Crab Species-Specific Excavation and Architecture of Burrows in Restored Mangrove Habitat
Abstract
1. Introduction
2. Materials and Methods
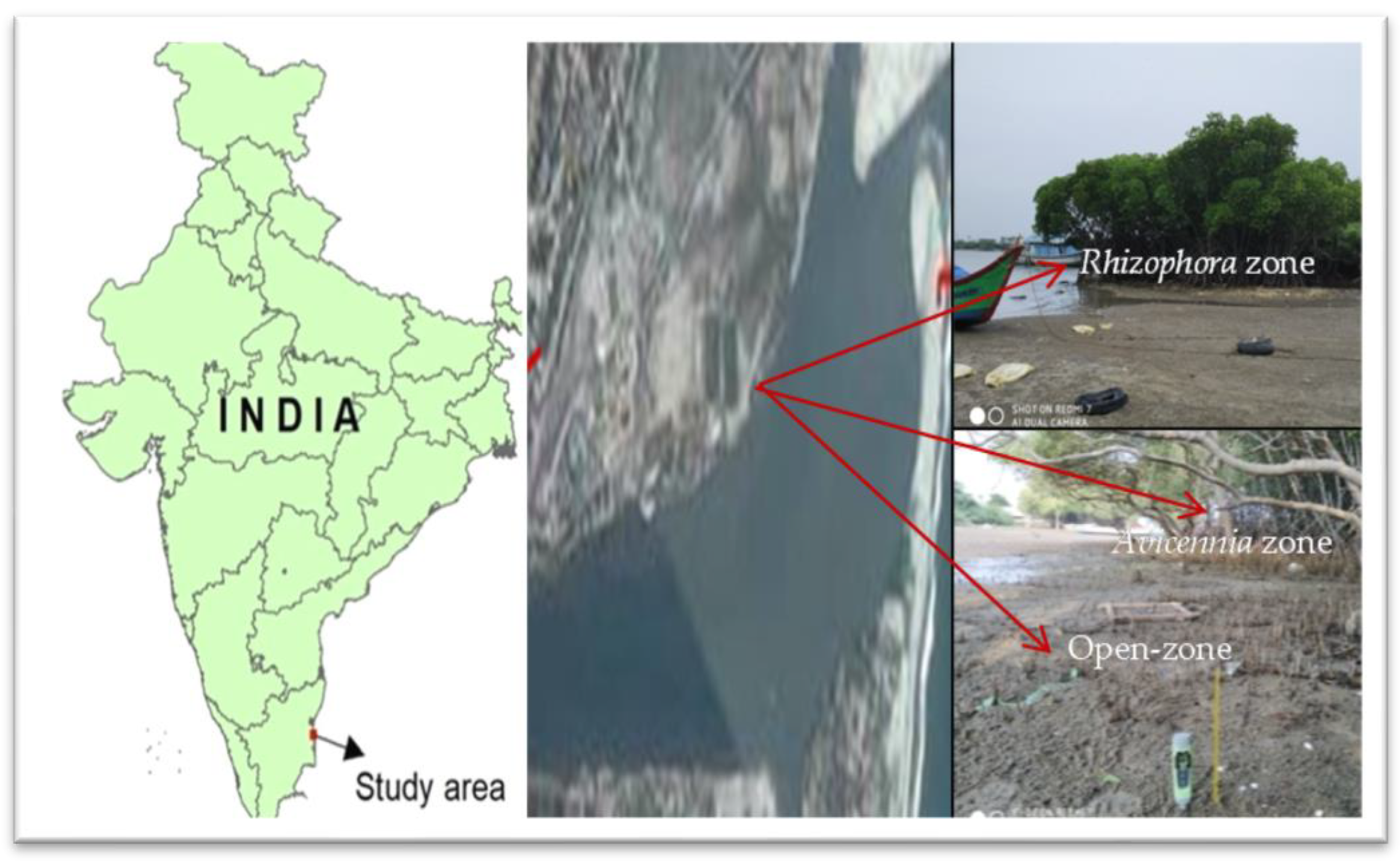
2.1. Study Area
2.2. Sampling and Analyses
3. Results
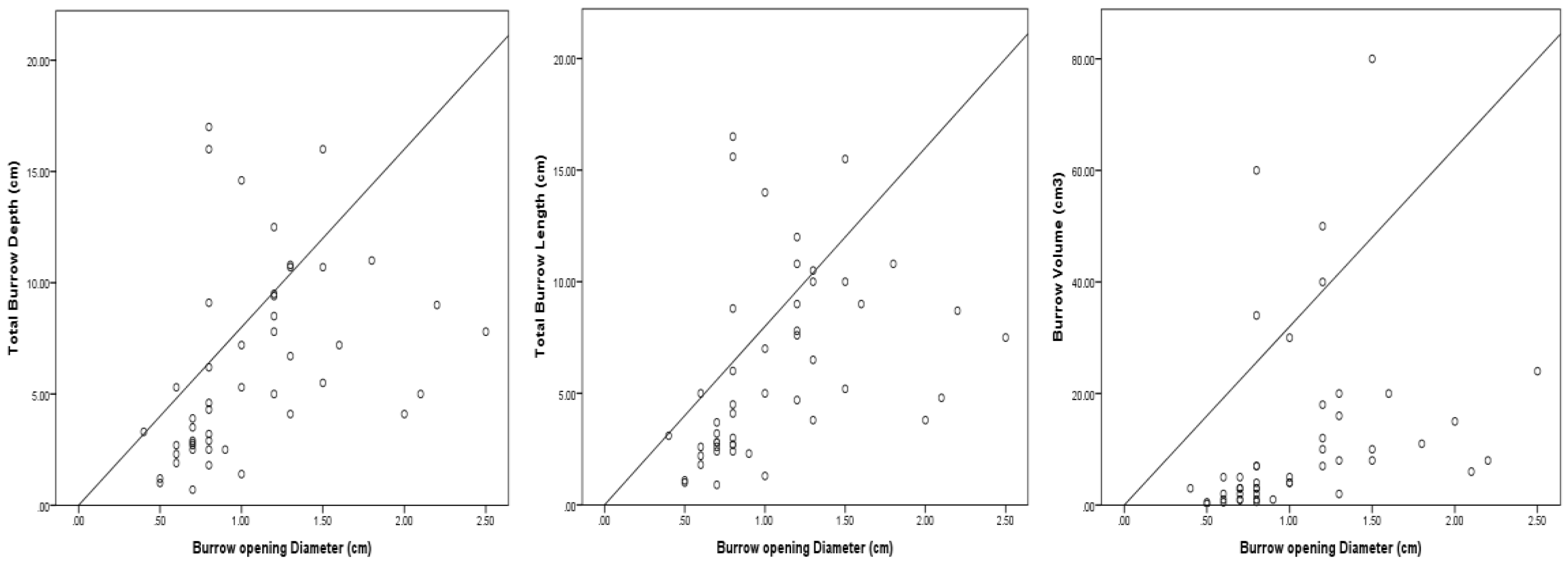
3.1. Burrow Types
3.2. Size of Crab Species
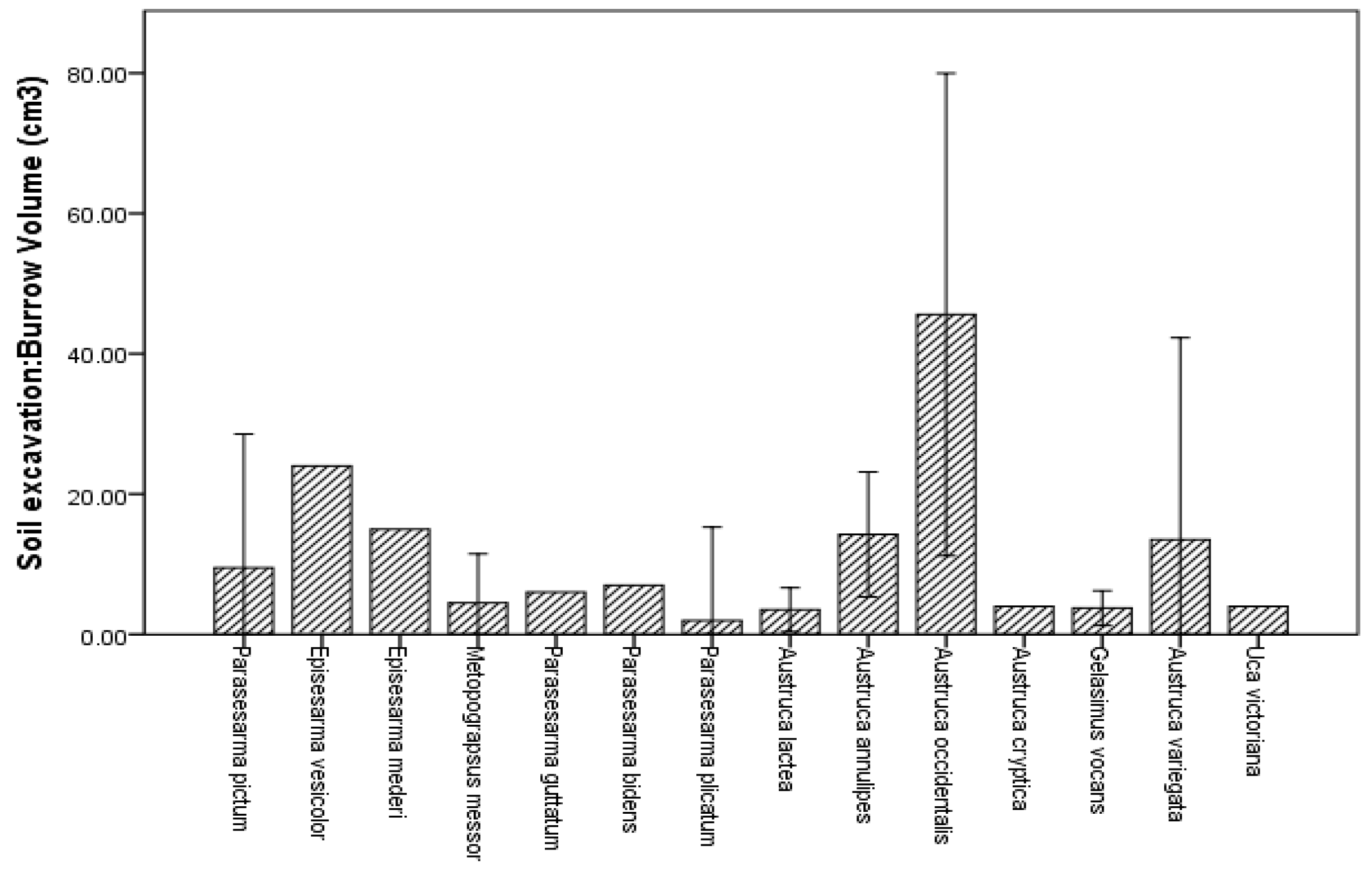
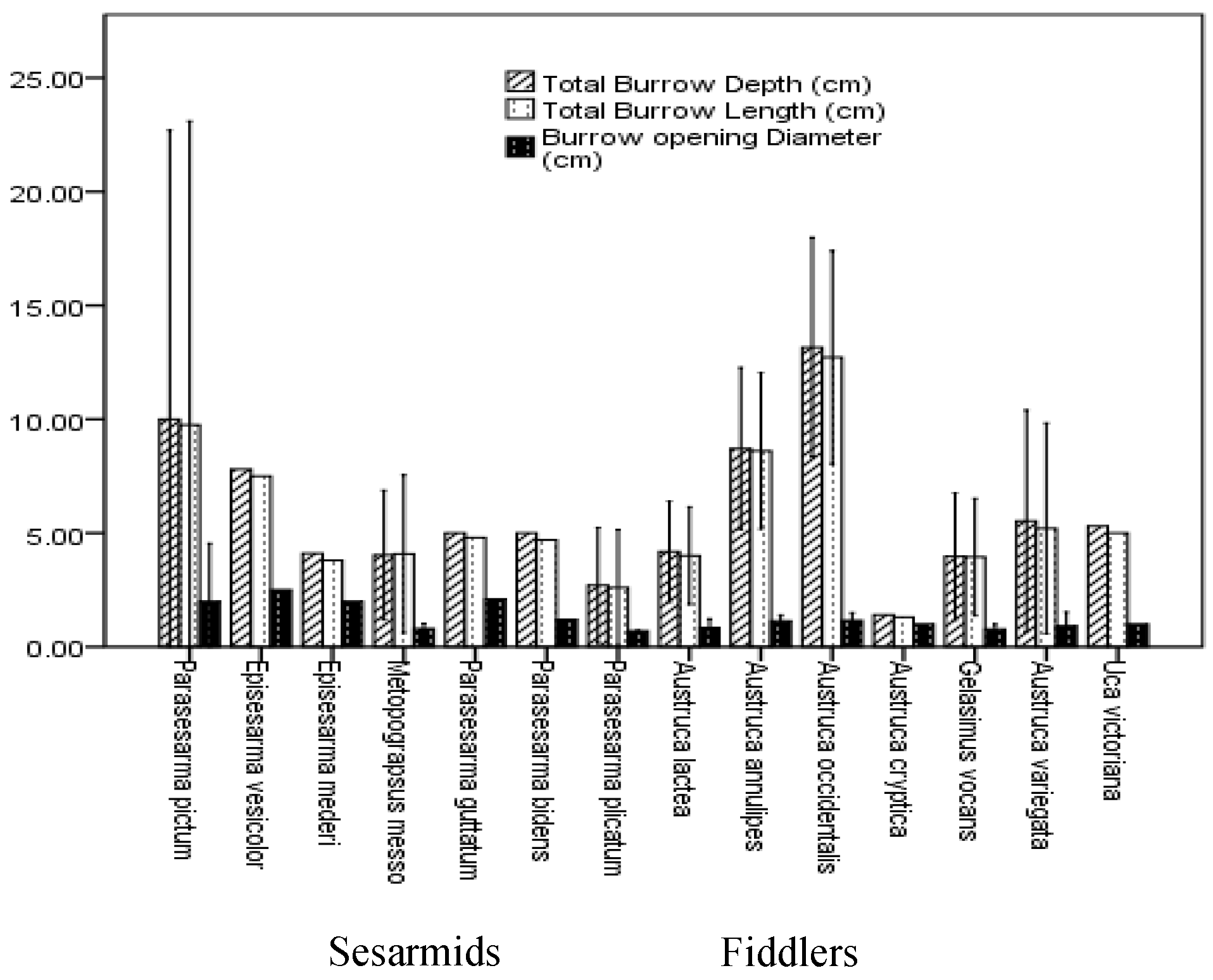
3.3. Burrow Architecture of Crab Species
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, T.J.; Boto, K.G.; Frusher, S.D.; Giddins, R.L. Keystone species and mangrove forest dynamics: The influence of burrowing by crabs on soil nutrient status and forest productivity. Estuar. Coast. Shelf Sci. 1991, 33, 419–432. [Google Scholar] [CrossRef]
- Lee, S.Y. Ecological role of grapsid crabs in mangrove ecosystems: A review. Mar. Freshw. Res. 1998, 49, 335–343. [Google Scholar] [CrossRef]
- Sarker, S.; Md Masud-Ul-Alam; Hossain, M.S.; Chowdhury, S.R.; Sharifuzzaman, S.M. A review of bioturbation and sediment organic geochemistry in mangroves. Geol. J. 2020, 56, 2439–2450. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Verniert, M.; Cannici, S.; Kairo, J.G.; Track, J.F.; Koedam, M. An exploratory study on grapsid crab zonation in Kenyan mangroves. Wetl. Ecol. Manag. 2002, 10, 179–187. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; DahdouhGuebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Penha-Lopes, G.; Bartolini, F.; Limbu, S.; Cannicci, S.; Kristensen, E.; Paula, J. Are fiddler crabs potentially useful ecosystem engineers in mangrove wastewater wetlands? Mar. Pollut. Bull. 2009, 58, 1694–1703. [Google Scholar] [CrossRef]
- Morrisey, D.J.; DeWitt, T.H.; Roper, D.S.; Williamson, R.B. Variation in the depth and morphology of burrows of the mud crab Helice crassa among different types of intertidal sediment in New Zealand. Mar. Ecol. Prog. Ser. 1999, 182, 231–242. [Google Scholar] [CrossRef]
- Lim, S.S.L.; Diong, C.H. Burrow-morphological characters of the fiddler crab, Uca annulipes (H. Milne Edwards, 1837) and ecological correlates in a lagoonal beach on Pulau Hantu. Crustaceana 2003, 76, 1055–1069. [Google Scholar] [CrossRef]
- Lim, S.S.L. Fiddler crab burrow morphology: How do burrow dimensions and bioturbative activities compare in sympatric populations of Uca vocans (Linnaeus, 1758) and U. annulipes (H. Milne Edwards, 1837). Crustaceana 2006, 79, 525–540. [Google Scholar] [CrossRef]
- Sen, S.; Homechaudhuri, S. Comparative Burrow Architectures of Resident Fiddler Crabs (Ocypodidae) in Indian Sundarban Mangroves to Assess Their Suitability as Bioturbating Agents. Proc. Zool. Soc. 2016, 71, 17–24. [Google Scholar] [CrossRef]
- Kristensen, E.; Alongi, D.M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 2006, 51, 1557–1571. [Google Scholar] [CrossRef]
- Machado, G.B.O.; Gusmao, J.; Joao, B.L.; Costa, T.M. Burrow morphology of Ucauruguayensis and Ucaleptodactylus (Decapoda: Ocypodidae) from a subtropical mangrove forest in the western Atlantic. Integr. Zool. 2013, 8, 307–314. [Google Scholar] [CrossRef]
- Agusto, L.E.; Sara, F.; Jimenez, P.J.; Quadros, A.; Cannicci, S. Structural characteristics of crab burrows in Hong Kong mangrove forests and their role in ecosystem engineering. Estuar. Coast. Shelf Sci. 2020, 248, 106973. [Google Scholar] [CrossRef]
- Kathirean, K.; Bingham, B.L. Biology of mangroves & mangrove ecosystem. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Kathiresan, K.; Qasim, S.Z. Biodiversity of Mangrove Ecosystems; Hindustan Publishing Corporation: New Delhi, India, 2005; 251p. [Google Scholar]
- Li, W.; Cui, L.; Zhang, M.; Wang, Y.; Zhang, Y.; Lei, Y.; Zhao, X. Effect of mangrove restoration on crab burrow density in Luoyangjiang Estuary, China. For. Ecosyst. 2015, 2, 21. [Google Scholar] [CrossRef]
- Ajmal Khan, S.; Raffi, S.M.; Lyla, P.S. Brachyuran crab diversity in natural (Pitchavaram) and artificially developed mangroves (Vellar estuary). Curr. Sci. 2005, 88, 1316–1324. [Google Scholar]
- Warburg, M.R.; Shuchman, E. Experimental studies on burrowing of Ocypode cursor L. (Crustacea; Ocypodidae) in response to sandmoisture. Mar. Behav. Physiol. 1978, 6, 147–156. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Kamanu, C.P. Burrowing in the East African mangrove crab, Chiromantes ortmanni (Crosnier, 1965) (Decapoda, Brachyura, Sesarmidae). Crustaceana 2005, 78, 1273–1275. [Google Scholar]
- Thongtham, N.; Kristensen, E. Physical and chemical characteristics of mangrove crab (Neoepisesarma versicolor) burrows in the Bangrong mangrove forest, Phuket, Thailand; with emphasis on behavioural response to changing environmental conditions. Vie Milieu 2003, 53, 141–151. [Google Scholar]
- Wang, J.; Bertness, M.D.; Li, B.; Chen, J.; Lü, W. Plant effects on burrowing crab morphology in a Chinese salt marsh: Native vs. exotic plants. Ecol. Eng. 2015, 74, 376–384. [Google Scholar] [CrossRef]
- Micheli, F.; Gherardi, F.; Vannini, M. Feeding and burrowing ecology of two East African mangrove crabs. Mar. Biol. 1991, 111, 247–254. [Google Scholar] [CrossRef]
- Stieglitz, T.; Ridd, P.; Müller, P. Passive irrigation and functional morphology of crustacean burrows in a tropical mangrove swamp. Hydrobiologia 2000, 421, 69–76. [Google Scholar] [CrossRef]
- De, C. Descriptive Ichnology. In Mangrove Ichnology of the Bay of Bengal Coast, Eastern India; Springer International Publishing: Cham, Switzerland, 2019; pp. 49–158. [Google Scholar] [CrossRef]
- Al-Khayat, J.A.; Giraldes, B.W. Burrowing crabs in arid mangrove forests on the southwestern Arabian Gulf: Ecological and biogeographical considerations. Reg. Stud. Mar. Sci. 2020, 39, 101416. [Google Scholar] [CrossRef]



| Species Name | Season of Analysis | Intertidal Zone | Burrow Shape | Burrow Structural Complexity Ratio (TBL/TBD) | |
|---|---|---|---|---|---|
| 1 | Metopograpsus messor | Monsoon | Rhizophora | L | 0.93 |
| Metopograpsus messor | Summer | Avicennia | J | 1.14 | |
| Metopograpsus messor | Monsoon | Rhizophora | I | 0.93 | |
| Metopograpsus messor | Monsoon | Rhizophora | CS | 0.91 | |
| M. messor | Monsoon | Rhizophora | C | 0.94 | |
| M. messor | Post-monsoon | Avicennia | CL | 0.96 | |
| Mean | L, J. I, CS, C, CL | 0.97 | |||
| 2 | Parasesarma guttatum | Monsoon | Rhizophora | L | 0.96 |
| 3 | Parasesarma plicatum | Post-monsoon | Avicennia | L | 0.97 |
| P.plicatum | Summer | Avicennia | J | 0.96 | |
| Mean | L, J | 0.97 | |||
| 4 | Austruca occidentalis | Monsoon | Avicennia | L | 0.97 |
| A. occidentalis | Monsoon | Avicennia | L | 0.96 | |
| A. occidentalis | Monsoon | Open | Y | 0.96 | |
| A. occidentalis | Monsoon | Open | JU | 0.98 | |
| A. occidentalis | Monsoon | Open | X | 0.97 | |
| Mean | L, Y, JU, X | 0.97 | |||
| 5 | A. lactea | Monsoon | Avicennia | L | 0.97 |
| A. lactea | Monsoon | Avicennia | L | 0.94 | |
| A. lactea | Monsoon | Avicennia | I | 0.94 | |
| A. lactea | Post-monsoon | Avicennia | I | 0.95 | |
| A. lactea | Post-monsoon | Avicennia | I | 0.96 | |
| A. lactea | Summer | Avicennia | I | 0.96 | |
| Mean | L, I | 0.95 | |||
| 6 | Uca victoriana | Monsoon | Avicennia | L | 0.94 |
| 7 | Austruca variegata | Monsoon | Rhizophora | J | 0.92 |
| A. variegata | Monsoon | Avicennia | J | 0.97 | |
| A. variegata | Post-monsoon | Rhizophora | J | 0.92 | |
| A. variegata | Monsoon | Rhizophora | I | 0.94 | |
| Mean | I, J | 0.94 | |||
| 8 | Parasesarma pictum | Summer | Avicennia | J | 0.98 |
| Parasesarma pictum | Monsoon | Avicennia | LL | 0.97 | |
| Mean | J, LL | 0.98 | |||
| 9 | Gelasimus vocans | Post-monsoon | Avicennia | J | 0.92 |
| Gelasimus vocans | Summer | Avicennia | J | 1.03 | |
| Gelasimus vocans | Monsoon | Avicennia | I | 0.93 | |
| Gelasimus vocans | Monsoon | Avicennia | I | 0.95 | |
| Gelasimus vocans | Monsoon | Open | I | 0.98 | |
| Gelasimus vocans | Post-monsoon | Open | I | 1 | |
| Gelasimus vocans | Summer | Open | I | 1.29 | |
| Gelasimus vocans | Monsoon | Avicennia | S | 0.97 | |
| Gelasimus vocans | Summer | Avicennia | V | 1.5 | |
| Mean | J, I, S, V | 1.06 | |||
| 10 | Austruca annulipes | Summer | Avicennia | J | 0.97 |
| A.annulipes | Monsoon | Avicennia | I | 0.95 | |
| A. annulipes | Monsoon | Avicennia | I | 0.93 | |
| A. annulipes | Monsoon | Avicennia | I | 0.97 | |
| A. annulipes | Monsoon | Avicennia | I | 0.96 | |
| A. annulipes | Monsoon | Avicennia | I | 0.93 | |
| Austruca annulipes | Summer | Avicennia | Y | 1.25 | |
| A. annulipes | Monsoon | Open | LL | 0.97 | |
| Mean | J, I, Y, LL | 0.99 | |||
| 11 | A. cryptica | Summer | Avicennia | I | 0.93 |
| 12 | Episesarma vesicolor | Summer | Rhizophora | Y | 0.96 |
| 13 | Episesarma mederi | Post-monsoon | Rhizophora | V | 0.93 |
| 14 | Parasesarma bidens | Monsoon | Rhizophora | U | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, W.W.; Kandasamy, K.; Balakrishnan, B. Crab Species-Specific Excavation and Architecture of Burrows in Restored Mangrove Habitat. J. Mar. Sci. Eng. 2023, 11, 310. https://doi.org/10.3390/jmse11020310
Min WW, Kandasamy K, Balakrishnan B. Crab Species-Specific Excavation and Architecture of Burrows in Restored Mangrove Habitat. Journal of Marine Science and Engineering. 2023; 11(2):310. https://doi.org/10.3390/jmse11020310
Chicago/Turabian StyleMin, Wah Wah, Kathiresan Kandasamy, and Balasubramaniyan Balakrishnan. 2023. "Crab Species-Specific Excavation and Architecture of Burrows in Restored Mangrove Habitat" Journal of Marine Science and Engineering 11, no. 2: 310. https://doi.org/10.3390/jmse11020310
APA StyleMin, W. W., Kandasamy, K., & Balakrishnan, B. (2023). Crab Species-Specific Excavation and Architecture of Burrows in Restored Mangrove Habitat. Journal of Marine Science and Engineering, 11(2), 310. https://doi.org/10.3390/jmse11020310







