Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry
Abstract
1. Introduction Outline
2. Materials and Methods
2.1. Sampling Phytoplankton in the Western Channel by WaMS, WCO L4 and CPR Survey
2.2. Extraction of Microscopic Phytoplankton Datasets from the Western Channel
2.3. Enumeration of Phytoplankton and Bacteria by Flow Cytometry (FC)
2.4. WaMS Sample DNA Extraction for Diversity Assessment and Quantitative Assessments of Potential Harmful Algae
2.5. Quantitative Real-Time PCR-HRM Assays of Potentially Harmful Algae Species of WaMS Samples
2.6. DNA Amplification, High Throughput Sequencing (HTS) and Bioinformatic Analysis of WaMS DNA Samples from 2011–2012
2.7. Assessment of SST and Nutrient Seasonal Patterns in WCO L4 Stations
2.8. Evaluation of Taxa Found by Microscopy from WCO L4 and CPR Surveys Compared to and HTS- Generated Taxa from WaMS
3. Results
3.1. Nutrient and SST Trends from WCO_L4
3.2. HRM and Quantitative Real-Time PCR Assay Performance for Potentially Harmful Algae Taxa
3.3. Seasonal Patterns of Potential Harmful Algae Species in the WaMS Samples by HRM-qRT-PCR
3.4. Phytoplankton Seasonal Trends in the Western Channel from Microscopy, FC from WaMS and WCO L4 Samples
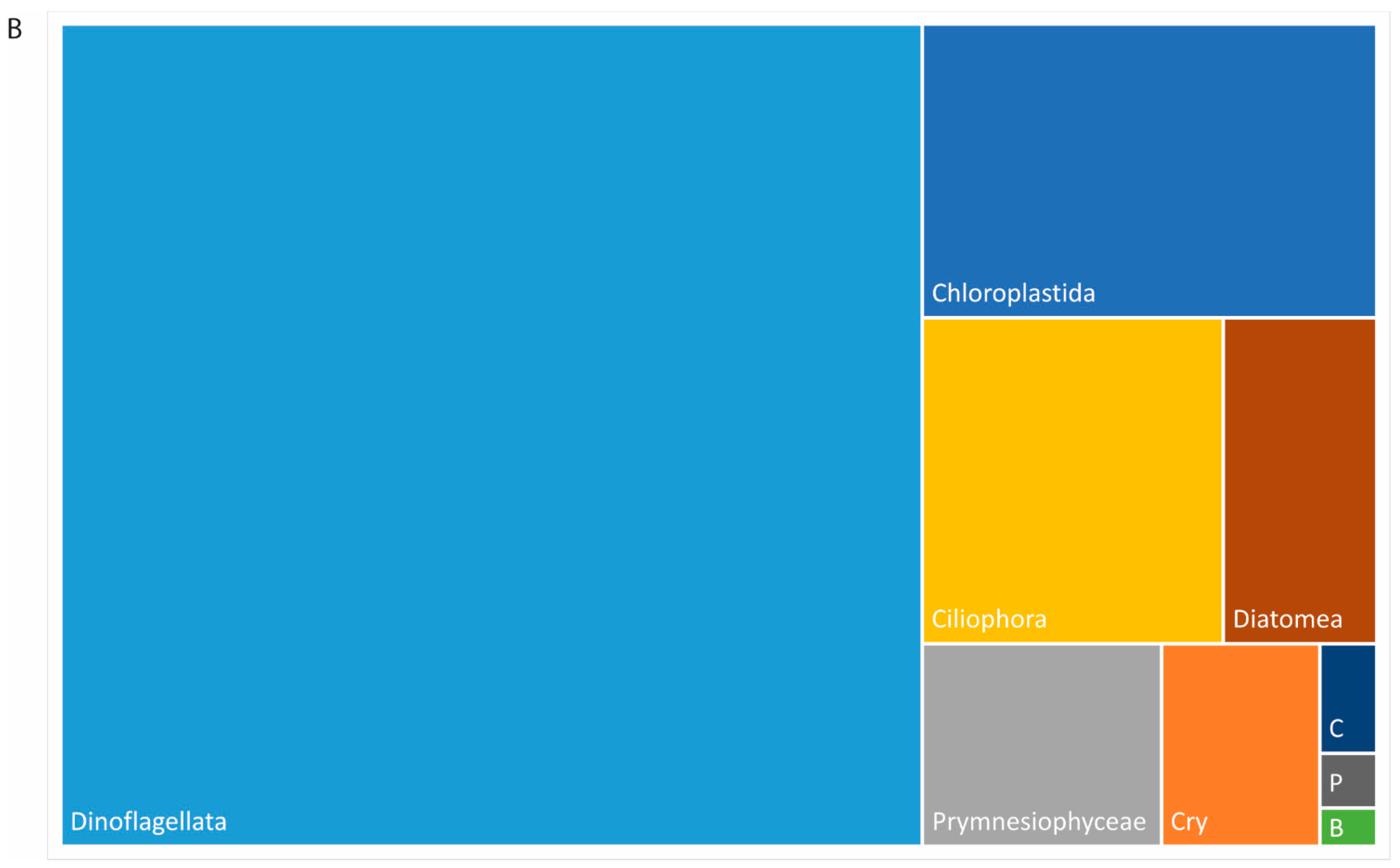
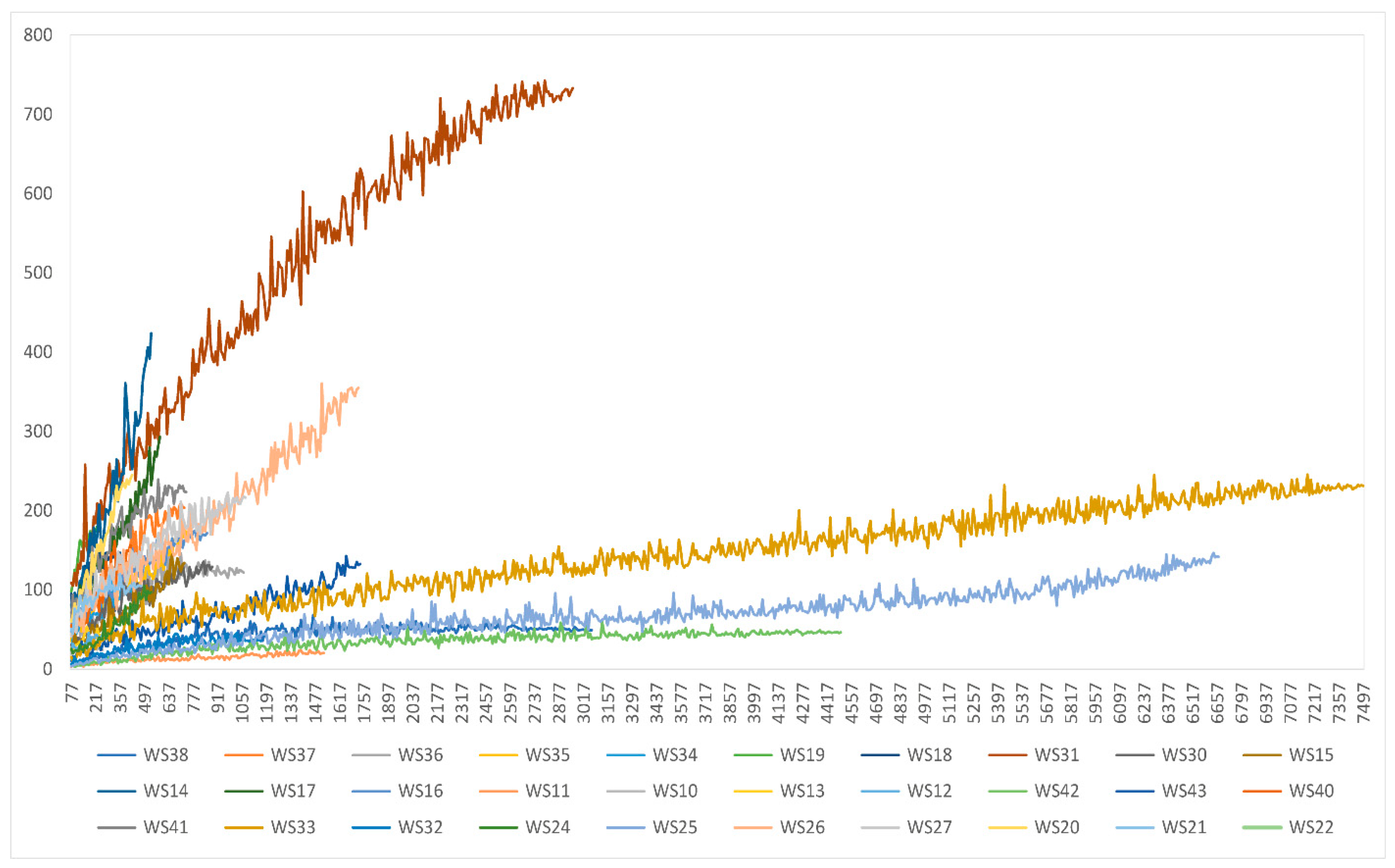
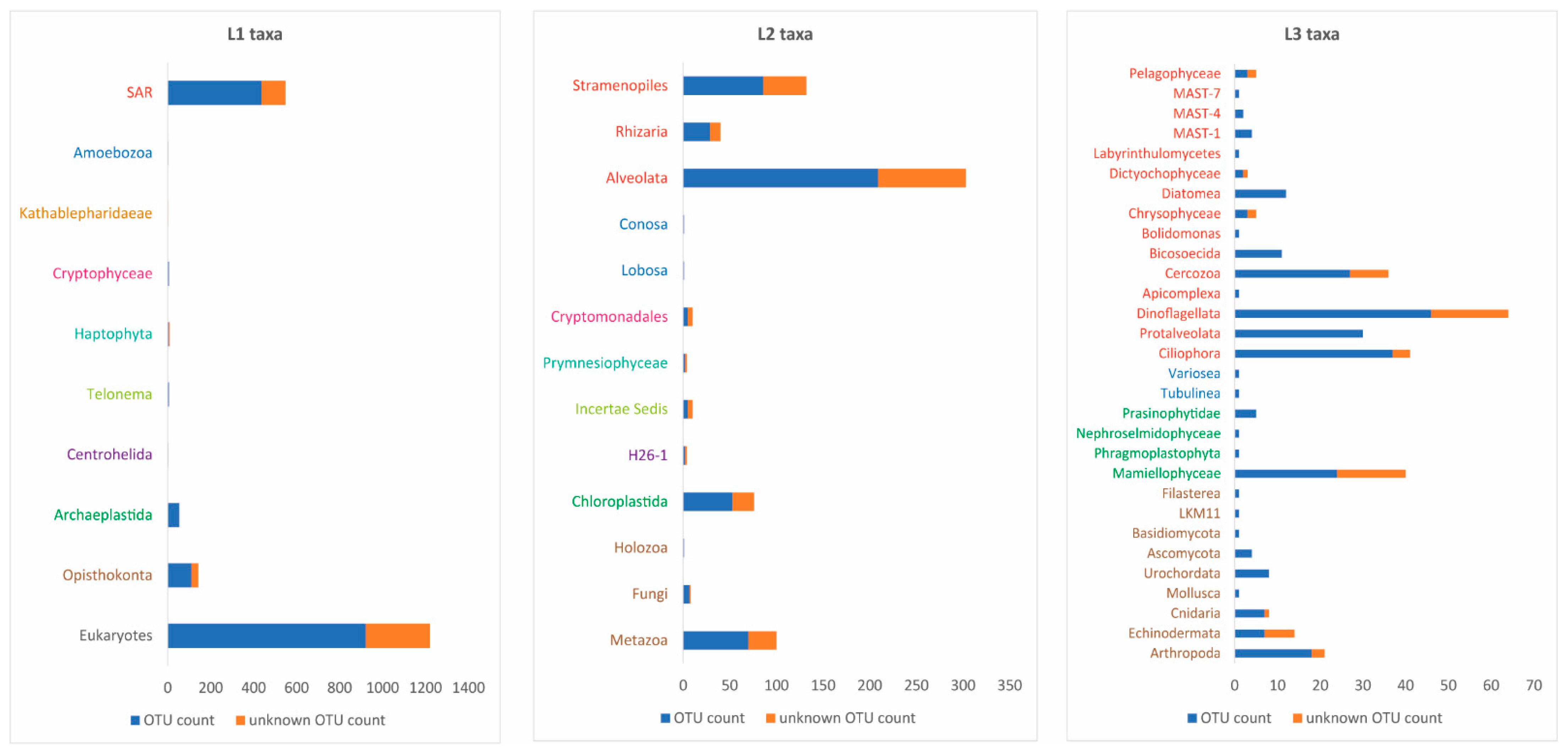
3.5. Plankton Diversity Trends from HTS of Partial 18S rDNA PCR Products from WaMS Samples, WS1−43
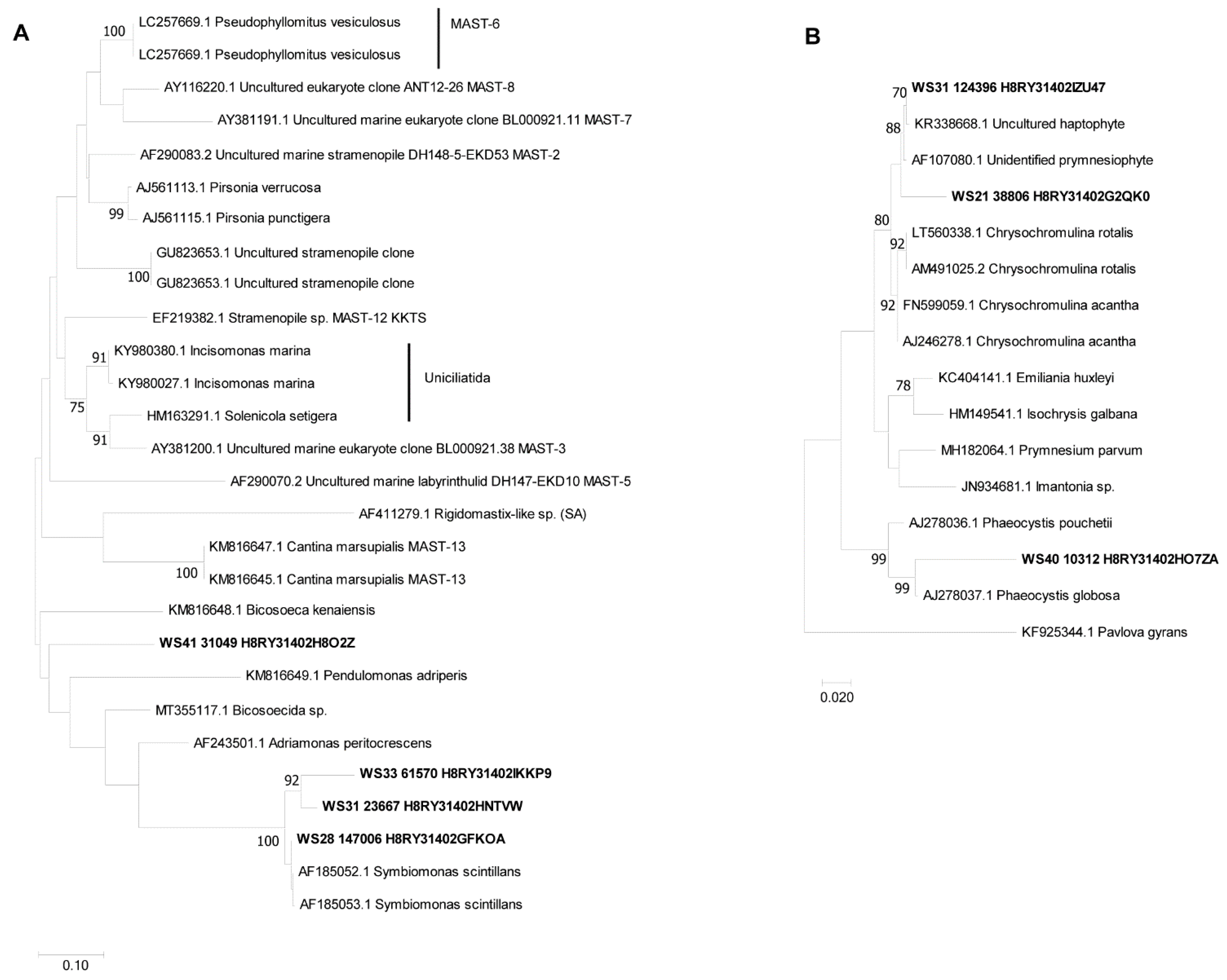
3.6. Phylogenetic Analysis of Partial 18S rDNA Reads of WaMS Samples, WS1−43
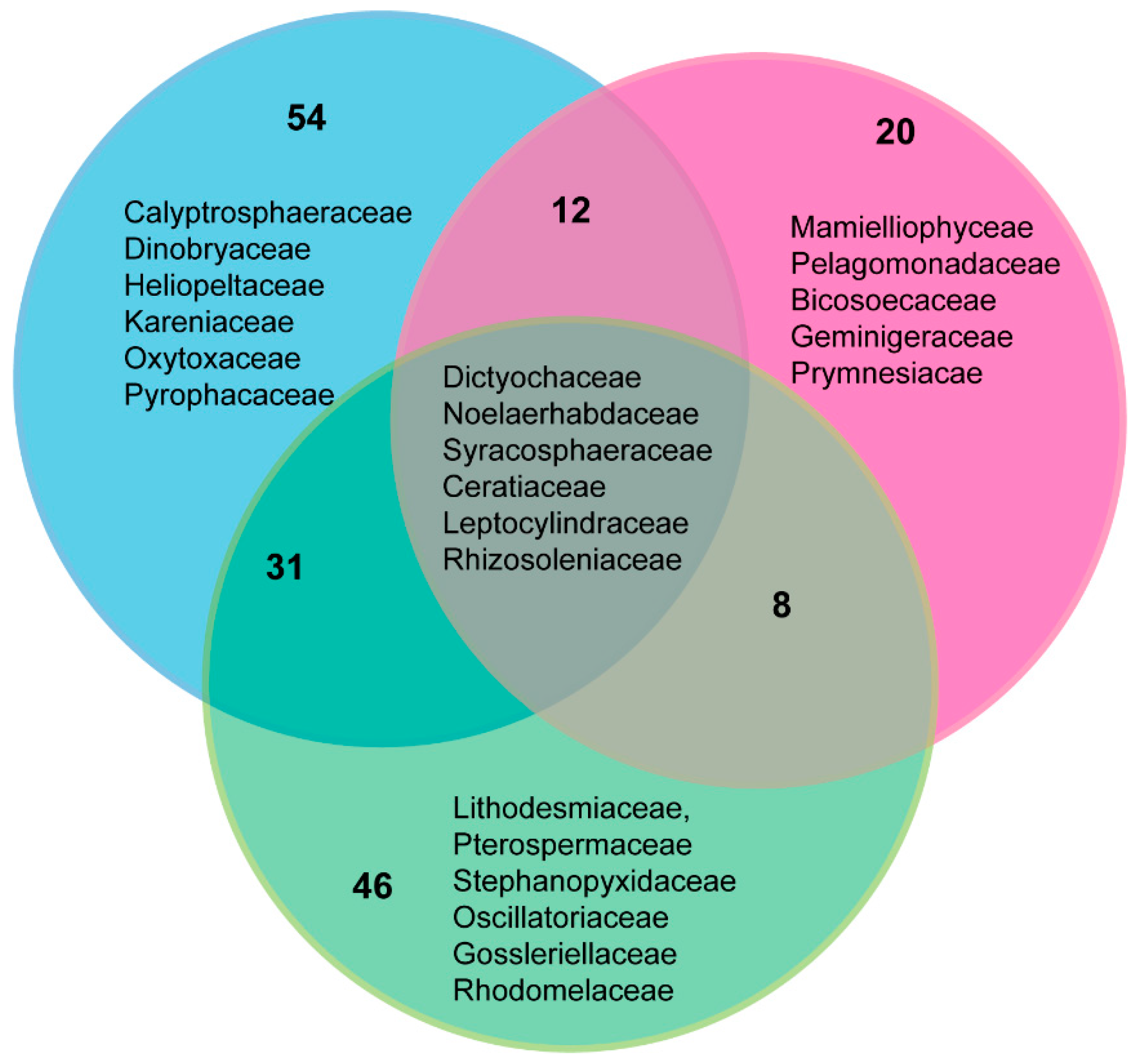
3.7. Comparison of Phytoplankton Diversity from WaMS 18S HTS Survey versus Microscopy Taxonomic Surveys in the Western Channel
4. Discussion
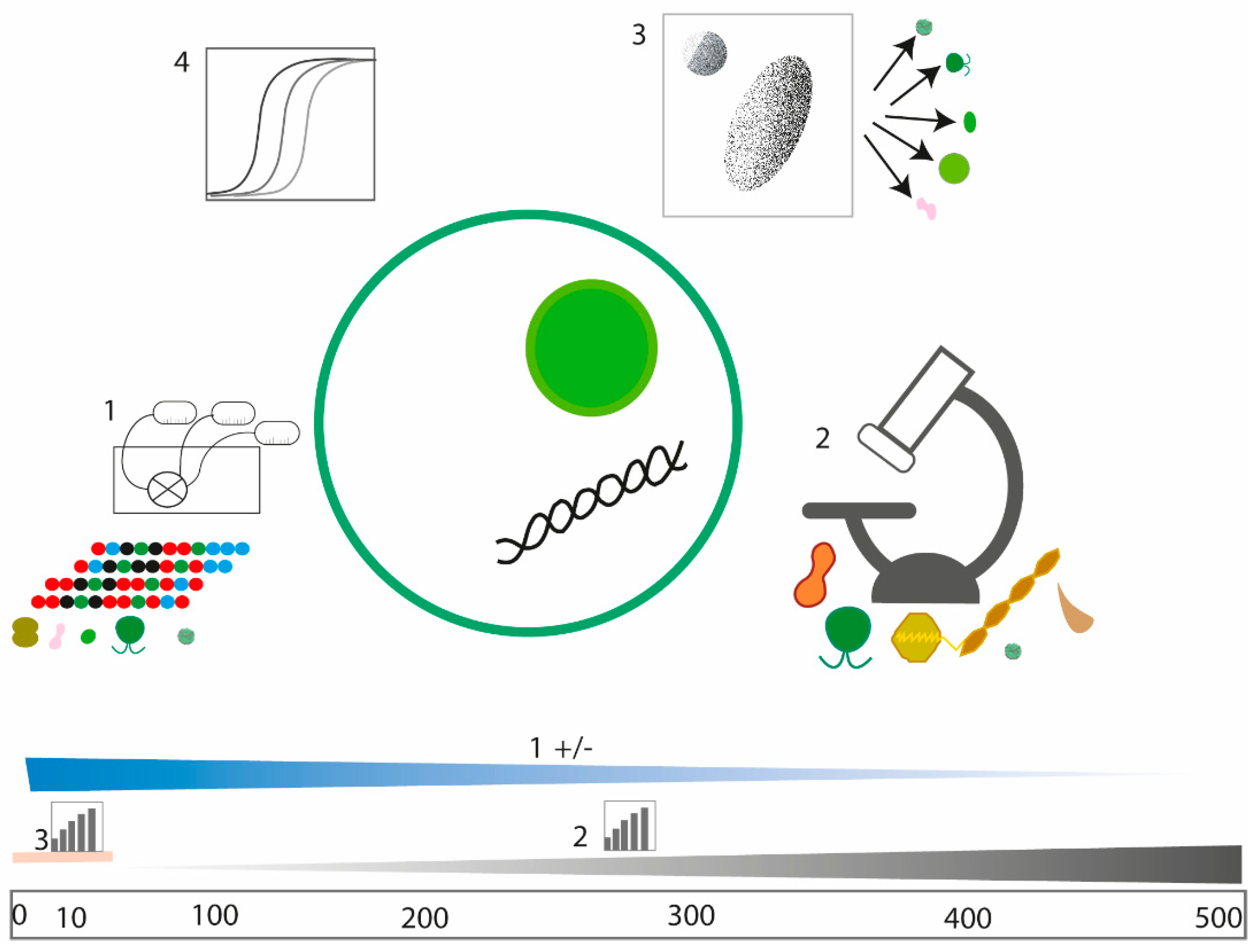
4.1. Performance of WaMS Sampling for Harmful Algae, Pico- and Nanoplankton Quantification
4.2. Seasonal Dynamics of P. delicatissima and A. anophagefferens in Comparison to FC- and Microscopy Measured Phytoplankton Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, F.; Ibarbalz, F.M.; Bowler, C. Global marine phytoplankton revealed by the Tara Oceans expedition. In Advances in Phytoplankton Ecology; Clementson, L.A., Eriksen, R.S., Willis, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–561. [Google Scholar]
- Falkowski, P.G.; Raven, J.A. Aquatic photosynthesis; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Falkowski, P. Ocean Science: The power of plankton. Nature 2012, 483, S17–S20. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2009, 32, 119–137. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Hanke, G.; Hoeppffner, N.; Palialexis, A.; Somma, F.; Stips, A.; Teixeira, H.; Tempera, F.; Tornero, V. D1 Biological Diversity. Available online: https://mcc.jrc.ec.europa.eu/main/dev.py?N=19&O=118&titre_chap=D1%20Biological%20diversity (accessed on 16 September 2022).
- Miloslavich, P.; Bax, N.J.; Simmons, S.E.; Klein, E.; Appeltans, W.; Aburto-Oropeza, O.; Andersen Garcia, M.; Batten, S.D.; Benedetti-Cecchi, L.; Checkley, D.M., Jr.; et al. Essential ocean variables for global sustained observations of biodiversity and ecosystem changes. Glob. Chang. Biol. 2018, 24, 2416–2433. [Google Scholar]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar]
- Vargas, C.d.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Bescot, N.L.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef]
- Hällfors, G.; Melvasalo, T.; Niemi, A.; Viljamaa, H. Effect of different fixatives and preservatives on phytoplankton counts. Pub. Water Res. Inst. 1979, 34, 25–34. [Google Scholar]
- Sosik, H.M.; Olson, R.J.; Armbrust, E.V. Flow Cytometry in Phytoplankton Research. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Prášil, O., Borowitzka, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 171–185. [Google Scholar] [CrossRef]
- Xiao, X.; Sogge, H.; Lagesen, K.; Tooming-Klunderud, A.; Jakobsen, K.S.; Rohrlack, T. Use of High Throughput Sequencing and Light Microscopy Show Contrasting Results in a Study of Phytoplankton Occurrence in a Freshwater Environment. PLoS ONE 2014, 9, e106510. [Google Scholar]
- Godhe, A.; Asplund, M.E.; Härnstörm, K.; Saravanan, V.; Tyagi, A.; Karunasagar, I. Quantification of Diatom and Dinoflagellate Biomasses in Coastal Marine Seawater Samples by Real-Time PCR. Appl. Environ. Microbiol. 2008, 74, 7174–7182. [Google Scholar]
- McQuatters-Gollop, A.; Guérin, L.; Arroyo, N.L.; Aubert, A.; Artigas, L.F.; Bedford, J.; Corcoran, E.; Dierschke, V.; Elliott, S.A.M.; Geelhoed, S.C.V.; et al. Assessing the state of marine biodiversity in the Northeast Atlantic. Ecol. Indic. 2022, 141, 109148. [Google Scholar]
- Uncles, R.J.; Stephens, J.A.; Harris, C. Physical processes in a coupled bay–estuary coastal system: Whitsand Bay and Plymouth Sound. Prog. Oceanogr. 2015, 137, 360–384. [Google Scholar]
- Barnes, M.K.; Tilstone, G.H.; Suggett, D.J.; Widdicombe, C.E.; Bruun, J.; Martinez-Vicente, V.; Smyth, T.J. Temporal variability in total, micro- and nano-phytoplankton primary production at a coastal site in the Western English Channel. Prog. Oceanogr. 2015, 137, 470–483. [Google Scholar]
- Napoléon, C.; Fiant, L.; Raimbault, V.; Riou, P.; Claquin, P. Dynamics of phytoplankton diversity structure and primary productivity in the English Channel. Mar. Ecol. Prog. Ser. 2014, 505, 49–64. [Google Scholar]
- Richardson, A.J.; Walne, A.W.; John, A.W.G.; Jonas, T.D.; Lindley, J.A.; Sims, D.W.; Stevens, D.; Witt, M. Using continuous plankton recorder data. Prog. Oceanogr. 2006, 68, 27–74. [Google Scholar]
- Southward, A.J.; Langmead, O.; Hardman-Mountford, N.J.; Aiken, J.; Boalch, G.T.; Dando, P.R.; Genner, M.J.; Joint, I.; Kendall, M.A.; Halliday, N.C.; et al. Long-Term Oceanographic and Ecological Research in the Western English Channel. Adv. Mar. Biol. 2005, 47, 1–105. [Google Scholar]
- Stern, R.F.; Picard, K.; Hamilton, K.M.; Walne, A.; McQuatters-Gollop, A.; Mills, D.; Edwards, M. An automated water sampler from Ships of Opportunity detects new boundaries of marine microbial biodiversity. Prog. Oceanogr. 2015, 137, 409–420. [Google Scholar] [CrossRef]
- Widdicombe, C.; Eloire, D.; Harbour, D.; Harris, R.; Somerfield, P. Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res. 2010, 32, 643–655. [Google Scholar]
- Hinder, S.L.; Hays, G.C.; Edwards, M.; Roberts, E.C.; Walne, A.W.; Gravenor, M.B. Changes in marine dinoflagellate and diatom abundance under climate change. Nat. Clim. Chang. 2012, 2, 271–275. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar]
- Hasle, G.; Lange, C.; Syvertsen, E. A review of Pseudo-nitzschia, with special reference to the Skagerrak, North Atlantic, and adjacent waters. Helgoländer Meeresunters. 1996, 50, 131–175. [Google Scholar]
- Downes-Tettmar, N.; Rowland, S.; Widdicombe, C.; Woodward, M.; Llewellyn, C. Seasonal variation in Pseudo-nitzschia spp. and domoic acid in the Western English Channel. Cont. Shelf Res. 2013, 53, 40–49. [Google Scholar]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- Sobrinho, B.F.; De Camargo, L.M.; Sandrini-Neto, L.; Kleemann, C.R.; Machado, E.d.C.; Mafra, L.L. Growth, Toxin Production and Allelopathic Effects of Pseudo-nitzschia multiseries under Iron-Enriched Conditions. Mar. Drugs 2017, 15, 331. [Google Scholar]
- Downes-Tettmar, N. Factors That Impact Pseudo-nitzschia spp. Occurence, Growth, and Toxin Production. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2012. [Google Scholar]
- Andree, K.B.; Fernández-Tejedor, M.; Elandaloussi, L.M.; Quijano-Scheggia, S.; Sampedro, N.; Garcés, E.; Camp, J.; Diogène, J. Quantitative PCR Coupled with Melt Curve Analysis for Detection of Selected Pseudo-nitzschia spp. (Bacillariophyceae) from the Northwestern Mediterranean Sea. Appl. Environ. Microbiol. 2011, 77, 1651–1659. [Google Scholar] [CrossRef]
- Popels, L.C.; Cary, S.C.; Hutchins, D.A.; Forbes, R.; Pustizzi, F.; Gobler, C.J.; Coyne, K.J. The use of quantitative polymerase chain reaction forthe detection and enumeration of the harmful algaAureococcus anophagef-ferensin environmental samples along the United States East Coast. Limnol. Oceanogr. Methods 2003, 1, 92–102. [Google Scholar]
- Gobler, C.J.; Berry, D.L.; Dyhrman, S.T.; Wilhelm, S.W.; Salamov, A.; Lobanov, A.V.; Zhang, Y.; Collier, J.L.; Wurch, L.L.; Kustka, A.B.; et al. Niche of harmful alga Aureococcus anophagefferen revealed through ecogenomics. Proc. Natl. Acad. Sci. USA 2011, 108, 4352–4357. [Google Scholar] [CrossRef]
- Doblin, M.A.; Popels, L.C.; Coyne, K.J.; Hutchins, D.A.; Cary, S.C.; Dobbs, F.C. Transport of the Harmful Bloom Alga Aureococcus anophagefferens by Oceangoing Ships and Coastal Boats. Appl. Environ. Microbiol. 2004, 70, 6495–6500. [Google Scholar] [CrossRef]
- Throndsen, J. Preservation and storage. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 69–74. [Google Scholar]
- Becker, S.; Aoyama, M.; Woodward, E.M.S.; Bakker, K.; Coverly, S.; Mahaffey, C.; Tanhua, T. GO-SHIP Repeat Hydrography Nutrient Manual: The Precise and Accurate Determination of Dissolved Inorganic Nutrients in Seawater, Using Continuous Flow Analysis Methods. Front. Mar. Sci. 2020, 7, 581790. [Google Scholar] [CrossRef]
- Reid, P.C.; Colebrook, J.M.; Matthews, J.B.L.; Aiken, J.; Continuous Plankton Recorder Team. The Continuous Plankton Recorder: Concepts and history, from Plankton Indicator to undulating recorders. Prog. Oceanogr. 2003, 58, 117–173. [Google Scholar]
- Widdicombe, C.E.; Harbour, D. Phytoplankton Taxonomic Abundance and Biomass Time-Series at Plymouth Station L4 in the Western English Channel, 1992–2020; British Oceanographic Data Centre NOC: Liverpool, UK, 2021. [Google Scholar]
- Utermöhl, H. Methods of collecting plankton for various purposes are discussed. SIL Commun. 1953–1996 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Winnepenninckx, B.; Backeljau, T.; De Wachter, R. Extraction of high molecular weight DNA from molluscs. Trends Genet. 1993, 9, 407. [Google Scholar]
- Walker, C.E. Molecular Identification of Pseudo-nitzschia Species in the English Channel; University of Plymouth: Plymouth, UK, 2014. [Google Scholar]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16s-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Pena, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar]
- Tamura, K.S.G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar]
- Skovgaard, A.; Karpov, S.A.; Guillou, L. The Parasitic Dinoflagellates Blastodinium spp. Inhabiting the Gut of Marine, Planktonic Copepods: Morphology, Ecology, and Unrecognized Species Diversity. Front. Microbiol. 2012, 3, 305. [Google Scholar]
- Steidinger, K.A.; Tangen, K. Dinoflagellates. In Identifying Marine Phytoplankton; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 387–584. [Google Scholar]
- Throndsen, J. The planktonic marine flagellates. In Identifying Marine Phytoplankton; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 633–644. [Google Scholar]
- Tomas, C.R. (Ed.) Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species; VLIZ: Ostend, Belgium, 2022. [Google Scholar]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar]
- Medin, L. Evolution of the diatoms: Major steps in their evolution and a review of the supporting molecular and morphological evidence. Phycologia 2016, 55, 79–103. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2022. [Google Scholar]
- Rachik, S.; Christaki, U.; Li, L.L.; Genitsaris, S.; Breton, E.; Monchy, S. Diversity and potential activity patterns of planktonic eukaryotic microbes in a mesoeutrophic coastal area (eastern English Channel). PLoS ONE 2018, 13, e0196987. [Google Scholar] [CrossRef]
- Ajani, P.A.; Verma, A.; Kim, J.H.; Woodcock, S.; Nishimura, T.; Farrell, H.; Zammit, A.; Brett, S.; Murray, S.A. Using qPCR and high-resolution sensor data to model a multi-species Pseudo-nitzschia (Bacillariophyceae) bloom in southeastern Australia. Harmful Algae 2021, 108, 102095. [Google Scholar]
- Lim, H.C.; Tan, S.N.; Teng, S.T.; Lundholm, N.; Orive, E.; David, H.; Quijano-Scheggia, S.; Leong, S.C.Y.; Wolf, M.; Bates, S.S.; et al. Phylogeny and species delineation in the marine diatom Pseudo-nitzschia (Bacillariophyta) using cox1, LSU, and ITS2 rRNA genes: A perspective in character evolution. J. Phycol. 2018, 54, 234–248. [Google Scholar]
- Turk Dermastia, T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo-nitzschia H. Peragallo in the northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar]
- Tajadini, M.; Panjehpour, M.; Javanmard, S.H. Comparison of SYBR Green and TaqMan methods in quantitative real-time polymerase chain reaction analysis of four adenosine receptor subtypes. Adv. Biomed. Res. 2014, 3, 85. [Google Scholar] [CrossRef]
- Smyth, T.J.; Fishwick, J.R.; AL-Moosawi, L.; Cummings, D.G.; Harris, C.; Kitidis, V.; Rees, A.; Martinez-Vicente, V.; Woodward, E.M.S. A broad spatio-temporal view of the Western English Channel observatory. J. Plankton Res. 2010, 32, 585–601. [Google Scholar] [CrossRef]
- Rodríguez, F.; Derelle, E.; Guillou, L.; Le Gall, F.; Vaulot, D.; Moreau, H. Ecotype diversity in the marine picoeukaryote Ostreococcus (Chlorophyta, Prasinophyceae). Environ. Microbiol. 2005, 7, 853–859. [Google Scholar]
- Frischkorn, K.R.; Harke, M.J.; Gobler, C.J.; Dyhrman, S.T. De novo assembly of Aureococcus anophagefferens transcriptomes reveals diverse responses to the low nutrient and low light conditions present during blooms. Front. Microbiol. 2014, 5, 375. [Google Scholar] [CrossRef]
- Husson, B.; Hernández-Fariñas, T.; Le Gendre, R.; Schapira, M.; Chapelle, A. Two decades of Pseudo-nitzschia spp. blooms and king scallop (Pecten maximus) contamination by domoic acid along the French Atlantic and English Channel coasts: Seasonal dynamics, spatial heterogeneity and interannual variability. Harmful Algae 2016, 51, 26–39. [Google Scholar] [CrossRef]
- Ward, B.B.; Rees, A.P.; Somerfield, P.J.; Joint, I. Linking phytoplankton community composition to seasonal changes in f-ratio. ISME J. 2011, 5, 1759–1770. [Google Scholar] [CrossRef]
- Cleary, A.C.; Durbin, E.G.; Rynearson, T.A.; Bailey, J. Feeding by Pseudocalanus copepods in the Bering Sea: Trophic linkages and a potential mechanism of niche partitioning. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 134, 181–189. [Google Scholar]
- McNichol, J.; Berube, P.M.; Biller, S.J.; Fuhrman, J.A. Evaluating and Improving Small Subunit rRNA PCR Primer Coverage for Bacteria, Archaea, and Eukaryotes Using Metagenomes from Global Ocean Surveys. mSystems 2021, 6, e0056521. [Google Scholar] [CrossRef]
- Liem, M.; Regensburg-Tuïnk, T.; Henkel, C.; Jansen, H.; Spaink, H. Microbial diversity characterization of seawater in a pilot study using Oxford Nanopore Technologies long-read sequencing. BMC Res. Notes 2021, 14, 42. [Google Scholar] [CrossRef]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.; et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef]
- Stern, R.; Kraberg, A.; Bresnan, E.; Kooistra, W.H.C.F.; Lovejoy, C.; Montresor, M.; Morán, X.A.G.; Not, F.; Salas, R.; Siano, R.; et al. Molecular analyses of protists in long-term observation programmes—Current status and future perspectives. J. Plankton Res. 2018, 40, 519–536. [Google Scholar] [CrossRef]
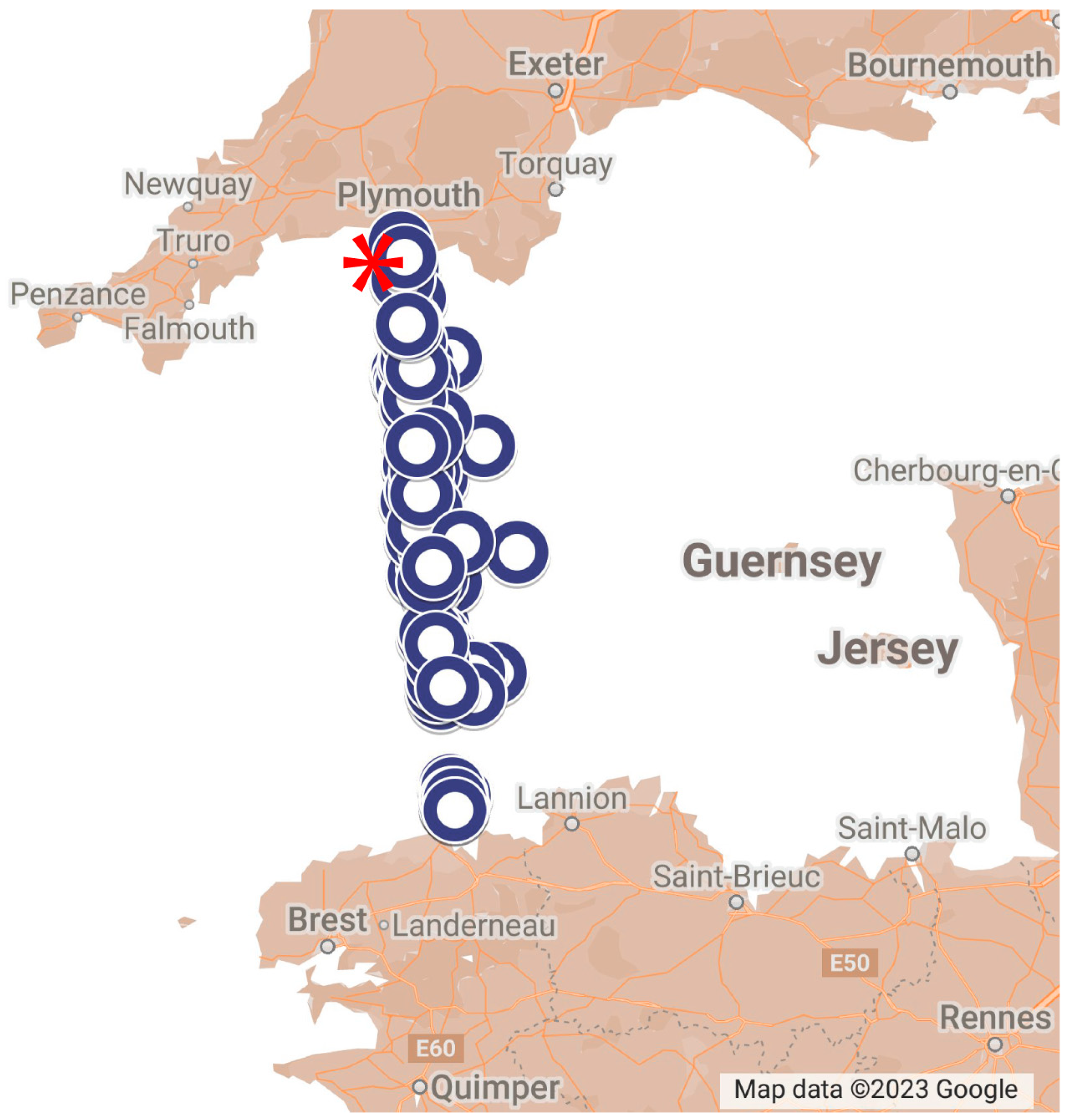
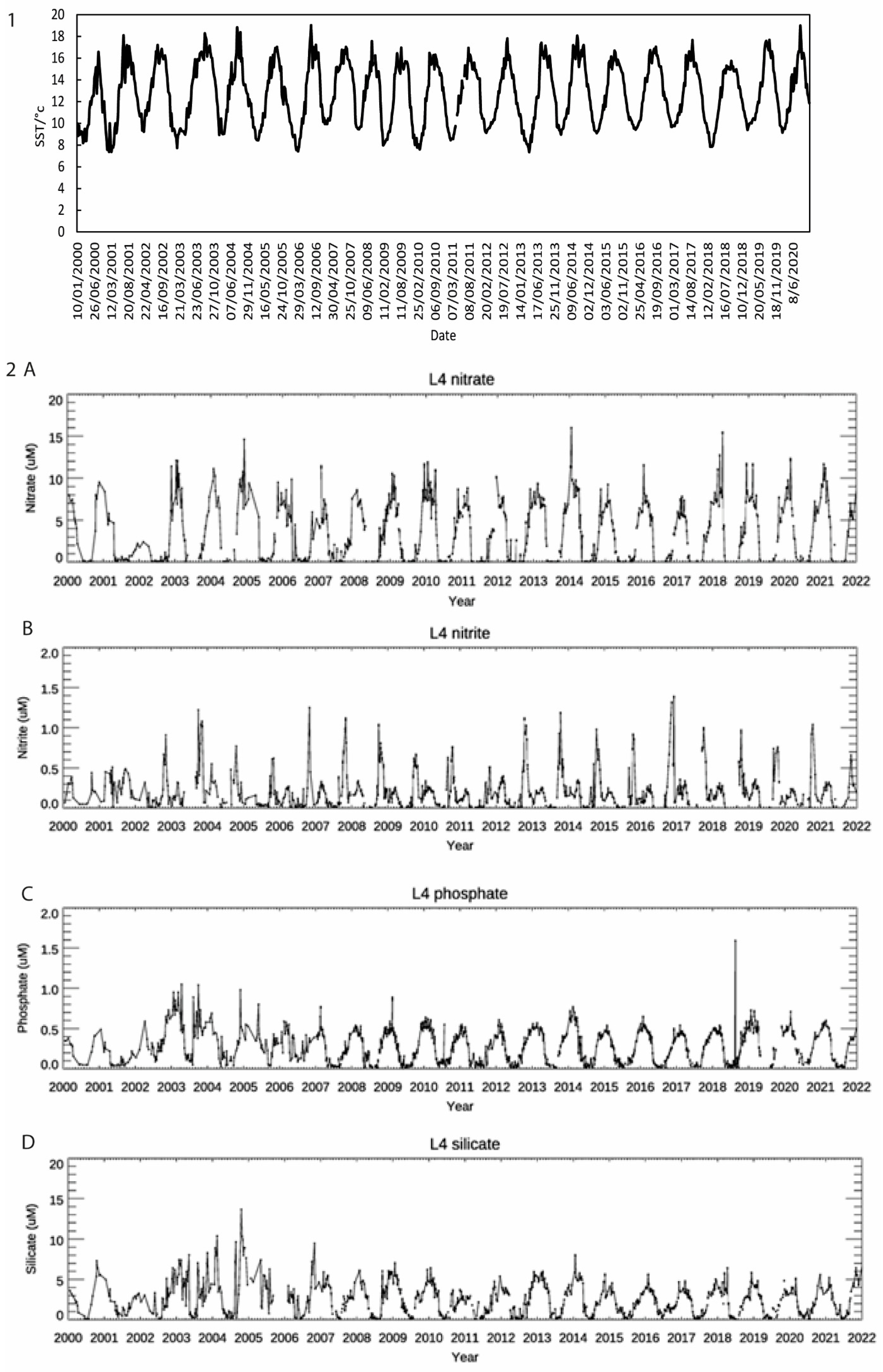
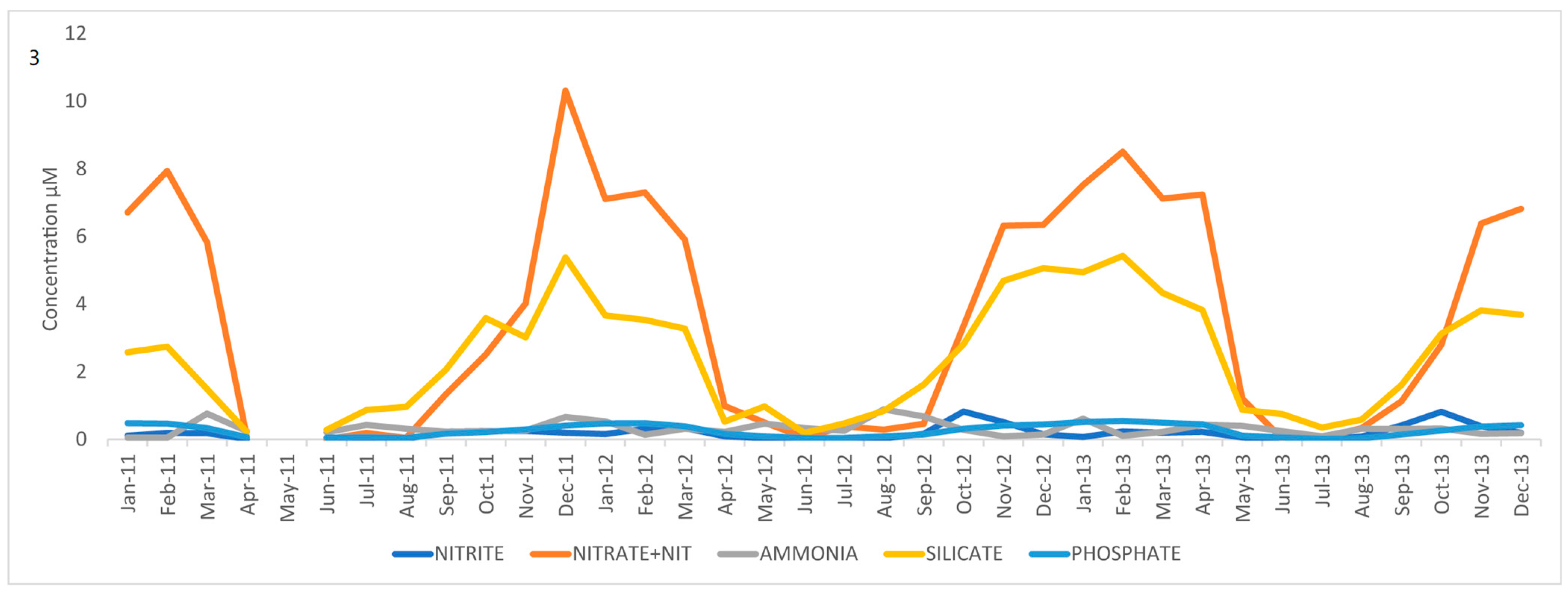
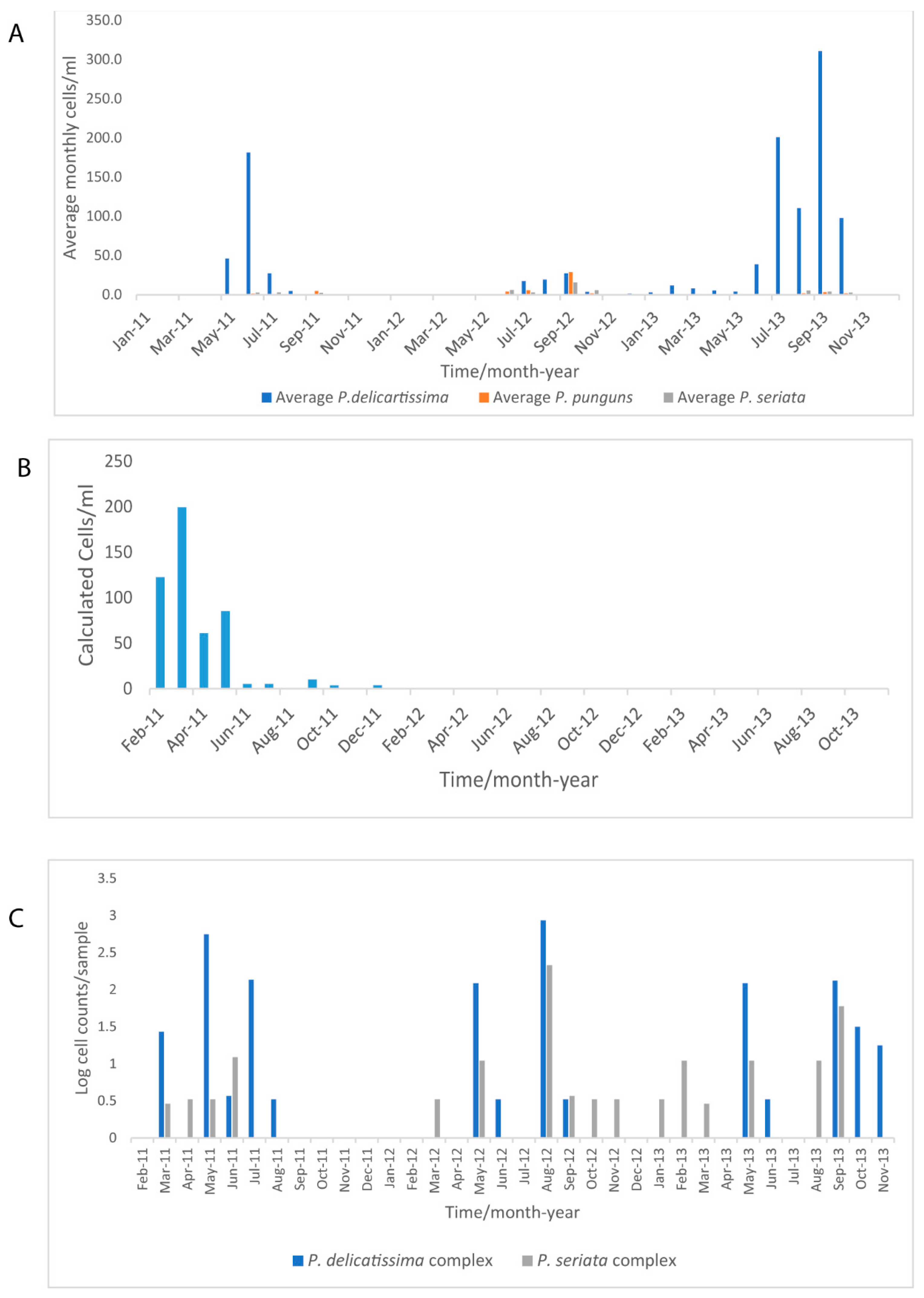



| Assay | Reference | Marker | Primers | Size of Product/bp | Standard Range Copies/µL |
|---|---|---|---|---|---|
| Pseudo-nitzschia fraudulenta | Andre et al. (2011) [28]: PN5. 8SF-HRM, QPfrauR-HRM | ITS1 | PN5.8SF-HRM 5′ CAGCGGTGGATGTCTAGGTTC−3′ QPfrauR-HRM 5′ CCGCTGCTAGAGCGGTCAGAG 3′ | 225 | 3.18 × 108–3.17 × 106 |
| Pseudo-nitzschia multiseries | PMulsF (this study), PN5. 8SR-HRM (Andre et al., 2011) [28] | ITS2 | PMulsF-HRM 5′ CTAGACTACTGTAGTCAAACTTAACCGGCAAC 3′ PN5.8SR-HRM 5′ GAACCTAGACATCCACCGCTG 3′ | 201 | 5.75 × 108–5.75 × 104 |
| Pseudo-nitzschia delicatissima | QPdelRa2F (this study), PN5. 8SR-HRM (Andre et al., 2011) [28] | ITS | QPdelRa2F GTGCAATACTTTGTTGGGTTTCG PN5.8SR-HRM 5′ GAACCTAGACATCCACCGCTG 3′ | 182 | 2.5 × 102 to 2.5 × 107 |
| Aureococcus anophagefferens | Aa1685f, Popels et al., 2003, [29] Euk B (Medlin et al., 1988) [39] | 18S | Aa1685f ACCTCCGGACTGGGGTT, EukB | 118 | 102–106 |
| HRM-qRT-PCR Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPR Tow | Pn | Y | M | Lat | Lon | T. | FC | Seq Sample | AA | PD | PF | PM |
| 344PR | E1 | 2011 | 2 | 48.03 | −3.83 | 9.56 | Yes | WS1 | Yes | Yes | Yes | |
| 2011 | 2 | |||||||||||
| 2011 | 2 | |||||||||||
| 2011 | 2 | |||||||||||
| E5 | 2011 | 2 | 49.94 | −4.12 | 9.31 | Yes | WS2 | Yes | Yes | Yes | ||
| 345PR | 2011 | 3 | ||||||||||
| E2 | 2011 | 3 | 49.28 | −4.02 | 9.75 | Yes | WS3 | Yes | Yes | Yes | ||
| 2011 | 3 | |||||||||||
| 2011 | 3 | |||||||||||
| E5 | 2011 | 3 | 49.78 | −4.12 | 9.78 | Yes | WS4 | Yes | Yes | Yes | ||
| 346PR | E1 | 2011 | 4 | 48.80 | −3.96 | 10.5 | Yes | WS5 | Yes | Yes | Yes | |
| E2 | 2011 | 4 | 49.08 | −4.01 | 10.25 | Yes | WS6 | Yes | Yes | Yes | ||
| E3 | 2011 | 4 | 49.37 | −4.04 | 10.08 | Yes | WS7 | Yes | Yes | Yes | ||
| E4 | 2011 | 4 | 49.67 | −4.11 | 10.17 | Yes | WS8 | Yes | Yes | Yes | ||
| E5 | 2011 | 4 | 49.97 | −4.17 | 10.18 | Yes | WS9 | Yes | Yes | Yes | ||
| 347PR | E1 | 2011 | 5 | 48.82 | −3.93 | N/A | Yes | N/A | Yes | Yes | Yes | |
| E2 | 2011 | 5 | 49.11 | −3.98 | 11.95 | Yes | WS10 | Yes | Yes | Yes | ||
| E3 | 2011 | 5 | 49.40 | −3.97 | 12.4 | Yes | WS11 | Yes | Yes | Yes | ||
| E4 | 2011 | 5 | 49.68 | −4.03 | 12.56 | Yes | WS12 | Yes | Yes | Yes | ||
| E5 | 2011 | 5 | 49.95 | −4.09 | 12.38 | Yes | WS13 | Yes | Yes | Yes | ||
| 348PR | E1 | 2011 | 6 | 48.78 | −3.96 | 13.7 | yes | WS14 | Yes | Yes | Yes | |
| E2 | 2011 | 6 | 49.10 | −4.02 | 12.9 | yes | WS15 | Yes | Yes | Yes | ||
| E3 | 2011 | 6 | 49.42 | −4.10 | 13.9 | yes | WS16 | Yes | Yes | Yes | ||
| E4 | 2011 | 6 | 49.68 | −4.10 | 13.9 | yes | WS17 | Yes | Yes | Yes | ||
| E5 | 2011 | 6 | 49.96 | −4.13 | 13.9 | yes | WS18 | Yes | Yes | Yes | ||
| 349PR | E1 | 2011 | 7 | 48.83 | −3.96 | 14.5 | yes | WS19 | Yes | Yes | Yes | |
| E2 | 2011 | 7 | 49.16 | −4.02 | 14.4 | yes | WS20 | Yes | Yes | Yes | ||
| E3 | 2011 | 7 | 49.41 | −4.04 | 15.3 | yes | WS21 | Yes | Yes | Yes | ||
| E4 | 2011 | 7 | 49.70 | −4.09 | 15.7 | yes | WS22 | Yes | Yes | Yes | ||
| E5 | 2011 | 7 | 49.97 | −4.13 | 15.7 | yes | WS23 | Yes | Yes | Yes | ||
| 351PR | E1 | 2011 | 9 | 48.81 | −3.93 | N/A | yes | WS24 | Yes | Yes | Yes | |
| E2 | 2011 | 9 | 49.15 | −3.78 | N/A | yes | WS25 | Yes | Yes | Yes | ||
| E3 | 2011 | 9 | 49.48 | −3.69 | N/A | yes | WS26 | Yes | Yes | Yes | ||
| E4 | 2011 | 9 | 49.77 | −3.83 | N/A | yes | WS27 | Yes | Yes | Yes | ||
| E5 | 2011 | 9 | 50.01 | −3.97 | N/A | yes | WS28 | Yes | Yes | Yes | ||
| 352PR | E1 | 2011 | 10 | 48.81 | −3.95 | 14.7 | yes | WS29 | Yes | Yes | Yes | |
| E2 | 2011 | 10 | 49.13 | −4.00 | 14.9 | yes | WS30 | Yes | Yes | Yes | ||
| E3 | 2011 | 10 | 49.39 | −4.05 | 15.0 | yes | WS31 | Yes | Yes | Yes | ||
| E4 | 2011 | 10 | 49.79 | −4.08 | 14.9 | yes | WS32 | Yes | Yes | Yes | ||
| E5 | 2011 | 10 | 49.93 | −4.10 | 14.7 | yes | WS33 | Yes | Yes | Yes | ||
| 354PR | E1 | 2011 | 12 | 48.79 | −3.96 | 13.0 | yes | WS34 | Yes | Yes | Yes | |
| E2 | 2011 | 12 | 49.15 | −4.01 | 12.7 | yes | WS35 | Yes | Yes | Yes | ||
| E3 | 2011 | 12 | 49.41 | −4.03 | 12.5 | yes | WS36 | Yes | Yes | Yes | ||
| E4 | 2011 | 12 | 49.70 | −4.06 | 12.3 | yes | WS37 | Yes | Yes | Yes | ||
| E5 | 2011 | 12 | 50.00 | −4.09 | 12.0 | yes | WS38 | Yes | Yes | Yes | ||
| 355PR | E1 | 2012 | 2 | 48.31 | −3.95 | 10.3 | yes | WS39 | Yes | Yes | Yes | |
| E2 | 2012 | 2 | 49.12 | −4 | 10.6 | yes | WS40 | Yes | Yes | Yes | ||
| E3 | 2012 | 2 | 49.44 | −4.06 | 10.3 | yes | WS41 | Yes | Yes | Yes | ||
| E4 | 2012 | 2 | 49.72 | −4.12 | 10.2 | yes | WS42 | Yes | Yes | Yes | ||
| E5 | 2012 | 2 | 49.95 | −4.16 | 9.7 | yes | WS43 | Yes | Yes | Yes | ||
| 356PR | E1 | 2012 | 3 | 48.82 | −3.96 | 10.40 | yes | Yes | Yes | |||
| E2 | 2012 | 3 | 49.15 | −3.98 | 10.70 | yes | Yes | Yes | ||||
| E3 | 2012 | 3 | 49.44 | −4.06 | 10.10 | yes | Yes | Yes | ||||
| E4 | 2012 | 3 | 49.72 | −4.12 | 10.30 | yes | Yes | Yes | ||||
| E5 | 2012 | 3 | 49.95 | −4.16 | 10.10 | yes | Yes | Yes | ||||
| 358PR | E1 | 2012 | 5 | 48.81 | −3.96 | 11.9 | yes | Yes | Yes | |||
| E2 | 2012 | 5 | 49.25 | −4.02 | 11.6 | yes | Yes | Yes | ||||
| E3 | 2012 | 5 | 49.61 | −4.06 | 11.6 | yes | Yes | Yes | ||||
| E4 | 2012 | 5 | 49.95 | −4.07 | 12.2 | yes | Yes | Yes | ||||
| E5 | 2012 | 5 | 50.27 | −4.17 | 11.4 | yes | Yes | Yes | ||||
| 359PR | E1 | 2012 | 6 | 48.80 | −3.96 | 13.41 | yes | Yes | Yes | |||
| E2 | 2012 | 6 | 49.20 | −4.02 | 13.22 | yes | Yes | Yes | ||||
| E3 | 2012 | 6 | 49.62 | −4.13 | 13.6 | yes | Yes | Yes | ||||
| E4 | 2012 | 6 | 49.94 | −4.15 | 13.8 | yes | Yes | Yes | ||||
| E5 | 2012 | 6 | 50.25 | −4.16 | 14.2 | yes | Yes | Yes | ||||
| 360PR | E1 | 2012 | 7 | 48.80 | −3.96 | 14.65 | yes | Yes | Yes | |||
| E2 | 2012 | 7 | 49.25 | −4.06 | 14.90 | yes | Yes | Yes | ||||
| E3 | 2012 | 7 | 49.65 | −4.11 | 15.05 | yes | Yes | Yes | ||||
| E4 | 2012 | 7 | 49.99 | −4.14 | 14.87 | yes | Yes | Yes | ||||
| 2012 | 7 | |||||||||||
| 361PR | E1 | 2012 | 8 | 48.80 | −3.96 | yes | Yes | Yes | ||||
| E2 | 2012 | 8 | 49.26 | −4.05 | yes | Yes | Yes | |||||
| E3 | 2012 | 8 | 49.66 | −4.09 | yes | Yes | Yes | |||||
| E4 | 2012 | 8 | 50.00 | −4.13 | yes | Yes | Yes | |||||
| E5 | 2012 | 8 | 50.32 | −4.18 | yes | Yes | Yes | |||||
| 362PR | 2012 | 9 | 48.8 | −3.96 | Yes | |||||||
| 2012 | 9 | 49.26 | −4.05 | Yes | ||||||||
| 2012 | 9 | 49.66 | −4.09 | Yes | ||||||||
| 2012 | 9 | 50.00 | −4.13 | Yes | ||||||||
| 2012 | 9 | 50.33 | −4.18 | Yes | ||||||||
| 364PR | E1 | 2012 | 10 | 48.84 | −3.97 | 14.40 | yes | Yes | Yes | |||
| E2 | 2012 | 10 | 49.21 | −3.99 | 14.5 | yes | Yes | Yes | ||||
| E3 | 2012 | 10 | 49.55 | −4.02 | 14.5 | yes | Yes | Yes | ||||
| E4 | 2012 | 10 | 49.9 | −4.13 | 14.3 | yes | Yes | Yes | ||||
| E5 | 2012 | 10 | 50.25 | −4.15 | 14.5 | yes | Yes | Yes | ||||
| Additional | E1 | 2012 | 10 | 48.84 | −3.97 | 14.40 | yes | Yes | Yes | |||
| E2 | 2012 | 10 | 49.21 | −3.99 | 14.5 | yes | Yes | Yes | ||||
| E3 | 2012 | 10 | 49.55 | −4.02 | 14.5 | yes | Yes | Yes | ||||
| E4 | 2012 | 10 | 49.9 | −4.13 | 14.3 | yes | Yes | Yes | ||||
| E5 | 2012 | 10 | 50.25 | −4.15 | 14.5 | yes | Yes | Yes | ||||
| 365PR | E1 | 2012 | 11 | 48.83 | −3.96 | 13 | yes | Yes | Yes | |||
| E2 | 2012 | 11 | 49.22 | −4.01 | 13.5 | yes | Yes | Yes | ||||
| E3 | 2012 | 11 | 49.6 | −4.05 | 13.6 | yes | Yes | Yes | ||||
| E4 | 2012 | 11 | 49.92 | −4.09 | 13.2 | yes | Yes | Yes | ||||
| 2012 | 11 | yes | ||||||||||
| 366PR | E1 | 2012 | 12 | 48.8 | −3.96 | 11.3 | yes | Yes | Yes | |||
| E2 | 2012 | 12 | 49.19 | −4.01 | 12 | yes | Yes | Yes | ||||
| E3 | 2012 | 12 | 49.52 | −4.11 | 11.8 | yes | Yes | Yes | ||||
| E4 | 2012 | 12 | 49.88 | −4.13 | 11.6 | yes | Yes | Yes | ||||
| E5 | 2012 | 12 | yes | |||||||||
| 367PR | E1 | 2013 | 1 | 48.78 | −3.96 | yes | Yes | Yes | ||||
| E2 | 2013 | 1 | 49.15 | −4.02 | yes | Yes | Yes | |||||
| E3 | 2013 | 1 | 49.49 | −4.05 | yes | Yes | Yes | |||||
| E4 | 2013 | 1 | 49.84 | −4.01 | yes | Yes | Yes | |||||
| E5 | 2013 | 1 | 50.17 | −4.12 | yes | Yes | Yes | |||||
| 368PR | E1 | 2013 | 2 | 48.8 | −3.96 | yes | Yes | Yes | ||||
| E2 | 2013 | 2 | 49.13 | −4.02 | yes | Yes | Yes | |||||
| E3 | 2013 | 2 | 49.4 | −4.06 | yes | Yes | Yes | |||||
| E4 | 2013 | 2 | 49.7 | −4.09 | yes | Yes | Yes | |||||
| E5 | 2013 | 2 | 50.05 | −4.12 | yes | Yes | Yes | |||||
| 371PR | 2013 | 5 | 48.78 | −3.94 | ||||||||
| 2013 | 5 | 49.15 | −3.87 | |||||||||
| 2013 | 5 | 49.51 | −3.92 | |||||||||
| E4 | 2013 | 5 | 49.79 | −4.05 | Yes | Yes | ||||||
| E5 | 2013 | 5 | 50.1 | −4.14 | Yes | Yes | ||||||
| 372PR | E1 | 2013 | 6 | 48.78 | −3.94 | Yes | Yes | |||||
| E2 | 2013 | 6 | 49.15 | −3.87 | Yes | Yes | ||||||
| E3 | 2013 | 6 | 49.51 | −3.92 | Yes | Yes | ||||||
| E4 | 2013 | 6 | 49.79 | −4.05 | Yes | Yes | ||||||
| E5 | 2013 | 6 | 50.1 | −4.14 | Yes | Yes | ||||||
| 374PR | E1 | 2013 | 7 | 48.80 | −3.95 | yes | Yes | Yes | ||||
| E2 | 2013 | 7 | 49.18 | −4.02 | yes | Yes | Yes | |||||
| E3 | 2013 | 7 | 49.55 | −4.10 | yes | Yes | Yes | |||||
| E4 | 2013 | 7 | 49.90 | −4.12 | yes | Yes | Yes | |||||
| E5 | 2013 | 7 | 50.22 | −4.17 | yes | Yes | Yes | |||||
| 375PR | E1 | 2013 | 8 | 48.77 | −3.95 | yes | Yes | Yes | ||||
| E2 | 2013 | 8 | 49.23 | −4.03 | yes | Yes | Yes | |||||
| E3 | 2013 | 8 | 49.64 | −4.09 | yes | Yes | Yes | |||||
| E4 | 2013 | 8 | 49.98 | −4.11 | yes | Yes | Yes | |||||
| E5 | 2013 | 8 | 50.28 | −4.16 | yes | Yes | Yes | |||||
| 376PR | E1 | 2013 | 9 | Yes | Yes | |||||||
| E2 | 2013 | 9 | 49.09 | −3.87 | yes | Yes | Yes | |||||
| E3 | 2013 | 9 | 49.51 | −3.92 | yes | Yes | Yes | |||||
| E4 | 2013 | 9 | 49.79 | −4.05 | yes | Yes | Yes | |||||
| E5 | 2013 | 9 | 50.09 | −4.14 | yes | Yes | Yes | |||||
| E6 | 2013 | 9 | ||||||||||
| E7 | 2013 | 9 | ||||||||||
| E8 | 2013 | 9 | ||||||||||
| E9 | 2013 | 9 | ||||||||||
| E10 | 2013 | 9 | ||||||||||
| 378PR | E1 | 2013 | 11 | 48.78 | −3.95 | yes | Yes | Yes | ||||
| E2 | 2013 | 11 | 49.12 | −3.98 | yes | Yes | Yes | |||||
| E3 | 2013 | 11 | 49.45 | −4.04 | yes | Yes | Yes | |||||
| E4 | 2013 | 11 | 49.78 | −4.11 | yes | Yes | Yes | |||||
| E5 | 2013 | 11 | 50.11 | −4.15 | yes | Yes | Yes | |||||
| PD 2011 | PD 2012 | PD 2013 | AA 2011 | AA 2012 | AA 2013 pt1 | AA 2013 pt2 | |
|---|---|---|---|---|---|---|---|
| R | 0.999 | 0.999 | 0.996 | 0.994 | 0.994 | 0.998 | 0.984 |
| R2 | 0.985 | 0.997 | 0.992 | 0.987 | 0.9888 | 0.995 | 0.969 |
| M | −3.935 | −2.900 | −3.266 | −3.502 | −3.858 | −3.979 | −3.223 |
| B | 36.361 | 28.489 | 34.76 | 43.483 | 43.900 | 38.268 | 29.398 |
| E | 0.872 | 1.212 | 1.023 | 0.9302 | 0.8163 | 0.7837 | 0.6076 |
| Y | M | SR | #/Sample | # OTU | +/− M | Above/Below Median | PDM | C1M | SM |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2 | 1 | WS1 | 132 | −1035.452 | −560 | 4.867548 | 36.0 | 24.100 |
| 2011 | 2 | 1 | WS2 | 346 | −821.452 | −346 | 1.6509 | 22.0 | 9.300 |
| 2011 | 3 | 1 | WS3 | 69 | −1098.452 | −623 | 4.633776 | 93.1 | 24.500 |
| 2011 | 3 | 1 | WS4 | 263 | −904.452 | −429 | 1.586147 | 9.5 | 7.200 |
| 2011 | 4 | 1 | WS5 | 182 | −985.452 | −510 | 2.120118 | 39.8 | 18.600 |
| 2011 | 4 | 1 | WS6 | 655 | −512.452 | −37 | 3.218702 | 29.3 | 14.900 |
| 2011 | 4 | 1 | WS8 | 930 | −237.452 | 238 | 1.08946 | 15.7 | 9.500 |
| 2011 | 4 | 1 | WS9 | 924 | −243.452 | 232 | 0.78551 | 9.9 | 6.400 |
| 2011 | 5 | 1 | WS10 | 257 | −910.452 | −435 | 0.68059 | 22.0 | 12.400 |
| 2011 | 5 | 1 | WS11 | 1535 | 367.548 | 843 | 0.154481 | 2.7 | 2.500 |
| 2011 | 5 | 1 | WS12 | 386 | −781.452 | −306 | 1.175051 | 23.2 | 14.100 |
| 2011 | 5 | 1 | WS13 | 268 | −899.452 | −424 | 2.10681 | 27.6 | 15.100 |
| 2011 | 6 | 2 | WS14 | 544 | −623.452 | −148 | 1.46735 | 62.5 | 23.900 |
| 2011 | 6 | 2 | WS15 | 729 | −438.452 | 37 | 1.561149 | 23.6 | 13.600 |
| 2011 | 6 | 2 | WS16 | 861 | −306.452 | 169 | 2.271352 | 56.7 | 25.300 |
| 2011 | 6 | 2 | WS17 | 592 | −575.452 | −100 | 2.49747 | 43.4 | 20.900 |
| 2011 | 6 | 2 | WS18 | 358 | −809.452 | −334 | 3.344375 | 74.0 | 25.200 |
| 2011 | 7 | 2 | WS19 | 237 | −930.452 | −455 | 3.779641 | 105.0 | 35.700 |
| 2011 | 7 | 2 | WS20 | 427 | −740.452 | −265 | 1.589128 | 32.9 | 27.300 |
| 2011 | 7 | 2 | WS21 | 484 | −683.452 | −208 | 1.889013 | 48.0 | 17.100 |
| 2011 | 7 | 2 | WS22 | 242 | −925.452 | −450 | 2.368124 | 57.5 | 18.400 |
| 2011 | 7 | 2 | WS23 | 445 | −722.452 | −247 | 0.71281 | 38.0 | 15.000 |
| 2011 | 9 | 2 | WS24 | 540 | −627.452 | −152 | 0.650258 | 22.1 | 12.500 |
| 2011 | 9 | 2 | WS25 | 6674 | 5506.548 | 5982 | 0.319075 | 4.5 | 2.900 |
| 2011 | 9 | 2 | WS26 | 1734 | 566.548 | 1042 | 1.622526 | 29.2 | 11.400 |
| 2011 | 9 | 2 | WS27 | 1080 | −87.452 | 388 | 1.786829 | 32.9 | 14.200 |
| 2011 | 9 | 2 | WS28 | 240 | −927.452 | −452 | 3.088336 | 79.0 | 24.000 |
| 2011 | 10 | 2 | WS29 | 928 | −239.452 | 236 | 2.057396 | 39.3 | 16.000 |
| 2011 | 10 | 2 | WS30 | 893 | −274.452 | 201 | 0.256501 | 36.7 | 12.100 |
| 2011 | 10 | 2 | WS31 | 2966 | 1798.548 | 2274 | 2.601002 | 89.1 | 31.000 |
| 2011 | 10 | 2 | WS32 | 1175 | 7.548 | 483 | 0.316267 | 7.1 | 4.300 |
| 2011 | 10 | 2 | WS33 | 7500 | 6332.548 | 6808 | 0.431583 | 12.9 | 6.500 |
| 2011 | 12 | 2 | WS34 | 451 | −716.452 | −241 | 0.772125 | 26.0 | 11.700 |
| 2011 | 12 | 2 | WS35 | 766 | −401.452 | 74 | 1.965868 | 51.0 | 13.100 |
| 2011 | 12 | 2 | WS36 | 1072 | −95.452 | 380 | 1.2185 | 20.4 | 9.800 |
| 2011 | 12 | 2 | WS37 | 736 | −431.452 | 44 | 2.471535 | 25.9 | 13.400 |
| 2011 | 12 | 2 | WS38 | 3072 | 1904.548 | 2380 | 0.046794 | 4.0 | 3.200 |
| 2012 | 2 | 2 | WS39 | 902 | −265.452 | 210 | 2.747683 | 83.8 | 18.600 |
| 2012 | 2 | 2 | WS40 | 455 | −712.452 | −237 | 0.916246 | 23.0 | 13.500 |
| 2012 | 2 | 2 | WS41 | 738 | −429.452 | 46 | 1.699598 | 66.8 | 19.200 |
| 2012 | 2 | 2 | WS42 | 4505 | 3337.548 | 3813 | 0 | 4.4 | 3.500 |
| 2012 | 2 | 2 | WS43 | 1740 | 572.548 | 1048 | 0.67496 | 10.8 | 7.200 |
| Total | 49,033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, R.; Picard, K.; Clarke, J.; Walker, C.E.; Martins, C.; Marshall, C.; Amorim, A.; Woodward, E.M.S.; Widdicombe, C.; Tarran, G.; et al. Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry. J. Mar. Sci. Eng. 2023, 11, 480. https://doi.org/10.3390/jmse11030480
Stern R, Picard K, Clarke J, Walker CE, Martins C, Marshall C, Amorim A, Woodward EMS, Widdicombe C, Tarran G, et al. Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry. Journal of Marine Science and Engineering. 2023; 11(3):480. https://doi.org/10.3390/jmse11030480
Chicago/Turabian StyleStern, Rowena, Kathryn Picard, Jessica Clarke, Charlotte E. Walker, Claudia Martins, Clare Marshall, Ana Amorim, E. Malcolm S. Woodward, Claire Widdicombe, Glen Tarran, and et al. 2023. "Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry" Journal of Marine Science and Engineering 11, no. 3: 480. https://doi.org/10.3390/jmse11030480
APA StyleStern, R., Picard, K., Clarke, J., Walker, C. E., Martins, C., Marshall, C., Amorim, A., Woodward, E. M. S., Widdicombe, C., Tarran, G., & Edwards, M. (2023). Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry. Journal of Marine Science and Engineering, 11(3), 480. https://doi.org/10.3390/jmse11030480






