Long-Term Ultraviolet Treatment for Macrofouling Control in Northern and Southern Hemispheres
Abstract
1. Introduction
2. Materials and Methods
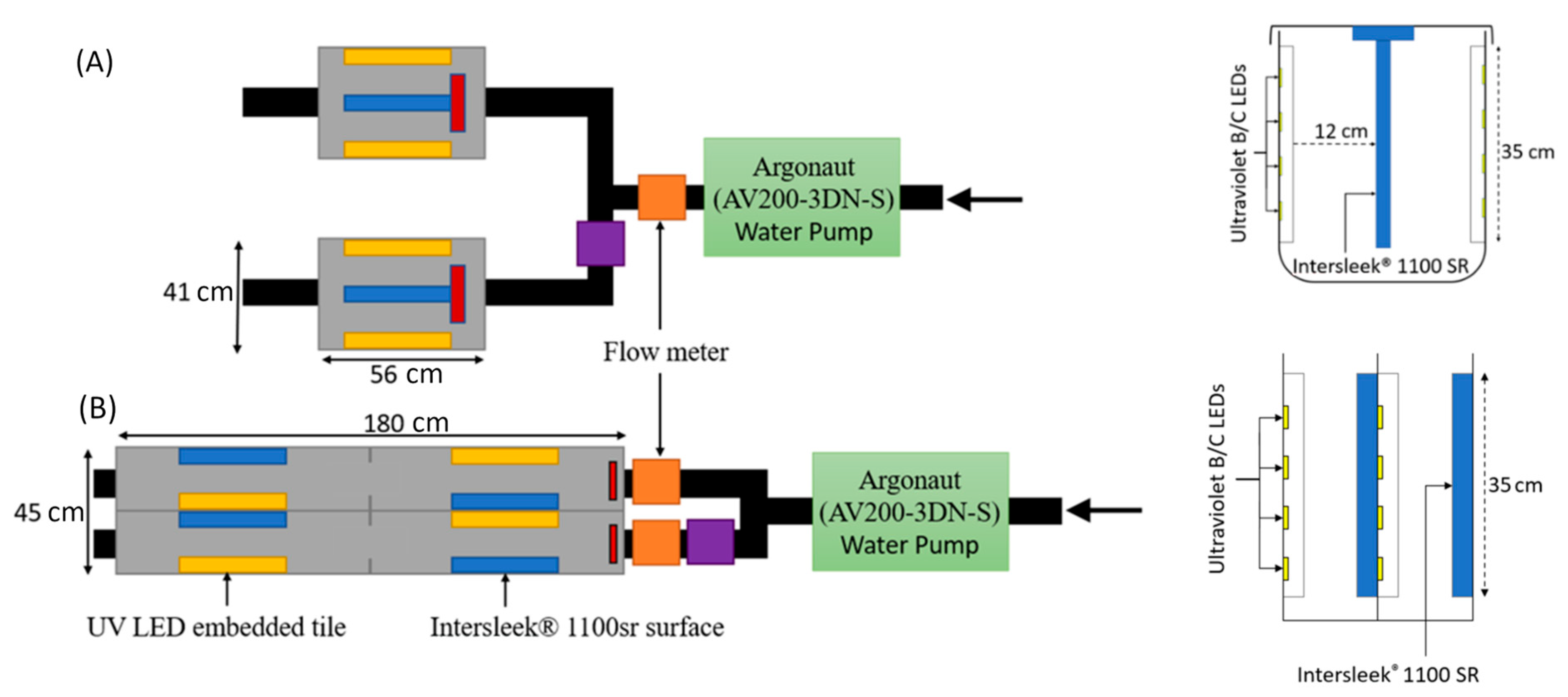
2.1. Test Chamber and Substrate Design
2.2. Environmental Sampling
- During step 5.B.2., the swabs were found to reabsorb the supernatant after centrifugation, requiring an additional step to release the lysed DNA. The silicate membrane was removed from the sterile spin columns and spin columns were placed within clean collection tubes. The sponges were added to the spin column and centrifuged at 13,000× g for 1 min. This allowed all lysed material to be spun down through the spin column into the collection tube below while the sponge remained in the upper spin column.
- Initial extractions were difficult to quantify via NanoDrop, owing to high salt contamination. To resolve this, steps 12 and 13 were repeated and the solutions were used at −20 °C.
- Step 16 was split into two elutions of 50 µL rather than one 100 µL elution. This provided higher DNA yields.
2.3. Image Capture and Analysis
3. Results
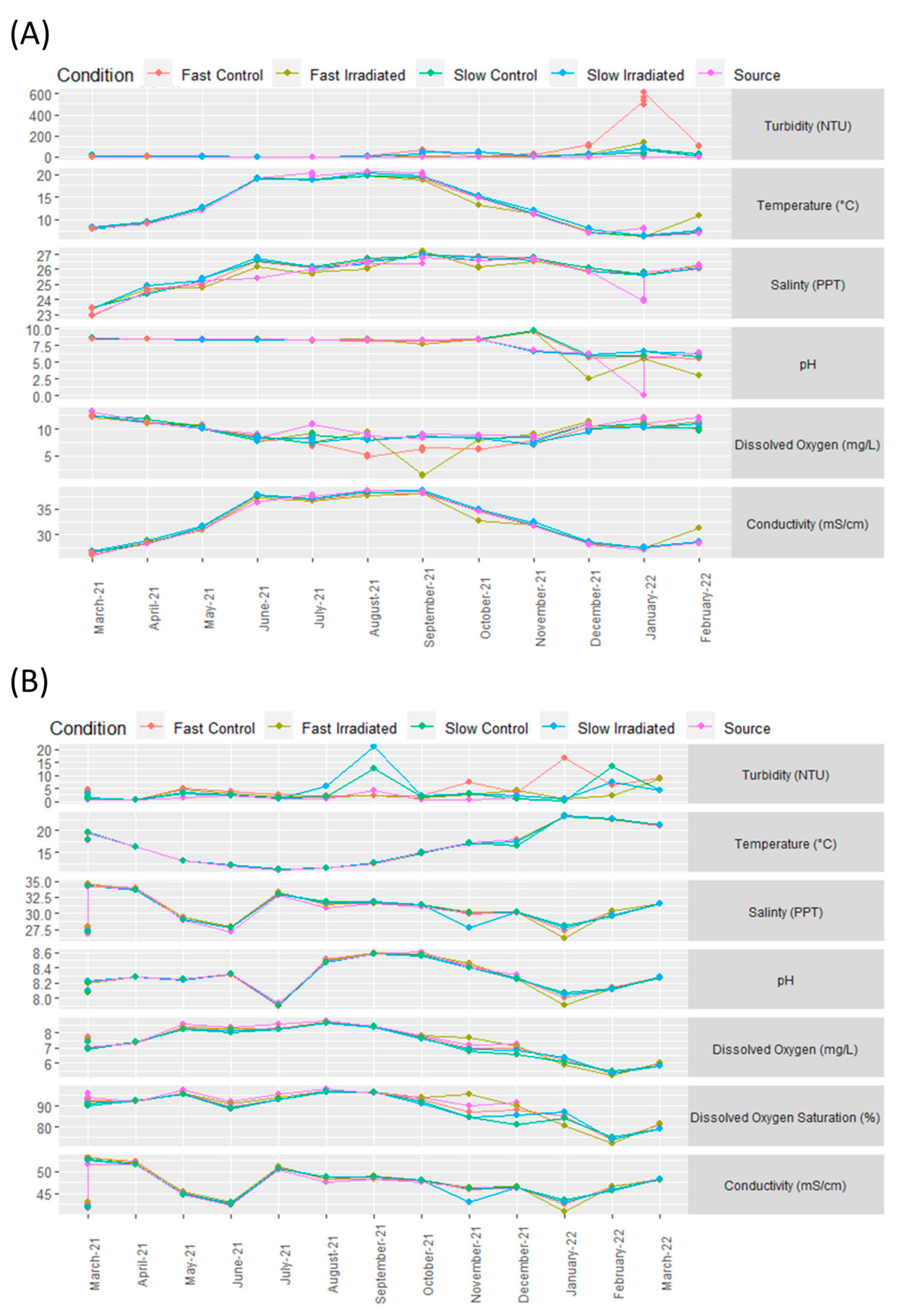
3.1. Environmental Conditions at the Test Sites
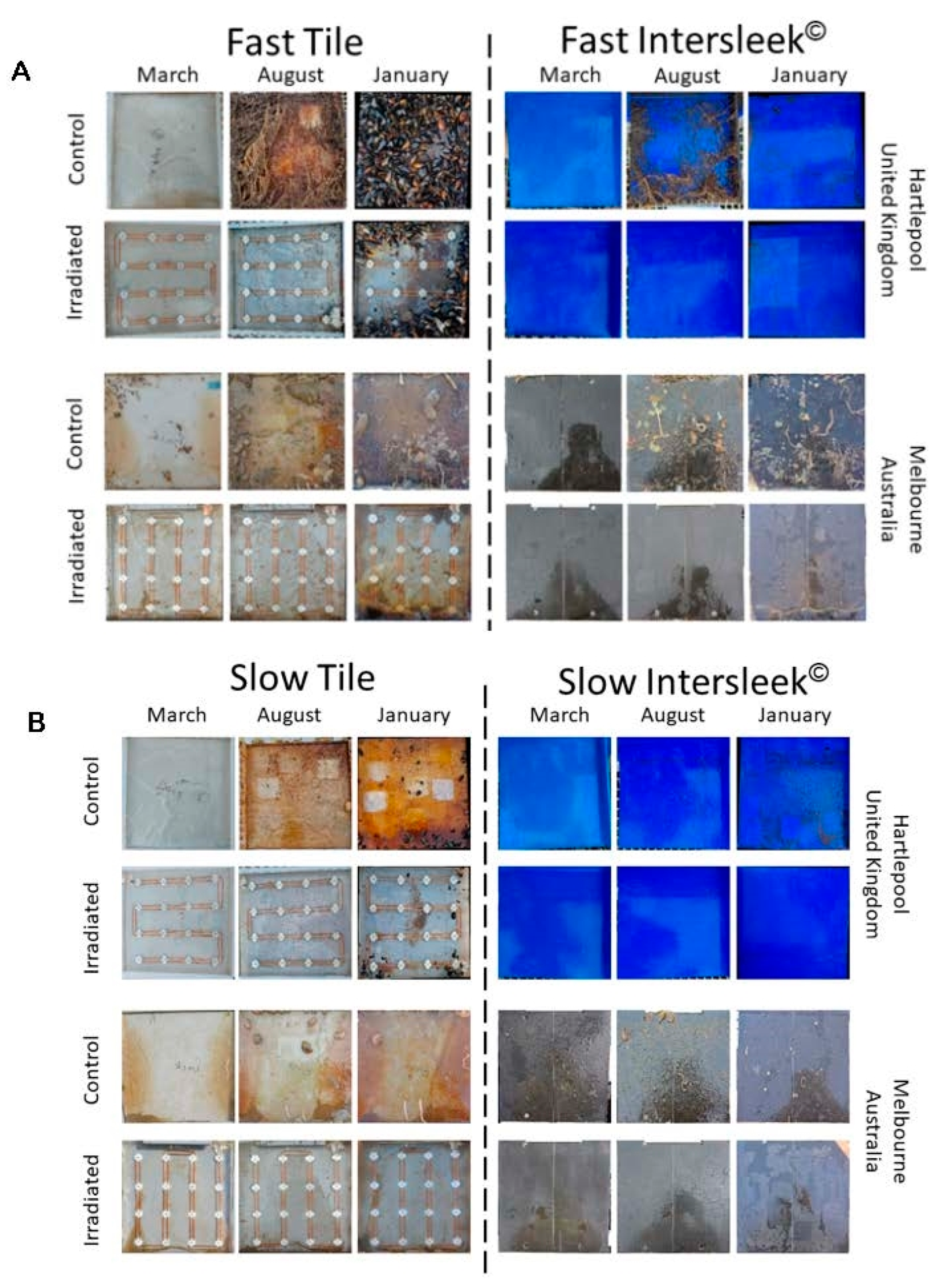
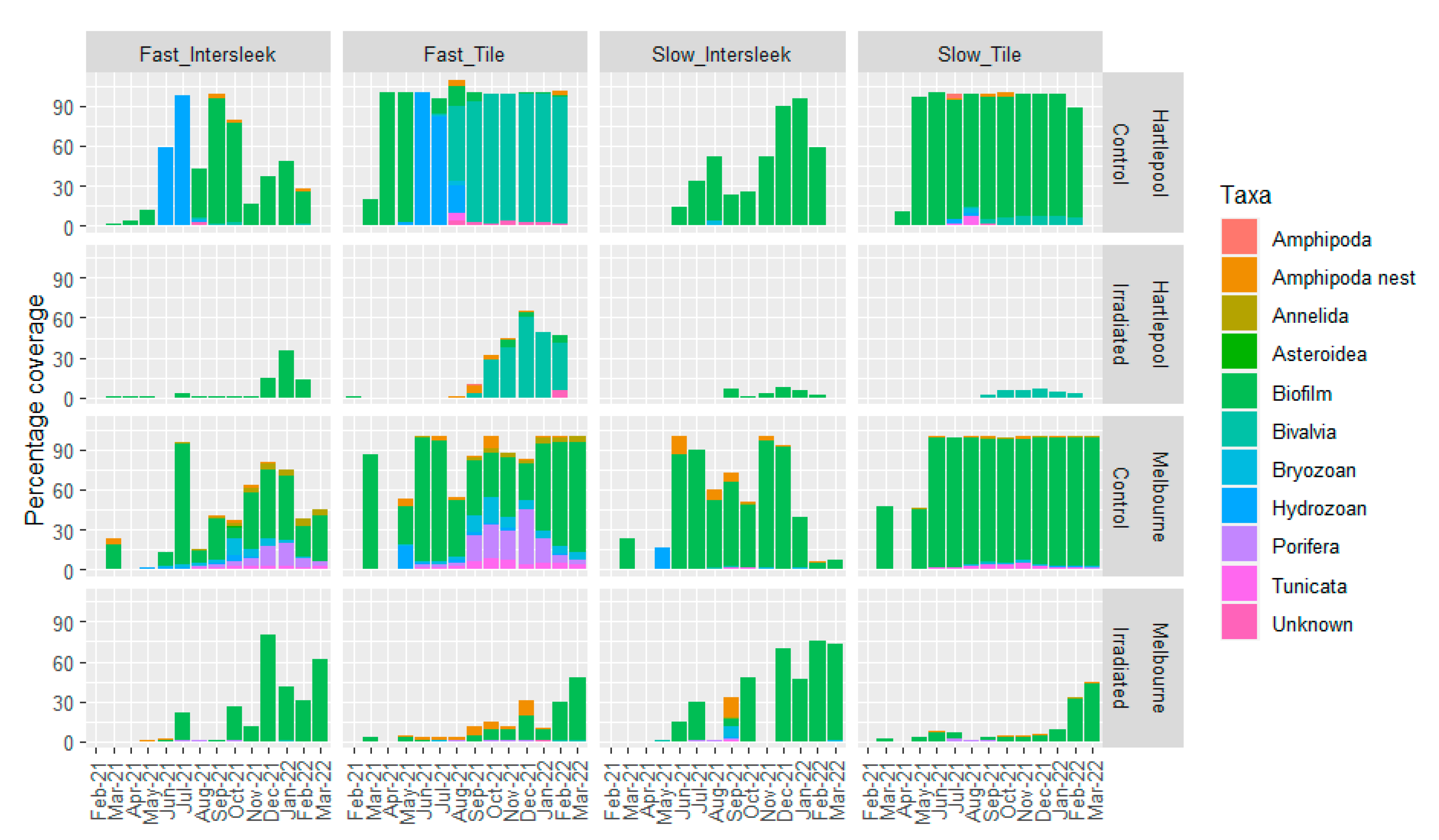
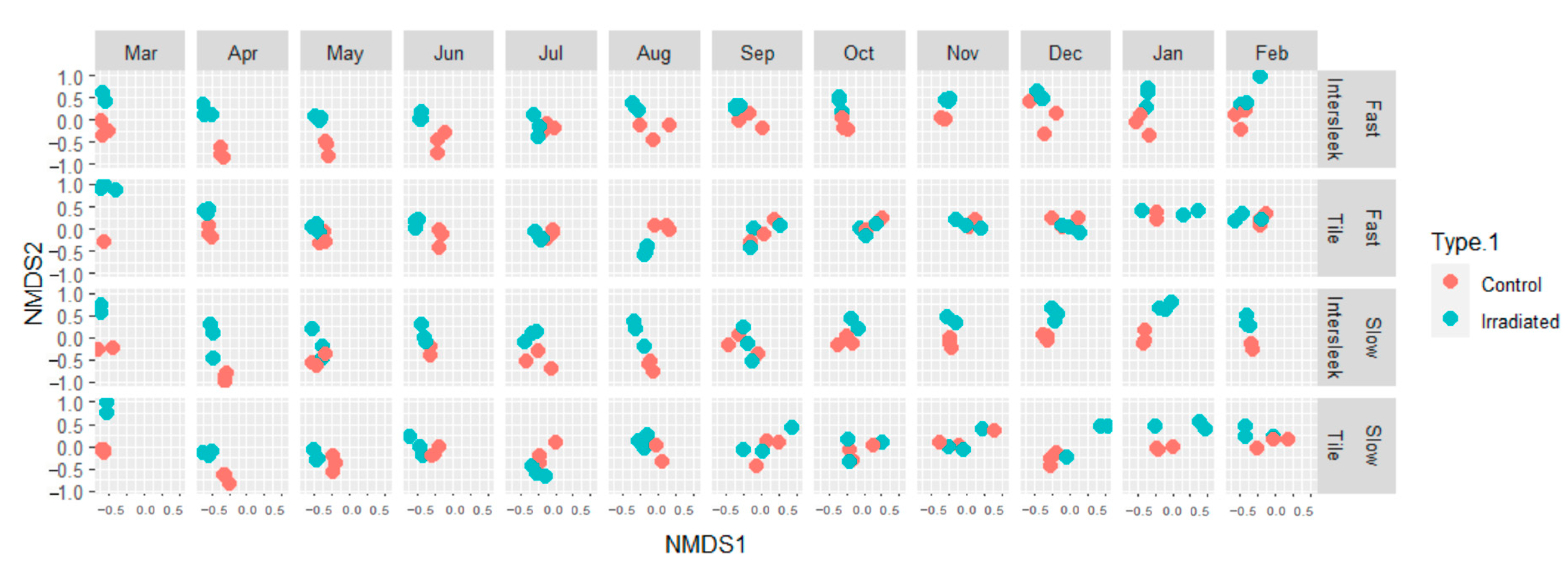
3.2. Imaging-Based Analysis of Fouling Communities
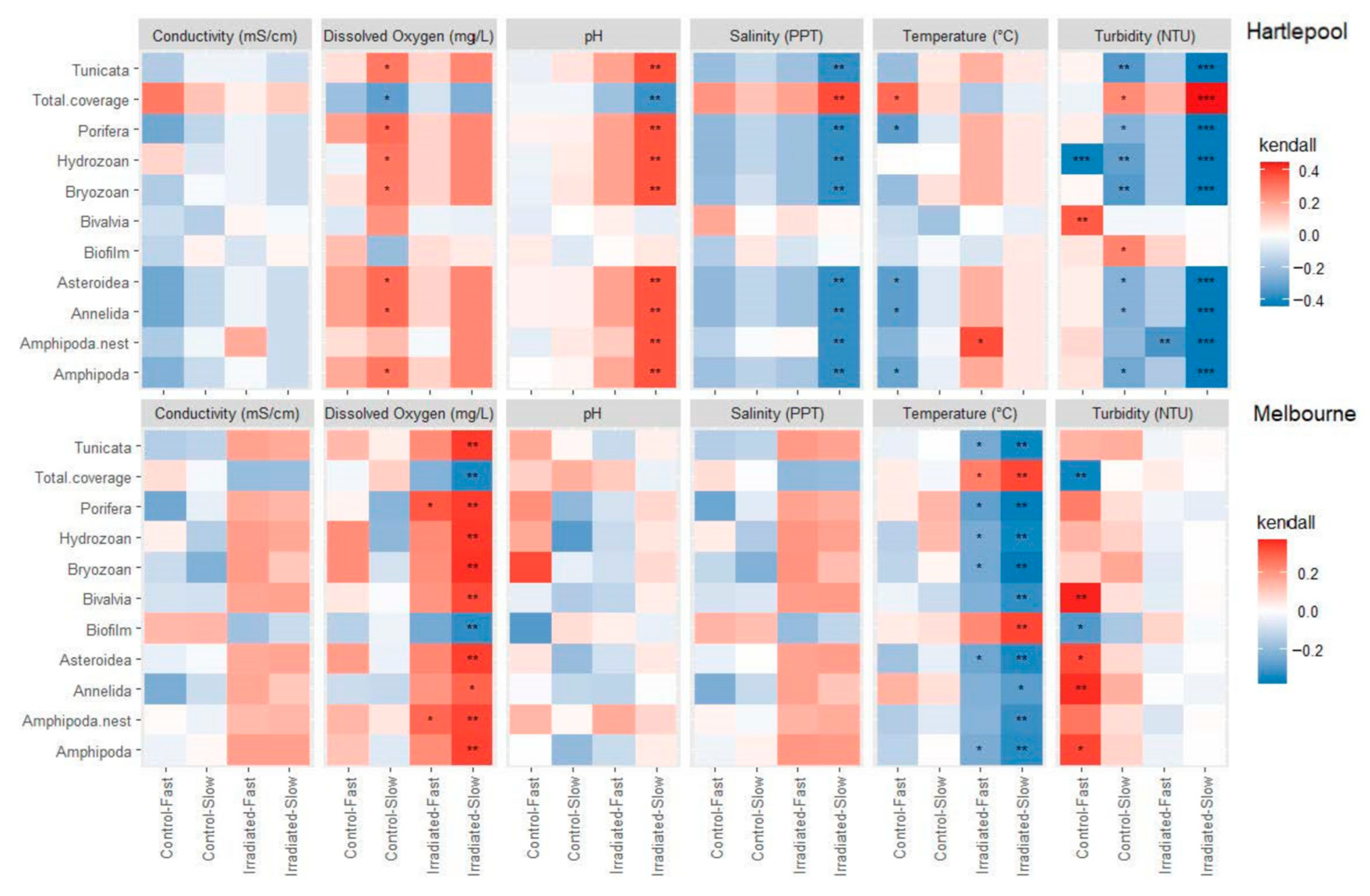
3.3. Community and Environmental Correlations
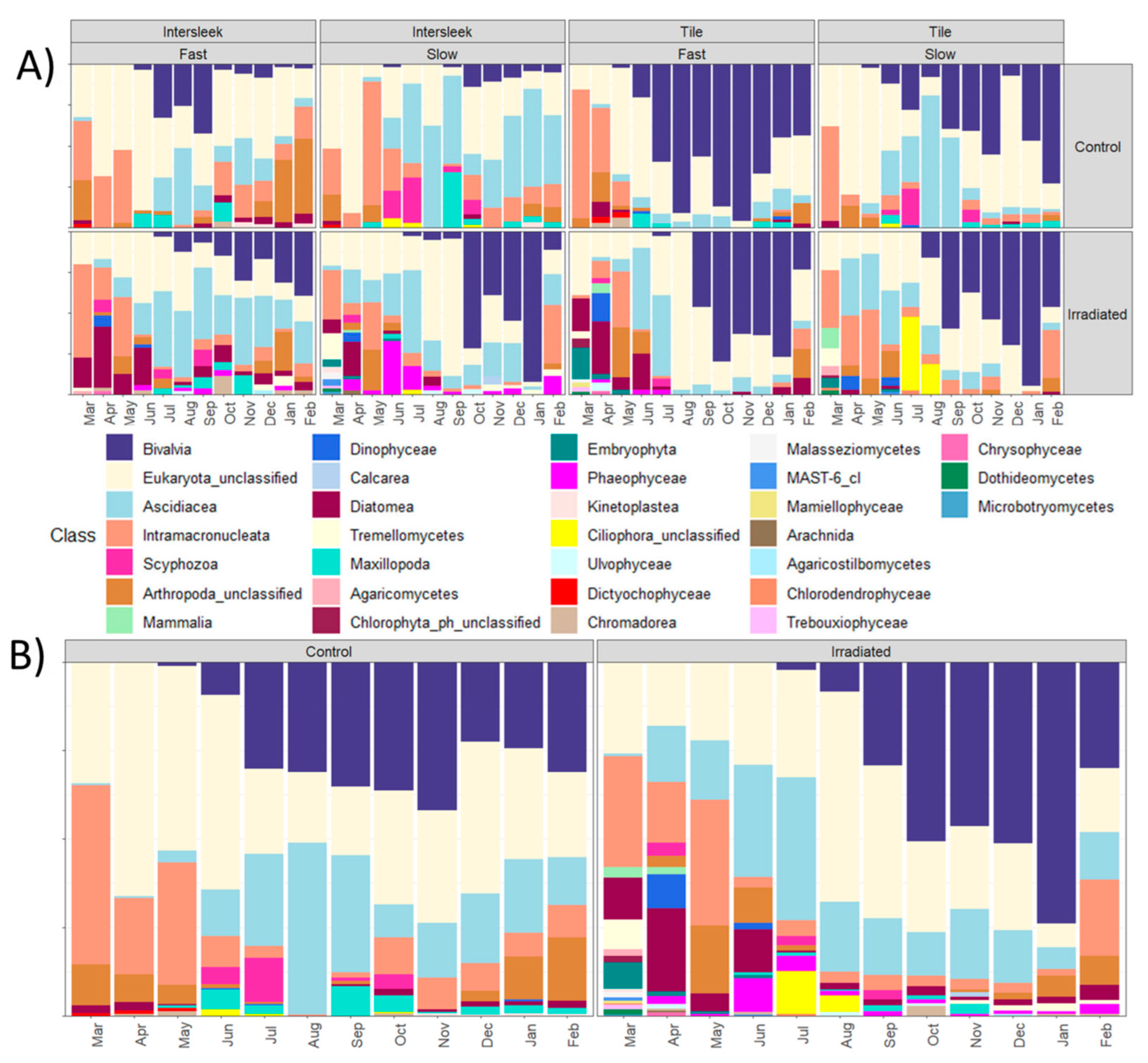
3.4. Amplicon-Based Analysis of Biofouling Communities at Hartlepool
3.5. Seasonal Fouling Community Differences
4. Discussion
4.1. UV Effects on Community Ecology
4.2. Indirect and Environmental Effects
4.3. Specific Effects of UV on Different Taxonomic Groups
4.4. Hardware Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Readman, J.W. Development, Occurrence and Regulation of Antifouling Paint Biocides: Historical Review and Future Trends. In Antifouling Paint Biocides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–15. [Google Scholar]
- Qin, L.; Lu, S.; Liu, J.; Wu, Y.; Ma, Z.; Mawignon, F.J.; Dong, G. Bionic Non-Smooth Epoxy Resin Coating with Corrosion Inhibitor for Drag-Reduction and Durability. Prog. Org. Coat. 2022, 173, 107176. [Google Scholar] [CrossRef]
- Yang, H.; Qin, L.; Zhao, W.; Mawignon, F.J.; Guo, H.; Wu, Y.; Zhang, Y.; Dong, G. Eco-Friendly Polysaccharide Coatings for Antifouling and Drag-Reduction and Potential Application for Marine Devices. Friction 2023, 12, 1–19. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine Paints: The Particular Case of Antifouling Paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Munk, T.; Kane, D.; Yebra, D.M. The Effects of Corrosion and Fouling on the Performance of Ocean-Going Vessels: A Naval Architectural Perspective. In Advances in Marine Antifouling Coatings and Technologies; Woodhead Publishing: Sawston, UK, 2009; pp. 148–176. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, H.; Jiang, R.; Zhou, X. Research Progress of Environmentally Friendly Marine Antifouling Coatings. Polym. Chem. 2021, 12, 3702–3720. [Google Scholar] [CrossRef]
- Finlay, J.A.; Franco, S.C.; Daniels, J.; Hadfield, M.G.; Stafslien, S.J.; Webster, D.C.; Wendt, D.E.; Brewer, L.; Clare, A.S.; Teo, S.L.M. Fouling-Release Performance of Silicone Oil-Modified SiloxanePolyurethane Coatings. ACS Appl. Mater. Interfaces 2016, 8, 29025–29036. [Google Scholar]
- Clare, A.S.; Rittschof, D.; Gerhart, D.J.; Maki, J.S. Molecular Approaches to Nontoxic Antifouling. Invertebr. Reprod. Dev. 1992, 22, 67–76. [Google Scholar] [CrossRef]
- Davidson, I.; Ashton, G.; Ruiz, G.; Scianni, C.; Brown, C.; Pagenkopp-Lohan, K.; Fleischer, R. Richness, Extent, Condition, Reproductive Status, and Parasitism of Fouling Communities on Commercial Vessels; Report to the California State Lands Commission Marine Invasive Species Program; Portland State University: Portland, OR, USA, 2013. [Google Scholar]
- Growcott, A.; Kluza, D.; Georgiades, E.; Science, B. Technical Advice: Evaluation of in-Water Systems to Reactively Treat or Remove Biofouling within Vessel Internal Niche Areas; Ministry for Primary Industries: Wellington, New Zealand, 2019; Volume 4, ISBN 9781988594194.
- Rahman, S. Implementation of Ballast Water Management Plan in Ships through Ballast Water Exchange System. Procedia Eng. 2017, 194, 323–329. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Taylor, M.D. A Preliminary Investigation of Biosecurity Risks Associated with Biofouling on Merchant Vessels in New Zealand. New Zeal. J. Mar. Freshw. Res. 2004, 38, 215–229. [Google Scholar] [CrossRef]
- Frey, M.A.; Simard, N.; Robichaud, D.D.; Martin, J.L.; Therriault, T.W. Fouling around: Vessel Sea-Chests as a Vector for the Introduction and Spread of Aquatic Invasive Species. Manag. Biol. Invasions 2014, 5, 21–30. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Buisman, E.; et al. Costs and Benefits to European Shipping of Ballast-Water and Hull-Fouling Treatment: Impacts of Native and Non-Indigenous Species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Sylvester, F.; MacIsaac, H.J. Is Vessel Hull Fouling an Invasion Threat to the Great Lakes? Divers. Distrib. 2010, 16, 132–143. [Google Scholar] [CrossRef]
- Georgiades, E.; Kluza, D.; Bates, T.; Lubarsky, K.; Brunton, J.; Growcott, A.; Smith, T.; McDonald, S.; Gould, B.; Parker, N.; et al. Regulating Vessel Biofouling to Support New Zealand’s Marine Biosecurity System—A Blue Print for Evidence-Based Decision Making. Front. Mar. Sci. 2020, 7, 390. [Google Scholar] [CrossRef]
- Cacabelos, E.; Ramalhosa, P.; Canning-Clode, J.; Troncoso, J.S.; Olabarria, C.; Delgado, C.; Dobretsov, S.; Gestoso, I. The Role of Biofilms Developed under Different Anthropogenic Pressure on Recruitment of Macro-Invertebrates. Int. J. Mol. Sci. 2020, 21, 2030. [Google Scholar] [CrossRef]
- Grant, K.R. Next-Generation Amplicon Sequencing: A Cost-Effective Method for Exploring Microbial Biodiversity. In Molecular Genetics and Genomics Tools in Biodiversity Conservation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 203–236. [Google Scholar]
- Comeau, A.M.; Lagunas, M.G.; Scarcella, K.; Varela, D.E.; Lovejoy, C. Nitrate Consumers in Arctic Marine Eukaryotic Communities: Comparative Diversities of 18S RRNA, 18S RRNA Genes, and Nitrate Reductase Genes. Appl. Environ. Microbiol. 2019, 85, e00247-19. [Google Scholar] [CrossRef]
- Weber, S.D.; Hofmann, A.; Pilhofer, M.; Wanner, G.; Agerer, R.; Ludwig, W.; Schleifer, K.-H.; Fried, J. The Diversity of Fungi in Aerobic Sewage Granules Assessed by 18S RRNA Gene and ITS Sequence Analyses. FEMS Microbiol. Ecol. 2009, 68, 246–254. [Google Scholar] [CrossRef]
- Chen, C.L.; Maki, J.S.; Rittschof, D.; Teo, S.L.M. Early Marine Bacterial Biofilm on a Copper-Based Antifouling Paint. Int. Biodeterior. Biodegrad. 2013, 83, 71–76. [Google Scholar] [CrossRef]
- de Vries, H.J.; Kleibusch, E.; Hermes, G.D.A.; van den Brink, P.; Plugge, C.M. Biofouling Control: The Impact of Biofilm Dispersal and Membrane Flushing. Water Res. 2021, 198, 117163. [Google Scholar] [CrossRef]
- Angelova, A.G.; Ellis, G.A.; Wijesekera, H.W.; Vora, G.J. Microbial Composition and Variability of Natural Marine Planktonic and Biofouling Communities from the Bay of Bengal. Front. Microbiol. 2019, 10, 2738. [Google Scholar] [CrossRef]
- Takada, K.; Shiba, T.; Yamaguchi, T.; Akane, Y.; Nakayama, Y.; Soda, S.; Inoue, D.; Ike, M. Cake Layer Bacterial Communities during Different Biofouling Stages in Full-Scale Membrane Bioreactors. Bioresour. Technol. 2018, 259, 259–267. [Google Scholar] [CrossRef]
- Von Ammon, U.; Wood, S.A.; Laroche, O.; Zaiko, A.; Tait, L.; Lavery, S.; Inglis, G.J.; Pochon, X. Combining Morpho-Taxonomy and Metabarcoding Enhances the Detection of Non-Indigenous Marine Pests in Biofouling Communities. Sci. Rep. 2018, 8, 16290. [Google Scholar] [CrossRef]
- Chiellini, C.; Iannelli, R.; Modeo, L.; Bianchi, V.; Petroni, G. Biofouling of Reverse Osmosis Membranes Used in River Water Purification for Drinking Purposes: Analysis of Microbial Populations. Biofouling 2012, 28, 969–984. [Google Scholar] [CrossRef]
- Chessa, D.; Murgia, M.; Sias, E.; Deligios, M.; Mazzarello, V.; Fiamma, M.; Rovina, D.; Carenti, G.; Ganau, G.; Pintore, E. Metagenomics and Microscope Revealed T. Trichiura and Other Intestinal Parasites in a Cesspit of an Italian Nineteenth Century Aristocratic Palace. Sci. Rep. 2020, 10, 12656. [Google Scholar] [CrossRef]
- Satjarak, A.; Graham, L.E.; Piotrowski, M.J.; Trest, M.T.; Wilcox, L.W.; Cook, M.E.; Knack, J.J.; Arancibia-Avila, P. Shotgun Metagenomics and Microscopy Indicate Diverse Cyanophytes, Other Bacteria, and Microeukaryotes in the Epimicrobiota of a Northern Chilean Wetland Nostoc (Cyanobacteria). J. Phycol. 2021, 57, 39–50. [Google Scholar] [CrossRef]
- Cheng, N.; Moe, P. Inactivation of Enveloped Viruses (Coronavirus, H5N1 Virus) and Disinfection of the Air with Legionella-X100 via Ultraviolet Germicidal Irradiation (UVGI); Academia.Edu: San Francisco, CA, USA, 2020; pp. 1–18. [Google Scholar]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Sarathy, S.R.; Mohseni, M. An Overview of UV-Based Advanced Oxidation Processes for Drinking Water Treatment. IUVA News 2006, 8, 16–27. [Google Scholar]
- Bukhari, Z.; Hargy, T.M.; Bolton, J.R.; Dussert, B.; Clancy, J.L. Medium-pressure UV for Oocyst Inactivation. J. Am. Water Work. Assoc. 1999, 91, 86–94. [Google Scholar] [CrossRef]
- Craik, S.A.; Weldon, D.; Finch, G.R.; Bolton, J.R.; Belosevic, M. Inactivation of Cryptosporidium Parvum Oocysts Using Medium-and Low-Pressure Ultraviolet Radiation. Water Res. 2001, 35, 1387–1398. [Google Scholar] [CrossRef]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C Irradiation: An Alternative Antimicrobial Approach to Localized Infections? Expert. Rev. Anti. Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef]
- Braga, C.; Hunsucker, K.; Erdogan, C.; Gardner, H.; Swain, G. The Use of a Uvc Lamp Incorporated with an ROV to Prevent Biofouling: A Proof-of-Concept Study. Mar. Technol. Soc. J. 2020, 54, 76–83. [Google Scholar] [CrossRef]
- Hunsucker, K.Z.; Braga, C.; Gardner, H.; Jongerius, M.; Hietbrink, R.; Salters, B.; Swain, G. Using Ultraviolet Light for Improved Antifouling Performance on Ship Hull Coatings. Biofouling 2019, 35, 658–668. [Google Scholar] [CrossRef]
- Richard, K.N.; Hunsucker, K.Z.; Gardner, H.; Hickman, K.; Swain, G. The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling. J. Mar. Sci. Eng. 2021, 9, 662. [Google Scholar] [CrossRef]
- Dobretsov, S.V.; Gosselin, L.; Qian, P.Y. Effects of Solar PAR and UV Radiation on Tropical Biofouling Communities. Mar. Ecol. Prog. Ser. 2010, 402, 31–43. [Google Scholar] [CrossRef]
- Whitworth, P.; Aldred, N.; Reynolds, K.J.; Plummer, J.; Duke, P.; Clare, A.S. Importance of Duration, Duty-Cycling and Thresholds for the Implementation of Ultraviolet C in Marine Biofouling Control. Front. Mar. Sci. 2022, 8, 809011. [Google Scholar] [CrossRef]
- Lakretz, A.; Ron, E.Z.; Mamane, H. Biofouling Control in Water by Various UVC Wavelengths and Doses. Biofouling 2010, 26, 257–267. [Google Scholar] [CrossRef]
- Pinnacle, F. Foul Free for over Two Years-Presentation of Results from AML Oceanographic UV•Xchange Biofouling Control Experiment at Folger Pinnacle. In Proceedings of the OCEANS 2016 MTS/IEEE Monterey, Monterey, CA, USA, 19–23 September 2016; pp. 19–23. [Google Scholar]
- Piola, R.; Salters, B.; Grandison, C.; Ciacic, M.; Hietbrink, R. Assessing the Use of Low Voltage UV-Light Emitting Miniature LEDs for Marine Biofouling Control; DST-Group-TR-3266; Australian Government Department of Defense: Canberra, Australia, 2016; pp. 2–28.
- Bueley, C. UV Irradiation: A New Kind of Antifoulant. Ocean News & Technology, 4 March 2014. [Google Scholar]
- Bueley, C.; Olender, D.; Brocking, B. The (in)Fluence of Light. J. Ocean. Technol. 2014, 9, 48–67. [Google Scholar]
- MacKenzie, A.F.; Maltby, E.A.; Harper, N.; Bueley, C.; Olender, D.; Wyeth, R.C. Periodic Ultraviolet-C Illumination for Marine Sensor Antifouling. Biofouling 2019, 35, 483–493. [Google Scholar] [CrossRef]
- Phukan, T.; Rai, A.N.; Syiem, M.B. Dose Dependent Variance in UV-C Radiation Induced Effects on Carbon and Nitrogen Metabolism in the Cyanobacterium Nostoc muscorum Meg1. Ecotoxicol. Environ. Saf. 2018, 155, 171–179. [Google Scholar] [CrossRef]
- Meunier, S.M.; Todorovic, B.; Dare, E.V.; Begum, A.; Guillemette, S.; Wenger, A.; Saxena, P.; Campbell, J.L.; Sasges, M.; Aucoin, M.G. Impact of Dissolved Oxygen during UV-Irradiation on the Chemical Composition and Function of CHO Cell Culture Media. PLoS ONE 2016, 11, e0150957. [Google Scholar] [CrossRef]
- Ishii, H.; Ohba, T.; Kobayashi, T. Effects of Low Dissolved Oxygen on Planula Settlement, Polyp Growth and Asexual Reproduction of Aurelia aurita. Plankt. Benthos Res. 2008, 3, 107–113. [Google Scholar] [CrossRef]
- Braga, C.R. Lighting a Path to Biofouling Prevention: Investigating the Effect of Ultraviolet Light on Biofouling. Photosynthetica 2018, 2, 1–13. [Google Scholar]
- Crimaldi, J.P.; Thompson, J.K.; Rosman, J.H.; Lowe, R.J.; Koseff, J.R. Hydrodynamics of Larval Settlement: The Influence of Turbulent Stress Events at Potential Recruitment Sites. Limnol. Oceanogr. 2002, 47, 1137–1151. [Google Scholar] [CrossRef]
- Dobretsov, S. Expected Effect of Climate Change on Fouling Communities and Its Impact on Antifouling Research. In Advances in Marine Antifouling Coatings and Technologies; Woodhead Publishing: Sawston, UK, 2009; pp. 222–239. [Google Scholar] [CrossRef]
- Raikow, D.F.; Reid, D.F.; Blatchley, E.R.; Jacobs, G.; Landrum, P.F. Effects of Proposed Physical Ballast Tank Treatments on Aquatic Invertebrate Resting Eggs. Environ. Toxicol. Chem. 2007, 26, 717–725. [Google Scholar] [CrossRef]
- Hung, O.S.; Thiyagarajan, V.; Wu, R.S.S.; Qian, P.Y. Effect of Ultraviolet Radiation on Biofilms and Subsequent Larval Settlement of Hydroides Elegans. Mar. Ecol. Prog. Ser. 2005, 304, 155–166. [Google Scholar] [CrossRef]
- Montero, C.I.; Johnson, M.R.; Chou, C.-J.; Conners, S.B.; Geouge, S.G.; Tachdjian, S.; Nichols, J.D.; Kelly, R.M. Responses of Wild-Type and Resistant Strains of the Hyperthermophilic Bacterium Thermotoga maritima to Chloramphenicol Challenge. Appl. Environ. Microbiol. 2007, 73, 5058–5065. [Google Scholar] [CrossRef]
- Habeebu, S.S.; Liu, J.; Liu, Y.; Klaassen, C.D. Metallothionein-Null Mice Are More Susceptible than Wild-Type Mice to Chronic CdCl2-Induced Bone Injury. Toxicol. Sci. 2000, 56, 211–219. [Google Scholar] [CrossRef][Green Version]
- Huesemann, M.H.; Hausmann, T.S.; Bartha, R.; Aksoy, M.; Weissman, J.C.; Benemann, J.R. Biomass Productivities in Wild Type and Pigment Mutant of Cyclotella sp.(Diatom). Appl. Biochem. Biotechnol. 2009, 157, 507–526. [Google Scholar] [CrossRef]
- Bonhivers, M.; Carbrey, J.M.; Gould, S.J.; Agre, P. Aquaporins in Saccharomyces: Genetic and Functional Distinctions between Laboratory and Wild-Type Strains. J. Biol. Chem. 1998, 273, 27565–27572. [Google Scholar] [CrossRef]
- Kozich, J.; Schloss, P.; Baxter, N.; Jenior, M.; Koumpouras, C.; Bishop, L. 16S RRNA Sequencing with the Illumina MiSeq: Library Generation, QC, & Sequencing; Novogene: Guangzhou, China, 2013. [Google Scholar]
- Amaral-Zettler, L.A.; Bauer, M.; Berg-Lyons, D.; Betley, J.; Caporaso, J.G.; Ducklow, W.; Walters, W.A. EMP 18S Illumina Amplicon Protocol. Protocols.io. 2018. Available online: https://www.protocols.io/view/emp-18s-illumina-amplicon-protocol-ewov1b6pgr24/v1?version_warning=no (accessed on 7 September 2021).
- Langenkämper, D.; Zurowietz, M.; Schoening, T.; Nattkemper, T.W. BIIGLE 2.0—Browsing and Annotating Large Marine Image Collections. Front. Mar. Sci. 2017, 4, 83. [Google Scholar] [CrossRef]
- Wickham, H.; Navarro, D.; Pedersen, T.L. Elegant Graphics for Data Analysis (Ggplot2); Springer: New York, NY, USA, 2016. [Google Scholar]
- Rivero, N.K.; Dafforn, K.A.; Coleman, M.A.; Johnston, E.L. Environmental and Ecological Changes Associated with a Marina. Biofouling 2013, 29, 803–815. [Google Scholar] [CrossRef]
- Floerl, O.; Pool, T.K.; Inglis, G.J. Positive Interactions between Nonindigenous Species Facilitate Transport by Human Vectors. Ecol. Appl. 2004, 14, 1724–1736. [Google Scholar] [CrossRef]
- Mineur, F.; Cook, E.J.; Minchin, D.; Bohn, K.; MacLeod, A.; Maggs, C.A. Changing Coasts: Marine Aliens and Artifcial Structures. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2012; pp. 198–243. ISBN 0429109385. [Google Scholar]
- Tait, L.; Inglis, G.; Seaward, K.; Spong, K.; Wilkens, S. Optimising Settlement Arrays for Surveillance of Non-Indigenous Biofouling Species; MPI Information—Paper No: 2016/71; Ministry for Primary Industries: Wellington, New Zealand, 2016; p. 74.
- Pierri, C.; Colangelo, P.; Del Pasqua, M.; Longo, C.; Giangrande, A. Consequences of the Experimental Removal of Sabella Spallanzanii (Gmelin, 1791) from the Fouling Assemblage of a Mediterranean Harbour. Mediterr. Mar. Sci. 2019, 20, 476–486. [Google Scholar] [CrossRef]
- Trani, R.; Corriero, G.; de Pinto, M.C.; Mercurio, M.; Pazzani, C.; Pierri, C.; Scrascia, M.; Longo, C. Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. J. Mar. Sci. Eng. 2021, 9, 178. [Google Scholar] [CrossRef]
- Charles, F.; Grémare, A.; Amouroux, J.-M.; Cahet, G. Filtration of the Enteric Bacteria Escherichia Coli by Two Filter-Feeding Bivalves, Venus verrucosa and Mytilus galloprovincialis. Mar. Biol. 1992, 113, 117–124. [Google Scholar] [CrossRef]
- Morton, B. The Biology and Functional Morphology of Mytilopsis sallei (Recluz)(Bivalvia: Dreissenacea) Fouling Visakhapatnam Harbour, Andhra Pradesh, India. J. Molluscan Stud. 1981, 47, 25–42. [Google Scholar] [CrossRef]
- Nair, P.S.R.; Krishnamurthy, K.; Mawatari, S.F. Salinity Tolerance in Four Estuarine Species of Bryozoa. Mar. Fouling 1992, 9, 15–20. [Google Scholar] [CrossRef][Green Version]
- Mann, R.; Harding, J.M. Salinity Tolerance of Larval Rapana venosa: Implications for Dispersal and Establishment of an Invading Predatory Gastropod on the North American Atlantic Coast. Biol. Bull. 2003, 204, 96–103. [Google Scholar] [CrossRef]
- Astudillo, J.C.; Bonebrake, T.C.; Leung, K.M.Y. The Recently Introduced Bivalve Xenostrobus securis Has Higher Thermal and Salinity Tolerance than the Native Brachidontes variabilis and Established Mytilopsis sallei. Mar. Pollut. Bull. 2017, 118, 229–236. [Google Scholar] [CrossRef]
- Lowe, A.J. Microcosmus Sqamiger, a Solitary Ascidian Introduced to Southern California Harbors and Marinas: Salinity Tolerance and Phylogenetic Analysis; California State University: Fullerton, CA, USA, 2002. [Google Scholar]
- Lesser, M.P. Oxidative Stress Causes Coral Bleaching during Exposure to Elevated Temperatures. Coral Reefs 1997, 16, 187–192. [Google Scholar] [CrossRef]
- Garcia-Corral, L.S.; Martinez-Ayala, J.; Duarte, C.M.; Agusti, S. Experimental Assessment of Cumulative Temperature and UV-B Radiation Effects on Mediterranean Plankton Metabolism. Front. Mar. Sci. 2015, 2, 48. [Google Scholar] [CrossRef][Green Version]
- Icoglu Aksakal, F.; Ciltas, A. The Impact of Ultraviolet B (UV-B) Radiation in Combination with Different Temperatures in the Early Life Stage of Zebrafish (Danio rerio). Photochem. Photobiol. Sci. 2018, 17, 35–41. [Google Scholar] [CrossRef]
- Clarke, A.; Fraser, K.P.P. Why Does Metabolism Scale with Temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef]
- Vernberg, W.B.; Vernberg, F.J. The Synergistic Effects of Temperature, Salinity, and Mercury on Survival and Metabolism of the Adult Fiddler Crab, Uca pugilator. Fish. Bull. 1972, 70, 415–420. [Google Scholar]
- Wang, J.; Rong, H.; Zhang, C. Evaluation of the Impact of Dissolved Oxygen Concentration on Biofilm Microbial Community in Sequencing Batch Biofilm Reactor. J. Biosci. Bioeng. 2018, 125, 532–542. [Google Scholar] [CrossRef]
- Skolimowski, M.; Nielsen, M.W.; Emnéus, J.; Molin, S.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic Dissolved Oxygen Gradient Generator Biochip as a Useful Tool in Bacterial Biofilm Studies. Lab. Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef]
- Mullineaux, L.S.; Butman, C.A. Initial Contact, Exploration and Attachment of Barnacle (Balanus amphitrite) Cyprids Settling in Flow. Mar. Biol. 1991, 110, 93–103. [Google Scholar] [CrossRef]
- Jonsson, P.R.; Berntsson, K.M.; Larsson, A.I. Linking Larval Supply to Recruitment: Flow-mediated Control of Initial Adhesion of Barnacle Larvae. Ecology 2004, 85, 2850–2859. [Google Scholar] [CrossRef]
- Larsson, A.I.; Jonsson, P.R. Barnacle Larvae Actively Select Flow Environments Supporting Post-settlement Growth and Survival. Ecology 2006, 87, 1960–1966. [Google Scholar] [CrossRef]
- Eckman, J.E.; Savidge, W.B.; Gross, T.F. Relationship between Duration of Cyprid Attachment and Drag Forces Associated with Detachment of Balanus amphitrite Cyprids. Mar. Biol. 1990, 107, 111–118. [Google Scholar] [CrossRef]
- Abelson, A.; Denny, M. Settlement of Marine Organisms in Flow. Annu. Rev. Ecol. Syst. 1997, 28, 317–339. [Google Scholar] [CrossRef]
- Walters, L.J.; Miron, G.; Bourget, E. Endoscopic Observations of Invertebrate Larval Substratum Exploration and Settlement. Mar. Ecol. Prog. Ser. 1999, 182, 95–108. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Bourget, E. Settlement Success, Spatial Pattern and Behavior of Mussel Larvae Mytilus spp. in Experimental’downwelling’systems of Varying Velocity and Turbulence. Mar. Ecol. Prog. Ser. 2003, 260, 125–140. [Google Scholar] [CrossRef]
- Lindegarth, M.; Jonsson, P.R.; André, C. Physical and Numerical Modeling of the Role of Hydrodynamic Processes on Adult-Larval Interactions of a Suspension-Feeding Bivalve. J. Mar. Res. 2002, 60, 499–516. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, L.; Deng, Y.; Zhi, X.; Jiang, Y.-H.; Tu, Q.; Xie, J.; Van Nostrand, J.D.; He, Z.; Yang, Y. Reproducibility and Quantitation of Amplicon Sequencing-Based Detection. ISME J. 2011, 5, 1303–1313. [Google Scholar] [CrossRef]
- Gregory, T.R. Coincidence, Coevolution, or Causation? DNA Content, Cell Size, and the C-Value Enigma. Biol. Rev. 2001, 76, 65–101. [Google Scholar] [CrossRef]
- Nocker, A.; Cheung, C.-Y.; Camper, A.K. Comparison of Propidium Monoazide with Ethidium Monoazide for Differentiation of Live vs. Dead Bacteria by Selective Removal of DNA from Dead Cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef]
- Andruszkiewicz Allan, E.; Zhang, W.G.; C Lavery, A.; F Govindarajan, A. Environmental DNA Shedding and Decay Rates from Diverse Animal Forms and Thermal Regimes. Environ. DNA 2021, 3, 492–514. [Google Scholar] [CrossRef]
- Shick, W.; Dunlap, J.M. Mycosporine-like Amino Acids and Related Gradusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Hirose, E.; Ohtsuka, K.; Ishikura, M.; Maruyama, T. Ultraviolet Absorption in Ascidian Tunic and Ascidian-Prochloron Symbiosis. J. Mar. Biol. Assoc. UK 2004, 84, 789–794. [Google Scholar] [CrossRef]
- Miller, M.A.; Gardner, I.A.; Kreuder, C.; Paradies, D.M.; Worcester, K.R.; Jessup, D.A.; Dodd, E.; Harris, M.D.; Ames, J.A.; Packham, A.E. Coastal Freshwater Runoff Is a Risk Factor for Toxoplasma gondii Infection of Southern Sea Otters (Enhydra lutris nereis). Int. J. Parasitol. 2002, 32, 997–1006. [Google Scholar] [CrossRef]
- Bellora, N.; Moliné, M.; David-Palma, M.; Coelho, M.A.; Hittinger, C.T.; Sampaio, J.P.; Gonçalves, P.; Libkind, D. Comparative Genomics Provides New Insights into the Diversity, Physiology, and Sexuality of the Only Industrially Exploited Tremellomycete: Phaffia rhodozyma. BMC Genom. 2016, 17, 901. [Google Scholar] [CrossRef]
- Chang, C.F.; Lee, C.F.; Liu, S.M. Cystobasidium keelungensis sp. Nov., a Novel Mycosporine Producing Carotenogenic Yeast Isolated from the Sea Surface Microlayer in Taiwan. Arch. Microbiol. 2019, 201, 27–33. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassan, M.H.A.; Alhadrami, H.A.; Hassan, H.M.; Goodfellow, M.; Rateb, M.E. Extreme Environments: Microbiology Leading to Specialized Metabolites. J. Appl. Microbiol. 2020, 128, 630–657. [Google Scholar] [CrossRef]
- Thomas, A. Microorganisms in Sea Ice Melt Pools as a Source of Ultraviolet Radiation Absorbing Metabolites. Master’s Thesis, University of Prince Edward Island, Charlottetown, PE, Canada, 2016. [Google Scholar]
- Jebaraj, C.S.; Forster, D.; Kauff, F.; Stoeck, T. Molecular Diversity of Fungi from Marine Oxygen-Deficient Environments (ODEs). In Biology of Marine Fungi; Springer: Berlin/Heidelberg, Germany, 2012; pp. 189–208. [Google Scholar]
- Lobban, C.S.; Hallam, S.J.; Mukherjee, P.; Petrich, J.W. Photophysics and Multifunctionality of Hypericin-like Pigments in Heterotrich Ciliates: A Phylogenetic Perspective. Photochem. Photobiol. 2007, 83, 1074–1094. [Google Scholar] [CrossRef]
- Buonanno, F.; Saltalamacchia, P.; Miyake, A. Defence Function of Pigmentocysts in the Karyorelictid Ciliate Loxodes striatus. Eur. J. Protistol. 2005, 41, 151–158. [Google Scholar] [CrossRef]
- Miyake, A.; Harumoto, T.; Salvi, B.; Rivola, V. Defensive Function of Pigment Granules in Blepharisma japonicum. Eur. J. Protistol. 1990, 25, 310–315. [Google Scholar] [CrossRef]
- Ellegaard, M.; Lenau, T.; Lundholm, N.; Maibohm, C.; Friis, S.M.M.; Rottwitt, K.; Su, Y. The Fascinating Diatom Frustule—Can It Play a Role for Attenuation of UV Radiation? J. Appl. Phycol. 2016, 28, 3295–3306. [Google Scholar] [CrossRef]
- Dahms, H.-U.; Dobretsov, S.; Qian, P.-Y. The Effect of Bacterial and Diatom Biofilms on the Settlement of the Bryozoan Bugula neritina. J. Exp. Mar. Bio. Ecol. 2004, 313, 191–209. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Krohne, G.; Gerdts, G. Mature Biofilm Communities on Synthetic Polymers in Seawater-Specific or General? Mar. Environ. Res. 2018, 142, 147–154. [Google Scholar] [CrossRef]
- Seed, R.; Richardson, C.A. Evolutionary Traits in Perna viridis (Linnaeus) and Septifer virgatus (Wiegmann) (Bivalvia: Mytilidae). J. Exp. Mar. Bio. Ecol. 1999, 239, 273–287. [Google Scholar] [CrossRef]
- Karentz, D. Chemical Defenses of Marine Organisms against Solar Radiation Exposure: UV-Absorbing Mycosporine-like Amino Acids and Scytonemim. In Marine Chemical Ecololgy; CRC Press: Boca Raton, FL, USA, 2001; pp. 481–520. [Google Scholar]
- Shick, J.M.; Dunlap, W.C.; Pearse, J.S.; Pearse, V.B. Mycosporine-like Amino Acid Content in Four Species of Sea Anemones in the Genus Anthopleura Reflects Phylogenetic but Not Environmental or Symbiotic Relationships. Biol. Bull. 2002, 203, 315–330. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Pitt, K.A.; Gillanders, B.M. Management of Jellyfish Fisheries, with Special Reference to the Order Rhizostomeae. Oceanogr. Mar. Biol. Annu. Rev. 2000, 38, 85–156. [Google Scholar]
- Pitt, K.A.; Kingsford, M.J. Reproductive Biology of the Edible Jellyfish Catostylus mosaicus (Rhizostomeae). Mar. Biol. 2000, 137, 791–799. [Google Scholar] [CrossRef]
- Klein, S.G.; Pitt, K.A.; Carroll, A.R. Surviving but Not Thriving: Inconsistent Responses of Zooxanthellate Jellyfish Polyps to Ocean Warming and Future UV-B Scenarios. Sci. Rep. 2016, 6, 28859. [Google Scholar] [CrossRef]
- Carroll, A.K.; Shick, J.M. Dietary Accumulation of UV-Absorbing Mycosporine-like Amino Acids (MAAs) by the Green Sea Urchin (Strongylocentrotus droebachiensis). Mar. Biol. 1996, 124, 561–569. [Google Scholar] [CrossRef]
- Isomura, N.; Nishihira, M. Size Variation of Planulae and Its Effect on the Lifetime of Planulae in Three Pocilloporid Corals. Coral Reefs 2001, 20, 309–315. [Google Scholar] [CrossRef]
- Tiemeyer, M.; Selleck, S.B.; Esko, J.D. Arthropoda. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- van den Broecke, L.; Martens, K.; Pieri, V.; Schön, I. Ostracod Valves as Efficient UV Protection. J. Limnol. 2012, 71, 119–124. [Google Scholar] [CrossRef]
- Smith, R.J.; Facts, A. The Ostracod Carapace. Available online: https://www.biwahaku.jp/smith/ostracod_carapace.html (accessed on 27 July 2022).
- Barnard, J.L.; Thomas, J.D.; Sandved, K.B. Behavior of Gammaridean Amphipoda: Corophium, Grandidierella, Podocerus, and Gibberosus (American Megaluropus) in Florida. Crustac. Suppl. 1988, 13, 234–244. [Google Scholar]
- Poore, A.G.B.; Steinberg, P.D. Preference–Performance Relationships and Effects of Host Plant Choice in an Herbivorous Marine Amphipod. Ecol. Monogr. 1999, 69, 443–464. [Google Scholar]
- Barnard, J.L.; Sandved, K.; Thomas, J.D. Tube-Building Behavior in Grandidierella, and Two Species of Cerapus. Hydrobiologia 1991, 223, 239–254. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Fernandez-Gonzalez, V.; Sanchez-Jerez, P.; Roura, A. Epibiontic Associations between Apostomid Ciliates Conidophrys Spp. and Amphipods Associated with Fish Farms Fouling in the Western Mediterranean Sea. Helgol. Mar. Res. 2016, 70, 12. [Google Scholar] [CrossRef][Green Version]
- Song, K.; Mohseni, M.; Taghipour, F. Application of Ultraviolet Light-Emitting Diodes (UV-LEDs) for Water Disinfection: A Review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Song, L.; Li, W.; He, J.; Li, L.; Li, T.; Gu, D.; Tang, H. Development of a Pulsed Xenon Ultraviolet Disinfection Device for Real-Time Air Disinfection in Ambulances. J. Healthc. Eng. 2020, 2020, 6053065. [Google Scholar] [CrossRef]
- Tonzani, S. Time to Change the Bulb. Nature 2009, 459, 312. [Google Scholar] [CrossRef]
- Vansteenbrugge, L.; Ampe, B.; De Troch, M.; Vincx, M.; Hostens, K. On the Distribution and Population Dynamics of the Ctenophore Mnemiopsis leidyi in the Belgian Part of the North Sea and Westerschelde Estuary. Mar. Environ. Res. 2015, 110, 33–44. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitworth, P.; Clare, A.S.; Finlay, J.A.; Piola, R.F.; Plummer, J.; Aldred, N. Long-Term Ultraviolet Treatment for Macrofouling Control in Northern and Southern Hemispheres. J. Mar. Sci. Eng. 2023, 11, 2211. https://doi.org/10.3390/jmse11122211
Whitworth P, Clare AS, Finlay JA, Piola RF, Plummer J, Aldred N. Long-Term Ultraviolet Treatment for Macrofouling Control in Northern and Southern Hemispheres. Journal of Marine Science and Engineering. 2023; 11(12):2211. https://doi.org/10.3390/jmse11122211
Chicago/Turabian StyleWhitworth, Paul, Anthony S. Clare, John A. Finlay, Richard F. Piola, Joseph Plummer, and Nick Aldred. 2023. "Long-Term Ultraviolet Treatment for Macrofouling Control in Northern and Southern Hemispheres" Journal of Marine Science and Engineering 11, no. 12: 2211. https://doi.org/10.3390/jmse11122211
APA StyleWhitworth, P., Clare, A. S., Finlay, J. A., Piola, R. F., Plummer, J., & Aldred, N. (2023). Long-Term Ultraviolet Treatment for Macrofouling Control in Northern and Southern Hemispheres. Journal of Marine Science and Engineering, 11(12), 2211. https://doi.org/10.3390/jmse11122211







