Abstract
Viral dynamics are the result of the balance between the rates of viral production and decay. Here, we have carried out independent measurements of viral production and decay rates in different depths of the southern East China Sea in summer (August and October 2021). In this study, the prevalence of viral abundance at the surface waters (14.2~27.6 × 105 viruses mL−1) was significantly higher than the bottom of the euphotic zone (2.9~12.6 × 105 viruses mL−1). As for viruses to bacteria ratio (VBR) values, we found a wide variability both at the surface (1.4 to 3.2) and bottom of the euphotic zone (2.1 to 16.2). The results of our study showed that at all stations examined, in the southern East China Sea, the values of gross viral production (GVP) were significantly higher in the sunlit surfaces compared to the bottom of the euphotic zone. In particular, our analysis indicates that no significant viral decay rates (VD) were observed in some regions at the bottom of the euphotic zone. Here, we also provide a budget for viral abundance and net viral production in different regions in the southern East China Sea. The GVP or VD is not applicable in our case to explain VBR is high at bottom of the euphotic zone. The mechanisms underlying VBR uncoupling, viral production, and viral loss in marine systems are still being investigated.
1. Introduction
The fact that free-living bacteria account for a considerable portion of the total carbon flux in marine systems is well documented [1,2]. It is well recognized that protozoan grazing and viral lysis are critical top-down controls of bacterial mortality in aquatic systems. When bacterial cells are grazed, energy is made available to higher trophic levels [3,4]. In recent years, it has been established that viral infections can contribute significantly to the loss of bacterial biomass, and viral lysis leads to a recycling of nutrients within the microbial loop [5,6,7,8,9].
There are several implications for aquatic systems when large numbers of bacteria are lysed by viruses, including effects on population size and diversity, or the transfer of genetic material [10,11,12,13,14]. However, the release of organic matter by the viral lysis of bacteria has shown that viral lysis stimulates the growth of bacteria both in simulations and in experimental studies [15,16]. Although viruses play a vital role in the ecosystems of the sea, we still have a very limited understanding of their distribution and impact on their hosts in the bottom waters.
In light-limited environments, there is sparse knowledge of viral lysis and its impact on bacterial growth [17]. The grazing rate of deep-water organisms is often low [16,18], so other mechanisms, such as viral lysis, must be involved in bacterial mortality. There is an association between viral abundance and the trophic status of an ecosystem [16,19], so its variability is correlated with other biophysical parameters such as phytoplankton [20] and bacterial abundance [16,21,22]. In some areas, virus abundance correlated more strongly with chlorophyll a than bacterial abundance [20,23,24]. These observations could be explained by an increase in phytoplankton biomass and viral infection rates. Under light-limiting conditions, the chlorophyll a concentration in deep waters is very low, making bacterial abundance a better predictor of viral abundance. Studies also indicate that viruses have a greater impact on bacteria in deeper water than at the surface [18,25,26].
As an indicator of the relationship between bacteria and viruses, the virus-to-bacteria ratio (VBR) is often used. When combined with studies on freshwater and marine habitats, VBRs range from 0.03 to 80, providing support for the notion that viruses are a dynamic component of aquatic food webs [20]. VBR has been shown in previous studies to be >10, which indicates a high viral infection rate contributing to bacterial mortality [20,27]. The differences in VBR have been noted in different marine environments; for example, the VBR in the surface waters of the Arctic Ocean is about 10 [28], as opposed to deep water in the Atlantic Ocean, where the VBR is often greater than 100 [29]. VBR is helpful for constructing theories on how viral infection affects aquatic bacterial communities, but it is essential to consider that many factors contribute to the production and loss of viruses and bacteria. Detailed analyses of factors affecting viral production and decay, as well as the viral balance, are necessary to better understand viral dynamics in marine environments. Furthermore, both viral production and decay rates should be estimated independently for a thorough analysis of viral dynamics. Viral production is primarily determined by the abundance, metabolic activity, and burst size of the prokaryotic hosts [29,30,31], a complex interaction of physical, chemical, and biological factors determines viral decay rates [12,20]. In addition, biological pumps export carbon from the euphotic zone, which is an important component of marine carbon cycles. In this regard, it is crucial to understand where viral activity occurs within the water column, based on the depth and strength of the pycnocline, since this determines whether lysed material will be recycled in the euphotic zone or exported to the deep sea.
In order to test the effect of different environmental conditions on viral dynamics, we carried out independent measurements of viral production and decay rates in different depths of the southern East China Sea. Considering the physicochemical changes associated with depth, we hypothesized that levels of bacterial, viral abundance, viral production, as well as viral decay, will characteristically differ between the surface and bottom of the euphotic zone. Here, we present vertical differences in viral and bacterial abundances in the southern East China Sea, collecting samples from the surface and bottom of the euphotic zone waters in summer (August and October 2021).
2. Materials and Methods
2.1. Study Site and Samplings
Samples were collected at 4 different stations (St. A, B, C, and D) located in the southern East China Sea (Figure 1). Stations were set up in the China Coastal Water (CCW; St. A), the Taiwan Current Warm Water (TCWW; St. B), the Upwelled Water (UW; St. C), and the Kuroshio Water (KW; St. D). For the incubation experiments, samples were collected in August (St. A and B) and October 2021 (St. C and D), at a surface depth of 3~5 m and the bottom depths of 30~200 m at the four established stations (Table 1) onboard the New R/V Ocean Research II. During the cruises, seawater samples were collected in 12L Teflon-coated Go-Flo bottles. Temperature, salinity, and photosynthetically active radiation (PAR) vertical profiles were obtained using the SBE 9/11plus CTD (Sea-Bird Scientific, Bellevue, WA, USA). For the detection of Chl a in water samples, a 25 mm GF/F filter and an in vitro fluorometer (Turner Designs, Inc., San Jose, CA, USA) were used [32]. For analysis of nutrient content, samples of seawater were collected in polypropylene bottles and frozen in liquid nitrogen as soon as possible. As a standard method, molybdenum blue was used to determine the phosphate concentration with a measurement precision of ±0.01 μM [32]. We collected 1 mL subsamples every 2 h from each incubation set-up, fixed them in paraformaldehyde (1% final concentration), and then froze them in liquid nitrogen. Virus and bacterial samples were preserved at −80 °C before flow cytometry (FCM) analysis.
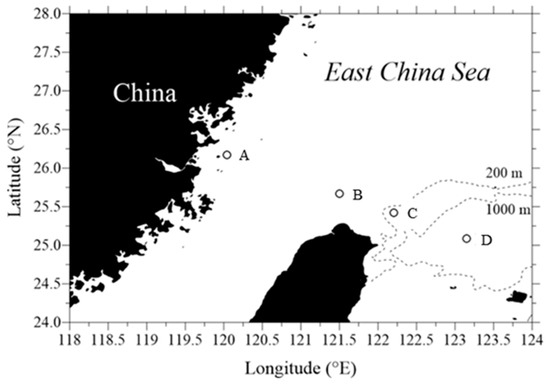
Figure 1.
Map of the southern East China Sea showing the location of the sampling sites.

Table 1.
Temperature, salinity, photosynthetically active radiation (PAR), chlorophyll a concentration, mean values (±SD) of viral abundance, bacterial abundance, and virus-to-bacteria ratio (VBR) at the sampling stations and depths.
A CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis, IN, USA) equipped with an air-cooled argon-ion laser at 488 nm, a filter at 525 nm, and an SYBR signal trigger was used to analyze viral and bacterial samples. In order to minimize interference from high particle density, virus samples were diluted 1:10 in TE buffer (pH 8.0, EM grade) before staining. Incubate the diluted samples at 80 °C for 10 min in the dark with SYBR Green I (final concentration of 50,000 of commercial stock). The samples were cooled in an ice bath to 25 °C after staining and then processed through FCM following the method of Brussaard [33]. To detect and eliminate noise in the buffer, blank controls were run on TE buffer stained with the same concentration of SYBR Green I. As described by Hammes and Egli [34], bacteria samples were stained with SYBR Green I (final concentration 1:10,000) for 15 min in the dark before processing through FCM.
2.2. Experiments on Viral Production and Decay
A track-etched polycarbonate filter membrane (Whatman, Maidstone, Kent, England) with a pore size of 2 μm and a 47 mm diameter was vacuum-filtered to produce grazer-free water. Grazer-free seawater was filtered through a Minimate TFF Capsule (Pall), which had a molecular weight cutoff of 30 kDa, to produce 200 mL of virus-free water. The virus-free water was diluted by adding 200 mL to 50 mL of grazer-free water, maintaining the bacterial and viral abundance at 20% of the in situ seawater density. Afterward, we thoroughly mixed the incubation water and filled 50 mL polycarbonate bottles with it. Following preparation of the bottles, neutral-density plastic sheets (Lee Filters, Hampshire, UK) were applied to the bottles in a thermo-controlled incubator to simulate the light intensity at the sampling depth. Furthermore, all treatments were conducted in triplicate, including incubation at the in situ temperature for bottom water samples. The linear regression between viral density and incubation time was used to determine net viral production (NVP) (viruses mL−1 h−1) [35]. The viral decay rate (VD) of water samples was assessed by filtering them through polycarbonate filters with pore sizes of 0.2 μm to exclude prokaryotes and particles larger than 0.2 μm [36]. The slope of the line is the net viral production (NVP) and decay (VD) rate [31]. We estimated gross viral production (GVP) as the sum of NVP and VD based on the patterns we observed in experiments (the equation is “Net viral production (NVP) = Gross viral production (GVP)-Decay rate (VD)”). Furthermore, in the 20% diluted samples (virus-free water diluted with 2 μm filtered water), bacteria were measured. This study determined the bacterial growth rate by plotting ln (bacterial abundance) against time as a slope of linear regression.
2.3. Statistical Analysis
Linear regression analysis was used to examine the relationship between viral abundance and incubation time for triplicate samples. The slopes of linear regressions calculated from time-course experiments were analyzed using ANOVA to test for statistical differences between viral production and viral decay rates. The statistical operations were performed with STATISTICA 7.0 software. The significance level was determined by a probability value of <0.05.
3. Results
3.1. Environmental Dynamics
In this study, surface water temperatures varied from 25.8 to 29.1 °C, while the bottom of the euphotic zone temperatures varied from 16.9 to 23.8 °C and salinity throughout the water column varied from 33.6 to 34.8 psu (Table 1, Figure 2). In Figure 2, the vertical values of the CTD data are plotted on a T–S diagram, while typical Kuroshio water (KW) is shown on a reference curve. It was found that KW primarily occurred at St. D and the upwelled Kuroshio subsurface water (UKSW) affected the environmental characteristics of St. C. (Figure 2). Direct measurement of light intensity at 30 m of St. A showed that it declined to 3% of its value at the surface, and <1% at 200 m of St. C and D (Table 1). Furthermore, a higher chl a concentration (0.4 µg L−1) was observed at St. C due to the location being near the upwelling region (Table 1).
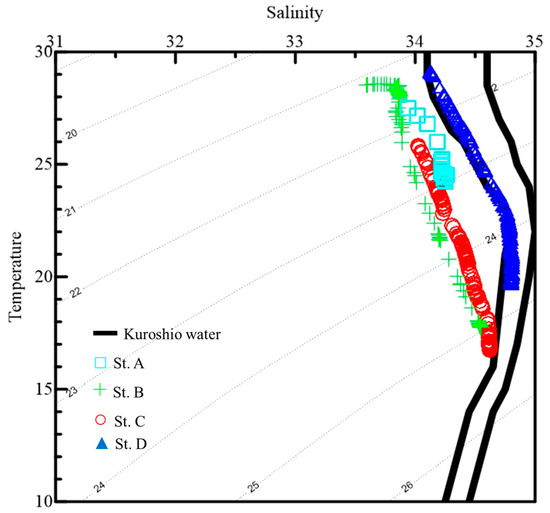
Figure 2.
Vertical variations of temperature (°C) and salinity (psu) at the study stations (A, B, C, and D). Typical Kuroshio water (KW) is used as the reference in a temperature–salinity (T–S) diagram of the processed CTD data (E).
Based on flow cytometry analysis, we found a relatively high abundance of viral and bacterial abundances at the surface waters in the southern East China Sea (Table 1). Overall, viral abundance ranged from 14.2 ± 2.6 to 27.6 ± 1.9 × 105 viruses mL−1, and bacterial abundance ranged from 5.8 ± 1.6 to 19.1 ± 0.9 × 105 cells mL−1 in the surface waters, respectively (Table 1). In the surface layer, the highest values of viral and bacterial abundance were observed at St. A. These values could have been affected by the proximity of the site to the coastal area. Furthermore, we observed a relatively low abundance of viral and bacterial abundances at the bottom of the euphotic zone (Table 1), with the viral abundance ranging from 2.9 ± 0.7 to 12.6 ± 1.4 × 105 viruses mL−1, and the bacterial abundance ranging from 0.3 ± 0.1 to 6.1 ± 0.9 × 105 cells mL−1 at the bottom of the euphotic zone, respectively. As for VBR values, we found a wide variability both at the surface (1.4 to 3.2) and bottom of the euphotic zone (2.1 to 16.2) (Table 2).

Table 2.
Mean values (±SD) of net viral production (NVP), viral decay rate (VD), and gross viral production (GVP) at the sampling stations and depths. ND is non-detectable value.
3.2. Viral Production and Decay Rate in Surface and Bottom of the Euphotic Zone
The slope of linear regression plotted against viral abundance and incubation time was used in the present study to estimate viral production rates. The variations in viral abundance in surface water at St. A remained stable during the 24 h time period and did not change significantly in our dilution experiment (ANOVA, p > 0.05). However, at the bottom of the euphotic zone at St. A, the slope decreased by 0.05 (Figure 3A). At St. B, the surface water exhibited a decreasing pattern of NVP with a value of −0.07 for the slope (Figure 3B). At the bottom of the euphotic zone at St. B, an increase in the viral abundance was observed over time with a rate of 0.11 × 105 viruses mL−1 h−1 (Figure 3B). At St. C, the value of NVP was about −0.05 × 105 viruses mL−1 h−1 in surface waters. Furthermore, an increasing pattern of NVP can be observed at St. D in surface water with 0.05 × 105 viruses mL−1 h−1 (Figure 3C, Table 2). As a result of the dilution experiments, we found that the viral abundance at the bottom of the euphotic zone at St. C and D did not change significantly (ANOVA, p > 0.05), and a non-significant change in NVP was calculated (Figure 3).
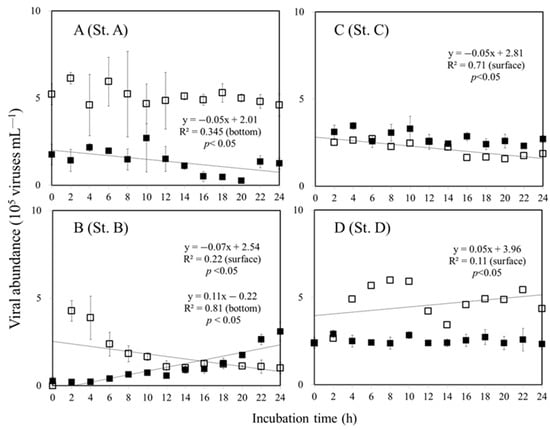
Figure 3.
Viral dynamics over the dilution experiment at the surface (□) and bottom of the euphotic zone (■) collected at the study stations A, B, C, and D. Error bars represent SD values. (—) is the regression line for sunlit surface and bottom of the euphotic zone.
Virus decay rates were estimated using a linear regression model that decreased in slope as viral abundance decreased with incubation time. On the surface, the highest VD was found at St. B, with a value of 0.23 × 105 viruses mL−1 h−1, while the lowest value was found at St. A, with a value of 0.09 × 105 viruses mL−1 h−1 (Figure 4). We did not observe a significant change in the viral abundance with incubation time at the bottom of the euphotic zone at St. B (depth of 100 m), C (depth of 200 m), and D (depth of 200 m), (Figure 4, Table 2). A different pattern was observed at the bottom of the euphotic zone (30 m) at St. A, showing a significant (p < 0.05) decline in the viral abundance over the incubation period (Figure 4).
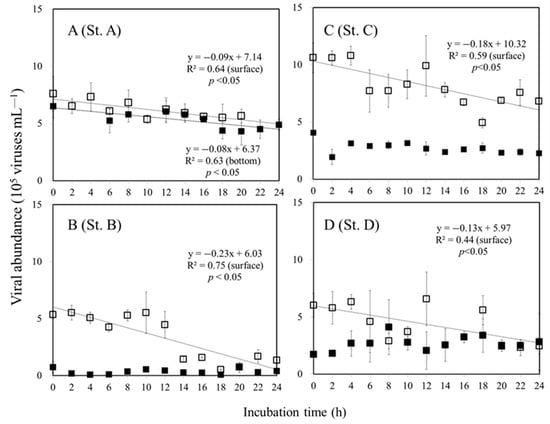
Figure 4.
Viral decay in the grazer-free water (<0.2 μm fraction) at the surface (□) and bottom of the euphotic zone (■) at the study stations A, B, C, and D. Error bars represent SD values. (—) is the regression line for sunlit surface and bottom of the euphotic zone.
Next, we calculated the gross viral production (GVP) based on the estimations of NVP and VD. According to the results of the study, the GVP ranged from 0.09 to 0.18 × 105 viruses mL−1 h−1. The highest GVP was observed in the surface water at St. D at a value of 0.18 × 105 viruses mL−1 h−1 (Table 2).
3.3. Variations in the Bacterial Growth in the Surface and Bottom Water
The bacterial growth rate was between 0.030 and 0.077 h−1 in the surface waters of the study region and between 0.022 and 0.138 h−1 at the bottom of the euphotic zone of the treatment with virus-diluted experiments (Figure 5, Table 2). Results of the study indicate a higher bacterial rate below the euphotic zone (0.138 h−1) than in the surface water at St. B (Figure 5B). Furthermore, no significant difference was observed between the growth rate of bacteria in the surface and bottom of the euphotic zone at St. D (Figure 5D; F-test, p > 0.05).
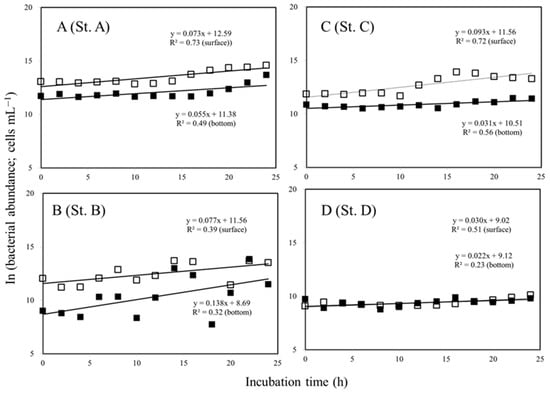
Figure 5.
Dynamics of the bacterial abundance in the surface (□) and bottom of the euphotic zone (■) layers at the study stations A, B, C, and D during the dilution experiment. (—) is the regression line for sunlit surface and bottom of the euphotic zone.
4. Discussion
A system of strong hydrographic gradients exists in the ECS, which ranges from the coastal region influenced by the China Coastal Current and oligotrophic Taiwan Warm Current to the offshore region influenced by the Kuroshio intrusion [32]. Based on our study of environmental variables in the southern ECS, our results indicate that the marine environment is typical of a summer water column. From the Taiwan Strait, warm oligotrophic Taiwan Current Warm Water mixes with the southern ECS, affecting the pelagic ecosystem throughout the surface water column. There is a persistent upwelling system known to contain significant amounts of nutrients near Kuroshio. Summer southwestern monsoons cause a strong Taiwan Strait Warm Current to move toward the shelf break and cover upwelling water in the area [37]. Furthermore, the low Chl a concentrations observed in this study may be attributed to the low nutrients (Table 1), previous study showed that nutrient-limiting conditions prevailed in strongly stratified water columns, preventing nutrients from being supplied upward from the subsurface. Subsurface chlorophyll a maximum was most prevalent during summer on offshore waters [32]. Little is known about how viruses affect bacterial communities at various depths in the southern East China Sea. A previous study investigated the abundance of bacteria and viruses, bacterial growth rate, and viral production at the surface (3 m) and bottom of the euphotic zone (130 m) of the ocean in this study area using preliminary samples from a single site [16]. Their study, however, did not rule out sampling different water masses for independent measurements of viral production and decay.
4.1. Variations in Virus to Bacteria Ratio (VBR)
There has been an increased prevalence of viruses in marine environments [38], often exceeding bacterial numbers by an order of magnitude [39]. As shown in Table 1, in the present study, the prevalence of viral abundance in the surface waters (14.2~27.6 × 105 viruses mL−1) was significantly higher than the bottom of the euphotic zone (2.9~12.6 × 105 × 105 viruses mL−1) (Table 1). Magiopoulos and Pitta [20] found viral abundance to be significantly higher in epipelagic compared to mesopelagic areas [26]. Based on the theory that virus abundance indicates viral activity [20], the increase in viral abundance must have been due to either an increase in algal biomass or an increase in bacterial activity. Moreover, previous studies reported that the distribution of virus abundances is determined by the planktonic processes, which are related to bacterial abundance, Chl a concentration [25,40]. Based on our current study, we confirm such observations of relatively high viral abundance with higher chlorophyll a concentrations and bacterial abundance in ocean surface waters (Table 1). In our study, viral abundance did not correlate significantly with chlorophyll a concentration in the surface waters (r = 0.085), but viral abundance did positively correlate with bacterial abundance (r = 0.89). The bacterial abundance in surface waters has been identified as a significant parameter in a regression analysis explaining viral abundance changes. Apart from these biological factors, the temperature may also play a role in determining the vertical variation in viral abundance because the temperature affects bacterial growth [4,24].
The virus-to-bacteria ratio (VBR) provides insight into the interaction between bacteria and viruses. VBR has been reported in both oceanic and freshwater environments to range from 0.03 to 80, suggesting that viruses are a highly dynamic component of the aquatic food web [20]. In a previous study by Wilcox and Fuhrman [41], low values of VBR < 1 imply low levels of viral-mediated bacterial mortality. A large percentage of bacterial mortality may be explained by viral infection when the VBR exceeds 10 [20,27]. Interestingly, Table 1 shows that VBR was significantly higher at the bottom of the euphotic zone (t-test, p < 0.05) than in surface marine waters. The high VBR value at the bottom of the euphotic zone cannot be attributed to bacterial mortality caused by a viral infection in our case since gross viral production (GVP) is low in the study area (Table 2). Viral production, virus migration, decay rates, and sinking particles are all factors affecting VBR values [31,42] and are discussed below.
4.2. Variations in Viral Production
Net viral production and viral decay experiments allowed us to estimate gross viral production (the sum of net viral production and viral decay), which may be a representation of the total number of viruses produced by infected bacteria. Our study estimated viral production at a dilution level of approximately 20% of bacterial and viral abundance. Because these incubated treatments involve a 20% function of viral lysis on bacteria, it is possible that our viral production rates may be underestimated. The factors that limit viral production in aquatic systems have remained elusive despite the growing interest in viral ecology in recent years [43,44]. The level of viral production is primarily determined by the metabolic activity, abundance of prokaryotic hosts, and burst size of those hosts [29,31]. The results of our study showed that at all stations examined in the southern East China Sea, the values of gross viral production (GVP) at St. C (depth of 200 m) and D (depth of 200 m) were not detectable (Table 2). A study in Lake Bourget (France) showed clear seasonal variation in viral production, ranging from 0.3 × 105 viruses mL−1 h−1 (July) to 20 × 105 viruses mL−1 h−1 (February and April) [45]. The values of GVP (0.09~0.18 × 105 viruses mL−1 h−1) in our study are close to the viral production measured in summer in Lake Bourget (France). However, our GVP values in the southern East China Sea are lower than the study in the North Adriatic Sea, which can up to 10 × 105 viruses mL−1 h−1 [46]. The eutrophic conditions of the northern Adriatic induced a higher growth rate of bacteria, which then supported higher viral production. Furthermore, as a result of the higher bacterial abundance in the surface environment of the East China Sea, there is higher viral production, resulting in high viral abundance in the surface water (Table 1 and Table 2). In similar studies, it has also been shown that the abundance and biomass of bacteria appear to be linked to the production of viruses [47,48]. Despite the low nutrients that may have limited bacterial growth at St. D (KW), higher virus production (0.18 × 105 viruses mL−1 h−1) was observed in the surface waters (Table 2). As Holmfeldt et al. [49] also found, the amount of viral production in Bothnian Bay was higher than that found elsewhere in the Northern Baltic Sea, although bacterial abundance and production were lower. It is possible to speculate that the low nutrient levels and low bacterial production in Bothnian Bay suggest a high proportion of lysogenic bacteria [49]. In their article, Holmfeldt et al. [49] also argued that viral production in aquatic environments cannot be entirely attributed to bacterial growth and abundance.
In our study, the removal of nanoflagellates and most potential viral lysis led to bacterial abundance increasing substantially within 24 h (Figure 5). Viral lysis, however, may release all of the contents of the host cell into the DOM pool, benefiting uninfected bacteria [5]. A previous study in the East China Sea showed that the bacterial growth rate was lower in virus-diluted treatments than in unfiltered treatments [16]. As such, our bacterial rates could be partially underestimated in this study.
4.3. Variations in Viral Decay
It is crucial to measure both viral production and decay rates independently in order to adequately analyze viral dynamics. A growing number of studies are being performed on viral production in pelagic systems, but there is little information available on viral decay rates [12].
The rate of viral decay is influenced by a complex interaction between physical, chemical, and biological factors [12,20]. Our analysis indicated that the decay rates at St. B (depth of 100 m), C (depth of 200 m), and D (depth of 200 m) at the bottom of the euphotic zone were not detectable (Table 2). It has been shown that the decay rate of viruses increases with light and temperature [48]. It would therefore make sense that the dark ocean would decay at a slower rate than the warmer surface waters due to its low temperature and radiation level [29]. There is evidence that sunlight plays a major role in causing the loss of viral infectivity [36,46]. When sunlit surface waters are exposed to high levels of solar radiation (especially ultraviolet radiation), viral DNA can be damaged, which reduces the abundance and activity of viruses [46]. This research led us to hypothesize that abiotic factors, such as UV damage to viruses, may play a major role in removing viruses from sunlit surface waters. As for spatial variations in VD in the surface waters, the lowest VD was found at St. A (coastal waters) in this study. Due to the penetration of UV into the water column, UV has a greater impact on virus survival in oligotrophic waters than in more eutrophic systems. In the case of these samples, we did not measure the effect of UV on viruses, so we are unable to make any conclusions in this regard.
Experimental work has demonstrated that there is a wide range of virus removal rates depending on the type of marine virus and the environmental factors [36,50,51]. Besides the effects of ultraviolet light, other factors, such as particulate matter and enzymes, are thought to influence the rate at which viruses are removed [20]. This method of estimating viral decay [36] involves removing suspended particles, bacteria, and organic molecules or aggregates to estimate viral decay, which may result in a lower percentage of viruses removed from the system. This method can therefore partially underestimate the viral decay rate.
The results of the measurements of GVP and VD at four stations in the southern East China Sea can be used to imagine the dynamics of viral transmission in that area (Table 2 and Figure 6). Here we provide, for the first time, a budget for viral abundance and net production in different regions in the southern East China Sea (Figure 6). According to our results, different causative agents are primarily responsible for the removal of viruses from the water at different depths (Figure 6). As for spatial variations in VD, a higher VD was found in the surface waters than at the bottom of the euphotic zone. These results indicated that environmental conditions, such as UV damage to viruses, promoted significantly higher viral decay rates compared to deeper marine systems. Furthermore, viral decay (0.08 × 105 viruses mL−1 h−1) at St. A clearly exceeded viral production (0.03 × 105 viruses mL−1 h−1) at 30 m, causing an apparent net negative balance of viral abundance and showing a lower ratio of virus to total bacterial abundance (2.1) (Table 1). A number of factors could cause virus loss in marine waters. We were able to discount these factors in our incubation experiments, including enzymatic degradation and adsorption to colloidal substances [36], settling particle scavenging [31], and solar irradiance [49]. During the study period, total suspended matter at the bottom of the euphotic zone was generally higher (3.52 mg L−1) than that in the surface waters (1.52 mg L−1) at Station A. According to some studies, the concentration of suspended matter increases the sedimentation rate of viruses attached to suspended matter while sinking along with them [24,50]. An exception may exist in some phytoplankton, where virus-infected cells sink rapidly, increasing their transport to deeper waters [52]. In these pathways, we believe that viruses attached to particles derived from surface waters could also be partially responsible for deep-water viruses, due to there being higher values of VBR at the bottom of the euphotic zone than that at surface waters. Furthermore, biological pumps export carbon from the euphotic zone, which is an important component of marine carbon cycles. In this regard, it is crucial to understand where viral activity occurs within the water column, based on the depth and strength of the pycnocline, since this determines whether lysed material will be recycled in the euphotic zone or exported to the deep sea. In this study, the values of GVP and VD at 200 m depth were not determined in the bottom waters at St. C and D. Despite this, the values of VBR (13.9~16.2) were extremely high at the bottom of the euphotic zone, suggesting that there may be other sources of viruses exported to the deep sea, as well as the fact that most viruses tend to sediment more quickly when they attach to particles from the upper waters (Figure 6). Testing the steady-state hypothesis and understanding how viruses respond to different environmental conditions requires an accurate analysis of the balance between viral production and decay rates.
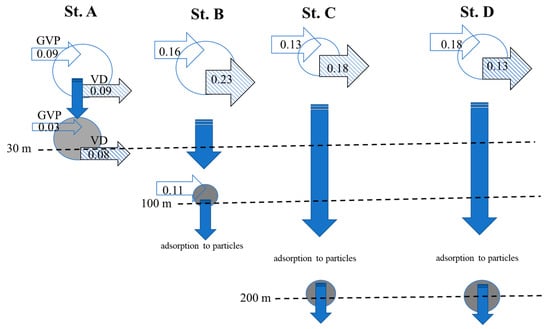
Figure 6.
Budgets for gross viral production (GVP, 105 viruses mL−1 h−1), viral decay (VD, 105 viruses mL−1 h−1), and functions of viral adsorption to particles in different regions of the southern East China Sea.
In summary, the high viral abundance observed in the surface waters of the East China Sea was due to high bacterial abundance. In order to determine the viral dynamics in marine environments, detailed analyses are required of the factors influencing viral production, decay, and viral balance. In this study, GVP and VD were not detectable at 200 m depth. The GVP or VD is not applicable in our case to explain the high VBR value at bottom of the euphotic zone. The mechanisms underlying VBR uncoupling, viral production, and viral loss in marine systems are still being investigated.
Author Contributions
Conceptualization, A.-Y.T.; methodology, A.-Y.T., P.W.-Y.C. and M.O.; validation, A.-Y.T.; formal analysis, P.W.-Y.C., A.-Y.T. and M.O.; investigation, A.-Y.T., P.W.-Y.C. and M.O.; resources, A.-Y.T.; data curation, A.-Y.T.; writing—original draft preparation, A.-Y.T. and P.W.-Y.C.; writing—review and editing, A.-Y.T. and V.M.; funding acquisition, A.-Y.T. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was conducted in the frame of the Russian state assignments No. 121040600178-6, 121121700354-9 (the program ‘Prioritet-2030’ of Sevastopol State University) and supported by RFBR project 21-55-52001, and the Ministry of Science and Technology, ROC (Taiwan), grant number NSC 109-2611-M-019-013 and MOST 111-2119-M-019-002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are provided in the main text.
Acknowledgments
We appreciate the language editing and helpful comments related to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Fuhrman, J.A.; Noble, R.T. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 1995, 40, 1236–1242. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Chiang, K.P.; Chang, J.; Gong, G.C. Seasonal diel variations of picoplankton and nanoplankton in a subtropical western Pacific coastal ecosystem. Limnol. Oceanogr. 2005, 50, 1221–1231. [Google Scholar] [CrossRef]
- Pasulka, A.L.; Samo, T.J.; Landry, M.R. Grazer and viral impacts on microbial growth and mortality in the southern California Current Ecosystem. J. Plankton Res. 2015, 37, 320–336. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Höfle, M.G. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 1998, 64, 431–438. [Google Scholar] [CrossRef]
- Lymer, D.; Lindstrom, E.S.; Vrede, K. Changing importance of viral induced bacterial mortality in lakes along gradients in trophic status and humic content. Freshw. Biol. 2008, 53, 1101–1113. [Google Scholar] [CrossRef]
- Taira, Y.; Uchimiya, M.; Kudo, I. Simultaneous estimation of viral lysis and protozoan grazing on bacterial mortality using a modified virus-dilution method. Mar. Ecol. Prog. Ser. 2009, 379, 23–32. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Chao, C.F. Contribution of viral lysis and nanoflagellate grazing to bacterial mortality at surface waters and deeper depths in the coastal ecosystem of subtropical Western Pacific. Estuar. Coasts 2016, 39, 1357–1366. [Google Scholar] [CrossRef]
- Tsai, A.Y. Effects of bacteria-virus interaction on Synechococcus spp. growth in the coastal waters. Terr. Atmo. Ocean. Sci. 2020, 31, 691–696. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Weinbauer, M.; Chen, B.; Jiao, N. Viral dynamics in the surface water of the western South China Sea in summer 2007. Aquat. Microb. Ecol. 2011, 63, 145–160. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Rassoulzadegan, F. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 2004, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Dell’Anno, A.; Corinaldesi, C.; Magagnini, M.; Noble, R.; Tamburini, C.; Weinbauer, M. Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 2008, 454, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Middelboe, M. Viral lysis of Phaeocystis pouchetii: Implications for algal population dynamics and heterotrophic C, N and P cycling. ISME J. 2009, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Shelford, E.J.; Middelboe, M.; Moller, E.F.; Suttle, C.A. Virus-driven nitrogen cycling enhances phytoplankton growth. Aquat. Microb. Ecol. 2012, 66, 41–46. [Google Scholar] [CrossRef]
- Salter, I.; Böttjet, D.; Christaki, U. The effect of inorganic particle concentration on bacteria–virus–nanoflagellate dynamics. Environ. Microbiol. 2011, 13, 2768–2777. [Google Scholar] [CrossRef]
- Tsai, A.-Y.; Lin, Y.-T.; Gong, G.-C. Effect of the presence of Virus-like Particles on Bacterial Growth in Sunlit Surface and Dark Deep Ocean Environments in the Southern East China Sea. Water 2021, 13, 2934. [Google Scholar] [CrossRef]
- Gobler, C.J.; Davis, T.W.; Deonarine, S.N.; Saxton, M.A.; Lavrentyev, P.J.; Jochem, F.J.; Wilhelm, S.W. Grazing and virus induced mortality of microbial populations before and during the onset of annual hypoxia in Lake Erie. Aquat. Microb. Ecol. 2008, 51, 117–128. [Google Scholar] [CrossRef]
- Colombet, J.; Sime-Ngando, T.; Cauchie, H.M.; Fonty, G.; Hoffmann, L.; Demeure, G. Depth-related gradients of viral activity in Lake Pavin. Appl. Environ. Microbiol. 2006, 72, 4440–4445. [Google Scholar] [CrossRef]
- He, L.; Yin, K.; Yuan, X.; Li, D.; Zhang, D.; Harrison, P.J. Spatial distribution of viruses, bacteria and chlorophyll in the northern South China Sea. Aquat. Microb. Ecol. 2009, 54, 153–162. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Alonso, M.C.; Jimenez-Gomez, F.; Rodriguez, J.; Borrego, J.J. Distribution of virus-like particles in an oligotrophic marine environment (Alboran Sea, western Mediterranean). Microb. Ecol. 2001, 42, 407–415. [Google Scholar] [CrossRef]
- Culley, A.I.; Welschmeyer, N.A. The abundance, distribution, and correlation of viruses, phytoplankton, and prokaryotes along a Pacific Ocean transect. Limnol. Oceanogr. 2002, 47, 1508–1513. [Google Scholar] [CrossRef]
- Jiang, S.C.; Paul, J.H. Seasonal and diel abundance of viruses and the occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar. Ecol. Prog. Ser. 1994, 104, 163–172. [Google Scholar] [CrossRef]
- Maranger, R.; Bird, D.F. Viral abundances in aquatic systems: A comparison between marine and fresh waters. Mar. Ecol. Prog. Ser. 1995, 121, 217–226. [Google Scholar] [CrossRef]
- Taylor, G.T.; Hein, C.; Iabichella, M. Temporal variations in viral distributions in the anoxic Cariaco Basin. Aquat. Microb. Ecol. 2003, 30, 103–116. [Google Scholar] [CrossRef]
- Magiopoulos, I.; Pitta, P. Viruses in a deep oligotrophic sea: Seasonal distribution of marine viruses in the epi-, meso- and bathypelagic waters of the Eastern Mediterranean Sea. Deep Sea Res. Part I 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Yager, P.L.; Connelly, T.L.; Mortazavi, B.; Wommack, K.E.; Bano, N.; Bauer, J.E.; Opsahl, S.; Hollibaugh, J.T. Dynamic bacterial and viral response to an algal bloom at subzero temperatures. Limnol. Oceanogr. 2001, 46, 790–801. [Google Scholar] [CrossRef]
- Clasen, J.L.; Brigden, S.M.; Payet, J.P.; Suttle, C.A. Evidence that viral abundance across oceans and lakes is driven by different biological factors. Freshw. Biol. 2008, 53, 1090–1100. [Google Scholar] [CrossRef]
- Parada, V.; Sintes, E.; Van Aken, H.M.; Weinbauer, M.G.; Herndl, N.J. Viral abundance, decay, and diversity in the meso- and bathypelagic waters of the north atlantic. Appl. Environ. Microbiol. 2007, 73, 4429–4438. [Google Scholar] [CrossRef]
- Wei, W.; Chen, X.; Weinbauer, M.G.; Jiao, N.; Zhang, R. Reduced bacterial mortality and enhanced viral productivity during sinking in the ocean. ISME J. 2022, 16, 1668–1675. [Google Scholar] [CrossRef]
- Mei, M.L.; Danovaro, R. Virus production and life strategies in aquatic sediments. Limnol. Oceanogr. 2004, 49, 459–470. [Google Scholar] [CrossRef]
- Gong, G.C.; Shiah, F.K.; Liu, K.K.; Wen, Y.H.; Liang, M.H. Spatial and temporal variation of chlorophyll a, primary productivity and chemical hydrography in the southern East China Sea. Cont. Shelf. Res. 2000, 20, 411–436. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 2004, 70, 1506–1513. [Google Scholar] [CrossRef]
- Hammes, F.; Egli, T. Cytometric methods for measuring bacteria in water: Advantages, pitfalls and applications. Anal. Bioanal. Chem. 2010, 397, 1083–1095. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Bridgen, S.M.; Suttle, C.A. A dilution technique for the direct measurement of viral production: A comparison in stratified and tidally mixed coastal waters. Microb. Ecol. 2002, 43, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.T.; Fuhrman, J.A. Viral decay and its causes in coastal waters. Appl. Environ. Microbiol. 1997, 63, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.L.; Tseng, Y.H.; Jan, S. The formation and dynamics of the cold-dome off northeastern Taiwan. J. Mar. Syst. 2011, 86, 10–27. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Auguet, J.C.; Montanié, H.; Delmas, D.; Hartmann, H.J.; Huet, V. Dynamic of virioplankton abundance and its environmental control in the Charente Estuary (France). Microb. Ecol. 2005, 50, 337–349. [Google Scholar] [CrossRef]
- Boehme, J.; Frischer, M.E.; Jiang, S.C.; Kellogg, C.A.; Pichard, S.; Rose, J.B.; Steinway, C.; Paul, J.H. Viruses, bacterioplankton, and phytoplankton in the southeastern Gulf of Mexico: Distribution and contribution to oceanic DNA pools. Mar. Ecol. Prog. Ser. 1993, 97, 1–10. [Google Scholar] [CrossRef]
- Wilcox, R.M.; Fuhrman, J.A. Bacterial viruses in coastal seawater: Lytic rather than lysogenic production. Mar. Ecol. Prog. Ser. 1994, 114, 35–45. [Google Scholar] [CrossRef]
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; Lennon, J.T.; Middelboe, M.; Suttle, C.A.; Stock, C. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016, 1, 15024. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.D.; Wilhelm, S.W.; Thingstad, F.; Weinbauer, M.G.; Bratbak, G.; Heldal, M.; Kimmance, S.A.; Middelboe, M.; Nagasaki, K.; Schroeder, D.C.; et al. Global-scale processes with a nanoscale drive: The role of marine viruses. ISME J. 2008, 2, 575–578. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Matteson, A.R. Freshwater and marine virioplankton: A brief overview of commonalities and differences. Freshw. Biol. 2008, 53, 1076–1089. [Google Scholar] [CrossRef]
- Thomas, R.; Berdjeb, L.; Sime-Ngando, T.; Jacquet, S. Viral abundance, production, decay rates and life strategies (lysogeny versus lysis) in Lake Bourget (France). Environ. Microbiol. 2011, 13, 616–630. [Google Scholar] [CrossRef]
- Bongiorni, L.; Magagnini, M.; Armeni, M.; Noble, R.; Danovaro, R. Viral production, decay rates, and life strategies along a trophic gradient in the north Adriatic sea. Appl. Environ. Microbiol. 2005, 71, 6644–6650. [Google Scholar] [CrossRef]
- Steward, G.F.; Wikner, J.; Cochlan, W.P.; Smith, D.C.; Azam, F. Estimation of virus production in the sea: II. Field results. Mar. Microb. Food Webs 1992, 6, 79–90. [Google Scholar]
- Weinbauer, M.G.; Fuks, D.; Peduzzi, P. Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl. Environ. Microbiol. 1993, 59, 4074–4082. [Google Scholar] [CrossRef]
- Holmfeldt, K.; Titelman, J.; Riemann, L. Virus productive and lysate recycling in different sub-basins of the Northern Baltic Sea. Microb. Ecol. 2010, 60, 572–580. [Google Scholar] [CrossRef]
- Suttle, C.A.; Chen, F. Mechanisms and rates of decay of manne viruses in seawater. Appl. Environ. Mlcrobiol. 1992, 58, 3721–3729. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Weinbauer, M.G.; Suttle, C.A.; Jeffrey, W.H. The role of sunlight in the removal and repair of viruses in the sea. Limnol. Oceanogr. 1998, 43, 586–592. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Chan, A.M.; Suttle, C.A. Viruses causing lysis of the toxic bloom-forming alga, Heterosigma akashiwo (Raphidophyceae), are widespread in coastal sediments of British Columbia, Canada. Limnol. Oceanogr. 2002, 47, 545–550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).