A Comparative Study on the Difference in Temperature and Salinity Tolerance of Crassostrea nippona and C. gigas Spat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Experimental Design
2.2.1. Experiment 1: Thermal and Salinity Tolerance Limits of Spat
2.2.2. Experiment 2: Growth and Survival Rates of Spat
2.3. Statistical Analyses
3. Results
3.1. Thermal and Salinity Tolerance Limits of Spat
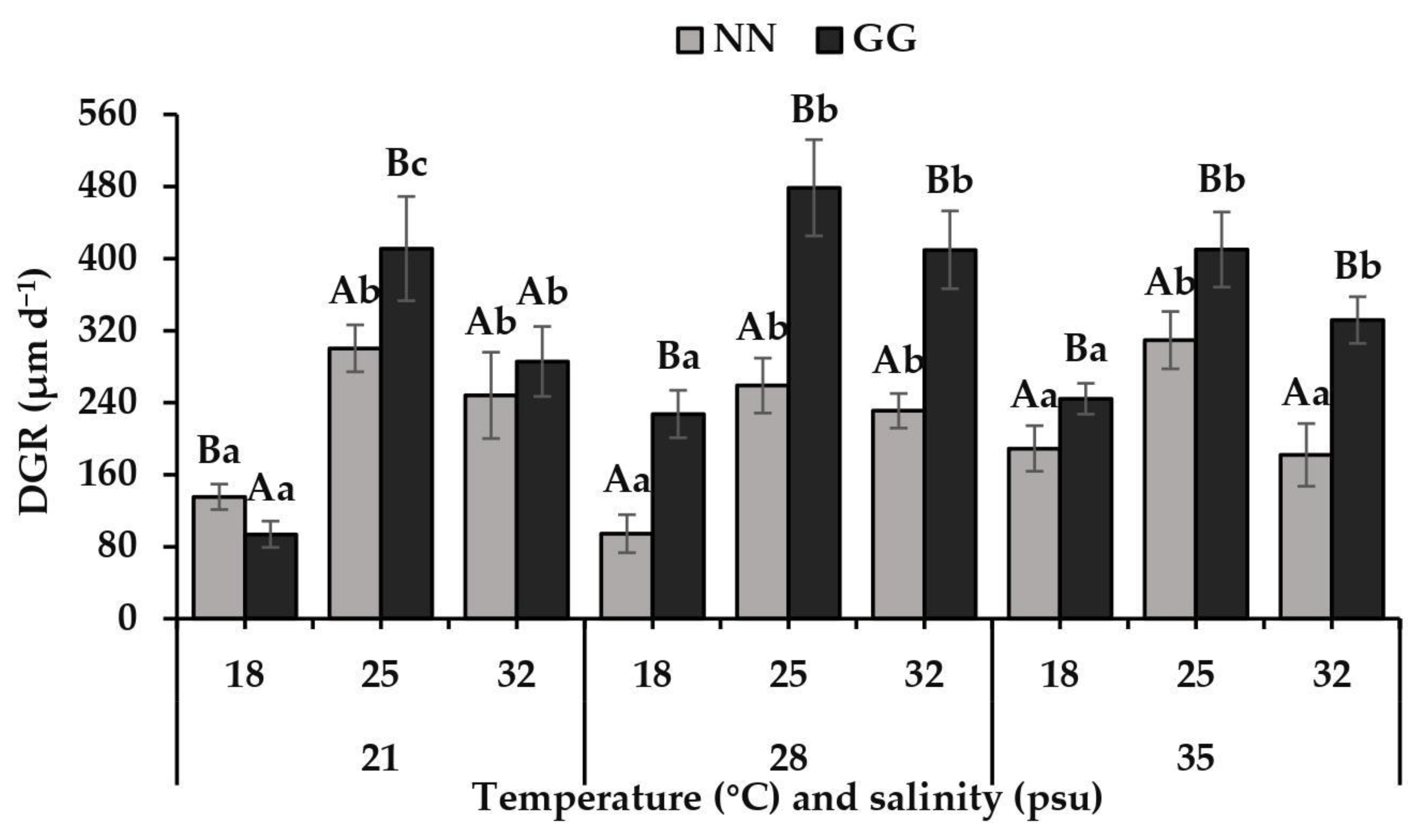
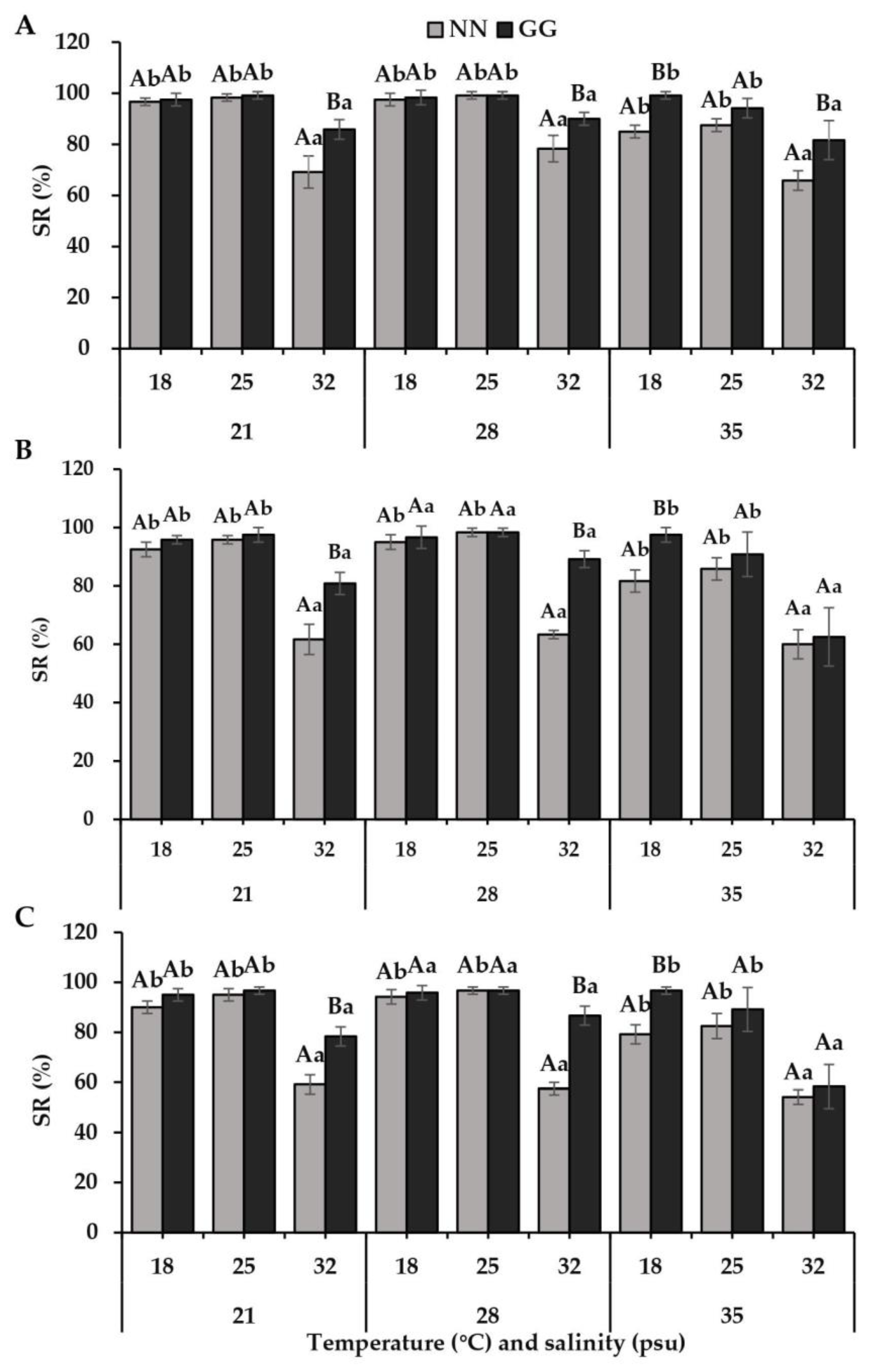
3.2. Growth and Survival Rates of Spat
4. Discussion
4.1. Differences in Thermal and Salinity Tolerance Limits
4.2. Differences in Growth and Survival Rates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Itoh, N.; Tun, K.L.; Komiyama, H.; Ueki, N.; Ogawa, K. An ovarian infection in the Iwagaki oyster, Crassostrea nippona, with the protozoan parasite Marteilioides chungmuensis. J. Fish. Dis. 2004, 27, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.Y.; Ke, C.H.; Zhu, A.J.; Wang, W.X. Oyster-based national mapping of trace metals pollution in the Chinese coastal waters. Environ. Pollut. 2017, 224, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Nobuaki, M.; Hitoshi, S.; Yoshihiro, K. Seasonal changes in glycogen contents of the Iwagaki oyster, Crassostrea nippona from the coast of Toga Bay, Konoura, Tomari, and Oki Islands [Japan] in the Sea of Japan. Bull. Jap. Soc. Sci. Fish. 2005, 71, 363–368. [Google Scholar] [CrossRef]
- Masahiro, Y.; Kawabeta, K.; Eguchi, A.; Abe, H.; Yamashita, E.; Koba, K.; Tominaga, M. Characterization of taste and micronutrient content of rock oysters (Crassostrea nippona) and Pacific oysters (Crassostrea gigas) in Japan. Int. J. Gastron. Food S 2018, 13, 52–57. [Google Scholar] [CrossRef]
- Semura, H.; Ishida, K.; Nakagami, H.; Hayashi, I. First maturation from 0-year-old to 1-year-old Iwagaki oyster Crassostrea nippona, cultured by hanging method in Uragou-Bay, Oki, Shimane Prefecture, Japan. Bull. Soc. Sea Water Sci. Jpn. 2010, 64, 39–43. (In Japanese) [Google Scholar]
- Hermabessiere, L.; Fabioux, C.; Lassudrie, M.; Boullot, F.; Long, M.; Lambert, C.; Le Goïc, N.; Gouriou, J.; Le Gac, M.; Chapelle, A. Influence of gametogenesis pattern and sex on paralytic shellfish toxin levels in triploid Pacific oyster Crassostrea gigas exposed to a natural bloom of Alexandrium minutum. Aquaculture 2016, 455, 118–124. [Google Scholar] [CrossRef]
- Houssin, M.; Trancart, S.; Denechere, L.; Oden, E.; Adeline, B.; Lepoitevin, M.; Pitel, P.-H. Abnormal mortality of triploid adult Pacific oysters: Is there a correlation with high gametogenesis in Normandy, France? Aquaculture 2019, 505, 63–71. [Google Scholar] [CrossRef]
- Sasaki, T. Study on Resource Regeneration and Aquaculture of the Commercially Valuable Shellfish Species in Rocky Shore of Shimane Prefecture; Shimane Prefectural Fisheries Technology Center: Uyagawa, Japan, 2022. [Google Scholar]
- Xu, H.Q.; Li, Q.; Han, Z.Q.; Liu, S.K.; Yu, H.; Kong, L.F. Fertilization, survival and growth of reciprocal crosses between two oysters, Crassostrea gigas and Crassostrea nippona. Aquaculture 2019, 507, 91–96. [Google Scholar] [CrossRef]
- Hu, Y.M.; Li, Q.; Xu, C.X.; Liu, S.; Kong, L.F.; Yu, H. Response to selection for growth in successive mass selected generations of Iwagaki oyster Crassostrea nippona. Aquaculture 2022, 560, 738575. [Google Scholar] [CrossRef]
- Li, S.Y.; Qin, Y.P.; Shi, G.P.Y.; Wan, W.T.; Liao, Q.L.; Ma, H.T.; Li, J.; Yu, Z.N.; Pan, Y.; Zhang, Y.H. Feasibility of interspecific hybridization between Iwagaki oyster, Crassostrea nippona and Portuguese oyster, Crassostrea angulata. Aquaculture 2022, 563, 739002. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q. Effects of salinity and temperature on growth and survival of juvenile Iwagaki oyster Crassostrea nippona. J. Ocean Univ. China 2018, 17, 941–946. [Google Scholar] [CrossRef]
- Takahashi, K.G.; Yanai, H.; Muroga, K.; Mori, K. Morphology and phagocytic activity of hemocytes from the Japanese rock oyster Crassostrea nippona and the Pacific oyster C. gigas. Aquacult. Sci. 2005, 53, 53–59. (In Japanese) [Google Scholar]
- Eguchi, T.; Chijiwa, Y. Maturation of the Iwagaki oyster, Crassostrea nippona, by hanging culture in karatsu bay. Bull. Saga Genkai Fish Res. Dev. Cent. 2012, 5, 57–59. (In Japanese) [Google Scholar]
- Kawasaki, T.; Shimizu, Y.; Iwasa, M.; Yoshida, S.; Kuwahara, Y. Identification of oysters in Okushiri Island by mitochondrial 16S rRNA analysis. Sci. Rep. Hokkaido Fish Res. Inst. 2014, 86, 137–144. (In Japanese) [Google Scholar]
- Miossec, L.; Deuff, R.-M.L.; Goulletquer, P. Alien Species Alert: Crassostrea gigas (Pacific oyster); ICES Cooperative Research Reports (CRR); ICES: Copenhagen, Denmark, 2009. [Google Scholar] [CrossRef]
- Hu, Y.M.; Li, Q.; Xu, C.X.; Liu, S.K.; Kong, L.F.; Yu, H. Genetic variability of mass-selected and wild populations of Iwagaki oyster (Crassostrea nippona) revealed by microsatellites and mitochondrial COI sequences. Aquaculture 2022, 561, 738737. [Google Scholar] [CrossRef]
- Christophersen, G.; Strand, Ø. Effect of reduced salinity on the great scallop (Pecten maximus) spat at two rearing temperatures. Aquaculture 2003, 215, 79–92. [Google Scholar] [CrossRef]
- Cerón-Ortiz, A.N.; Cordero, B.; Arredondo-Vega, B.O.; Voltolina, D. Effect of algal diet and temperature on survival, growth and biochemical composition of spat of the lion‘s paw scallop Nodipecten subnodosus. Aquaculture 2009, 298, 64–69. [Google Scholar] [CrossRef]
- Van der Walt, K.-A.; Porri, F.; Potts, W.M.; Duncan, M.I.; James, N.C. Thermal tolerance, safety margins and vulnerability of coastal species: Projected impact of climate change induced cold water variability in a temperate African region. Mar. Environ. Res. 2021, 169, 105346. [Google Scholar] [CrossRef]
- Guo, X.M. Use and exchange of genetic resources in molluscan aquaculture. Rev. Aquac. 2009, 1, 251–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Mu, H.W.; Li, J.; Zhang, Y.H.; Xu, F.J.; Xiang, Z.M.; Qian, P.-Y.; Qiu, J.-W.; Yu, Z.N. Proteomic basis of stress responses in the gills of the Pacific oyster Crassostrea gigas. J. Proteome Res. 2015, 14, 304–317. [Google Scholar] [CrossRef]
- Zhang, G.F.; Fang, X.D.; Guo, X.M.; Li, L.; Luo, R.B.; Xu, F.; Yang, P.C.; Zhang, L.L.; Wang, X.T.; Qi, H.G. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Gong, J.W.; Li, Q.; Yu, H.; Liu, S.K.; Kong, L.F. Effects of low salinity on hemolymph osmolality and transcriptome of the Iwagaki oyster, Crassostrea nippona. Fish Shellfish Immunol. 2022, 126, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kinne, O. The effects of temperature and salinity on marine and brackish water animals: II. Salinity and temperature-salinity combinations. Oceanogr. Mar. Biol. Ann. Rev. 1964, 2, 281–339. [Google Scholar]
- O‘Connor, W.A.; Lawler, N.F. Salinity and temperature tolerance of embryos and juveniles of the pearl oyster, Pinctada imbricata Röding. Aquaculture 2004, 229, 493–506. [Google Scholar] [CrossRef]
- Nell, J.A.; Holliday, J.E. Effects of salinity on the growth and survival of Sydney rock oyster (Saccostrea commercialis) and Pacific oyster (Crassostrea gigas) larvae and spat. Aquaculture 1988, 68, 39–44. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q. Effects of temperature, salinity and body size on the physiological responses of the Iwagaki oyster Crassostrea nippona. Aquacult. Res. 2020, 51, 728–737. [Google Scholar] [CrossRef]
- Flores-Vergara, C.; Cordero-Esquivel, B.; Cerón-Ortiz, A.N.; Arredondo-Vega, B.O. Combined effects of temperature and diet on growth and biochemical composition of the Pacific oyster Crassostrea gigas (Thunberg) spat. Aquac. Res. 2004, 35, 1131–1140. [Google Scholar] [CrossRef]
- Degremont, L.; Ernande, B.; Bédier, E.; Boudry, P. Summer mortality of hatchery-produced Pacific oyster spat (Crassostrea gigas). I. Estimation of genetic parameters for survival and growth. Aquaculture 2007, 262, 41–53. [Google Scholar] [CrossRef]
- Noro, T.; Musashi, T.; Inoguchi, N. Influence of lower water temperature on survival of the Iwagaki oyster, Crassostrea nippona. Saibai Gyogyo Gijutsu Kaihatsu Kenkyu 2006, 34, 59–65. (In Japanese) [Google Scholar]
- Utting, S.; Millican, P. Techniques for the hatchery conditioning of bivalve broodstocks and the subsequent effect on egg quality and larval viability. Aquaculture 1997, 155, 45–54. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.Z.; Liu, S.K.; Kong, L.F. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fish Sci. 2011, 77, 643–648. [Google Scholar] [CrossRef]
- Wang, Y.P.; Guo, X.M. ITS length polymorphism in oysters and its use in species identification. J. Shellfish Res. 2008, 27, 489–493. [Google Scholar] [CrossRef]
- Liu, K.K.; Li, Q. Fast discrimination of Crassostrea nippona and C. gigas based on PCR-RFLP of mtDNA J. Ocean Univ. China 2018, 48, 17–22. (In Chinese) [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, W.C. Salinity tolerance of Haliotis diversicolor supertexta at different salinity and temperature levels. Aquaculture 2000, 181, 191–203. [Google Scholar] [CrossRef]
- Wu, Y.D.; Yu, X.K.; Suo, N.; Bai, H.Q.; Ke, Q.Z.; Chen, J.; Pan, Y.; Zheng, W.Q.; Xu, P. Thermal tolerance, safety margins and acclimation capacity assessments reveal the climate vulnerability of large yellow croaker aquaculture. Aquaculture 2022, 561, 738665. [Google Scholar] [CrossRef]
- Chu, P.; Chen, Y.C.; Kununaka, A. Seasonal Variability of the Yellow Sea/East China Sea Surface Fluxes and Thermohaline Structure. Adv. In. Atm. Sci. 2005, 22, 1–20. [Google Scholar] [CrossRef]
- Hu, M.Y.; Li, Q.; Li, L. Effect of salinity and temperature on salinity tolerance of the sea cucumber Apostichopus japonicus. Fish Sci. 2010, 76, 267–273. [Google Scholar] [CrossRef]
- Vinagre, C.; Leal, I.; Mendonça, V.; Flores, A.A. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. J. Therm. Biol. 2015, 47, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.Y.; Cheng, S.Y.; Chen, J.C. Temperature and salinity tolerances of yellowfin sea bream, Acanthopagrus latus, at different salinity and temperature levels. Aquacult. Res. 2003, 34, 175–185. [Google Scholar] [CrossRef]
- Meng, G.W.; Li, Q.; Xu, C.X.; Liu, S.K. Effects of high-temperature stress on survival and five immune indicators of Pacific oyster ‘Haida No. 3’. J. Fish Sci. China 2019, 26, 738–744. (In Chinese) [Google Scholar] [CrossRef]
- Xu, F.; Guo, X.M.; Li, L.; Zhang, G.F. Effects of salinity on larvae of the oysters Crassostrea ariakensis, C. sikamea and the hybrid cross. Mar. Biol. Res. 2011, 7, 796–803. [Google Scholar] [CrossRef]
- Lenz, M.; da Gama, B.A.; Gerner, N.V.; Gobin, J.; Gröner, F.; Harry, A.; Jenkins, S.R.; Kraufvelin, P.; Mummelthei, C.; Sareyka, J. Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: Results from a globally replicated study. Environ. Res. 2011, 111, 943–952. [Google Scholar] [CrossRef] [PubMed]
- de Bravo, M.S.; Chung, K.; Perez, J. Salinity and temperature tolerances of the green and brown mussels, Perna viridis and Perna perna (Bivalvia: Mytilidae). Rev. Biol. Trop. 1998, 46, 121–125. [Google Scholar]
- Fu, J.Q.; Chen, C.; Chu, Y.L. Spatial–temporal variations of oceanographic parameters in the Zhoushan sea area of the East China Sea based on remote sensing datasets. Reg. Stud. Mar. Sci. 2019, 28, 100626. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Zhang, J.X.; Yu, R.H. Effects of salinity, stocking density, and algal density on growth and survival of Iwagaki oyster Crassostrea nippona larvae. Aquac. Int. 2018, 26, 947–958. [Google Scholar] [CrossRef]
- Laing, I. Effect of salinity on growth and survival of king scallop spat (Pecten maximus). Aquaculture 2002, 205, 171–181. [Google Scholar] [CrossRef]
- Bayne, B.L. Biology of Oysters. Developments in Aquaculture and Fisheries Science; Academic Press: London, UK, 2017; Volume 41. [Google Scholar]
- Mann, R.L.; Burreson, E.M.; Baker, P.K. The decline of the Virginia Oyster fishery in Chesapeake Bay considerations for introduction of a non-endemic species, Crassostrea gigas (Thunberg, 1793). J. Shellfish Res. 1991, 10, 379–388. [Google Scholar]
- Langdon, C.J.; Robinson, A.M. Aquaculture potential of the Suminoe oyster (Crassostrea ariakensis Fugita 1913). Aquaculture 1996, 144, 321–338. [Google Scholar] [CrossRef]
- Mao, Y.Z.; Zhou, Y.; Yang, H.S.; Wang, R.C. Seasonal variation in metabolism of cultured Pacific oyster, Crassostrea gigas, in Sanggou Bay, China. Aquaculture 2006, 253, 322–333. [Google Scholar] [CrossRef]
- Meng, L.X.; Li, Q.; Xu, C.X.; Liu, S.K.; Kong, L.F.; Yu, H. Hybridization improved stress resistance in the Pacific oyster: Evidence from physiological and immune responses. Aquaculture 2021, 545, 737227. [Google Scholar] [CrossRef]
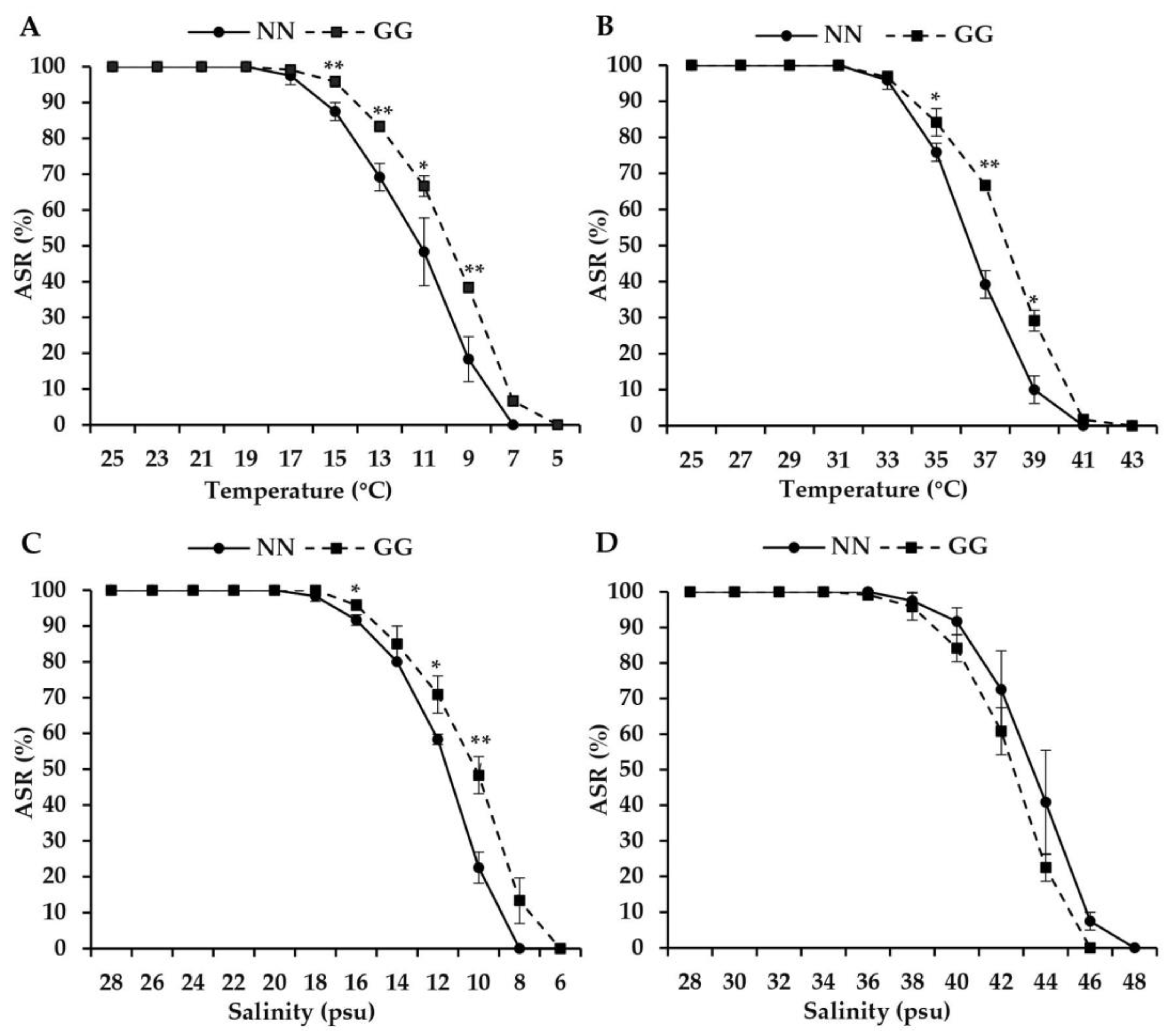
| Physiological Indices | Temperature (°C) | Salinity (psu) | ||
|---|---|---|---|---|
| NN | GG | NN | GG | |
| CMin | 7 | 5 | 8 | 6 |
| 50% CMin | 11.61 ± 0.42 b | 10.24 ± 0.09 a | 12.03 ± 0.08 b | 10.78 ± 0.40 a |
| SMin | 19.33 | 18 | 18.33 | 17.67 |
| CMax | 41 | 42.33 | 48 | 46 |
| 50% CMax | 36.32 ± 0.19 a | 37.51 ± 0.14 b | 43.02 ± 0.54 b | 42.06 ± 0.23 a |
| SMax | 32.33 | 33 | 36.67 | 36 |
| Source of Variation | Df | Daily Growth Rate (μm d−1) | Survival Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean Squares | F-Value | p-Value | Mean Squares | F-Value | p-Value | ||
| Temperature (Te) | 2 | 177,412.604 | 151.918 | <0.001 | 4248.727 | 258.514 | <0.001 |
| Salinity (Sa) | 2 | 7432.998 | 6.365 | 0.004 | 639.005 | 38.880 | <0.001 |
| Species (Sp) | 1 | 148,419.893 | 127.091 | <0.001 | 1204.167 | 73.268 | <0.001 |
| Te × Sa | 4 | 7768.062 | 6.652 | <0.001 | 37.269 | 2.268 | 0.081 |
| Te × Sp | 2 | 11,096.771 | 9.502 | <0.001 | 250.347 | 15.232 | <0.001 |
| Sa × Sp | 2 | 22,573.074 | 19.329 | <0.001 | 3.125 | 0.190 | 0.828 |
| Te × Sa × Sp | 4 | 1883.387 | 1.613 | 0.192 | 178.472 | 10.859 | <0.001 |
| Error | 36 | 1167.820 | 16.435 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Li, Q.; Xu, C.; Liu, S.; Kong, L.; Yu, H. A Comparative Study on the Difference in Temperature and Salinity Tolerance of Crassostrea nippona and C. gigas Spat. J. Mar. Sci. Eng. 2023, 11, 284. https://doi.org/10.3390/jmse11020284
Hu Y, Li Q, Xu C, Liu S, Kong L, Yu H. A Comparative Study on the Difference in Temperature and Salinity Tolerance of Crassostrea nippona and C. gigas Spat. Journal of Marine Science and Engineering. 2023; 11(2):284. https://doi.org/10.3390/jmse11020284
Chicago/Turabian StyleHu, Yiming, Qi Li, Chengxun Xu, Shikai Liu, Lingfeng Kong, and Hong Yu. 2023. "A Comparative Study on the Difference in Temperature and Salinity Tolerance of Crassostrea nippona and C. gigas Spat" Journal of Marine Science and Engineering 11, no. 2: 284. https://doi.org/10.3390/jmse11020284
APA StyleHu, Y., Li, Q., Xu, C., Liu, S., Kong, L., & Yu, H. (2023). A Comparative Study on the Difference in Temperature and Salinity Tolerance of Crassostrea nippona and C. gigas Spat. Journal of Marine Science and Engineering, 11(2), 284. https://doi.org/10.3390/jmse11020284








