Different Microsatellite Mutation Models May Lead to Contrasting Demographic Inferences through Genealogy-Based Approaches: A Case Study of the Finless Porpoise off the East Asian Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Locations and Sample Information
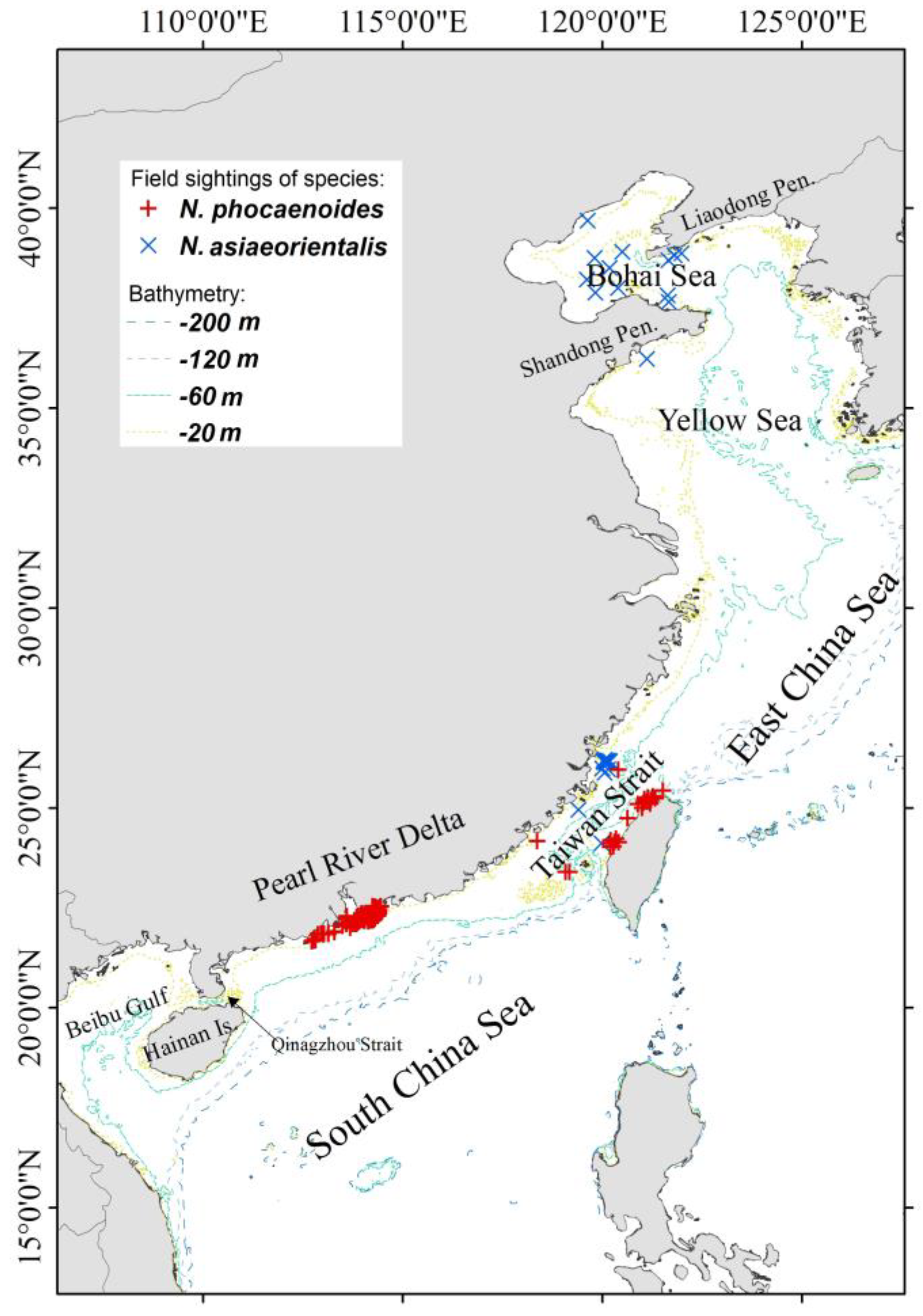
2.2. DNA Preparation and STR Amplification
2.3. Demographic Reconstruction Using MSVAR
2.4. Demographic Reconstruction Using VarEff
2.5. Demographic Reconstruction Using MIGRAINE
2.6. Ecological Niche Modeling
3. Results
3.1. Data Summary
3.2. Microsatellite Genotype and Diversity
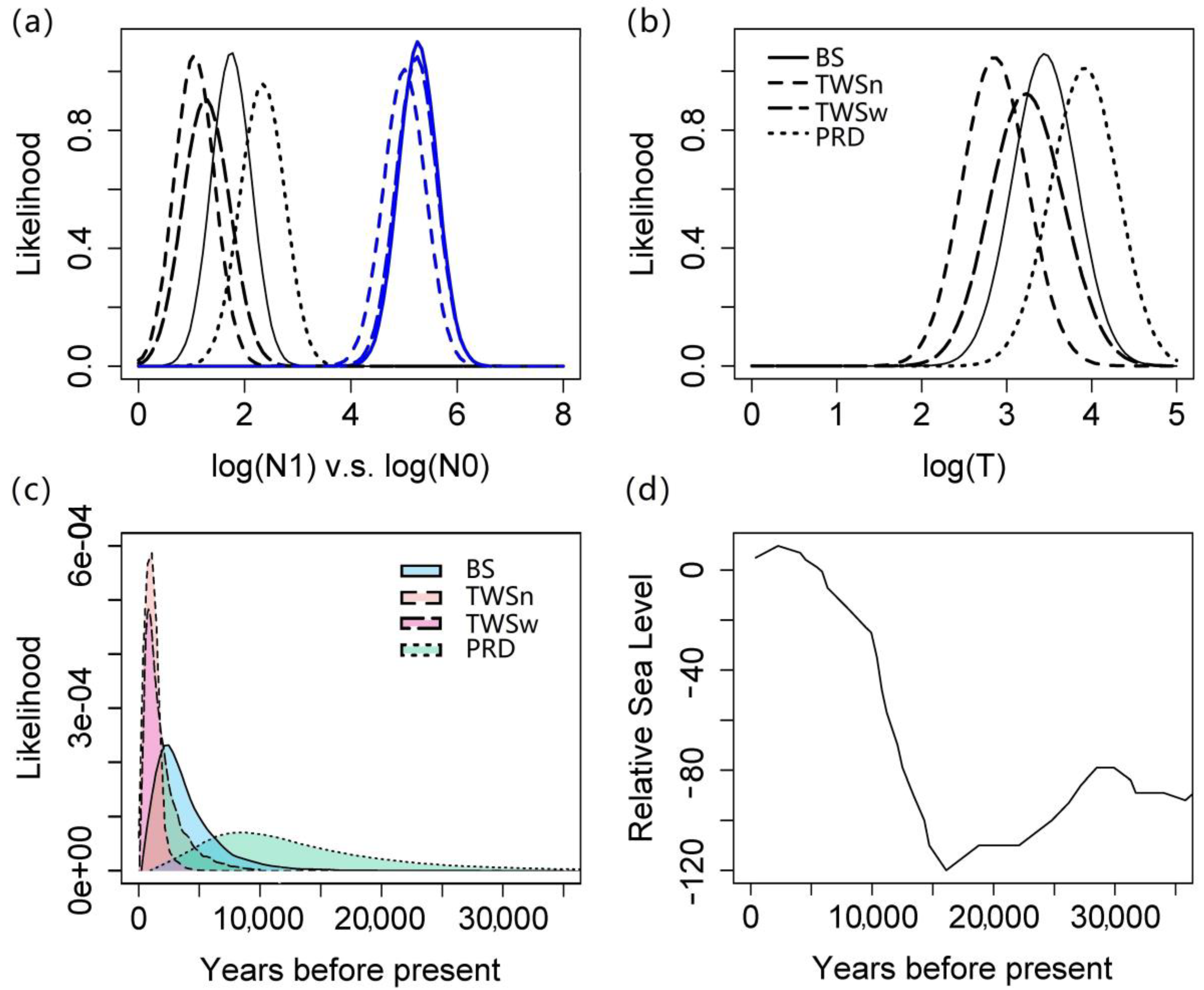
3.3. Demographic History Based on MSVAR
3.4. Demographic History Based on VarEff
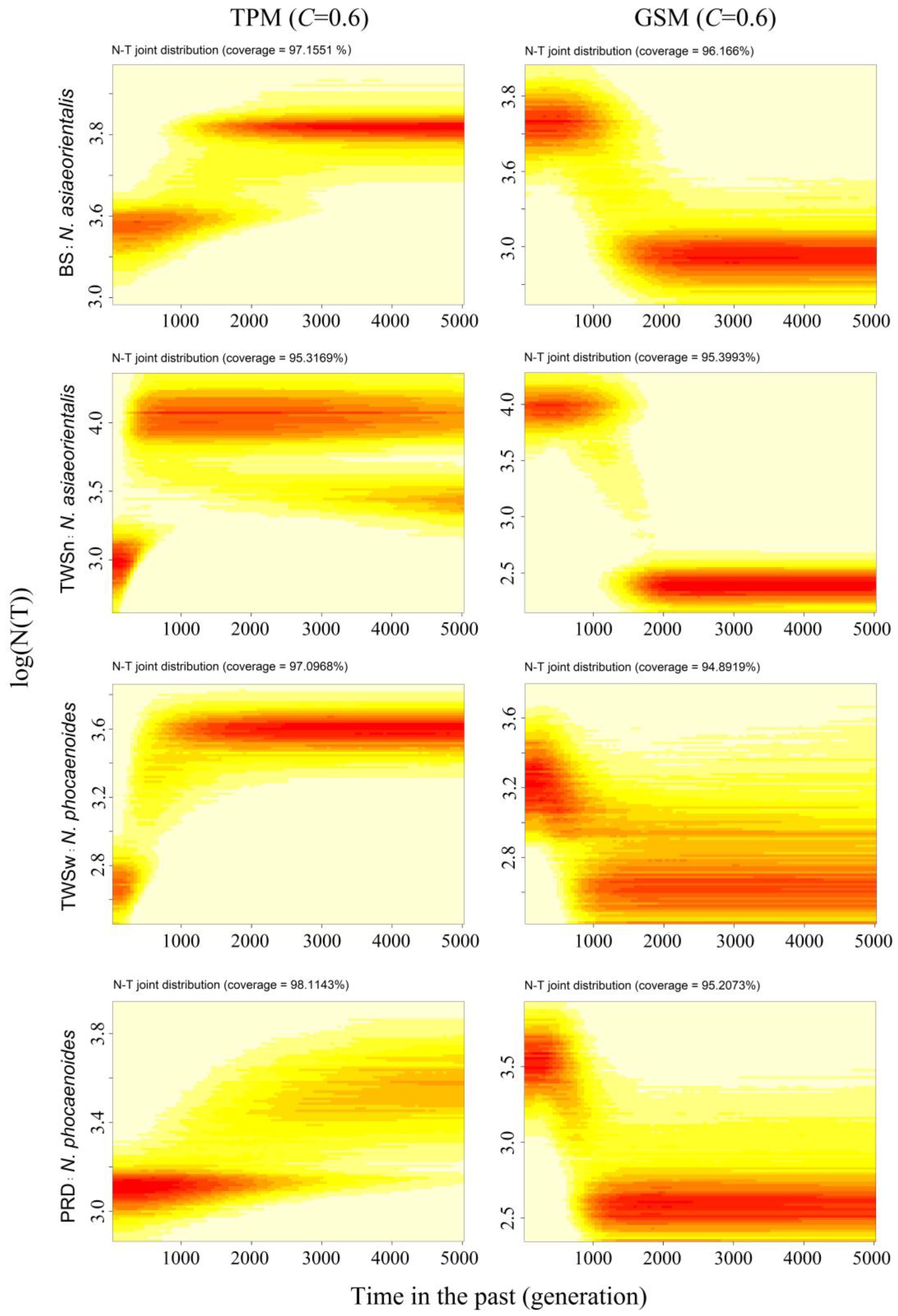
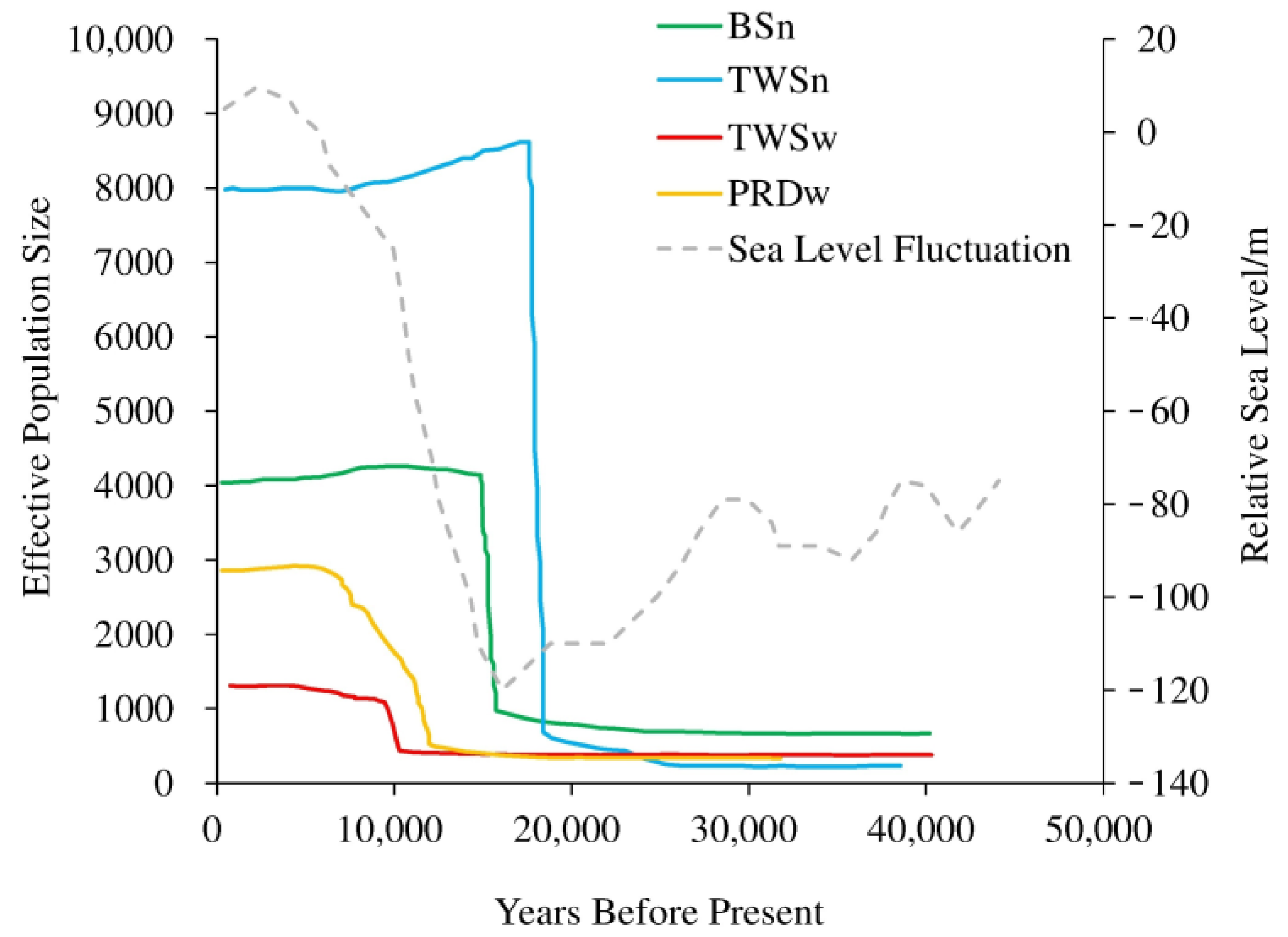
3.5. Demographic History Based on MIGRAINE
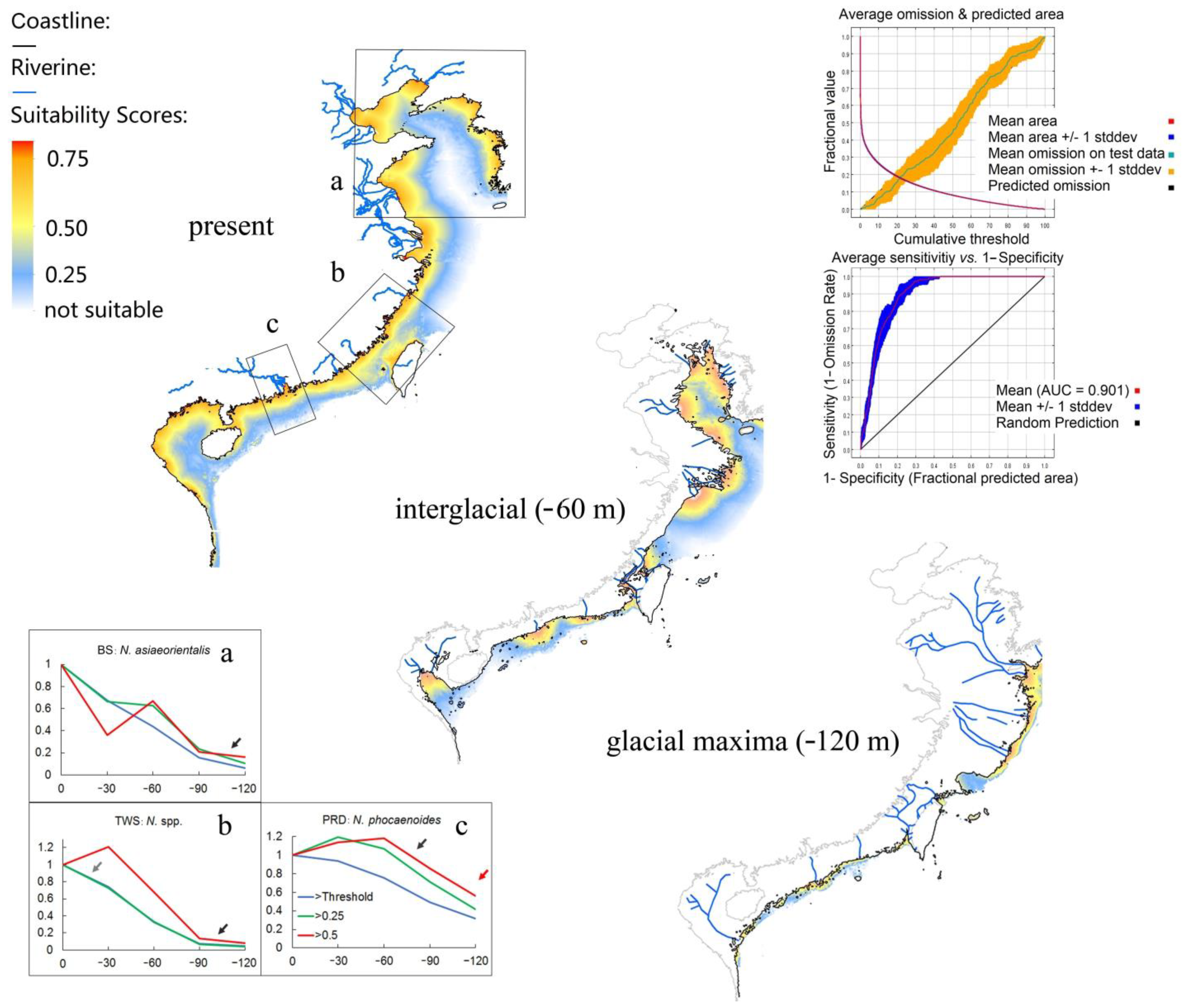
3.6. Ecological Niche Modeling
4. Discussion
4.1. Robustness of Demographic Inferences
4.2. Niche Suitability Modeling
4.3. Change in Habitat Size vs. Demographic Change
4.4. Change in Habitat Patchiness through the Sea Level Fluctuations
4.5. Limitations of the Present Study
5. Closing Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chikhi, L.; Sousa, V.C.; Luisi, P.; Goossens, B.; Beaumont, M.A. The confounding effects of population structure, genetic diversity and the sampling scheme on the detection and quantification of population size changes. Genetics 2010, 186, 983–995. [Google Scholar] [CrossRef]
- Garza, J.C.; Williamson, E.G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 2001, 10, 305–318. [Google Scholar] [CrossRef]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting FST. Nature Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef]
- Girod, C.; Vitalis, R.; Leblois, R.; Fréville, H. Inferring population decline and expansion from microsatellite data: A simulation-based evaluation of the Msvar Method. Genetics 2011, 188, 165–179. [Google Scholar] [CrossRef]
- Navascués, M.; Leblois, R.; Burgarella, C. Demographic inference through approximate-Bayesian-computation skyline plots. PeerJ 2017, 5, e3530. [Google Scholar] [CrossRef]
- Neel, M.C.; McKelvey, K.; Ryman, N.; Lloyd, M.W.; Short Bull, R.; Allendorf, F.W.; Schwartz, M.K.; Waples, R.S. Estimation of effective population size in continuously distributed populations: There goes the neighborhood. Heredity 2013, 111, 189–199. [Google Scholar] [CrossRef]
- Nikolic, N.; Chevalet, C. Detecting past changes of effective population size. Evol. Appl. 2014, 7, 663–681. [Google Scholar] [CrossRef]
- Lin, W.; Karczmarski, L.; Xia, J.; Zhang, X.; Yu, X.; Wu, Y. Increased human occupation and agricultural development accelerates the population contraction of an estuarine delphinid. Sci. Rep. 2016, 6, 35713. [Google Scholar] [CrossRef]
- Jordi, S.; Rasmus, H.; Erwan, Q.; Lounès, C. Climate change and human colonization triggered habitat loss and fragmentation in Madagascar. Mol. Ecol. 2017, 26, 5203–5222. [Google Scholar] [CrossRef]
- Li, Y.C.; Korol, A.B.; Fahima, T.; Beiles, A.; Nevo, E. Microsatellites: Genomic distribution, putative functions and mutational mechanisms: A review. Mol. Ecol. 2002, 11, 2453–2465. [Google Scholar] [CrossRef]
- Leblois, R.; Pudlo, P.; Néron, J.; Bertaux, F.; Reddy Beeravolu, C.; Vitalis, R.; Rousset, F. Maximum-likelihood inference of population size contractions from microsatellite data. Mol. Biol. Evol. 2014, 31, 2805–2823. [Google Scholar] [CrossRef] [PubMed]
- Janko, K.; Lecointre, G.; DeVries, A.; Couloux, A.; Cruaud, C.; Marshall, C. Did glacial advances during the Pleistocene influence differently the demographic histories of benthic and pelagic Antarctic shelf fishes?—Inferences from intraspecific mitochondrial and nuclear DNA sequence diversity. BMC Evol. Biol. 2007, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Ludt, W.B.; Rocha, L.A. Shifting seas: The impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa. J. Biogeogr. 2015, 42, 25–38. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, R.; Porter, L.; Chen, J.; Wu, Y. Evolution of Sousa chinensis: A scenario based on mitochondrial DNA study. Mol. Phylogenet. Evol. 2010, 57, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Cassens, I.; Van Waerebeek, K.; Best, P.B.; Tzika, A.; Van Helden, A.L.; Crespo, E.A.; Milinkovitch, M.C. Evidence for male dispersal along the coasts but no migration in pelagic waters in dusky dolphins (Lagenorhynchus obscurus). Mol. Ecol. 2005, 14, 107–121. [Google Scholar] [CrossRef]
- Whitehead, H. Gene–culture coevolution in whales and dolphins. Proc. Natl. Acad. Sci. USA 2017, 114, 7814–7821. [Google Scholar] [CrossRef]
- He, L.; Zhang, A.; Zhu, C.; Weese, D.; Qiao, Z. Phylogeography of the mud crab (Scylla serrata) in the Indo-West Pacific reappraised from mitochondrial molecular and oceanographic clues: Transoceanic dispersal and coastal sequential colonization. Mar. Ecol. 2011, 32, 52–64. [Google Scholar] [CrossRef]
- Yang, S.; Lai, X.; Sheng, G.; Wang, S. Deep genetic divergence within a “living fossil” brachiopod Lingula anatina. J. Paleontol. 2013, 87, 902–908. [Google Scholar] [CrossRef]
- Tsoi, K.H.; Wang, Z.Y.; Chu, K.H. Genetic divergence between two morphologically similar varieties of the kuruma shrimp Penaeus Japonicus. Mar. Biol. 2005, 147, 367–379. [Google Scholar] [CrossRef]
- Wei, K.Y.; Lee, M.Y.; Duan, W.; Chen, C.; Wang, C.H. Palaeoceanographic change in the northeastern South China Sea during the last 15000 years. J. Quat. Sci. 1998, 13, 55–64. [Google Scholar] [CrossRef]
- Hu, Z.-M.; Uwai, S.; Yu, S.-H.; Komatsu, T.; Ajisaka, T.; Duan, D. Phylogeographic heterogeneity of the brown macroalga Sargassum horneri (Fucaceae) in the northwestern Pacific in relation to late Pleistocene glaciation and tectonic configurations. Mol. Ecol. 2011, 20, 3894–3909. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Wang, J.Y. Revision of the taxonomy of finless porpoises (genus Neophocaena): The existence of two species. J. Mar. Anim. Ecol. 2011, 4, 3–16. [Google Scholar]
- Wang, J.Y.; Yang, S.C.; Wang, B.J.; Wang, L.S. Distinguishing between two species of finless porpoises (Neophocaena phocaenoides and N. asiaeorientalis) in areas of sympatry. Mammalia 2010, 74, 305–310. [Google Scholar] [CrossRef]
- Wang, J.Y.; Frasier, T.R.; Yang, S.C.; White, B.N. Detecting recent speciation events: The case of the finless porpoise (genus Neophocaena). Heredity 2008, 101, 145. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Perrin, W.F.; Reeves, R.R.; Rosel, P.E.; Wang, J.Y.; Cipriano, F.; Scott Baker, C.; Brownell, R.L., Jr. Why we should develop guidelines and quantitative standards for using genetic data to delimit subspecies for data-poor organisms like cetaceans. Mar. Mammal Sci. 2017, 33, 12–26. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Tzika, A.C.; Zhu, Q.; Van Doninck, K.; Milinkovitch, M.C. Analysis of global and local population stratification of finless porpoises Neophocaena phocaenoides in Chinese waters. Mar. Biol. 2011, 158, 1791–1804. [Google Scholar] [CrossRef]
- Lee, S.-M.; Park, K.J.; Kim, B.-Y.; Min, M.-S.; Lee, H.; Lee, M.-Y. Genetic diversity and population demography of narrow-ridged finless porpoises from South Korea on the basis of mitochondrial DNA variation: Implications for its conservation in East Asia. Mar. Mammal Sci. 2018, 35, 574–594. [Google Scholar] [CrossRef]
- Lin, W.; Karczmarski, L.; Wu, Y. Phylogeography of the finless porpoise and potential implications for the taxonomy of Neophocaena spp. Mamm. Biol. Z. Für Säugetierkd. 2017, 86, 92–101. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, L.; Chen, S.; Sakornwimon, W.; Aierken, R.; Kittiwattanawong, K.; Wang, X.; Li, S. Novel insights into the spatial genetic patterns of the finless porpoise from East to Southeast Asia. Water Biol. Secur. 2023, 2, 100094. [Google Scholar] [CrossRef]
- Yoshida, H.; Yoshioka, M.; Shirakihara, M.; Chow, S. Population structure of finless porpoises (Neophocaena phocaenoides) in coastal waters of Japan based on mitochondrial DNA sequences. J. Mammal. 2001, 82, 123–130. [Google Scholar] [CrossRef]
- Taylor, B.L.; Archer, F.I.; Martien, K.K.; Rosel, P.E.; Hancock-Hanser, B.L.; Lang, A.R.; Leslie, M.S.; Mesnick, S.L.; Morin, P.A.; Pease, V.L.; et al. Guidelines and quantitative standards to improve consistency in cetacean subspecies and species delimitation relying on molecular genetic data. Mar. Mammal Sci. 2017, 33, 132–155. [Google Scholar] [CrossRef]
- Lin, W.; Karczmarski, L.; Li, J.; Chan, S.C.Y.; Guo, L.; Wu, Y. Differential population dynamics of a coastal porpoise correspond to the fishing effort in a large estuarine system. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 223–234. [Google Scholar] [CrossRef]
- Lee, Y.R.; An, Y.R.; Park, K.J.; Sohn, H.; An, D.H.; Kim, S.A. Age and reproduction of the finless porpoises, Neophocaena asiaeorientalis, in the Yellow Sea, Korea. Anim. Cells Syst. 2013, 17, 366–373. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Hung, S.K.; Robertson, K.M.; Archer, F.I. Life history of the Indo-Pacific humpback dolphin in the Pearl River Estuary, southern China. Mar. Mammal Sci. 2012, 28, 84–104. [Google Scholar] [CrossRef]
- Hashimoto, M.; Shirakihara, K.; Shirakihara, M.; Hiramatsu, K. Estimating the rate of increase for the finless porpoise with special attention to predictions for the Inland Sea population in Japan. Popul. Ecol. 2013, 55, 441–449. [Google Scholar] [CrossRef]
- Lin, W.; Frère, C.H.; Karczmarski, L.; Xia, J.; Gui, D.; Wu, Y. Phylogeography of the finless porpoise (genus Neophocaena): Testing the stepwise divergence hypothesis in the northwestern Pacific. Sci. Rep. 2014, 4, 6572. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Lin, W.; Gui, D.; Karczmarski, L.; Wu, Y. Molecular evidence reveals the distinctiveness of Indo-Pacific finless porpoises (Neophocaena phocaenoides) in the Pearl River Estuary and insights into genus Neophocaena’s origin. Mar. Biol. 2014, 161, 1919–1930. [Google Scholar] [CrossRef]
- Zhou, X.; Guang, X.; Sun, D.; Xu, S.; Li, M.; Seim, I.; Jie, W.; Yang, L.; Zhu, Q.; Xu, J.; et al. Population genomics of finless porpoises reveal an incipient cetacean species adapted to freshwater. Nat. Commun. 2018, 9, 1276. [Google Scholar] [CrossRef]
- Brüniche-Olsen, A.; Westerman, R.; Kazmierczyk, Z.; Vertyankin, V.V.; Godard-Codding, C.; Bickham, J.W.; DeWoody, J.A. The inference of gray whale (Eschrichtius robustus) historical population attributes from whole-genome sequences. BMC Evol. Biol. 2018, 18, 87. [Google Scholar] [CrossRef]
- Voris, H.K. Maps of Pleistocene sea levels in Southeast Asia: Shorelines, river systems and time durations. J. Biogeogr. 2000, 27, 1153–1167. [Google Scholar] [CrossRef]
- Bintanja, R.; van de Wal, R.S.W. North American ice-sheet dynamics and the onset of the 100,000-year glacial cycles. Nature 2008, 454, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Huang, G.; Switzer, A.D.; Yu, F.; Yim, W.W.-S. An evolutionary model for the Holocene formation of the Pearl River delta, China. Holocene 2009, 19, 129–142. [Google Scholar] [CrossRef]
- Nan, Q.; Li, T.; Chen, J.; Nigma, R.; Yu, X.; Xu, Z.; Yang, Z. Late Holocene (~2 ka) East Asian Monsoon variations inferred from river discharge and climate interrelationships in the Pearl River Estuary. Quat. Res. 2014, 81, 240–250. [Google Scholar] [CrossRef]
- Yao, Y.; Harff, J.; Meyer, M.; Zhan, W. Reconstruction of paleocoastlines for the northwestern South China Sea since the Last Glacial Maximum. Sci. China Ser. D Earth Sci. 2009, 52, 1127–1136. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Hung, S.K.; Würsig, B. Protecting small cetaceans from coastal development: Impact assessment and mitigation experience in Hong Kong. Mar. Policy 2009, 33, 305–311. [Google Scholar] [CrossRef]
- Berné, S.; Vagner, P.; Guichard, F.; Lericolais, G.; Liu, Z.; Trentesaux, A.; Yin, P.; Yi, H.I. Pleistocene forced regressions and tidal sand ridges in the East China Sea. Mar. Geol. 2002, 188, 293–315. [Google Scholar] [CrossRef]
- Xiao, S.; Li, A.; Jiang, F.; Li, T.; Wan, S.; Huang, P. The history of the Yangtze River entering sea since the last glacial maximum: A review and look forward. J. Coast. Res. 2004, 20, 599–604. [Google Scholar] [CrossRef]
- Ku, F.-C. No Recent Gene Flow among the Three Subspecies of Genus Neophocaena Revealed by Microsatellite Markers. Master’s Thesis, Taiwan University, Taiwan, China, 2006. [Google Scholar]
- Chen, L.; Bruford, M.; Yang, G. Isolation and characterization of microsatellite loci in the finless porpoise (Neophocaena phocaenoides). Mol. Ecol. Notes 2007, 7, 1129–1131. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. Development of tetranucleotide microsatellite loci for the finless porpoise (Neophocaena phocaenoides). Conserv. Genet. 2008, 9, 1033–1035. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. A set of polymorphic dinucleotide and tetranucleotide microsatellite markers for the Indo-Pacific humpback dolphin (Sousa chinensis) and cross-amplification in other cetacean species. Conserv. Genet. 2009, 10, 697–700. [Google Scholar] [CrossRef]
- Zheng, J.; Liao, X.; Tong, J.; Du, H.; Milinkovitch, M.; Wang, D. Development and characterization of polymorphic microsatellite loci in the endangered Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis). Conserv. Genet. 2008, 9, 1007–1009. [Google Scholar] [CrossRef]
- Storz, J.F.; Beaumont, M.A. Testing for genetic evidence of population expansion and contraction: An empirical analysis of microsatellite DNA variation using a hierarchical bayesian model. Evolution 2002, 56, 154–166. [Google Scholar] [PubMed]
- Fajardo-Mellor, L.; Berta, A.; Brownell, R.L.; Boy, C.C.; Natalie, P.; Goodall, R. The phylogenetic relationships and biogeography of true porpoises (Mammalia: Phocoenidae) based on morphological data. Mar. Mammal Sci. 2006, 22, 910–932. [Google Scholar] [CrossRef]
- Brooks, S.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Smith, B.J. BOA: An R package for MCMC output convergence assessment and posterior inference. J. Stat. Softw. 2007, 21, 1–37. [Google Scholar] [CrossRef]
- Beaumont, M.A. Detecting population expansion and decline using microsatellites. Genetics 1999, 153, 2013–2029. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, P.; Welch, P. Simulation run length control in the presence of an initial transient. Oper. Res. 1983, 31, 1109–1144. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, S.-L.; Hao, Y.; Turvey, S.T.; Gong, W.; Wang, D. Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 2012, 153, 192–200. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, K.; Gao, A.; Chang, Q. A study on the life table and dynamics of three finless porpoise populations in the Chinese waters. Acta Theriol. Sin. 1998, 18, 1–7. [Google Scholar]
- Park, K.J.; Sohn, H.; An, Y.R.; Kim, H.W.; An, D.H. A new abundance estimate for the finless porpoise Neophocaena asiaeorientalis on the west coast of Korea: An indication of population decline. Fish. Aquat. Sci. 2015, 18, 411–416. [Google Scholar] [CrossRef]
- Shirakihara, M.; Shirakihara, K. Finless porpoise bycatch in Ariake Sound and Tachibana Bay, Japan. Endargered Species Res. 2013, 21, 255–262. [Google Scholar] [CrossRef]
- Sun, J.X.; Helgason, A.; Masson, G.; Ebenesersdóttir, S.S.; Li, H.; Mallick, S.; Gnerre, S.; Patterson, N.; Kong, A.; Reich, D. A direct characterization of human mutation based on microsatellites. Nat. Genet. 2012, 44, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-J.; Kim, Z.-G.; Zhang, C.-I. Abundance estimation of the finless porpoise, Neophocaena phocaenoides, using models of the detection function in a line transect. Korean J. Fish. Aquat. Sci. 2007, 40, 201–209. [Google Scholar]
- Zuo, T.; Sun, J.; Shi, Y.; Wang, J. Primary survey of finless porpoise population in the Bohai Sea. Acta Theriol. Sin. 2018, 38, 551–561. [Google Scholar]
- Hung, S.K.Y. Monitoring of Marine Mammals in Hong Kong Waters—Data Collection (2009–2010). In Report to the Agriculture, Fisheries and Conservation Department of the Hong Kong SAR Government; AFCD/SQ/137/08; Hong Kong SAR Government: Hong Kong, China, 2010; 85p. [Google Scholar]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Williams, K.J.; Belbin, L.; Austin, M.P.; Stein, J.L.; Ferrier, S. Which environmental variables should I use in my biodiversity model? Int. J. Geogr. Inf. Sci. 2012, 26, 2009–2047. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Yoo, D.G.; Lee, G.S.; Kim, G.Y.; Kang, N.K.; Yi, B.Y.; Kim, Y.J.; Chun, J.H.; Kong, G.S. Seismic stratigraphy and depositional history of late Quaternary deposits in a tide-dominated setting: An example from the eastern Yellow Sea. Mar. Petrol. Geol. 2016, 73, 212–227. [Google Scholar] [CrossRef]
- Gardner, M.G.; Bull, C.M.; Cooper, S.J.B.; Duffield, G.A. Microsatellite mutations in litters of the Australian lizard Egernia stokesii. J. Evolution. Biol. 2000, 13, 551–560. [Google Scholar] [CrossRef]
- Peery, M.Z.; Kirby, R.; Reid, B.N.; Stoelting, R.; Doucet-Bëer, E.; Robinson, S.; VÁSquez-Carrillo, C.; Pauli, J.N.; Palsbøll, P.J. Reliability of genetic bottleneck tests for detecting recent population declines. Mol. Ecol. 2012, 21, 3403–3418. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, A.; Peterson, A.C.; Garza, J.C.; Valdes, A.M.; Slatkin, M.; Freimer, N.B. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci. USA 1994, 91, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Seielstad, M.T.; Perez-Lezaun, A.; Feldman, M.W. Population growth of human Y chromosome microsatellites. Mol. Biol. Evol. 1999, 16, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, D.Y.; Zeng, Y.F.; Bai, W.N. Recent demographic histories of temperate deciduous trees inferred from microsatellite markers. BMC Ecol. Evol. 2021, 21, 88. [Google Scholar] [CrossRef]
- Lucati, F.; Poignet, M.; Miró, A.; Trochet, A.; Aubret, F.; Barthe, L.; Bertrand, R.; Buchaca, T.; Calvez, O.; Caner, J.; et al. Multiple glacial refugia and contemporary dispersal shape the genetic structure of an endemic amphibian from the Pyrenees. Mol. Ecol. 2020, 29, 2904–2921. [Google Scholar] [CrossRef]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Shirakihara, K.; Shirakihara, M.; Yamamoto, Y. Distribution and abundance of finless porpoise in the Inland Sea of Japan. Mar. Biol. 2007, 150, 1025–1032. [Google Scholar] [CrossRef]
- Amano, M.; Nakahara, F.; Hayano, A.; Shirakihara, K. Abundance estimate of finless porpoises off the Pacific coast of eastern Japan based on aerial surveys. Mamm. Study 2003, 28, 103–110. [Google Scholar] [CrossRef]
- Krützen, M.; Beasley, I.; Ackermann, C.Y.; Lieckfeldt, D.; Ludwig, A.; Ryan, G.E.; Bejder, L.; Parra, G.J.; Wolfensberger, R.; Spencer, P.B.S. Demographic collapse and low genetic diversity of the Irrawaddy dolphin population inhabiting the Mekong River. PLOS ONE 2018, 13, e0189200. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Gao, T.X.; Wu, S.F.; Zhang, Y.P. Pleistocene isolation in the Northwestern Pacific marginal seas and limited dispersal in a marine fish, Chelon haematocheilus (Temminck & Schlegel, 1845). Mol. Ecol. 2010, 16, 275–288. [Google Scholar]
- Poommouang, A.; Kriangwanich, W.; Buddhachat, K.; Brown, J.L.; Piboon, P.; Chomdej, S.; Kampuansai, J.; Mekchay, S.; Kaewmong, P.; Kittiwattanawong, K.; et al. Genetic diversity in a unique population of dugong (Dugong dugon) along the sea coasts of Thailand. Sci. Rep. 2021, 11, 11624. [Google Scholar] [CrossRef] [PubMed]
- Tsandev, I.; Rabouille, C.; Slomp, C.; Cappellen, P.V. Shelf erosion and submarine river canyons: Implications for deep-sea oxygenation and ocean productivity during glaciation. Biogeosciences 2010, 7, 1973–1982. [Google Scholar] [CrossRef]
- Lin, W.; Chang, L.; Frère, C.H.; Zhou, R.; Chen, J.; Chen, X.; Wu, Y. Differentiated or not? An assessment of current knowledge of genetic structure of Sousa chinensis in China. J Exp. Mar. Biol. Ecol. 2012, 416–417, 17–20. [Google Scholar] [CrossRef]
- Chen, M.; Fontaine, M.C.; Chehida, Y.B.; Zheng, J.; Mei, Z.; Hao, Y.; Wang, K.; Wu, M.; Zhao, Q.; Wang, D. Genetic footprint of population fragmentation and contemporaneous decline in the endangered Yangtze finless porpoise. Sci. Rep. 2017, 7, 14449. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Wu, M.; Ruan, R.; Zhao, Q.; Wang, D. Genetic diversity and population structure of the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) as revealed by mitochondrial and microsatellite DNA. Int. J. Mol. Sci. 2014, 15, 11307–11323. [Google Scholar] [CrossRef]
- Koh, H.S.; Jo, J.E.; Ahn, N.H.; Lee, J.H.; Kim, K.S.; Jin, C.W. Preliminary study on genetic differences between two species of finless porpoises, genus Neophocaena, with lack of genetic divergence between two subspecies of the narrow-ridged finless porpoise, N. asiaeorientalis: Cytochrome b sequence analyses. J. Cetacean Res. Manag. 2013, 13, 195–199. [Google Scholar]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Edrén, S.M.C.; Wisz, M.S.; Teilmann, J.; Dietz, R.; Söderkvist, J. Modelling spatial patterns in harbour porpoise satellite telemetry data using maximum entropy. Ecography 2010, 33, 698–708. [Google Scholar] [CrossRef]
| Population | N | # of Loci | A | He | Ho |
|---|---|---|---|---|---|
| BSn | 16 | 19 | 6.6 | 0.73 | 0.60 |
| BSn [26] | 147 | 9 | 9.6 | 0.79 | 0.79 |
| TWSn [49] | 17 | 11 | 7.0 | 0.70 | 0.70 |
| TWSn [29] | 13 | 18 | 3.9 | 0.69 | 0.64 |
| TWSw [49] | 29 | 10 | 6.1 | 0.50 | 0.50 |
| TWSw [29] | 12 | 18 | 3.3 | 0.57 | 056 |
| PRDw | 112 | 19 | 10.5 | 0.65 | 0.50 |
| Population | Parameters | Convergence Test | Estimate | ||
|---|---|---|---|---|---|
| Estimate | 0.975 Quantile | Mode | 95% HPD | ||
| Pearl River Delta (N. phocaenoides) | μ | 1.003968 | 1.016194 | 0.00027 | 0.00011–0.00069 |
| N0 | 1.014079 | 1.066325 | 313 | 96–1560 | |
| N1 | 1.000077 | 1.000437 | 245,625 | 91,368–660,314 | |
| T | 1.021028 | 1.090848 | 6987 | 2266–22,653 | |
| Taiwan Strait (N. phocaenoides) | μ | 1.000183 | 1.001017 | 0.00026 | 0.00010–0.0006 |
| N0 | 1.068236 | 1.271684 | 19 | 5–75 | |
| N1 | 1.001211 | 1.005221 | 252,187 | 25,2187–784,675 | |
| T | 1.049098 | 1.205557 | 1722 | 493–6901 | |
| Taiwan Strait (N. asiaeorientalis) | μ | 1.000591 | 1.002447 | 0.00030 | 0.00012–0.00077 |
| N0 | 1.026537 | 1.109023 | 11 | 4–34 | |
| N1 | 1.003475 | 1.016309 | 101,903 | 31,184–324,717 | |
| T | 1.040346 | 1.171372 | 722 | 250–2209 | |
| Bohai Sea (N. asiaeorientalis) | μ | 1.000466 | 1.002282 | 0.00033 | 0.00013–0.00082 |
| N0 | 1.076863 | 1.293392 | 56 | 20–160 | |
| N1 | 1.000356 | 1.001944 | 183,364 | 67,306–489,324 | |
| T | 1.057863 | 1.234439 | 2789 | 952–8089 | |
| Slope | DS | DRM | |
|---|---|---|---|
| Slope | |||
| DS | 0.271 | ||
| DRM | 0.094 | 0.621 | |
| Depth | 0.619 | 0.386 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Karczmarski, L.; Zeng, C.; Luo, D.; Li, S. Different Microsatellite Mutation Models May Lead to Contrasting Demographic Inferences through Genealogy-Based Approaches: A Case Study of the Finless Porpoise off the East Asian Coast. J. Mar. Sci. Eng. 2023, 11, 524. https://doi.org/10.3390/jmse11030524
Lin W, Karczmarski L, Zeng C, Luo D, Li S. Different Microsatellite Mutation Models May Lead to Contrasting Demographic Inferences through Genealogy-Based Approaches: A Case Study of the Finless Porpoise off the East Asian Coast. Journal of Marine Science and Engineering. 2023; 11(3):524. https://doi.org/10.3390/jmse11030524
Chicago/Turabian StyleLin, Wenzhi, Leszek Karczmarski, Chen Zeng, Dingyu Luo, and Songhai Li. 2023. "Different Microsatellite Mutation Models May Lead to Contrasting Demographic Inferences through Genealogy-Based Approaches: A Case Study of the Finless Porpoise off the East Asian Coast" Journal of Marine Science and Engineering 11, no. 3: 524. https://doi.org/10.3390/jmse11030524
APA StyleLin, W., Karczmarski, L., Zeng, C., Luo, D., & Li, S. (2023). Different Microsatellite Mutation Models May Lead to Contrasting Demographic Inferences through Genealogy-Based Approaches: A Case Study of the Finless Porpoise off the East Asian Coast. Journal of Marine Science and Engineering, 11(3), 524. https://doi.org/10.3390/jmse11030524







