on microbenthic fauna in the shallow waters of the Gulf of Gabès (Tunisia, central Mediterranean Sea). Inter. J. Environ. Res. Public Health 2022, 19, 1282. [Google Scholar] [CrossRef]
- Boudaya, L.; Mosbahi, N.; Dauvin, J.C.; Neifar, L. Trophic and functional organization of the benthic macrofauna of an anthropogenic influenced area: The Skhira Bay (Gulf of Gabès, central Mediterranean Sea). Environ. Sci. Pollut. Res. 2019, 26, 13522–13538. [Google Scholar] [CrossRef]
- Antit, M.; Azzouna, A. Mollusques des milieux littoraux de la baie de Tunis. Soc. Esp. Malaco. Iberus 2012, 30, 107–133. [Google Scholar]
- Antit, M.; Daoulatli, A.; Rueda, J.L.; Salas, C. Temporal variation of the algae-associated molluscan assemblage of artificial substrata in the Bay of Tunis (Tunisia). Medit. Mar. Sci. 2013, 14, 390–402. [Google Scholar] [CrossRef]
- Zaouali, J. Les peuplements benthiques de la petite syrte, golfe de Gabès—Tunisie. Résultats de la campagne de prospection du mois de Juillet 1990. Etude préliminaire: Biocénose et thanatocénoses récentes. Mar. Life 1993, 3, 47–60. [Google Scholar]
- Afli, A.; Ayari, R.; Zaabi, S. Ecological quality of some Tunisian coast and lagoon locations, by using benthic community parameters and biotic indices. Estuar. Coast. Shelf Sci. 2008, 80, 269–280. [Google Scholar] [CrossRef]
- Afli, A.; Chakroun, R.; Ayari, R.; Aissa, P. Seasonal and spatial variability of the community and trophic structure of the benthic macrofauna within Tunisian lagoonal and marine coastal areas (southwestern Mediterranean). J. Coast. Res. 2009, 25, 1198–1209. [Google Scholar] [CrossRef]
- Afli, A.; Chakroun, R.; Ayari, R.; Aissa, P. Response of the benthic macrofauna to seasonal natural and anthropogenic constraints within Tunisian lagoonal and coastal areas (South-Western Mediterranean). Vie Milieu 2009, 59, 21–30. [Google Scholar]
- Rosso, J.C. Faune malacologique de la plate-forme tunisienne: Étude de quelques dragages et carottages effectués à l’intérieur ou au large du golfe de Gabès. Bull. Inst. Natn. Sci. Tech. Mer Salammbô 1978, 5, 17–41. [Google Scholar]
- Rosso, J.C. Mollusques testacés (Macrofaune). In La mer Pélagienne. Etude Sédimentologique et Écologique du Plateau Tunisien et du Golfe de Gabès; Burollet, P.F., Clairefond, P., Winnock, E., Eds.; Èditions de L’Université de Provence: Provence, France, 1979; Volume 6, pp. 143–170. [Google Scholar]
- Cecalupo, A.; Buzzurro, G.; Mariani, M. Contributo alla conoscenza della malacofauna del Golfo di Gabès (Tunisia). Quad. Civ. Staz. Idrobiol. Milano 2008, 31, 1–175. [Google Scholar]
- Dauvin, J.C.; Fersi, A.; Pezy, J.P.; Bakalem, A.; Neifar, L. Macrobenthic communities in the tidal channels around the Gulf of Gabès, Tunisia. Mar. Poll. Bull. 2021, 162, 111846. [Google Scholar] [CrossRef] [PubMed]
- Bali, M.; Gueddari, M. Les chenaux de marée autour des îles de Kneiss, Tunisie: Sédimentologie et évolution. Hydrol. Sci. J. 2011, 56, 498–506. [Google Scholar] [CrossRef]
- Béjaoui, B.; Ben Ismail, S.; Othmani, A.; Ben Abdallah-Ben Hadj Hamida, O.; Chevalier, C.; Feki-Sahnoun, W.; Harzallah, A.; Ben Hadj Hamida, N.; Bouaziz, R.; Dahech, S.; et al. Synthesis review of the Gulf of Gabes (eastern Mediterranean Sea, Tunisia): Morphological, climatic, physical oceanographic, biogeochemical and fisheries features. Estuar. Coast. Shelf Sci. 2019, 219, 395–408. [Google Scholar] [CrossRef]
- Hattab, T.; Ben Rais Lasram, F.; Albouy, C.; Salah Romdhane, M.; Jarboui, O. An ecosystem model of an exploited southern Mediterranean shelf region (Gulf of Gabès, Tunisia) and a comparison with other Mediterranean ecosystem model properties. J. Mar. Sys. 2013, 128, 159–174. [Google Scholar] [CrossRef]
- Sammari, C.; Koutitonsky, V.G.; Moussa, M. Sea level variability and tidal resonance in the Gulf of Gabes, Tunisia. Cont. Shelf Res. 2006, 26, 338–350. [Google Scholar] [CrossRef]
- Fersi, A.; Dauvin, J.C.; Pezy, J.P.; Neifar, L. Amphipods from tidal channels of the Gulf of Gabès (central Mediterranean Sea). Medit. Mar. Sci. 2018, 19, 430–443. [Google Scholar] [CrossRef]
- Antit, M.; Gofas, S.; Azzouna, A. Les Tricoliidae (Gastéropodes, Prosobranches) des côtes Nord-est de la Tunisie. Bull. Assoc. Franç. Conch. 2009, 125, 25–27. [Google Scholar]
- Gofas, S.; Salas, C. Small Nuculidae (bivalvia) with functional primary hinge in the adults. J. Conch. Lond. 1996, 35, 427–435. [Google Scholar]
- Gofas, S.; Moreno, D.; Salas, C. (Coordinadores). Moluscos Marinos de Andalucía; Servicio de Publicaciones e Intercambio Cientifico, Universidad de Malaga: Andalucia, Spain, 2011; Volume 1, pp. 1–342. [Google Scholar]
- Gofas, S.; Moreno, D.; Salas, C. (Coordinadores). Moluscos Marinos de Andalucía; Servicio de Publicaciones e Intercambio Cientifico, Universidad de Malaga: Andalucia, Spain, 2011; Volume 2, pp. 343–798. [Google Scholar]
- Graham, A. Molluscs: Prosobranch and Pyramidellid Gastropods: Keys and Notes for the Identification of the Species; Linnean Society: London UK, 1988; 662p. [Google Scholar]
- Nordsieck, F. Die œeuropäischen Meeres-Gehäuseschnecken: Prosobranchia: Vom Eismeer bis Kapverden und Mittelmee; Fischer: Stuttgart, Germany, 1968; 273p. [Google Scholar]
- Nordsieck, F. Die Europäischen Meeresmuscheln. Vom Eismeer bis Kapverden, Mittelmeer und Schwarzes Meer; Fischer: Stuttgart, Germany, 1969; 256p. [Google Scholar]
- Parenzan, P. Gastropodi. In Carta D’identita delle Conchiglie del Mediterraneo; Taras, B., Ed.; Bios Tara: Taranto, Italy, 1970; Volume 1, 283p. [Google Scholar]
- Parenzan, P. Bivalvi Prima Parte. In Carta D’identita delle Conchiglie del Mediterraneo; Taras, B., Ed.; Bios Tara: Taranto, Italy, 1974; Volume 2, 277p. [Google Scholar]
- Parenzan, P. Bivalvi Secunda Parte. In Carta D’identita delle Conchiglie del Mediterraneo; Taras, B., Ed.; Bios Tara: Taranto, Italy, 1976; Volume 2, 281p. [Google Scholar]
- Sabelli, B.; Giannuzzi Savelli, R.; Bedulli, D. Catalogo Annotato dei Molluschi Marini del Mediterraneo; Libreria Naturalistica Bolognese: Bologna, Italy, 1990; Volume 1, pp. 1–348. [Google Scholar]
- Sabelli, B.; Giannuzzi Savelli, R.; Bedulli, D. Catalogo Annotato dei Molluschi Marini del Mediterraneo; Libreria Naturalistica Bolognese: Bologna, Italy, 1992; Volume 2, pp. 349–498. [Google Scholar]
- Sabelli, B.; Giannuzzi Savelli, R.; Bedulli, D. Catalogo Annotato dei Molluschi Marini del Mediterraneo; Libreria Naturalistica Bolognese: Bologna, Italy, 1992; Volume 3, pp. 501–781. [Google Scholar]
- Tebble, N. British Bivalve Seashells: A Handbook for Identification; The British Museum (Natural History): London, UK, 1966; 212p. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Legendre, P.; Andersson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Holzknecht, M.; Albano, P.G. The molluscan assemblage of a pristine Posidonia oceanica meadow in the eastern Mediterranean. Mar. Biod. 2022, 52, 59. [Google Scholar] [CrossRef]
- Tlig-Zouari, S.; Maamouri-Mokhtar, F. Macrozoobenthic species composition and distribution in the Northern lagoon of Tunis. Trans. Water Bull. 2008, 2, 1–15. [Google Scholar]
- Afli, A.; Ben Mustapha, K.; Jarboui, O.; Bradai, M.N.; Hattour, A.; Langar, H.; Sadok, S. La Biodiversité Marine en Tunisie. Doc. République Tunis. Ministère de l’Environnement et du Développement Durable. Direction Générale de l’Environnement et de la Qualité de la vie (DGEQV), 20p. 2005. Available online: http://hdl.handle.net/1834/12470 (accessed on 15 December 2022).
- Russo, G.F.; Vinci, D.; Scadri, M.; Fresi, E. Mollusc syntaxon of foliar stratum along a depth gradient in a Posidonia oceanica bed: 3 a year’s cycle at Ischia Island. Posidonia Newsl. 1991, 4, 15–25. [Google Scholar]
- Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Depth and seasonal distribution of some groups of the vagile fauna of the Posidonia oceanica leaf stratum: Structural and trophic analyses. PSZNI Mar. Ecol. 1992, 13, 17–39. [Google Scholar] [CrossRef]
- Russo, G.F.; Chessa, L.A.; Vinci, D.; Fresi, E. Molluscs of Posidonia oceanica beds in the bay of Porto Conte (North-Western Sardania): Zonation pattern, seasonal variability and geographical comparison. Posidonia Newsl. 1991, 4, 5–14. [Google Scholar]
- Chemello, R.; Riggio, S. A up to date list of the Mollusca recorded in the Stagnone di Marsala (W. Sicily). In Proceedings of the Tenth International Malacoligical Congress, Tübingen, Germany, 17 August–2 September 1989; pp. 343–344. [Google Scholar]
- Dauvin, J.C.; Grimes, S.; Bakalem, A. Marine Biodiversity on the Algerian Continental Shelf (Mediterranean Sea). J. Nat. Hist. 2013, 47, 1745–1765. [Google Scholar] [CrossRef]
- Donnarumma, L.; Sandulli, R.; Appolloni, L.; Giovanni Fulvio Russo, G. Assessing molluscs functional diversity within different coastal habitats of Mediterranean marine protected areas. Ecol. Quest. 2018, 29, 35–51. [Google Scholar]
- Rueda, J.L.; Salas, C. Molluscs associated with a subtidal Zostera marina L. bed in southern Spain: Linking seasonal changes of fauna and environmental variables. Estuar. Coast. Shelf Sci. 2008, 79, 157–167. [Google Scholar] [CrossRef]
- Urra, J.; Mateo-Ramirez, A.; Marina, P.; Salas, C.; Gofas, S.; Rueda, J.L. Highly diverse molluscan assemblages of Posidonia oceanica meadows in northwestern Alboran Sea (W Mediterranean): Seasonal dynamics and environmental drivers. Estuar. Coast. Shelf Sci. 2013, 117, 136–147. [Google Scholar] [CrossRef]
- Afli, A.; Chaabane, K.I.; Chakroun, R.; Jabeur, C.; Ramos-Esplá, A.A. Specific diversity of the benthic macrofauna within the western coast f Tunis Bay and the Djerba Island coast (South-Western Mediterranean). Bull. Inst. Natn. Sci. Tech. Mer Salammbô 2013, 40, 51–62. [Google Scholar]
- Koutsoubas, D.; Arvanitidis, C.; Dounas, C.; Drummond, L. Community structure and dynamics of the molluscan fauna in a Mediterranean lagoon (Gialova lagoon, SW Greece). Belg. J. Zool. 2000, 130 (Suppl. 1), 135–142. [Google Scholar]
- Riggio, S.; Chemello, R. The role of coastal lagoons in the emerging and segregation of new marine taxa: Evidence from the Stagnone di Marsala Sound (Sicily). Bull. Inst. Oceanogr. Monaco 1992, 9, 1–19. [Google Scholar]
- Rueda, J.L.; Gofas, S.; Urra, J.; Salas, C. A highly diverse molluscan assemblage associated with eelgrass beds (Zostera marina L.) in the Alboran Sea: Microhabitat preference, feeding guilds and biogeographical distribution. Scientia Marina 2009, 73, 669–700. [Google Scholar] [CrossRef]
- Zenetos, A.; Galanidi, M. Mediterranean non-indigenous species at the start of the 2020s: Recent changes. Mar. Biodiver. Rec. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Ounifi-Ben Amor, K.; Rifi, M.; Ghanem, R.; Draief, I.; Zaouli, J.; Ben Souissi, J. Update of alien fauna and new records from Tunisian marine waters. Medit. Mar. Sci. 2016, 17, 124–139. [Google Scholar] [CrossRef]
- Crocetta, F.; Renda, W.; Vazzana, A. Alien Mollusca along the Calabrian shores of the Messina Strait area and a review of their distribution in the Italian seas. Boll. Malacol. 2009, 45, 15–30. [Google Scholar]
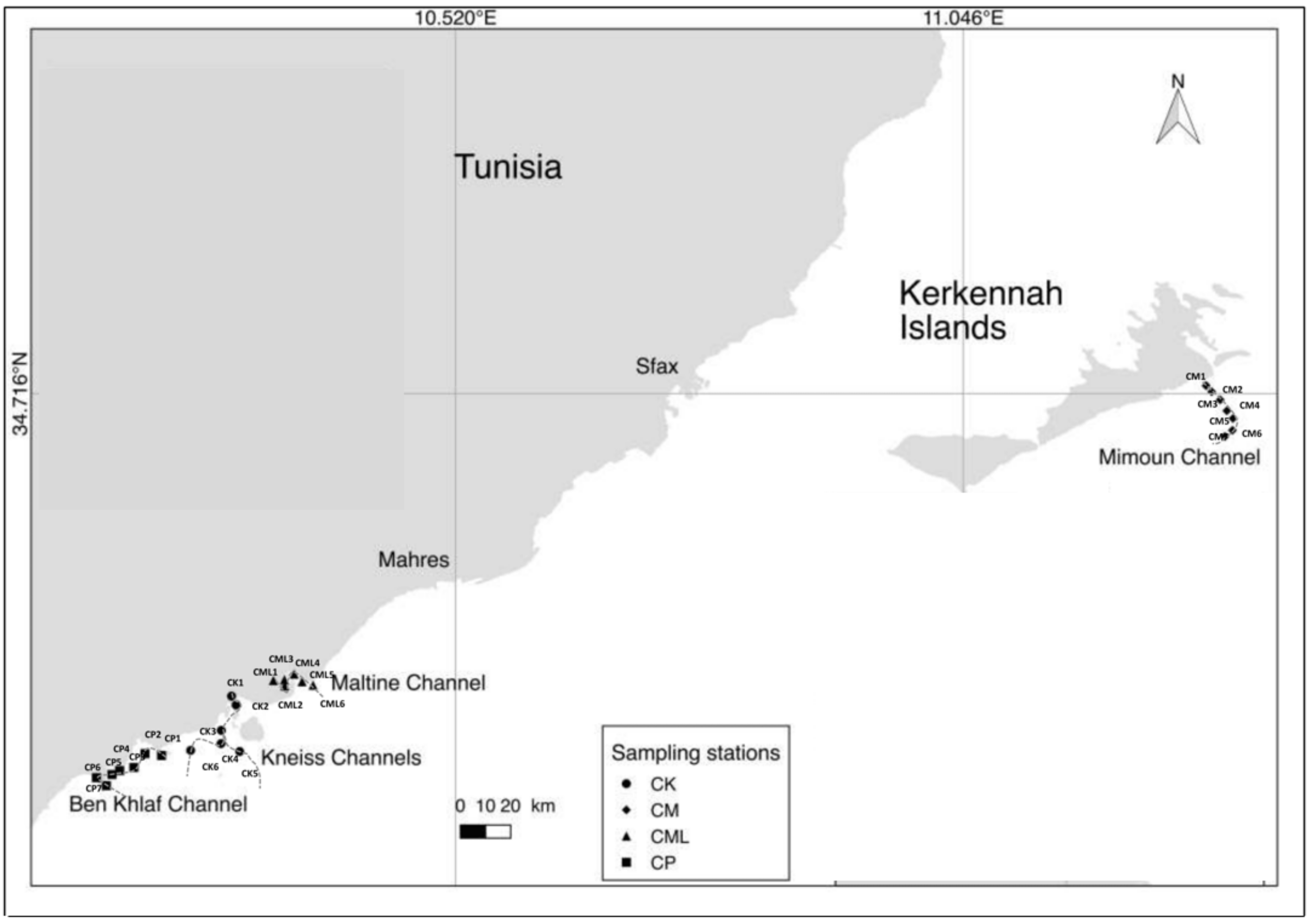
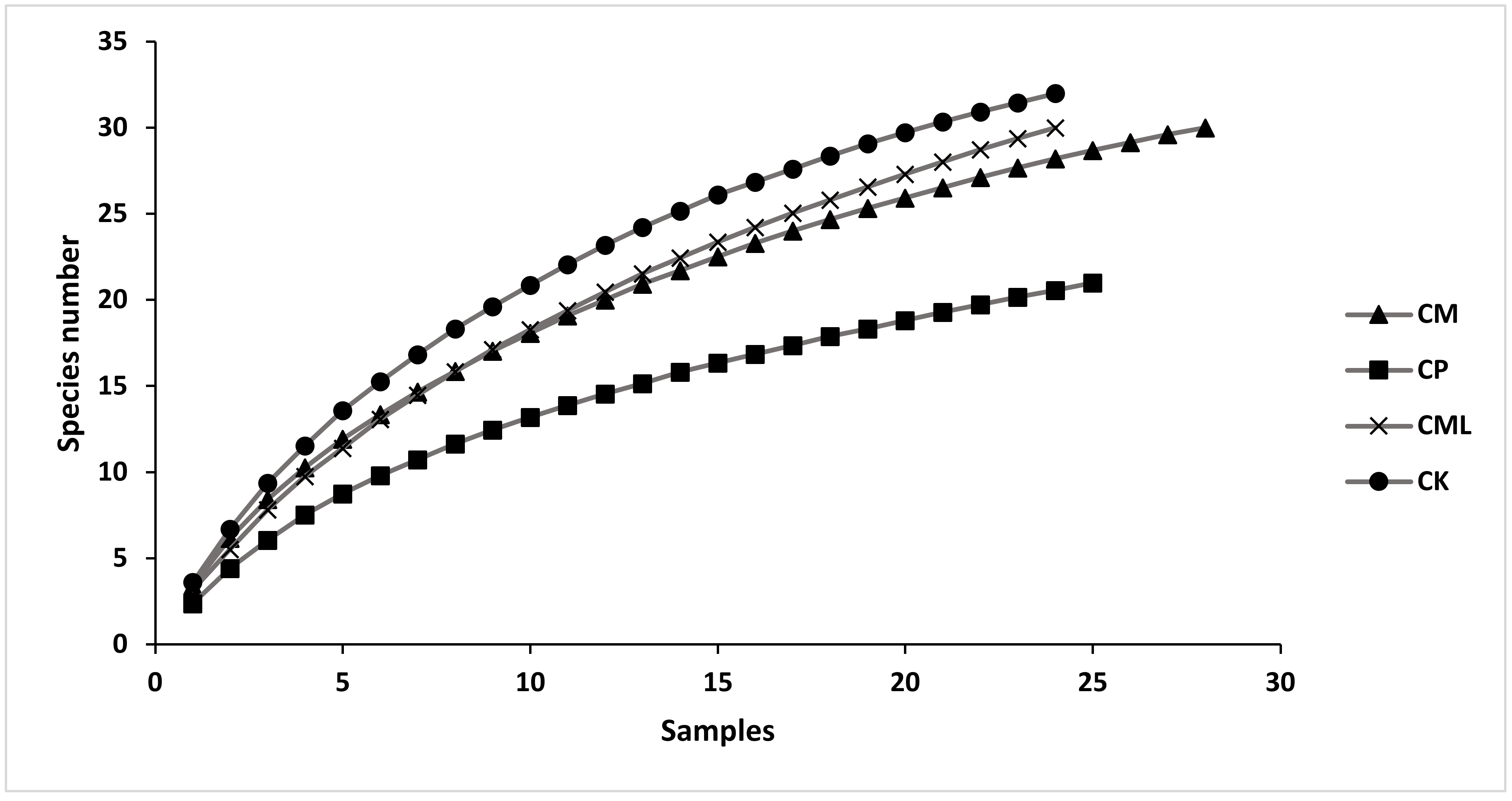

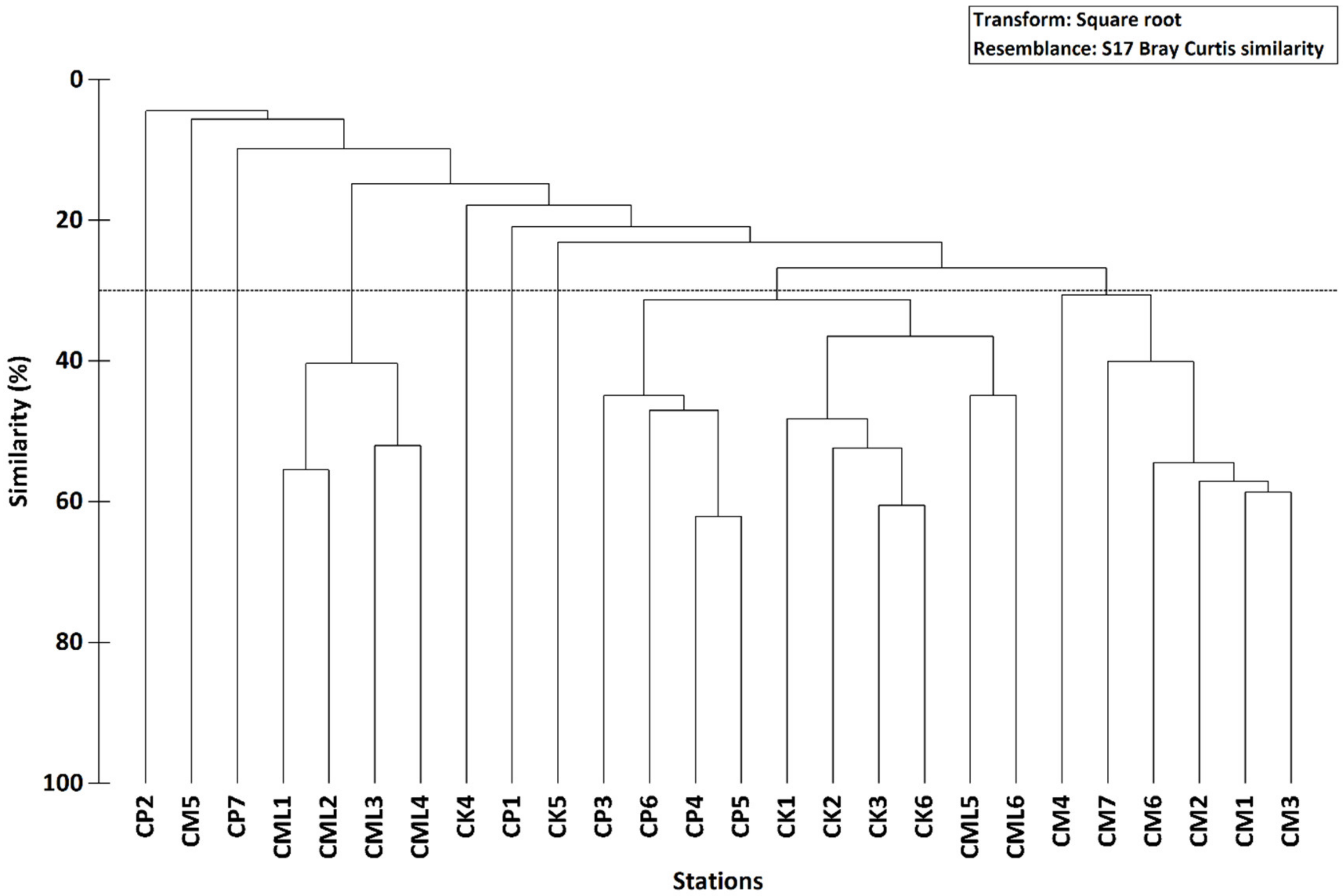
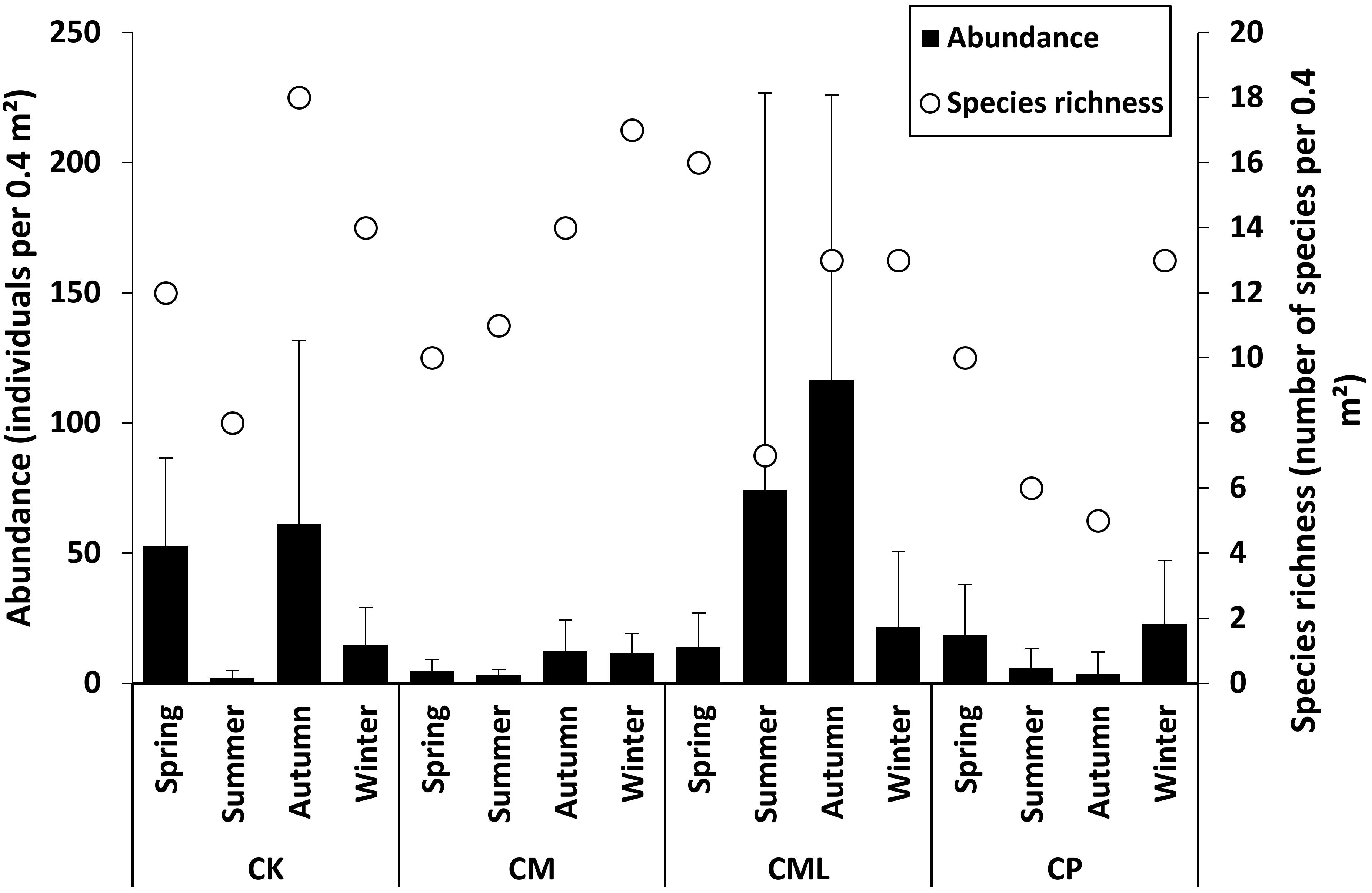
| Station | Depth (m) | Gravel (%) | Sand (%) | Silt-Clay (%) | OM % | Sediment Type | Phanerogams |
|---|---|---|---|---|---|---|---|
| CP 1 | 3.5 | 2.0 | 84.8 | 12.6 | 2.8 | Fine sand | Cymodocea nodosa, Zostera noltei |
| CP 2 | 2.8 | 1.7 | 96.6 | 1.5 | 0.9 | Fine sand | Cymodocea nodosa |
| CP 3 | 3.2 | 76.3 | 18.5 | 4.8 | 2.4 | Gravelly sand | Cymodocea nodosa |
| CP 4 | 6.1 | 7.9 | 87.8 | 3.8 | 1.1 | Fine sand | - |
| CP 5 | 7.6 | 3.7 | 83.6 | 12.3 | 1.9 | Fine sand | - |
| CP 6 | 11.9 | 2.1 | 62.4 | 34.9 | 7.3 | Silty sand | - |
| CP 7 | 0.9 | 2.0 | 95.3 | 2.4 | 1.9 | Fine sand | - |
| CML 1 | 1.0 | 22.2 | 49.8 | 27.6 | 9.3 | Silty sand | Zostera noltei |
| CML 2 | 2.1 | 57.2 | 35.4 | 7.0 | 3.1 | Shell and gravelly sand | Zostera noltei, Cymodocea nodosa, Halophila stipulacea |
| CML 3 | 2.1 | 67.6 | 26.4 | 5.8 | 1.7 | Shell and gravelly sand | Cymodocea nodosa |
| CML 4 | 3.1 | 80.6 | 17.8 | 1.5 | 1.5 | Gravelly sand | Halophila stipulacea, Posidonia oceanica |
| CML 5 | 4.4 | 4.3 | 93.5 | 1.7 | 1.4 | Fine sand | - |
| CML 6 | 3.7 | 30.7 | 66.9 | 2.0 | 1.5 | Coarse sand | Halophila stipulacea |
| CK 1 | 2.0 | 1.6 | 89.8 | 8.5 | 4.2 | Fine sand | Cymodocea nodosa, Halophila stipulacea |
| CK 2 | 8.5 | 18.1 | 78.5 | 3.1 | 0.7 | Medium sand | - |
| CK 3 | 5.3 | 2.3 | 90.3 | 6.9 | 2.4 | Fine sand | Cymodocea nodosa |
| CK 4 | 7.4 | 17.5 | 78.5 | 3.6 | 1.6 | Medium sand | - |
| CK 5 | 5.3 | 2.6 | 94.9 | 2.5 | 1.7 | Fine sand | Cymodocea nodosa, Zostera noltei |
| CK 6 | 8.3 | 4.0 | 80.9 | 14.4 | 5.8 | Silty sand | Cymodocea nodosa |
| CM 1 | 3.3 | 24.1 | 67.7 | 7.6 | 5.8 | Medium sand | - |
| CM 2 | 3.3 | 14.6 | 78.1 | 6.6 | 5.2 | Fine sand | Cymodocea nodosa |
| CM 3 | 3.6 | 35.8 | 57.4 | 6.1 | 4.9 | Medium sand | Posidonia oceanica |
| CM 4 | 4.1 | 62.8 | 36.5 | 0.4 | 1.7 | Gravelly sand | - |
| CM 5 | 10.0 | 61.6 | 36.6 | 1.5 | 1.8 | Gravelly sand | - |
| CM 6 | 13.5 | 75.7 | 21.6 | 2.4 | 2.5 | Gravelly sand | |
| CM 7 | 15.0 | 5.7 | 86.7 | 6.8 | 5.4 | Fine sand | Posidonia oceanica |
| Kerkennah (CM) | Maltine (CML) | Kneiss (CK) | Ben Kelaf (CP) | Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class/Family/Species | Spr | Sum | Aut | Win | Total | Spr | Sum | Aut | Win | Total | Spr | Sum | Aut | Win | Total | Spr | Sum | Aut | Win | Total | |
| Polyplacophora | |||||||||||||||||||||
| Tonicellidae Simroth, 1894 | |||||||||||||||||||||
| Lepidochitona cinerea (Linnaeus, 1767) | 21 | 21 | 21 | ||||||||||||||||||
| Acanthochitonidae Pilsbry, 1893 | |||||||||||||||||||||
| Acanthochitona crinita (Pennant, 1777) | 1 | 1 | 2 | 2 | |||||||||||||||||
| Gastropoda | |||||||||||||||||||||
| Fissurellidae Fleming, 1822 | |||||||||||||||||||||
| Diodora graeca (Linnaeus, 1758) | 1 | 2 | 3 | 3 | |||||||||||||||||
| Trochidae Rafinesque, 1815 | |||||||||||||||||||||
| Clanculus cruciatus (Linnaeus, 1758) | 1 | 3 | 4 | 4 | |||||||||||||||||
| Phasianellidae Swaison, 1840 | |||||||||||||||||||||
| Tricolia pullus (Linnaeus, 1758) | 1 | 1 | 2 | 2 | 3 | ||||||||||||||||
| Tricolia speciosa (Megerle von Mühlfeld, 1824) | 11 | 16 | 27 | 131 | 9 | 140 | 167 | ||||||||||||||
| Tricolia tenuis (Michaud, 1829) | 1 | 1 | 1 | ||||||||||||||||||
| Neritidae Rafinesque, 1815 | |||||||||||||||||||||
| Smaragdia viridis (Linnaeus, 1758) | 1 | 1 | 1 | 3 | 5 | 9 | 1 | 1 | 2 | 12 | |||||||||||
| Cerithiidae Fleming, 1822 | |||||||||||||||||||||
| Bittium reticulatum (da Costa, 1778) | 1 | 1 | 8 | 429 | 437 | 4 | 2 | 6 | 4 | 1 | 5 | 449 | |||||||||
| Cerithium scabridum Philippi, 1848 | 1 | 2 | 3 | 17 | 665 | 22 | 704 | 18 | 18 | 3 | 3 | 728 | |||||||||
| Littorinidae Children, 1834 | |||||||||||||||||||||
| Melarhaphe neritoides (Linnaeus, 1758) | 1 | 1 | 1 | ||||||||||||||||||
| Rissoidae Gray, 1847 | |||||||||||||||||||||
| Alvania sp | 1 | 1 | 1 | ||||||||||||||||||
| Rissoa auriscalpium (Linnaeus, 1758) | 5 | 5 | 5 | ||||||||||||||||||
| Setia sciutiana (Aradas & Benoit, 1874) | 4 | 4 | 4 | ||||||||||||||||||
| Velutinidae Gray, 1840 | |||||||||||||||||||||
| Lamellaria perspicua (Linnaeus, 1758) | 90 | 90 | 90 | ||||||||||||||||||
| Columbellidae Swaison, 1840 | |||||||||||||||||||||
| Amphissa acutecostata (Philippi, 1844) | 2 | 2 | 2 | ||||||||||||||||||
| Nassariidae Iredale, 1916 | |||||||||||||||||||||
| Tritia mutabilis (Linnaeus, 1758) | 2 | 2 | 2 | ||||||||||||||||||
| Tritia varicosa (W. Turton, 1825) | 4 | 4 | 4 | 1 | 5 | 9 | |||||||||||||||
| Muricidae Rafinesque, 1815 | |||||||||||||||||||||
| Bolinus brandaris (Linnaeus, 1758) | 4 | 4 | 4 | ||||||||||||||||||
| Hexaplex trunculus (Linnaeus, 1758) | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 4 | |||||||||||||
| Tudiclidae Cossmann, 1901 | |||||||||||||||||||||
| Euthria cornea (Linnaeus, 1758) | 1 | 1 | 1 | ||||||||||||||||||
| Mitridae Swaison, 1831 | |||||||||||||||||||||
| Episcomitra zonata (Marryat, 1819) | 1 | 1 | 1 | ||||||||||||||||||
| Conidae Fleming, 1822 | |||||||||||||||||||||
| Conus ventricosus Gmelin, 1791 | 1 | 1 | 1 | ||||||||||||||||||
| Bullidae Gray, 1822 | |||||||||||||||||||||
| Bulla striata Bruguière, 1792 | 1 | 1 | 1 | 1 | 2 | ||||||||||||||||
| Pyramidellidae Gray, 1840 | |||||||||||||||||||||
| Turbonilla lactea (Linnaeus, 1758) | 1 | 1 | 1 | ||||||||||||||||||
| Bivalvia | |||||||||||||||||||||
| Nuculidae Gray, 1824 | |||||||||||||||||||||
| Linucula hartvigiana (Dohrn, 1864) | 1 | 1 | 1 | 1 | 2 | ||||||||||||||||
| Nucula hanleyi Winckworth, 1931 | 1 | 1 | 1 | ||||||||||||||||||
| Nucula nitidosa Winckworth, 1930 | 2 | 2 | 1 | 1 | 3 | ||||||||||||||||
| Nucula nucleus (Linnaeus, 1758) | 29 | 29 | 29 | ||||||||||||||||||
| Solemyidae Gray, 1815 | |||||||||||||||||||||
| Solemya togata (Poli, 1791) | 13 | 3 | 2 | 18 | 1 | 1 | 2 | 20 | |||||||||||||
| Nuculanidae H. Adams & A. Adams, 1858 | |||||||||||||||||||||
| Lembulus pella (Linnaeus, 1758) | 1 | 1 | 4 | 2 | 6 | 7 | |||||||||||||||
| Mytilidae Rafinesque, 1815 | |||||||||||||||||||||
| Gregariella petagnae (Scacchi, 1832) | 2 | 3 | 20 | 11 | 36 | 5 | 5 | 2 | 2 | 6 | 6 | 49 | |||||||||
| Lioberus agglutinans (Cantraine, 1835) | 1 | 1 | 2 | 2 | |||||||||||||||||
| Musculus costulatus (Risso, 1826) | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 5 | 8 | |||||||||||
| Margaritidae Blainville, 1824 | |||||||||||||||||||||
| Pinctada radiata (Leach, 1814) | 33 | 13 | 46 | 1 | 1 | 2 | 1 | 2 | 3 | 2 | 2 | 53 | |||||||||
| Ostreidae Rafinesque, 1815 | |||||||||||||||||||||
| Magallana gigas (Thunberg, 1793) | 1 | 1 | 1 | ||||||||||||||||||
| Lucinidae J. Feming, 1828 | |||||||||||||||||||||
| Ctena decussata (O. G. Costa, 1829) | 1 | 1 | 2 | 1 | 1 | 2 | 4 | ||||||||||||||
| Loripes orbiculatus Poli, 1791 | 10 | 9 | 11 | 15 | 45 | 6 | 4 | 1 | 11 | 56 | 1 | 5 | 3 | 65 | 6 | 2 | 7 | 24 | 39 | 160 | |
| Cardiidae Lamarck, 1809 | |||||||||||||||||||||
| Cerastoderma glaucum (Bruguière, 1789) | 1 | 1 | 7 | 6 | 1 | 14 | 37 | 2 | 39 | 12 | 12 | 66 | |||||||||
| Papillicardium papillosum (Poli, 1791) | 2 | 2 | 2 | ||||||||||||||||||
| Parvicardium scriptum (Bucquoy, Dautzenberg & Dollfus, 1892) | 1 | 3 | 4 | 1 | 1 | 2 | 2 | 7 | |||||||||||||
| Chamidae Lamarck, 1809 | |||||||||||||||||||||
| Chama gryphoides Linnaeus, 1758 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 5 | |||||||||||||
| Tellinidae Blainville, 1814 | |||||||||||||||||||||
| Fabulina fabula (Gmelin, 1791) | 1 | 1 | 1 | ||||||||||||||||||
| Macomangulus tenuis (da Costa, 1778) | 1 | 1 | 1 | ||||||||||||||||||
| Moerella distorta (Poli, 1791) | 1 | 1 | 2 | 2 | 3 | 5 | 25 | 33 | 36 | ||||||||||||
| Moerella pulchella (Lamarck, 1818) | 12 | 12 | 1 | 1 | 13 | ||||||||||||||||
| Peronaea planata (Linnaeus, 1758) | 126 | 126 | 126 | ||||||||||||||||||
| Donacidae J. Fleming, 1828 | |||||||||||||||||||||
| Donax semistriatus Poli, 1795 | 1 | 1 | 1 | ||||||||||||||||||
| Semelidae Stoliczka, 1870 | |||||||||||||||||||||
| Abra alba (W. Wood, 1802) | 1 | 6 | 14 | 21 | 3 | 6 | 7 | 73 | 89 | 2 | 93 | 57 | 152 | 24 | 4 | 34 | 62 | 324 | |||
| Abra segmentum (Récluz, 1843) | 1 | 1 | 1 | ||||||||||||||||||
| Abra tenuis (Montagu, 1803) | 9 | 9 | 6 | 6 | 2 | 2 | 17 | ||||||||||||||
| Veneridae Rafinesque 1815 | |||||||||||||||||||||
| Gouldia minima (Montagu, 1803) | 1 | 5 | 6 | 1 | 2 | 3 | 2 | 2 | 4 | 1 | 1 | 2 | 15 | ||||||||
| Pitar rudis (Poli, 1795) | 1 | 1 | 1 | ||||||||||||||||||
| Ruditapes decussatus (Linnaeus, 1758) | 3 | 2 | 5 | 3 | 3 | 29 | 1 | 1 | 31 | 1 | 1 | 40 | |||||||||
| Corbulidae Lamarck, 1818 | |||||||||||||||||||||
| Varicorbula gibba (Olivi, 1792) | 1 | 2 | 3 | 1 | 1 | 16 | 1 | 8 | 2 | 27 | 87 | 4 | 7 | 8 | 106 | 137 | |||||
| Pharidae H. Adams & A. Adams, 1856 | |||||||||||||||||||||
| Pharus legumen (Linnaeus, 1758) | 1 | 1 | 1 | 1 | 43 | 43 | 45 | ||||||||||||||
| Total | 33 | 22 | 86 | 81 | 222 | 83 | 446 | 698 | 130 | 1357 | 317 | 13 | 357 | 89 | 786 | 110 | 36 | 24 | 160 | 330 | 2695 |
| df | F | p | Tukey Test | ||
|---|---|---|---|---|---|
| Species richness | Season | 3 | 3.11 | <0.05 | Sum ≠ Spr; Aut; Win |
| Channel | 3 | 2.07 | 0.11 | - | |
| Abundance | Season | 3 | 1.66 | 0.18 | - |
| Channel | 3 | 4.87 | <0.01 | CML ≠ CP; CM | |
| J’ | Season | 3 | 1.27 | 0.29 | - |
| Channel | 3 | 5.62 | <0.01 | CML ≠ CM | |
| H’ | Season | 3 | 1.43 | 0.24 | - |
| Channel | 3 | 3.48 | <0.05 | CM ≠ CP | |
| ∑ | 98 |
| Spring | Summer | Autumn | Winter | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CM | CP | CML | CK | CM | CP | CML | CK | CM | CP | CML | CK | CM | CP | CML | CK | |
| H’ | 2.46 | 1.34 | 3.28 | 2.62 | 2.86 | 1.65 | 0.32 | 2.78 | 2.71 | 2.13 | 0.41 | 2.39 | 3.44 | 2.83 | 2.13 | 2.10 |
| J’ | 0.74 | 0.40 | 0.82 | 0.73 | 0.82 | 0.64 | 0.11 | 0.93 | 0.71 | 0.92 | 0.11 | 0.57 | 0.84 | 0.76 | 0.57 | 0.55 |
| Study Area (Country) | Year | Depth (m) | Nature of Bottom-Sediment and Vegetation | Sampling Technique (Gear) | Sampled Area Per Station (m2) | Total Sampled Area (m2) | Taxonomic Richness | Abundance (ind./m2) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gulf of Gabès, Tunisia | Four Tidal Channels: Maltine Channel, Kneiss Islands Channel, Ben Khlaf Channel and Mimoun Channel | Seasonally: March, July, September 2016, January 2017 | 3 to 15 | Fine sand (FS), Gravelly sand with seagrass (Cymodosa nodosa, Zostera noltei) and macroalgae | Van Veen grab | 0.4 | 40.8 Maltine: 9.6 Kneiss: 9.6 Ben Khlaf: 11.2 Mimoun: 10.4 | 56 29 32 30 21 | 69 (mean) 142 82 30 22 | This study |
| Boughrara lagoon | Seasonally: February 2012 to November 2013 | 0.2 to 3.1 | FS | Scuba diving | 0.75 | 9.75 | 26 | 112 | 1 | |
| Kneiss Islands | January and March 2012 | Intertidal | FS-Medium sand (MS) with Zostera noltei | Hand corer | 0.032 | 0.608 | 24 | 639 | 2 | |
| Kneiss Islands and Maltine Wadi | Seasonally: July 2013 to April 2014 | Intertidal | FS-MS with Zostera noltei | Quadrat | 1.25 | 10 | 82 | 487 | 3 | |
| Kneiss Islands | September 2013 and December 2013 | Intertidal | FS-MS with Zostera noltei | Hand corer | 0.72 | 1.30 | No data | Minimum 804 Maximum 1913 | 4 | |
| Kneiss Islands | Spring 2013 and 2014 | Intertidal | FS-MS with Zostera noltei | Hand corer | 0.09 | 3.06 | 40 | 6000 | 5 | |
| Kneiss Islands | March and April 2015 | Intertidal | FS-MS with Zostera noltei | Hand corer | 0.18 | 0.72 | 17 | No data | 6 | |
| Kneiss Islands | April 2014 and Seasonally: October 2013, January, April and July 2014 | Intertidal | FS-MS with Zostera noltei | Hand corer | 0.09 | 3.06 | 46 | No data | 7 | |
| Zone of South Sfax | Seasonally: April, July and October 2015, January 2016 | 1.5 to 8 | FS-MS—Coarse sand Mud | Van Veen grab | 0.2 | 9.6 | 19 | No data | 8 | |
| Skhira Bay | April 2010 | 1.5 to 24 | FS-Coarse sand –Muddy sand | Van Veen grab | 0.2 | 5.6 | 64 | 115 | 21 | |
| Tunisia (others) | Bizerte lagoon (NE of Tunisia) | Monthly: September 2009 to September 2010 | 0.2 to 0.8 | Meadow of seagrass (Cymodosa nodosa) and macroalgae | Scrapping | 0.75 | 9 | 13 | 838 | 13 |
| Cap Zebib (NE of Tunisia) | Monthly: May 2007 to May 2008 | 3 and 12 | P. oceanica meadow | Scuba diving | 0.15 | 3.6 | 47 | 210 | 14 | |
| Tunis Bay (N Tunisia) Djerba Island (Gulf of Gabès, Tunisia) | May 2008 July 2009 | <5 10 to 34 | Sandy mud Sandy mud | Scuba diving Van Veen grab | 0.4 0.36 | 4 3.96 | 39 3 | No data | 18 | |
| Others Mediterranean Sites | Mellah lagoon (NE Algeria) | Seasonally: July 2008 to June 2009 | 1 to 5 | FS with Ruppia spp. | Van Veen grab | 0.3 | 18 | 11 | 188 | 9 |
| Smir lagoon (Mediterranean coast of Morocco) | Monthly: May 1999 to November 2000 | 1 to 2 | FS-MS with macroalgae and seagrass (Ruppia maritima, Zostera noltei) | Hand corer | 0.25 | 30 | 9 | 76 | 10 | |
| Canuelo bay (Southern Spain) | Seasonally: June, September, December 2004 and March 2005 | 12–14 | Zostera marina meadow | Scuba diving | 0.32 | 1.27 | 85 | 1896 | 20 | |
| Tyrrhenian Sea Ionian Sea (Southern Italia) | Spring 2015 summer 2015 | 10 to 20 | FS Posidonia oceanica meadow | Airlift pump | 0.48 0.75 | 1.92 3 | 36 38 | 355 135 | 15 | |
| Calaburas (Southern Spain) Calahonda (Southern Spain) | Seasonally: July, November 2007 and January, April 2008 | 2 to 3 | Posidonia oceanica meadow | Airlift sampler | 1.25 1.25 | 5 5 | 143 134 | 641 1101 | 11 | |
| Gialova lagoon (Greece) | Seasonally: June, September, December 1995 and March 1996 | 0 to 1 | Muddy sand with Cymodocea nodosa | Van Veen grab | 0.25 | 7 | 23 | 2970 | 12 |
| Gulf of Gabès | Others Sites Tunisia | Others Mediterranean Sites | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class/Family/Species | Present Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 21 | 13 | 14 | 17 | ## | 16 (Ghar El Melh) | 16 (Southern Lagoon Tunis) | 16 (Tunis Bay) | 19 (Coast of Dkhila) | 9 | 10 | 12 | 15 (FS) | 15 (HP) | 20 | 11 |
| Polyplacophora | |||||||||||||||||||||||||
| Chitonidae Rafinesque, 1815 | |||||||||||||||||||||||||
| Rhyssoplax olivacea (Spengler, 1797) | + | + | |||||||||||||||||||||||
| Scaphopoda | |||||||||||||||||||||||||
| Fustiariidae Steiner, 1991 | |||||||||||||||||||||||||
| Fustiaria rubescens (Deshayes, 1826) | + | ||||||||||||||||||||||||
| Dentaliidae (Children, 1834) | |||||||||||||||||||||||||
| Antalis vulgaris (da Costa, 1778) | + | ||||||||||||||||||||||||
| Gasteropoda | |||||||||||||||||||||||||
| Trochidae Rafinesque, 1815 | |||||||||||||||||||||||||
| Gibbula ardens (Salis Marschlins, 1793) | + | ||||||||||||||||||||||||
| Jujubinus exasperatus (Pennant, 1777) | + | ||||||||||||||||||||||||
| Jujubinus striatus (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Phorcus articulatus (Lamark, 1822) | + | ||||||||||||||||||||||||
| Steromphala racketti (Payraudeau, 1826) | + | ||||||||||||||||||||||||
| Steromphala umbilicaris (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Calliostomatidae Thiele, 1924 | |||||||||||||||||||||||||
| Calliostoma laugieri (Payraudeau, 1826) | + | ||||||||||||||||||||||||
| Calliostoma zizyphinum (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Phasianellidae Swaison, 1840 | |||||||||||||||||||||||||
| Tricolia pullus (Linnaeus, 1758) | + | + | + | ||||||||||||||||||||||
| Tricolia speciosa (Megerle von Mühlfeld, 1824) | + | + | + | + | + | + | + | ||||||||||||||||||
| Neritidae Rafinesque, 1815 | |||||||||||||||||||||||||
| Smaragdia viridis (Linnaeus, 1758) | + | + | + | + | |||||||||||||||||||||
| Cerithiidae Fleming, 1822 | |||||||||||||||||||||||||
| Bittium latreillii (Payeaudeau, 1826) | + | ||||||||||||||||||||||||
| Bittium reticulatum (da Costa, 1778) | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Cerithium scabridum Philippi, 1848 | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Cerithium vulgatum Bruguière, 1792 | + | + | + | + | |||||||||||||||||||||
| Potamididae H. Adams & A. Adams, 1854 | |||||||||||||||||||||||||
| Pirenella conica (de Blainville, 1829) | + | + | + | + | |||||||||||||||||||||
| Naticidae Guilding, 1834 | |||||||||||||||||||||||||
| Euspira nitida (Donovan, 1803) | + | ||||||||||||||||||||||||
| Neverita josephinia Risso, 1826 | + | ||||||||||||||||||||||||
| Rissoidae Gray, 1847 | |||||||||||||||||||||||||
| Rissoa auriscalpium (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Rissoa violacea Desmarest, 1914 | + | ||||||||||||||||||||||||
| Anabathridae Keen, 1951 | |||||||||||||||||||||||||
| Nodulus contortus (Jeffreys, 1856) | + | ||||||||||||||||||||||||
| Hydrobiidae Pruvot-Fol, 1937 | |||||||||||||||||||||||||
| Ecrobia ventrosa (Montagu, 1803) | + | + | |||||||||||||||||||||||
| Hydrobia acuta (Draparnaud, 1805) | + | + | + | + | |||||||||||||||||||||
| Cystiscidae Stimpson, 1865 | |||||||||||||||||||||||||
| Gibberula miliaria (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Columbellidae Swaison, 1840 | |||||||||||||||||||||||||
| Columbella rustica (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| NassariidaeIredale, 1916 | |||||||||||||||||||||||||
| Tritia corniculum (Olivi, 1792) | + | + | |||||||||||||||||||||||
| Tritia incrassata (Strøm, 1768) | + | + | |||||||||||||||||||||||
| Tritia mutabilis (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Tritia neritea (Linnaeus, 1758) | + | + | + | + | |||||||||||||||||||||
| Tritia nitida (Jeffreys, 1867) | + | ||||||||||||||||||||||||
| Tritia pellucida (Risso, 1827) | + | ||||||||||||||||||||||||
| Tritia reticulata (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Tritia varicosa (W. Turton, 1825) | + | ||||||||||||||||||||||||
| Pisaniidae Gray, 1857 | |||||||||||||||||||||||||
| Aplus dorbignyi (Payraudeau, 1826) | + | ||||||||||||||||||||||||
| Muricidae Rafinesque, 1815 | |||||||||||||||||||||||||
| Hexaplex trunculus (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Conidae Fleming, 1822 | |||||||||||||||||||||||||
| Conus ventricosus (Gmelin, 1791) | + | ||||||||||||||||||||||||
| Bullidae Gray, 1822 | |||||||||||||||||||||||||
| Bulla striata Bruguière, 1792) | + | ||||||||||||||||||||||||
| Haminoeidae Pilsbry, 1895 | |||||||||||||||||||||||||
| Haminoea navicula (da Costa, 1778) | + | ||||||||||||||||||||||||
| Pyramidellidae Gray, 1840 | |||||||||||||||||||||||||
| Parthenina juliae (de Folin, 1872) | + | ||||||||||||||||||||||||
| Bivalvia | |||||||||||||||||||||||||
| Nuculidae Gray, 1824 | |||||||||||||||||||||||||
| Nucula nitidosa (Winckworth, 1930) | + | ||||||||||||||||||||||||
| Solemyidae Gray, 1815 | |||||||||||||||||||||||||
| Solemya togata (Poli, 1791) | + | ||||||||||||||||||||||||
| Mytilidae Rafinesque, 1815 | |||||||||||||||||||||||||
| Musculus costulatus (Risso, 1826) | + | ||||||||||||||||||||||||
| Mytilaster marioni (Locard, 1889) | + | ||||||||||||||||||||||||
| Mytilaster minimus (Poli, 1795) | + | + | |||||||||||||||||||||||
| Mytilus galloprovincialis Lamark, 1819 | + | + | + | + | |||||||||||||||||||||
| Noetiidae Stewart, 1930 | |||||||||||||||||||||||||
| Striarca lactea (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Margaritidae Blainville, 1824 | |||||||||||||||||||||||||
| Pinctada radiata (Leach, 1814) | + | + | + | ||||||||||||||||||||||
| Lucinidae J. Feming, 1828 | |||||||||||||||||||||||||
| Loripes orbiculatus Poli, 1795 | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Lucinella divaricata (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Cardiidae Lamarck, 1809 | |||||||||||||||||||||||||
| Cerastoderma glaucum (Bruguière, 1789) | + | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Parvicardium exiguum (Gmelin, 1791) | + | ||||||||||||||||||||||||
| Parvicardium scriptum (Bucquoy, Dautzenberg & Dollfus, 1892) | + | ||||||||||||||||||||||||
| Cardita calyculata (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Glans trapezia (Linnaeus, 1767) | + | + | |||||||||||||||||||||||
| Mactridae Lamarck, 1809 | |||||||||||||||||||||||||
| Lutraria lutraria (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Tellinidae Blainville, 1814 | |||||||||||||||||||||||||
| Fabulina fabula (Gmelin, 1791) | + | ||||||||||||||||||||||||
| Gastrana fragilis (Linnaeus, 1758) | + | ||||||||||||||||||||||||
| Macomangulus tenuis (da Costa, 1778) | + | + | |||||||||||||||||||||||
| Moerella distorta (Poli, 1791) | + | ||||||||||||||||||||||||
| Moerella donacina (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Moerella pulchella Lamarck, 1818) | + | + | + | ||||||||||||||||||||||
| Semelidae Stoliczka, 1870 | |||||||||||||||||||||||||
| Abra alba (W. Wood, 1802) | + | + | + | + | |||||||||||||||||||||
| Abra segmentum (Récluz, 1843) | + | + | |||||||||||||||||||||||
| Abra tenuis (Montagu, 1803) | + | + | |||||||||||||||||||||||
| Scrobicularia plana (da Costa, 1778) | + | + | + | + | + | + | + | + | |||||||||||||||||
| Veneridae Rafinesque 1815 | |||||||||||||||||||||||||
| Chamelea gallina (Linnaeus, 1758) | + | + | |||||||||||||||||||||||
| Dosinia lupinus (Linnaeus, 1758) | + | + | + | + | |||||||||||||||||||||
| Polititapes aureus (Gmelin, 1791) | + | + | + | + | |||||||||||||||||||||
| Ruditapes decussatus (Linnaeus, 1758) | + | + | + | + | + | ||||||||||||||||||||
| Corbulidae Lamarck, 1818 | |||||||||||||||||||||||||
| Varicorbula gibba (Olivi, 1792) | + | + | + | ||||||||||||||||||||||
| Hiatellidae Gray, 1824 | |||||||||||||||||||||||||
| Hiatella arctica (Linnaeus, 1767) | + | ||||||||||||||||||||||||
| Pharidae H. Adams & A. Adams, 1856 | |||||||||||||||||||||||||
| Pharus legumen (Linnaeus, 1758) | + | ||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fersi, A.; Pezy, J.-P.; Bakalem, A.; Neifar, L.; Dauvin, J.-C. Molluscs from Tidal Channels of the Gulf of Gabès (Tunisia): Quantitative Data and Comparison with Other Lagoons and Coastal Waters of the Mediterranean Sea. J. Mar. Sci. Eng. 2023, 11, 545. https://doi.org/10.3390/jmse11030545
Fersi A, Pezy J-P, Bakalem A, Neifar L, Dauvin J-C. Molluscs from Tidal Channels of the Gulf of Gabès (Tunisia): Quantitative Data and Comparison with Other Lagoons and Coastal Waters of the Mediterranean Sea. Journal of Marine Science and Engineering. 2023; 11(3):545. https://doi.org/10.3390/jmse11030545
Chicago/Turabian StyleFersi, Abir, Jean-Philippe Pezy, Ali Bakalem, Lassad Neifar, and Jean-Claude Dauvin. 2023. "Molluscs from Tidal Channels of the Gulf of Gabès (Tunisia): Quantitative Data and Comparison with Other Lagoons and Coastal Waters of the Mediterranean Sea" Journal of Marine Science and Engineering 11, no. 3: 545. https://doi.org/10.3390/jmse11030545
APA StyleFersi, A., Pezy, J.-P., Bakalem, A., Neifar, L., & Dauvin, J.-C. (2023). Molluscs from Tidal Channels of the Gulf of Gabès (Tunisia): Quantitative Data and Comparison with Other Lagoons and Coastal Waters of the Mediterranean Sea. Journal of Marine Science and Engineering, 11(3), 545. https://doi.org/10.3390/jmse11030545







