Modeling Soil Organic Carbon at Coastal Sabkhas with Different Vegetation Covers at the Red Sea Coast of Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Soil Sampling, Analyses, and Functions
2.3. Modeling and Validation Indices
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil Analyses
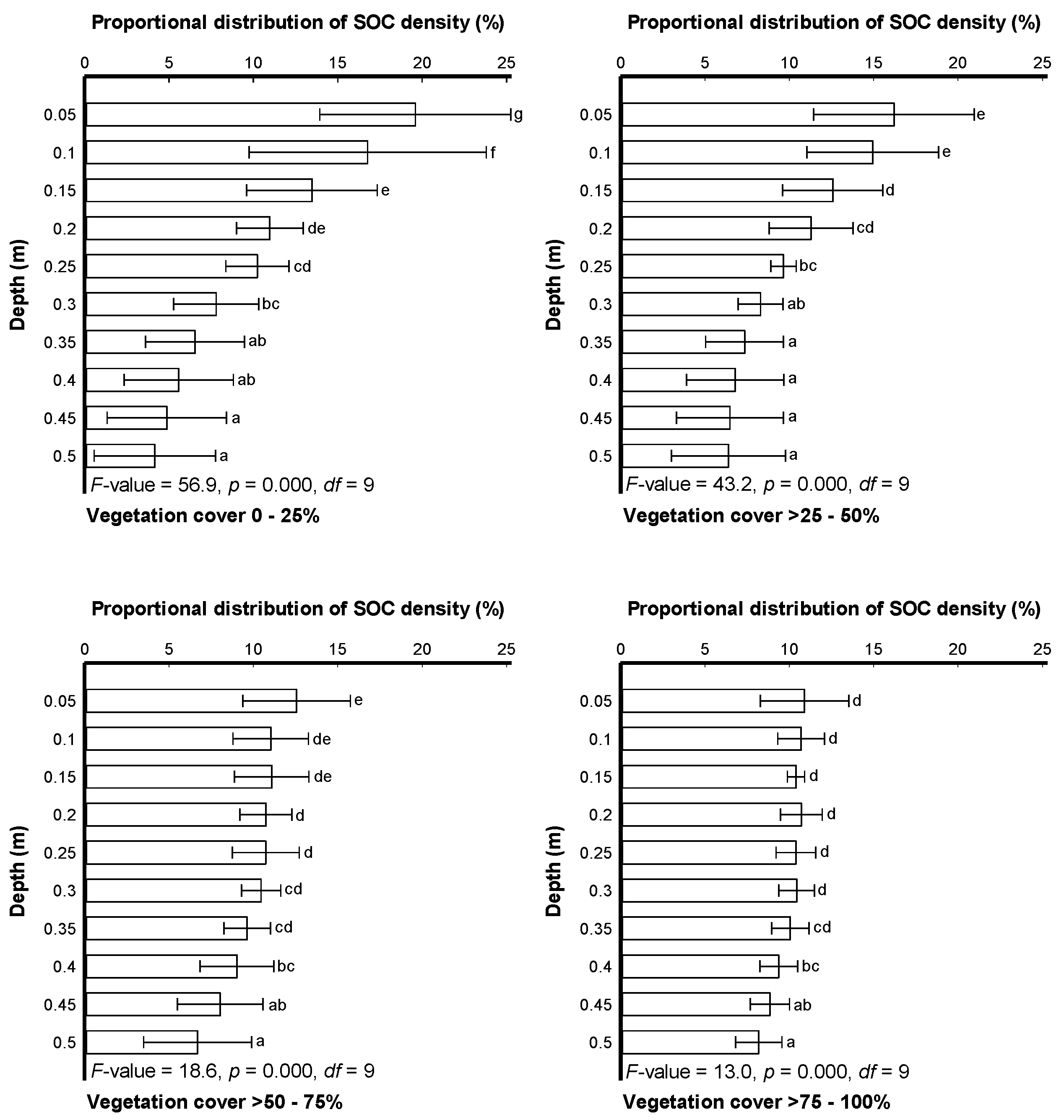
3.2. Modeling Depth Distribution of SOC Density and Stocks among Different Vegetation Covers
3.3. Prediction and Validation of SOC Density and Stocks among Different Vegetation Cover Classes
3.4. Soil Organic Carbon—Top versus Bottom Soil Profile (TCFs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giesler, R.J.; Cottier-Cook, E.J. Marine and Coastal Ecosystems. In Routledge Handbook of Biosecurity and Invasive Species; Barker, K., Francis, R.A., Eds.; Routledge: Abingdon, UK, 2021; pp. 142–160. [Google Scholar]
- Ayyam, V.; Palanivel, S.; Chandrakasan, S. Coastal Ecosystems and Services; Springer-Verlag Singapore Pte Ltd: Singapore, 2019; pp. 21–47. [Google Scholar]
- van den Belt, M.; Forgie, V.; Farley, J. Valuation of Coastal Ecosystem Services; Elsevier Academic Press Inc: San Diego, CA, USA, 2011; pp. 35–54. [Google Scholar]
- Joseph, A. The Protective Role of Coastal Ecosystems; Elsevier Academic Press Inc: San Diego, CA, USA, 2011; pp. 103–108. [Google Scholar]
- Burkett, V.; Codignotto, J.O.; Forbes, D.L.; Mimura, N.; Beamish, R.J.; Ittekkot, V. Coastal Zones and Marine Ecosystems; Cambridge University Press: Cambridge, UK, 2001; pp. 343–379. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Keshta, A.E.; Shaltout, K.H.; Baldwin, A.H.; El-Din, A.A.S. Sediment clays are trapping heavy metals in urban lakes: An indicator for severe industrial and agricultural influence on coastal wetlands at the Mediterranean coast of Egypt. Mar. Pollut. Bull. 2020, 151, 110816. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Falyouna, O.; Malloum, A.; Othmani, A.; Bornman, C.; Bedair, H.; Onyeaka, H.; Al-Sharify, Z.T.; Ajala, O.J.; Miri, T.; et al. A general review on the use of advance oxidation and adsorption processes for the removal of furfural from industrial effluents. Microporous Mesoporous Mat. 2022, 331, 14. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 12. [Google Scholar] [CrossRef]
- Kuwae, T.; Kanda, J.; Kubo, A.; Nakajima, F.; Ogawa, H.; Sohma, A.; Suzumura, M. Blue carbon in human-dominated estuarine and shallow coastal systems. Ambio 2016, 45, 290–301. [Google Scholar] [CrossRef]
- Van de Broek, M.; Temmerman, S.; Merckx, R.; Govers, G. Controls on soil organic carbon stocks in tidal marshes along an estuarine salinity gradient. Biogeosciences 2016, 13, 6611–6624. [Google Scholar] [CrossRef]
- Keshta, A.E.; Riter, J.C.A.; Shaltout, K.H.; Baldwin, A.H.; Kearney, M.; Sharaf El-Din, A.; Eid, E.M. Loss of Coastal Wetlands in Lake Burullus, Egypt: A GIS and Remote-Sensing Study. Sustainability 2022, 14, 4980. [Google Scholar] [CrossRef]
- Hilmi, N.; Chami, R.; Sutherland, M.D.; Hall-Spencer, J.M.; Lebleu, L.; Benitez, M.B.; Levin, L.A. The role of blue carbon in climate change mitigation and carbon stock conservation. Front. Clim. 2021, 102, 710546. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.; Eid, E.M.; El-Morsy, M.H.; Osman, H.E.; Keshta, A.E. Benefits of Blue Carbon Stocks in a Coastal Jazan Ecosystem Undergoing Land Use Change. Wetlands 2022, 42, 103. [Google Scholar] [CrossRef]
- Loughland, R.A.; Qasem, A.M.; Burwell, B.; Prihartato, P.K. Coastal Sabkha (Salt Flats) of the Southern and Western Arabian Gulf. In The Wetland Book: II: Distribution, Description, and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer Netherlands: Dordrecht, Germany, 2018; pp. 1173–1183. [Google Scholar]
- Boer, B.; Clusener-Godt, M.; Gul, B.; Khan, M.A.; Hameed, A. Sabkha Ecosystems Vol VI: Asia-Pacific—Summarizing the Story. In Sabkha Ecosystems, Vol Vi: Asia/Pacific; Gul, B., Boer, B., Khan, M.A., ClusenerGodt, M., Hameed, A., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2019; Volume 49, pp. 505–507. [Google Scholar]
- Ghazanfar, S.A.; Boer, B.; Al Khulaidi, A.W.; El-Keblawy, A.; Alateeqi, S. Plants of Sabkha Ecosystems of the Arabian Peninsula. In Sabkha Ecosystems, Vol Vi: Asia/Pacific; Gul, B., Boer, B., Khan, M.A., ClusenerGodt, M., Hameed, A., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2019; Volume 49, pp. 55–80. [Google Scholar]
- Gul, B. Sabkha Ecosystems Volume VI: Asia/Pacific Preface. In Sabkha Ecosystems, Vol Vi: Asia/Pacific; Gul, B., Boer, B., Khan, M.A., ClusenerGodt, M., Hameed, A., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2019; Volume 49, pp. VII–VIII. [Google Scholar]
- Nair-Bedouelle, S. Sabkha Ecosystems Volume VI: Asia/Pacific Foreword. In Sabkha Ecosystems, Vol Vi: Asia/Pacific; Gul, B., Boer, B., Khan, M.A., ClusenerGodt, M., Hameed, A., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2019; Volume 49, pp. V–VI. [Google Scholar]
- Chenchouni, H. Edaphic factors controlling the distribution of inland halophytes in an ephemeral salt lake "Sabkha ecosystem" at North African semi-arid lands. Sci. Total Environ. 2017, 575, 660–671. [Google Scholar] [CrossRef]
- Evans, G.; Kirkham, A. The Abu Dhabi sabkhat: Distribution of sabkhat in the Arabian Peninsula and adjacent countries. In Sabkha Ecosystems; Barth, H.-J.B.B., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2002; p. 353. [Google Scholar]
- Eid, E.M.; Keshta, A.E.; Shaltout, K.H.; Baldwin, A.H.; El-Din, S.; Ahmed, A. Carbon sequestration potential of the five Mediterranean lakes of Egypt. Fundam. Appl. Limnol. Arch. Für Hydrobiol. 2017, 190, 87–96. [Google Scholar] [CrossRef]
- Keshta, A.E.; Yarwood, S.A.; Baldwin, A.H. A new in situ method showed greater persistence of added soil organic matter in natural than restored wetlands. Restor. Ecol. 2021, 11, e13437. [Google Scholar] [CrossRef]
- Keshta, A.E. Hydrology, Soil Redox, and Pore-Water Iron Regulate Carbon Cycling in Natural and Restored Tidal Freshwater Wetlands in the Chesapeake Bay, Maryland, USA. Ph.D. Thesis, University of Maryland at College Park, University of Maryland Libraries, College Park, MD, USA, 2017. [Google Scholar]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marba, N.; et al. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Khedher, K.M.; Ayed, H.; Arshad, M.; Moatamed, A.; Mouldi, A. Evaluation of carbon stock in the sediment of two mangrove species, Avicennia marina and Rhizophora mucronata, growing in the Farasan Islands, Saudi Arabia. Oceanologia 2020, 62, 200–213. [Google Scholar] [CrossRef]
- Eid, E.M.; Arshad, M.; Shaltout, K.H.; El-Sheikh, M.A.; Alfarhan, A.H.; Pico, Y.; Barcelo, D. Effect of the conversion of mangroves into shrimp farms on carbon stock in the sediment along the southern Red Sea coast, Saudi Arabia. Environ. Res. 2019, 176, 7. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, L.; Zhang, W.Q.; Zhang, S.P.; Wang, H.L.; Fu, X.H.; Le, Y.Q. Variability of soil carbon sequestration capability and microbial activity of different types of salt marsh soils at Chongming Dongtan. Ecol. Eng. 2010, 36, 1754–1760. [Google Scholar] [CrossRef]
- Patoine, G.; Eisenhauer, N.; Cesarz, S.; Phillips, H.R.P.; Xu, X.F.; Zhang, L.H.; Guerra, C.A. Drivers and trends of global soil microbial carbon over two decades. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Eid, E.M.; Arshad, M.; Alrumman, S.A.; Al-Bakre, D.A.; Ahmed, M.T.; Almahasheer, H.; Keshta, A.E. Evaluation of Soil Organic Carbon Stock in Coastal Sabkhas under Different Vegetation Covers. J. Mar. Sci. Eng. 2022, 10, 1234. [Google Scholar] [CrossRef]
- Bai, J.H.; Zhang, G.L.; Zhao, Q.Q.; Lu, Q.Q.; Jia, J.; Cui, B.S.; Liu, X.H. Depth-distribution patterns and control of soil organic carbon in coastal salt marshes with different plant covers. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Ahmed, M.T.; Alrumman, S.A.; Ahmed, D.A.; Eid, E.M. Evaluation of the carbon sequestration capacity of arid mangroves along nutrient availability and salinity gradients along the Red Sea coastline of Saudi Arabia. Oceanologia 2020, 62, 56–69. [Google Scholar] [CrossRef]
- Morley, N. The coastal waters of the Red Sea. Bull. Mar. Reserach Cent. 1975, 5, 8–19. [Google Scholar]
- Hickey, B.; Goudie, A. The use of TOMS and MODIS to identify dust storm source areas: The Tokar delta (Sudan) and the Seistan basin (south west Asia). Geomorphol. Var. 2007, 37, 57. [Google Scholar]
- Bruckner, A.; Rowlands, G.; Riegl, B.; Purkis, S.; Williams, A.; Renaud, P. Atlas of Saudi Arabian Red Sea Marine Habitats; Panoramic Press: Phoenix, AZ, USA, 2013. [Google Scholar]
- Nabhan, A.I.; Yang, W. Modern sedimentary facies, depositional environments, and major controlling processes on an arid siliciclastic coast, Al qahmah, SE Red Sea, Saudi Arabia. J. Afr. Earth Sci. 2018, 140, 9–28. [Google Scholar] [CrossRef]
- PME, Presidncy of Metrology and Environmnetal Protection. Surface Annual Climatological Report. Jizan, Presidency of Metrology and Environment Protection; National Meteorology and Environment Center, Saudi Arabia: Riyadh, Saudi Arabia, 2012. [Google Scholar]
- Liang, S.; Wang, J. Advanced Remote Sensing: Terrestrial Information Extraction and Applications; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Peet, R.K.; Wentworth, T.R.; White, P.S. A flexible, multipurpose method for recording vegetation composition and structure. Castanea 1998, 262–274. [Google Scholar]
- Eid, E.M.; Shaltout, K.H. Distribution of soil organic carbon in the mangrove Avicennia marina (Forssk.) Vierh. along the Egyptian Red Sea Coast. Reg. Stud. Mar. Sci. 2016, 3, 76–82. [Google Scholar] [CrossRef]
- Wilke, B.M. Determination of chemical and physical soil properties. In Manual of Soil Analysis—Monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Heidelberg, Germany, 2005; Volume 5, pp. 47–95. [Google Scholar]
- Craft, C.B.; Seneca, E.D.; Broome, S.W. Loss on Ignition and Kjeldahl Digestion for Estimating Organic-Carbon and Total Nitrogen in Estuarine Marsh Soils—Calibration with Dry Combustion. Estuaries 1991, 14, 175–179. [Google Scholar] [CrossRef]
- Han, F.; Hu, W.; Zheng, J.; Du, F.; Zhang, X. Estimating soil organic carbon storage and distribution in a catchment of Loess Plateau, China. Geoderma 2010, 154, 261–266. [Google Scholar] [CrossRef]
- Osman, H.E.M.; Elaidarous, A.A.; El-Morsy, M.H.; Eid, E.M.; Keshta, A.E. Soils with more clay and dense vegetation were rich in soil carbon along Wadi Al-Sharaea, Makkah, Saudi Arabia. Heliyon 2023, 9, e12988. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 2—Chemical and Microbiological Properities, 2nd ed.; Page, A., Miller, R., Keeney, D., Eds.; American Society of Agronomy, Inc. & Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 2, pp. 539–579. [Google Scholar]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil Bulk Density as related to Soil Texture, Organic Matter Content and available total Nutrients of Coimbatore Soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Meersmans, J.; De Ridder, F.; Canters, F.; De Baets, S.; Van Molle, M. A multiple regression approach to assess the spatial distribution of Soil Organic Carbon (SOC) at the regional scale (Flanders, Belgium). Geoderma 2008, 143, 1–13. [Google Scholar] [CrossRef]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Novotna, M.; Mikes, O.; Komprdova, K. Development and comparison of regression models for the uptake of metals into various field crops. Environ. Pollut. 2015, 207, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Amundson, R.; Gong, P.; Yu, Q. Quantity and spatial variability of soil carbon in the conterminous United States. Soil Sci. Soc. Am. J. 2006, 70, 590–600. [Google Scholar] [CrossRef]
- IBM SPSS. IBM SPSS Statistics for Windows, version 20.0; IBM Corp.: New York, NY, USA, 2015; Volume 440, p. 394. [Google Scholar]
- Ballantine, K.; Schneider, R. Fifty-five years of soil development in restored freshwater depressional wetlands. Ecol. Appl. 2009, 19, 1467–1480. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods 2nd Edition; Klute, A.E., Ed.; American Society of Agronomy—Soil Science Society of America: Madison, WI, USA, 1986; Volume Agronomy Monograph 9, pp. 363–382. [Google Scholar]
- Bryant, J.C.; Chabreck, R.H. Effects of impoundment on vertical accretion of coastal marsh. Estuaries 1998, 21, 416–422. [Google Scholar] [CrossRef]
- Drexler, J.Z.; Krauss, K.W.; Sasser, M.C.; Fuller, C.C.; Swarzenski, C.M.; Powell, A.; Swanson, K.M.; Orlando, J. A Long-Term Comparison of Carbon Sequestration Rates in Impounded and Naturally Tidal Freshwater Marshes Along the Lower Waccamaw River, South Carolina. Wetlands 2013, 33, 965–974. [Google Scholar] [CrossRef]
- Garbout, A.; Munkholm, L.J.; Hansen, S.B. Tillage effects on topsoil structural quality assessed using X-ray CT, soil cores and visual soil evaluation. Soil Tillage Res. 2013, 128, 104–109. [Google Scholar] [CrossRef]
- Keshta, A.; Koop-Jakobsen, K.; Titschack, J.; Mueller, P.; Jensen, K.; Baldwin, A.; Nolte, S. Ungrazed salt marsh has well connected soil pores and less dense sediment compared with grazed salt marsh: A CT scanning study. Estuar. Coast. Shelf Sci. 2020, 245, 106987. [Google Scholar] [CrossRef]
- Eid, E.M.; El-Bebany, A.F.; Alrumman, S.A. Distribution of soil organic carbon in the mangrove forests along the southern Saudi Arabian Red Sea coast. Rend. Lincei-Sci. Fis. E Nat. 2016, 27, 629–637. [Google Scholar] [CrossRef]
- Almahasheer, H.; Serrano, O.; Duarte, C.M.; Arias-Ortiz, A.; Masque, P.; Irigoien, X. Low Carbon sink capacity of Red Sea mangroves. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, J.P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef]
- Craft, C.; Broome, S.; Campbell, C. Fifteen years of vegetation and soil development after brackish-water marsh creation. Restor. Ecol. 2002, 10, 248–258. [Google Scholar] [CrossRef]
- Wang, J.J.; Bai, J.H.; Zhao, Q.Q.; Lu, Q.Q.; Xia, Z.J. Five-year changes in soil organic carbon and total nitrogen in coastal wetlands affected by flow-sediment regulation in a Chinese delta. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Craft, C. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes. Limnol. Oceanogr. 2007, 52, 1220–1230. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.Y.; Zhu, T.S.; Long, X.H.; Gao, X.M.; Liu, Z.P.; Shao, H.B.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 12. [Google Scholar] [CrossRef]
- Anacker, B.L.; Seastedt, T.R.; Halward, T.M.; Lezberg, A.L. Soil carbon and plant richness relationships differ among grassland types, disturbance history and plant functional groups. Oecologia 2021, 196, 1153–1166. [Google Scholar] [CrossRef]
- Wan, Q.Z.; Zhu, G.F.; Guo, H.W.; Zhang, Y.; Pan, H.X.; Yong, L.L.; Ma, H.Y. Influence of Vegetation Coverage and Climate Environment on Soil Organic Carbon in the Qilian Mountains. Sci. Rep. 2019, 9, 9. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Radabaugh, K.; Moyer, R.; Chappel, A.; Powell, C.E.; Bociu, I.; Clark, B.C.; Smoak, J.M. Coastal Blue Carbon Assessment of Mangroves, Salt Marshes, and Salt Barrens in Tampa Bay, Florida, USA. Estuaries Coasts 2018, 41, 1496–1510. [Google Scholar] [CrossRef]
- Ellison, J.C.; Beasy, K.M. Sediment Carbon Accumulation in Southern Latitude Saltmarsh Communities of Tasmania, Australia. Biology 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Perera, N.; Lokupitiya, E.; Halwatura, D.; Udagedara, S. Quantification of blue carbon in tropical salt marshes and their role in climate change mitigation. Sci. Total Environ. 2022, 820, 153313. [Google Scholar] [CrossRef] [PubMed]
- Minasny, B.; McBratney, A.B.; Mendonca-Santos, M.L.; Odeh, I.O.A.; Guyon, B. Prediction and digital mapping of soil carbon storage in the Lower Namoi Valley. Aust. J. Soil Res. 2006, 44, 233–244. [Google Scholar] [CrossRef]
- Mestdagh, I.; Lootens, P.; Van Cleemput, O.; Carlier, L. Soil organic carbon stocks in Flemish grasslands: How accurate are they? Grass Forage Sci. 2004, 59, 310–317. [Google Scholar] [CrossRef]
- Mikhailova, E.; Post, C. Organic carbon stocks in the Russian Chernozem. Eur. J. Soil Sci. 2006, 57, 330–336. [Google Scholar] [CrossRef]
- Yang, R.M.; Zhang, G.L.; Yang, F.; Zhi, J.J.; Liu, F.; Zhao, Y.G.; Li, D.C. Precise estimation of soil organic carbon stocks in the northeast Tibetan Plateau. Sci. Rep. 2016, 6, 21842. [Google Scholar] [CrossRef]
- Kempen, B.; Brus, D.J.; Stoorvogel, J.J. Three-dimensional mapping of soil organic matter content using soil type-specific depth functions. Geoderma 2011, 162, 107–123. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Vogt, D.J.; Siccama, T.G.; Beard, K.H. Detecting nutrient pool changes in rocky forest soils. Soil Sci. Soc. Am. J. 2003, 67, 1282–1286. [Google Scholar] [CrossRef]
- Bernoux, M.; Arrouays, D.; Cerri, C.C.; Bourennane, H. Modeling vertical distribution of carbon in oxisols of the western Brazilian Amazon (Rondonia). Soil Sci. 1998, 163, 941–951. [Google Scholar] [CrossRef]
- Hu, W.; Shao, M.A.; Wang, Q.J.; Fan, J.; Reichardt, K. Spatial variability of soil hydraulic properties on a steep slope in the Loess Plateau of China. Sci. Agric. 2008, 65, 268–276. [Google Scholar] [CrossRef]
- Malone, B.P.; McBratney, A.B.; Minasny, B.; Laslett, G.M. Mapping continuous depth functions of soil carbon storage and available water capacity. Geoderma 2009, 154, 138–152. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Hofman, G. Estimates of carbon stock changes in Belgian cropland. Soil Use Manag. 2003, 19, 166–171. [Google Scholar] [CrossRef]
- Chen, C.; Hu, K.L.; Li, H.; Yun, A.P.; Li, B.G. Three-Dimensional Mapping of Soil Organic Carbon by Combining Kriging Method with Profile Depth Function. PLoS ONE 2015, 10, 15. [Google Scholar] [CrossRef]
- Mishra, U.; Lal, R.; Slater, B.; Calhoun, F.; Liu, D.S.; Van Meirvenne, M. Predicting Soil Organic Carbon Stock Using Profile Depth Distribution Functions and Ordinary Kriging. Soil Sci. Soc. Am. J. 2009, 73, 614–621. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Keshta, A.E.; Shaltout, K.H.; Baldwin, A.H.; Sharaf El-Din, A.; Eid, E.M. Variation in Plant Community Composition and Biomass to Macro and Micronutrients and Salinity across Egypt’s Five Major Coastal Lakes. Sustainability 2022, 14, 6180. [Google Scholar]
- Shaltout, K.H.; El-Beheiry, M.A.; El-Kady, H.F.; Keshta, A.E. Distribution of the naturalized species Dalbergia sissoo Roxb. ex DC. in Nile Delta, Egypt. Taeckholmia 2011, 30, 59–79. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Keshta, A.E. The biology of Egyptian woody perennials 4. Dalbergia sissoo roxb. ex DC. Assiut Univ. Bull. Environ. Res. 2011, 14, 131–152. [Google Scholar]
- Veldkamp, E.; Becker, A.; Schwendenmann, L.; Clark, D.A.; Schulte-Bisping, H. Substantial labile carbon stocks and microbial activity in deeply weathered soils below a tropical wet forest. Glob. Change Biol. 2003, 9, 1171–1184. [Google Scholar] [CrossRef]
- Padbhushan, R.; Sharma, S.; Kumar, U.; Rana, D.S.; Kohli, A.; Kaviraj, M.; Parmar, B.; Kumar, R.; Annapurna, K.; Sinha, A.K.; et al. Meta-Analysis Approach to Measure the Effect of Integrated Nutrient Management on Crop Performance, Microbial Activity, and Carbon Stocks in Indian Soils. Front. Environ. Sci. 2021, 9, 1171–1184. [Google Scholar] [CrossRef]
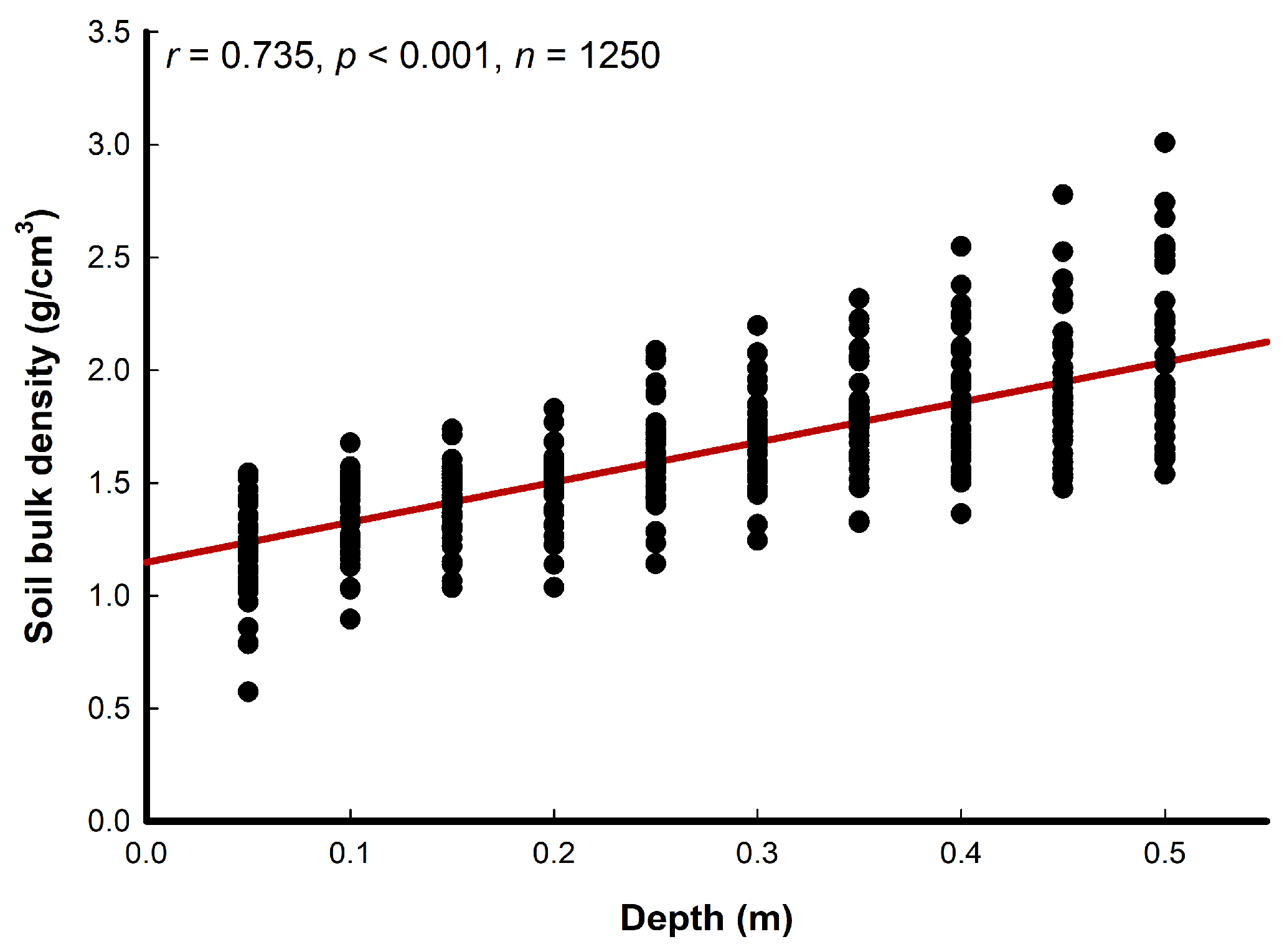
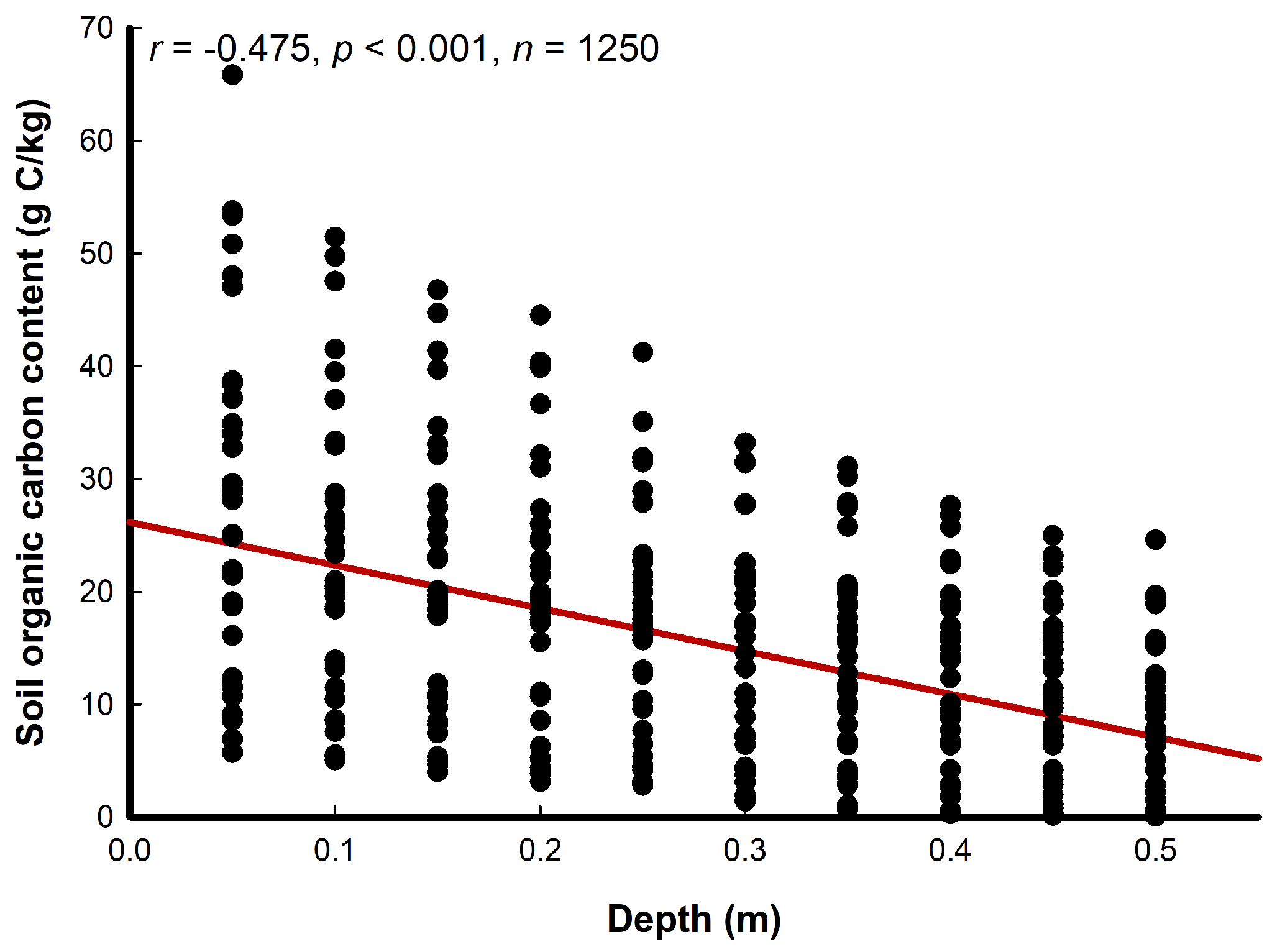

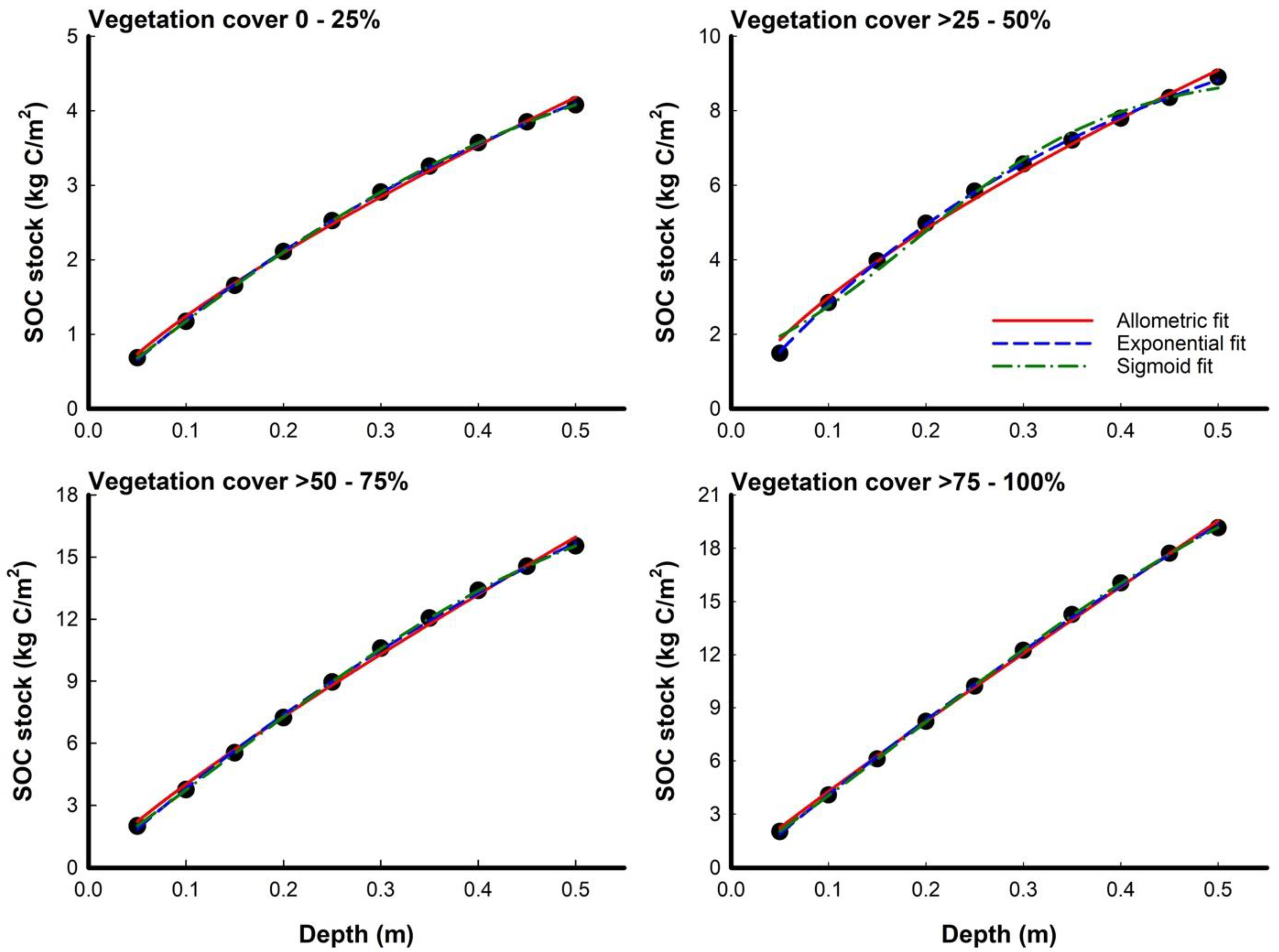
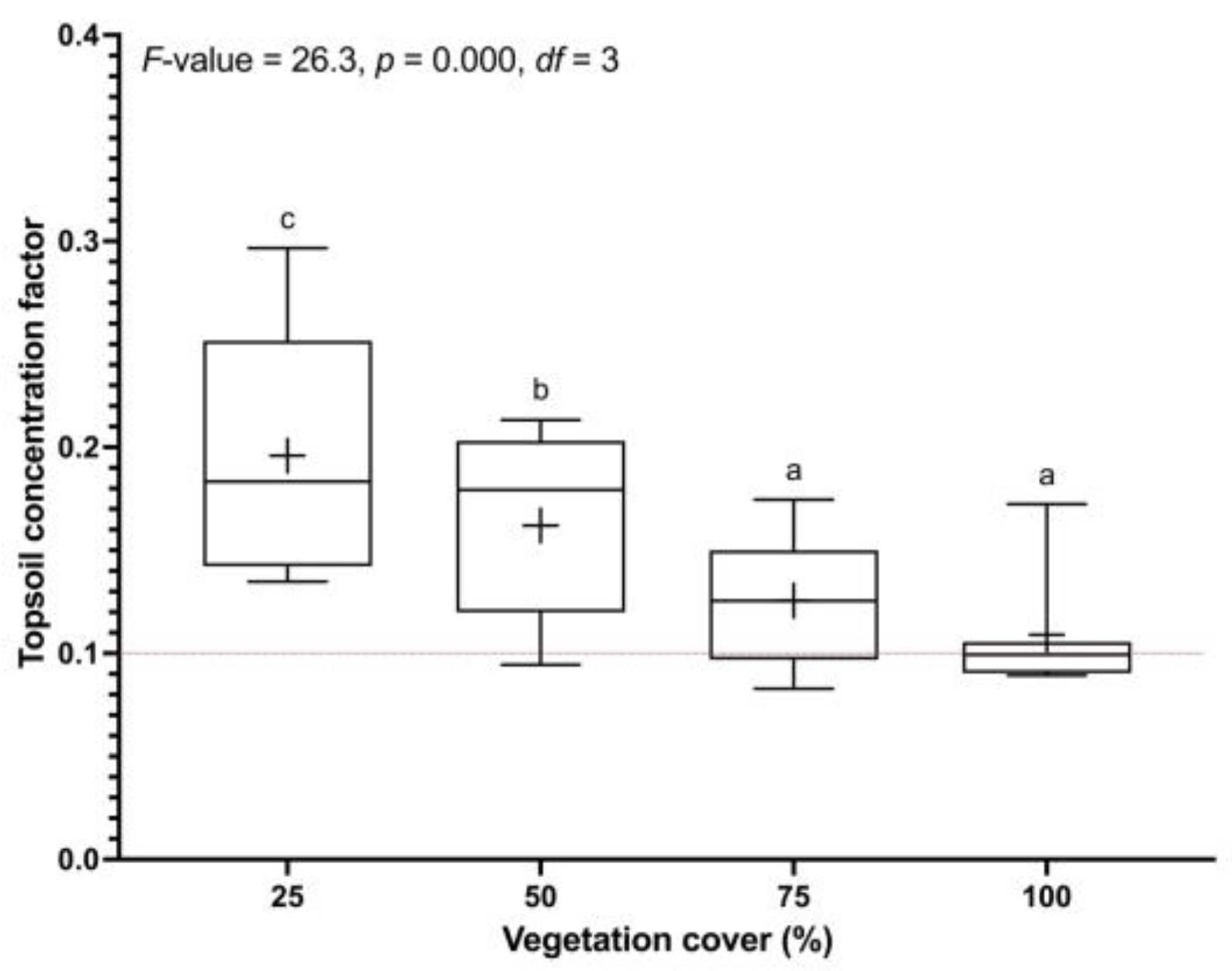
| Soil Depth (cm) | Vegetation Cover 0–25% | Vegetation Cover >25–50% | Vegetation Cover >50–75% | Vegetation Cover >75–100% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (n = 32) | Min | Max | CV (%) | Mean (n = 30) | Min | Max | CV (%) | Mean (n = 33) | Min | Max | CV (%) | Mean (n = 30) | Min | Max | CV (%) | |
| 0–5 | 1.33 | 1.16 | 1.52 | 9.9 | 1.24 | 0.79 | 1.55 | 18.6 | 1.24 | 0.97 | 1.41 | 10.1 | 0.97 | 0.57 | 1.16 | 18.2 |
| 5–10 | 1.46 | 1.39 | 1.53 | 3.4 | 1.40 | 1.13 | 1.68 | 13.6 | 1.35 | 1.24 | 1.47 | 5.6 | 1.14 | 0.89 | 1.27 | 10.1 |
| 10–15 | 1.51 | 1.45 | 1.57 | 3.2 | 1.47 | 1.14 | 1.74 | 12.8 | 1.43 | 1.31 | 1.54 | 5.0 | 1.25 | 1.03 | 1.42 | 9.9 |
| 15–20 | 1.55 | 1.49 | 1.61 | 3.2 | 1.54 | 1.14 | 1.83 | 12.3 | 1.50 | 1.38 | 1.68 | 6.3 | 1.37 | 1.04 | 1.55 | 11.4 |
| 20–25 | 1.74 | 1.23 | 2.09 | 12.9 | 1.72 | 1.40 | 2.05 | 13.1 | 1.61 | 1.52 | 1.71 | 4.2 | 1.46 | 1.14 | 1.60 | 9.8 |
| 25–30 | 1.83 | 1.67 | 2.20 | 9.5 | 1.75 | 1.45 | 2.08 | 12.3 | 1.64 | 1.56 | 1.73 | 4.1 | 1.57 | 1.24 | 1.85 | 12.3 |
| 30–35 | 1.90 | 1.71 | 2.32 | 10.1 | 1.83 | 1.48 | 2.23 | 14.3 | 1.68 | 1.32 | 1.87 | 12.6 | 1.66 | 1.56 | 1.76 | 4.7 |
| 35–40 | 2.01 | 1.74 | 2.55 | 12.3 | 1.91 | 1.50 | 2.38 | 16.4 | 1.78 | 1.36 | 2.11 | 12.6 | 1.69 | 1.56 | 1.81 | 5.8 |
| 40–45 | 2.08 | 1.77 | 2.78 | 14.2 | 1.98 | 1.52 | 2.53 | 18.6 | 1.88 | 1.48 | 2.17 | 13.1 | 1.72 | 1.56 | 1.86 | 7.0 |
| 45–50 | 2.25 | 1.80 | 3.01 | 17.1 | 2.06 | 1.54 | 2.68 | 20.8 | 2.04 | 1.65 | 2.48 | 14.0 | 1.76 | 1.61 | 1.92 | 7.2 |
| FVegetation cover = 125.5 ***, FDepth = 214.2 ***, FVegetation cover x Depth = 1.6 * | ||||||||||||||||
| Soil Depth (cm) | Vegetation Cover 0–25% | Vegetation Cover >25–50% | Vegetation Cover >50–75% | Vegetation Cover >75–100% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (n = 32) | Min | Max | CV (%) | Mean (n = 30) | Min | Max | CV (%) | Mean (n = 33) | Min | Max | CV (%) | Mean (n = 30) | Min | Max | CV (%) | |
| 0–5 | 11.7 | 5.7 | 21.9 | 47.1 | 21.7 | 12.3 | 32.8 | 36.3 | 31.5 | 19.1 | 50.9 | 28.0 | 44.0 | 29.0 | 65.9 | 25.8 |
| 5–10 | 8.7 | 5.1 | 18.5 | 47.8 | 18.6 | 11.5 | 28.7 | 39.1 | 24.9 | 18.5 | 47.5 | 33.8 | 36.6 | 26.4 | 51.5 | 23.7 |
| 10–15 | 6.6 | 4.0 | 9.7 | 30.6 | 15.0 | 10.6 | 23.1 | 33.8 | 23.8 | 17.8 | 46.8 | 36.0 | 32.5 | 24.6 | 44.7 | 21.5 |
| 15–20 | 5.2 | 3.1 | 8.5 | 30.2 | 13.1 | 8.6 | 19.5 | 30.6 | 21.9 | 17.2 | 39.9 | 29.9 | 30.6 | 22.2 | 44.5 | 24.3 |
| 20–25 | 4.2 | 2.8 | 6.5 | 28.2 | 10.7 | 7.7 | 13.0 | 18.9 | 19.1 | 15.7 | 31.9 | 22.9 | 27.8 | 21.5 | 41.2 | 22.8 |
| 25–30 | 3.3 | 1.4 | 6.4 | 49.7 | 8.9 | 7.1 | 11.0 | 17.8 | 17.6 | 13.2 | 21.8 | 14.1 | 25.8 | 20.7 | 33.2 | 19.0 |
| 30–35 | 2.8 | 0.6 | 6.4 | 66.3 | 7.7 | 3.7 | 10.2 | 30.8 | 15.6 | 11.3 | 19.8 | 17.3 | 23.5 | 15.5 | 31.1 | 23.0 |
| 35–40 | 2.4 | 0.3 | 6.4 | 82.4 | 7.0 | 1.7 | 10.1 | 41.8 | 13.9 | 6.7 | 18.9 | 27.4 | 20.7 | 12.3 | 27.6 | 24.8 |
| 40–45 | 2.2 | 0.1 | 6.4 | 97.6 | 6.5 | 0.8 | 10.1 | 48.4 | 12.1 | 3.3 | 18.9 | 37.2 | 18.6 | 9.7 | 25.0 | 26.0 |
| 45–50 | 1.8 | 0.1 | 5.0 | 102.8 | 6.3 | 0.4 | 10.0 | 52.1 | 9.6 | 1.6 | 18.9 | 56.1 | 15.9 | 7.8 | 24.6 | 30.0 |
| FVegetation cover = 1073.9 ***, FDepth = 159.9 ***, FVegetation cover x Depth = 6.5 *** | ||||||||||||||||
| Vegetation Cover | Allometric | Exponential | Sigmoid |
|---|---|---|---|
| 0–25% | y = 14.3028*(1 + x)–2.5525 | y = 2.6823 + 11.6364*exp–3.0919*x | y = 10.1039 + (–3.8894)/(1 + (exp(–(x – 0.2788)/0.0008))) |
| R2 = 0.935843 | R2 = 0.933853 | R2 = 0.622018 | |
| >25–50% | y = 35.3809*(1 + x)–3.1838 | y = 5.9543 + 29.9211*exp–3.9297*x | y = 10.0813 + (27.5375)/(1 + (exp(–(x – 0.1415)/–0.0975))) |
| R2 = 0.989351 | R2 = 0.991550 | R2 = 0.997783 | |
| >50–75% | y = 43.7382*(1 + x)–1.5030 | y = 42.8691*exp–1.2225*x | y = (39.1543)/(1 + (exp(–(x – 0.5052)/–0.1407))) |
| R2 = 0.855285 | R2 = 0.879740 | R2 = 0.961066 | |
| >75–100% | y = 45.0017*(1 + x)–0.6953 | y = 44.6879*exp–0.5735*x | y = 24.9748 + (16.0487)/(1 + (exp(–(x – 0.4475)/–0.0463))) |
| R2 = 0.578317 | R2 = 0.620824 | R2 = 0.962327 |
| Vegetation Cover | Allometric | Exponential | Sigmoid |
|---|---|---|---|
| 0–25% | y = 7.0520*x0.7540 | y = 6.9939–6.9104*exp–1.7446*x | y = –5.0340 + (10.4305)/(1 + (exp(–(x – 0.0003)/0.2579))) |
| R2 = 0.997466 | R2 = 0.999785 | R2 = 0.999988 | |
| >25–50% | y = 14.6802*x0.6905 | y = 12.1200–12.0570*exp–2.5960*x | y = (9.0968)/(1 + (exp(–(x – 0.1894)/0.1074))) |
| R2 = 0.994294 | R2 = 0.999694 | R2 = 0.990562 | |
| >50–75% | y = 28.9464*x0.8576 | y = 33.8656–34.0791*exp–1.2588*x | y = –9.6689 + (29.8449)/(1 + (exp(–(x – 0.1428)/0.2106))) |
| R2 = 0.997179 | R2 = 0.999385 | R2 = 0.999985 | |
| >75–100% | y = 37.5923*x0.9436 | y = 68.8010–69.1784*exp–0.6706*x | y = –12.8338 + (40.8197)/(1 + (exp(–(x – 0.1852)/0.2440))) |
| R2 = 0.998625 | R2 = 0.999571 | R2 = 0.999958 |
| Vegetation Cover | Equation | MNAE | MNB | Student’s t-Test | |
|---|---|---|---|---|---|
| t-Value | p | ||||
| 0–25% (n = 48) | Allometric | 4.132 | 0.833 | –2.857 | 0.016 |
| Exponential | 2.348 | 0.388 | –2.559 | 0.027 | |
| Sigmoid | 0.251 | –0.077 | 2.006 | 0.070 | |
| >25–50% (n = 45) | Allometric | 2.178 | 0.194 | –3.488 | 0.004 |
| Exponential | 1.156 | 0.134 | –2.170 | 0.048 | |
| Sigmoid | 0.091 | 0.059 | –1.639 | 0.123 | |
| >50–75% (n = 48) | Allometric | 0.814 | 0.264 | –3.003 | 0.005 |
| Exponential | 0.688 | 0.199 | –2.433 | 0.021 | |
| Sigmoid | 0.317 | 0.032 | –0.694 | 0.493 | |
| >75–100% (n = 45) | Allometric | 0.133 | 0.056 | –5.653 | 0.000 |
| Exponential | 0.121 | 0.123 | –2.401 | 0.031 | |
| Sigmoid | 0.107 | 0.075 | –1.454 | 0.168 | |
| Vegetation Cover | Equation | MNAE | MNB | Student’s t-Test | |
|---|---|---|---|---|---|
| t-Value | p | ||||
| 0–25% (n = 48) | Allometric | 0.162 | 0.137 | –3.425 | 0.006 |
| Exponential | 0.027 | 0.014 | –1.691 | 0.119 | |
| Sigmoid | 0.043 | –0.044 | 2.837 | 0.016 | |
| >25–50% (n = 45) | Allometric | 0.069 | 0.071 | –2.593 | 0.021 |
| Exponential | 0.031 | –0.004 | 0.361 | 0.723 | |
| Sigmoid | 0.098 | –0.092 | 4.893 | 0.000 | |
| >50–75% (n = 48) | Allometric | 0.063 | 0.051 | –2.668 | 0.012 |
| Exponential | 0.039 | 0.043 | –1.983 | 0.056 | |
| Sigmoid | 0.054 | –0.037 | 3.603 | 0.001 | |
| >75–100% (n = 45) | Allometric | 0.018 | 0.016 | –2.831 | 0.013 |
| Exponential | 0.022 | 0.012 | –1.491 | 0.158 | |
| Sigmoid | 0.064 | –0.047 | 2.325 | 0.036 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, E.M.; Keshta, A.E.; Alrumman, S.A.; Arshad, M.; Shaltout, K.H.; Ahmed, M.T.; Al-Bakre, D.A.; Alfarhan, A.H.; Barcelo, D. Modeling Soil Organic Carbon at Coastal Sabkhas with Different Vegetation Covers at the Red Sea Coast of Saudi Arabia. J. Mar. Sci. Eng. 2023, 11, 295. https://doi.org/10.3390/jmse11020295
Eid EM, Keshta AE, Alrumman SA, Arshad M, Shaltout KH, Ahmed MT, Al-Bakre DA, Alfarhan AH, Barcelo D. Modeling Soil Organic Carbon at Coastal Sabkhas with Different Vegetation Covers at the Red Sea Coast of Saudi Arabia. Journal of Marine Science and Engineering. 2023; 11(2):295. https://doi.org/10.3390/jmse11020295
Chicago/Turabian StyleEid, Ebrahem M., Amr E. Keshta, Sulaiman A. Alrumman, Muhammad Arshad, Kamal H. Shaltout, Mohamed T. Ahmed, Dhafer A. Al-Bakre, Ahmed H. Alfarhan, and Damia Barcelo. 2023. "Modeling Soil Organic Carbon at Coastal Sabkhas with Different Vegetation Covers at the Red Sea Coast of Saudi Arabia" Journal of Marine Science and Engineering 11, no. 2: 295. https://doi.org/10.3390/jmse11020295
APA StyleEid, E. M., Keshta, A. E., Alrumman, S. A., Arshad, M., Shaltout, K. H., Ahmed, M. T., Al-Bakre, D. A., Alfarhan, A. H., & Barcelo, D. (2023). Modeling Soil Organic Carbon at Coastal Sabkhas with Different Vegetation Covers at the Red Sea Coast of Saudi Arabia. Journal of Marine Science and Engineering, 11(2), 295. https://doi.org/10.3390/jmse11020295










